Copper-Based Silica Nanotubes as Novel Catalysts for the Total Oxidation of Toluene
Abstract
:1. Introduction
2. Results and Discussion
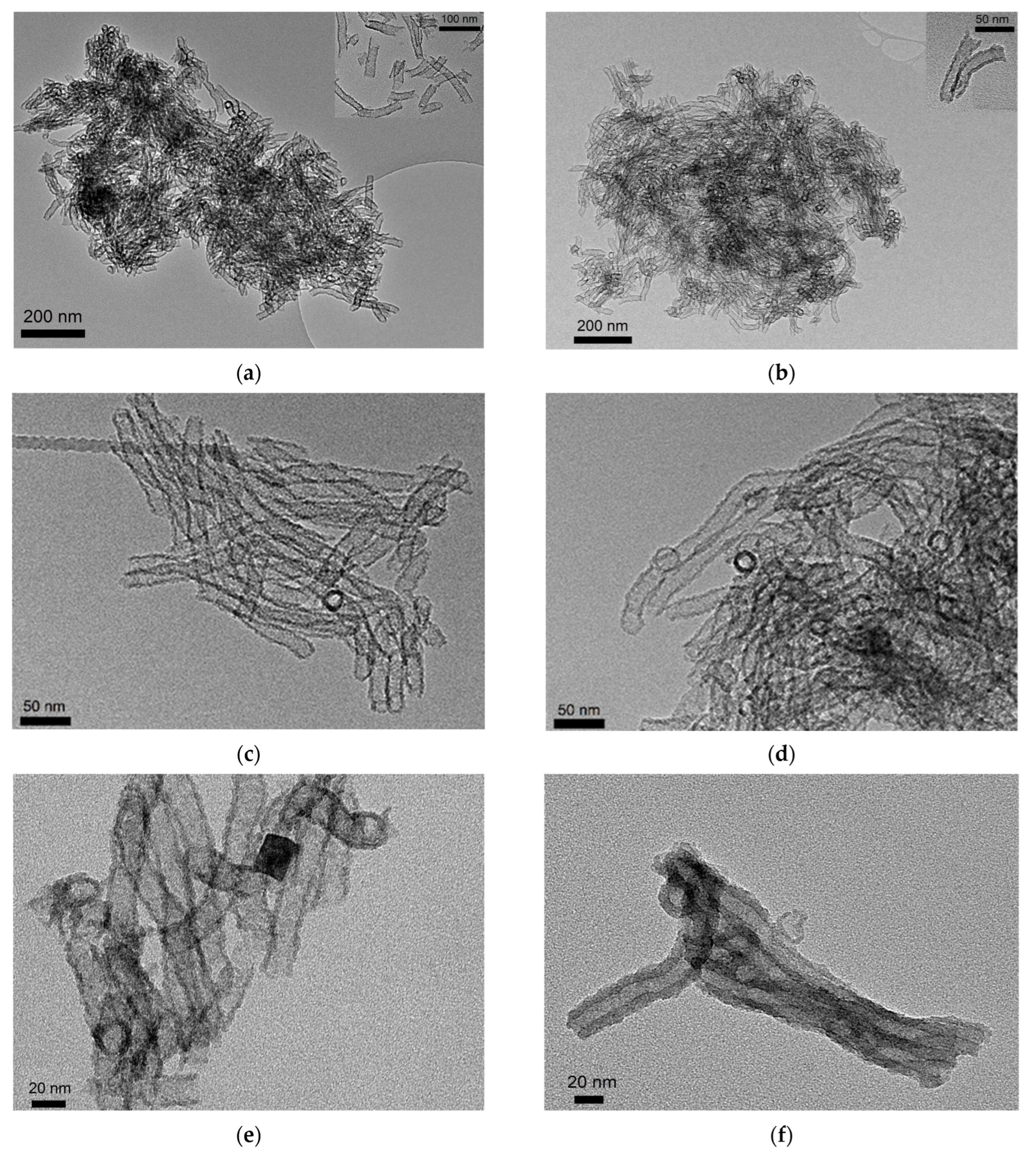
2.1. TEM
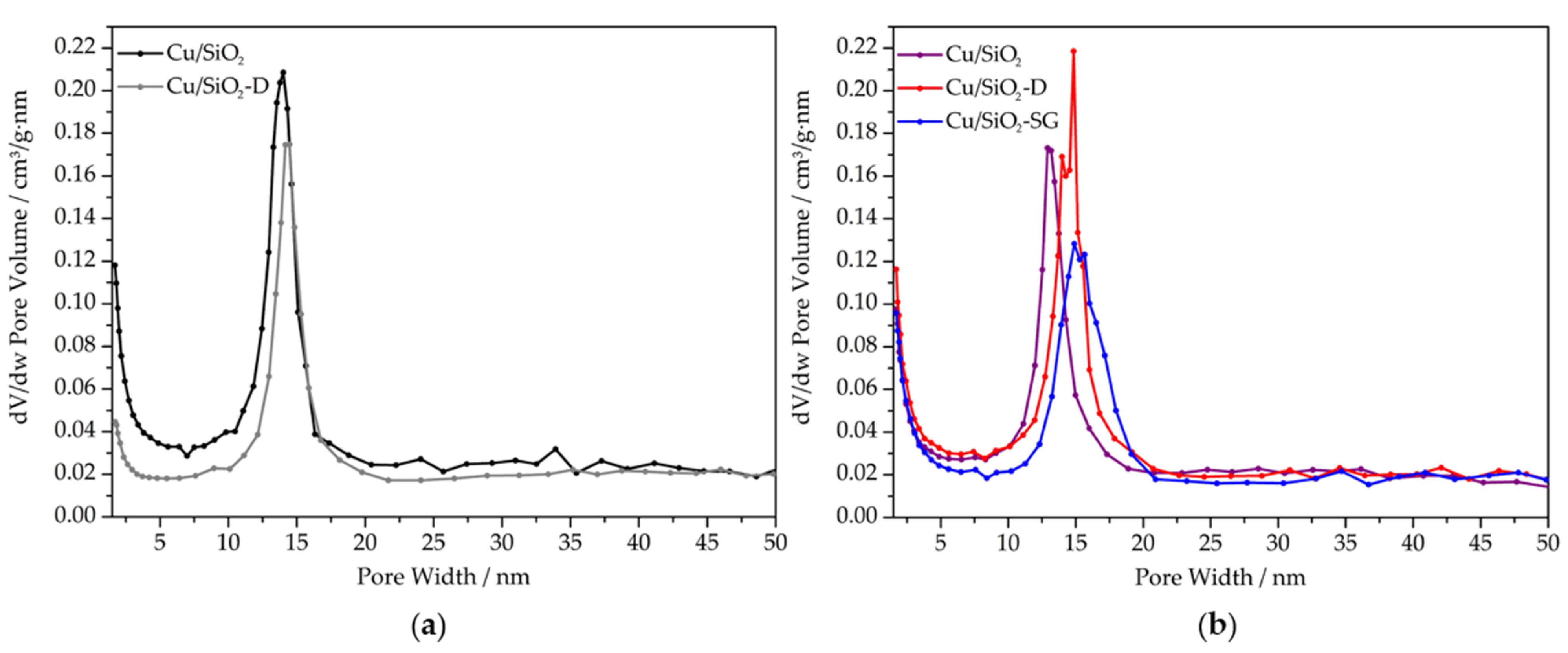
2.2. N2 Physisorption
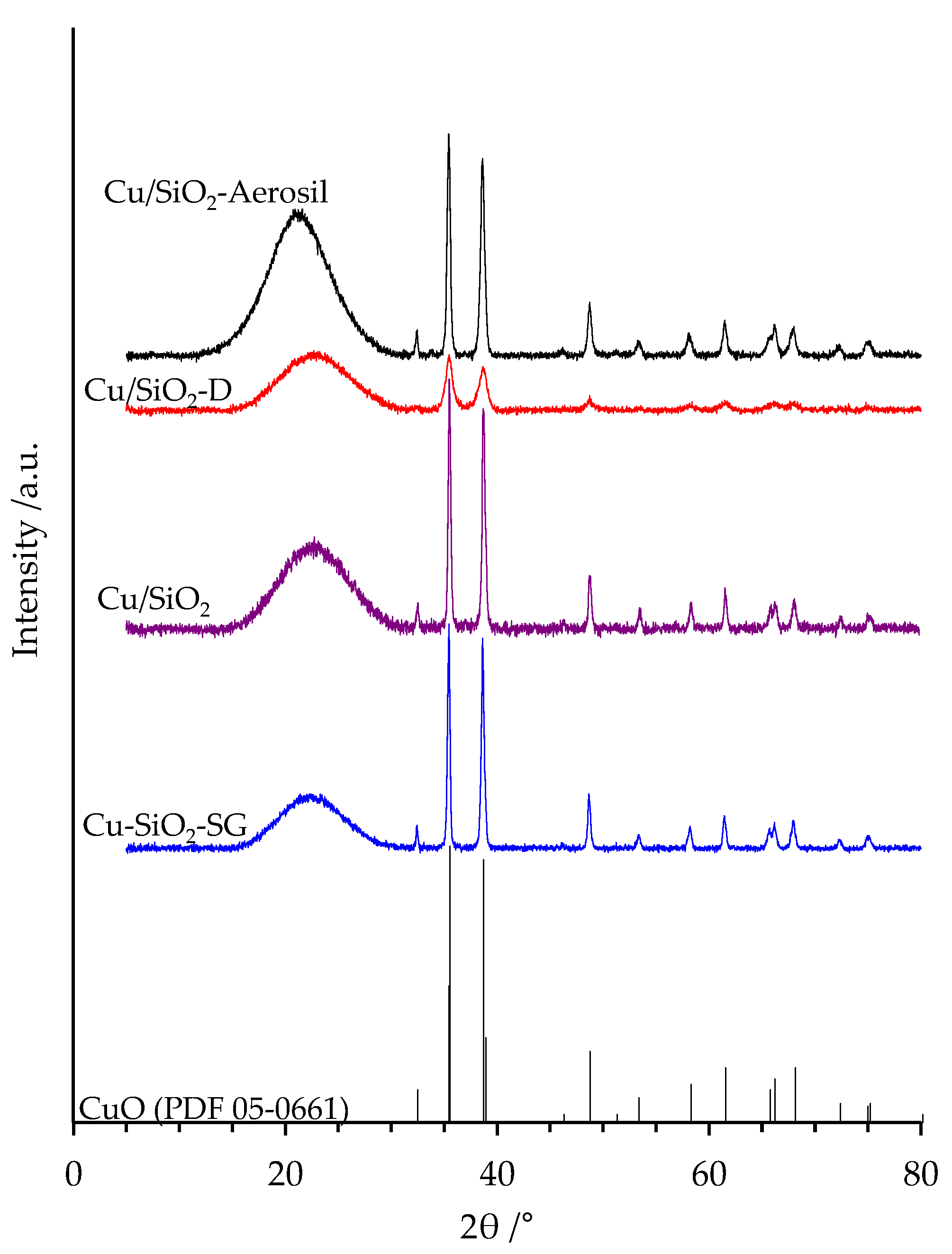
2.3. XRD
2.4. Raman
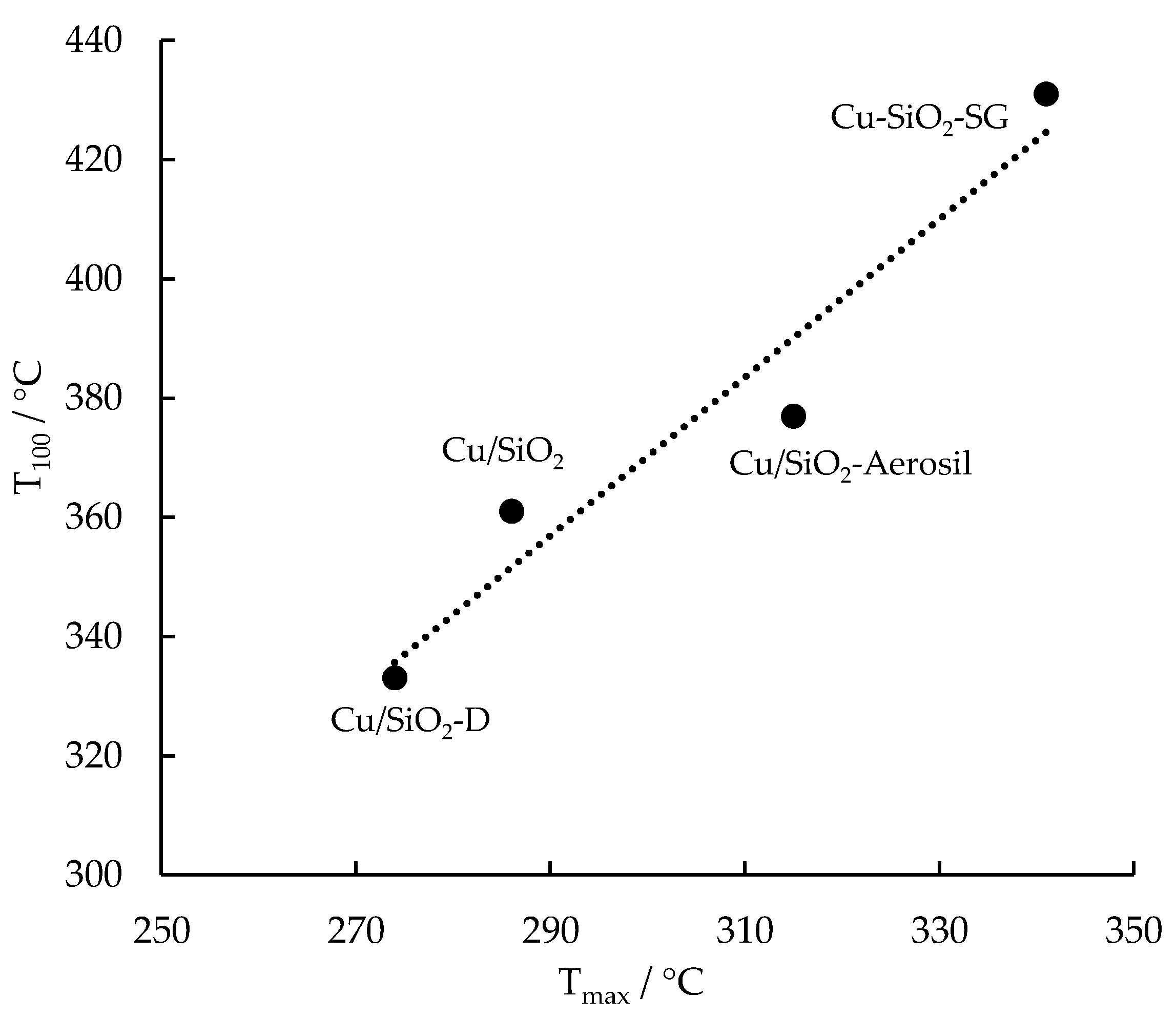
2.5. H2-TPR
2.6. XPS
2.7. Catalytic Test
3. Materials and Methods
3.1. Synthesis of Copper Silica-Based Materials
3.2. Characterization of Copper Silica-Based Materials
3.3. Catalytic Performance Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Zulkifli, M.F.H.; Hawari, N.S.S.L.; Latif, M.T.; Hamid, H.H.A.; Mohtar, A.A.A.; Idris, W.M.R.W.; Mustaffa, N.I.H.; Juneng, L. Volatile organic compounds and their contribution to ground-level ozone formation in a tropical urban environment. Chemosphere 2022, 302, 134852. [Google Scholar] [CrossRef]
- Sutradhar, M.; Marques, G.; Soliman, M.M.; da Silva, M.C.G.; Flores, D.S.; Granadeiro, C.M.; Balula, S.S.; Pombeiro, A.J.; Alegria, E.C. Vanadium(V) complexes supported on porous MIL-100(Fe) as catalysts for the selective oxidation of toluene. Microporous Mesoporous Mater. 2022, 341, 112091. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Rokicińska, A.; Natkański, P.; Michalik, M.; Kuśtrowski, P. Novel CuO-containing catalysts based on ZrO2 hollow spheres for total oxidation of toluene. Microporous Mesoporous Mater. 2019, 279, 446–455. [Google Scholar] [CrossRef]
- Zeng, Y.; Haw, K.-G.; Wang, Z.; Wang, Y.; Zhang, S.; Hongmanorom, P.; Zhong, Q.; Kawi, S. Double redox process to synthesize CuO–CeO2 catalysts with strong Cu–Ce interaction for efficient toluene oxidation. J. Hazard. Mater. 2021, 404, 124088. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Q.; Song, C.; Ji, N.; Ma, D.; Lu, X. Enhanced catalytic performance for VOCs oxidation on the CoAlO oxides by KMnO4 doped on facile synthesis. Chemosphere 2019, 218, 895–906. [Google Scholar] [CrossRef]
- Liotta, L.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marcì, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4–CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-R.; Zhang, W.-P.; Li, C.; Xiao, H.; He, C. Insight into the catalytic performance and reaction routes for toluene total oxidation over facilely prepared Mn-Cu bimetallic oxide catalysts. Appl. Surf. Sci. 2021, 550, 149179. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Garcia-Vargas, J.; Retailleau, L.; Descorme, C.; Liotta, L.F.; Valverde, J.L.; Gil, S.; Giroir-Fendler, A. Manganese oxide-based catalysts for toluene oxidation. Appl. Catal. B Environ. 2017, 209, 689–700. [Google Scholar] [CrossRef]
- Kim, S.C. The catalytic oxidation of aromatic hydrocarbons over supported metal oxide. J. Hazard. Mater. 2002, 91, 285–299. [Google Scholar] [CrossRef]
- Chlala, D.; Giraudon, J.-M.; Nuns, N.; Labaki, M.; Lamonier, J.-F.; Chlala, D. Highly Active Noble-Metal-Free Copper Hydroxyapatite Catalysts for the Total Oxidation of Toluene. ChemCatChem 2017, 9, 2275–2283. [Google Scholar] [CrossRef]
- Yang, J.S.; Jung, W.Y.; Lee, G.D.; Park, S.S.; Jeong, E.D.; Kim, H.G.; Hong, S.-S. Catalytic combustion of benzene over metal oxides supported on SBA-15. J. Ind. Eng. Chem. 2008, 14, 779–784. [Google Scholar] [CrossRef]
- Szegedi, Á.; Popova, M.; Lázár, K.; Klébert, S.; Drotár, E. Impact of silica structure of copper and iron-containing SBA-15 and SBA-16 materials on toluene oxidation. Microporous Mesoporous Mater. 2013, 177, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Tsoncheva, T.; Issa, G.; Blasco, T.; Dimitrov, M.; Popova, M.; Hernández, S.; Kovacheva, D.; Atanasova, G.; Nieto, J.M.L. Catalytic VOCs elimination over copper and cerium oxide modified mesoporous SBA-15 silica. Appl. Catal. A Gen. 2013, 453, 1–12. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Liu, L.-S.; Ren, J.; Hu, T.-P. Catalytic combustion of volatile organic compounds over CuO-CeO2 supported on SiO2-Al2O3 modified glass-fiber honeycomb. J. Fuel Chem. Technol. 2017, 45, 354–361. [Google Scholar] [CrossRef]
- Djinović, P.; Ristić, A.; Žumbar, T.; Dasireddy, V.D.; Rangus, M.; Dražić, G.; Popova, M.; Likozar, B.; Logar, N.Z.; Tušar, N.N. Synergistic effect of CuO nanocrystals and Cu-oxo-Fe clusters on silica support in promotion of total catalytic oxidation of toluene as a model volatile organic air pollutant. Appl. Catal. B Environ. 2020, 268, 118749. [Google Scholar] [CrossRef]
- Huang, L.; Kruk, M. Versatile Surfactant/Swelling-Agent Template for Synthesis of Large-Pore Ordered Mesoporous Silicas and Related Hollow Nanoparticles. Chem. Mater. 2015, 27, 679–689. [Google Scholar] [CrossRef]
- Bivona, L.A.; Vivian, A.; Fusaro, L.; Fiorilli, S.; Aprile, C. Design and catalytic applications of 1D tubular nanostructures: Improving efficiency in glycerol conversion. Appl. Catal. B Environ. 2019, 247, 182–190. [Google Scholar] [CrossRef]
- Soumoy, L.; Célis, C.; Debecker, D.P.; Armandi, M.; Fiorilli, S.; Aprile, C. Hafnium-doped silica nanotubes for the upgrading of glycerol into solketal: Enhanced performances and in-depth structure-activity correlation. J. Catal. 2022, 411, 41–53. [Google Scholar] [CrossRef]
- Zha, K.; Liu, H.; Xue, L.; Huang, Z.; Xu, H.; Shen, W. Co3O4 Nanoparticle-Decorated SiO2 Nanotube Catalysts for Propane Oxidation. ACS Appl. Nano Mater. 2021, 4, 8937–8949. [Google Scholar] [CrossRef]
- Farid, G.; Kruk, M. Silica Nanotubes with Widely Adjustable Inner Diameter and Ordered Silicas with Ultralarge Cylindrical Mesopores Templated by Swollen Micelles of Mixed Pluronic Triblock Copolymers. Chem. Mater. 2017, 29, 4675–4681. [Google Scholar] [CrossRef]
- Gunathilake, C.; Manchanda, A.S.; Ghimire, P.; Kruk, M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: Synthesis and CO2 sorption properties. Environ. Sci. Nano 2016, 3, 806–817. [Google Scholar] [CrossRef]
- Loverde, S.M.; Ortiz, V.; Kamien, R.D.; Klein, M.L.; Discher, D.E. Curvature-driven molecular demixing in the budding and breakup of mixed component worm-like micelles. Soft Matter 2010, 6, 1419. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Dong, Y.; Hu, X.; Feng, X.; Jia, A.; Xie, G.; Hu, G.; Lu, J.; Luo, M.; Fan, M. Tetraethylenepentamine-Modified Silica Nanotubes for Low- Temperature CO2 Capture. Energy Fuels 2013, 27, 7673–7680. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Guan, Z.; Liu, J.; Zhao, J.; Yang, Y.; Yang, Q. Organosilica nanotubes: Large-scale synthesis and encapsulation of metal nanoparticles. Chem. Commun. 2011, 47, 8073. [Google Scholar] [CrossRef]
- Wang, L.; Tomura, S.; Ohashi, F.; Maeda, M.; Suzuki, M.; Inukai, K. Synthesis of single silica nanotubes in the presence of citric acid. J. Mater. Chem. 2001, 11, 1465–1468. [Google Scholar] [CrossRef]
- Yin, Z.-H.; Liu, X.; Su, Z.-X. Novel fabrication of silica nanotubes using multi-walled carbon nanotubes as template. Bull. Mater. Sci. 2010, 33, 351–355. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, X.; Wang, T. Large-scale controlled synthesis of silica nanotubes using zinc oxide nanowires as templates. Nanotechnology 2005, 16, 1978–1982. [Google Scholar] [CrossRef]
- Ciotonea, C.; Dragoi, B.; Ungureanu, A.; Catrinescu, C.; Petit, S.; Alamdari, H.; Marceau, E.; Dumitriu, E.; Royer, S. Improved dispersion of transition metals in mesoporous materials through a polymer-assisted melt infiltration method. Catal. Sci. Technol. 2017, 7, 5448–5456. [Google Scholar] [CrossRef]
- Chirieac, A.; Dragoi, B.; Ungureanu, A.; Ciotonea, C.; Mazilu, I.; Royer, S.; Mamede, A.S.; Rombi, E.; Ferino, I.; Dumitriu, E. Facile synthesis of highly dispersed and thermally stable copper-based nanoparticles supported on SBA-15 occluded with P123 surfactant for catalytic applications. J. Catal. 2016, 339, 270–283. [Google Scholar] [CrossRef]
- Murthy, P.S.; Venugopalan, V.P.; Das, D.A.; Dhara, S.; Pandiyan, R.; Tyagi, A.K. Antibiofilm activity of nano sized CuO. In Proceedings of the International Conference on Nanoscience, Engineering and Technology (ICONSET 2011), Chennai, India, 28–30 November 2011; pp. 580–583. [Google Scholar] [CrossRef]
- Sonobe, K.; Tanabe, M.; Imaoka, T.; Chun, W.; Yamamoto, K. Low-Temperature H2 Reduction of Copper Oxide Subnanoparticles. Chem. Eur. J. 2021, 27, 8452–8456. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jin, M.; An, H.; Guo, Z.; Lv, Z. Hydrogenation Performance of Acetophenone to 1-Phenylethanol on Highly Active Nano Cu/SiO2 Catalyst. Catal. Lett. 2020, 150, 56–64. [Google Scholar] [CrossRef]
- Kong, Y.; Zhu, H.Y.; Yang, G.; Guo, X.F.; Hou, W.H.; Yan, Q.J.; Gu, M.; Hu, C. Investigation of the Structure of MCM-41 Samples with a High Copper Content. Adv. Funct. Mater. 2004, 14, 816–820. [Google Scholar] [CrossRef]
- Zapata, P.M.C.; Miranda, J.F.; Orellana, F.; Gonzo, E.; Bonini, N.A. Synthesis of Cu/SiO2(MCM-41) mesostructured catalysts. Effect of the preparation method on the textural properties and chemical structure. Mater. Chem. Phys. 2022, 287, 126232. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Han, X.; Cao, Y.; He, P.; Li, H. Selective hydrogenation of ethylene carbonate to methanol and ethylene glycol over Cu/SiO2 catalysts prepared by ammonia evaporation method. Int. J. Hydrogen Energy 2017, 42, 2144–2156. [Google Scholar] [CrossRef]
- Vaschetti, V.M.; Viola, B.M.; Barrera, D.; Sapag, K.; Eimer, G.A.; Cánepa, A.L.; Casuscelli, S.G. Improved template—Ion exchange synthesis of Cu-nanostructured molecular sieves. Microporous Mesoporous Mater. 2019, 284, 410–420. [Google Scholar] [CrossRef]
- Arunmetha, S.; Karthik, A.; Srither, S.R.; Vinoth, M.; Suriyaprabha, R.; Manivasakan, P.; Rajendran, V. Size-dependent physicochemical properties of mesoporous nanosilica produced from natural quartz sand using three different methods. RSC Adv. 2015, 5, 47390–47397. [Google Scholar] [CrossRef]
- Espinós, J.P.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J.P.; González-Elipe, A.R. Interface Effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS Determination of the Valence State of Copper in Cu/SiO2 and Cu/ZrO2 Catalysts. J. Phys. Chem. B 2002, 106, 6921–6929. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Hart, B.R.; Polack, R.; Kobe, B.A.; Smart, R.S. Analysis of mineral surface chemistry in flotation separation using imaging XPS. Miner. Eng. 2007, 20, 152–162. [Google Scholar] [CrossRef]
- Santos, V.; Pereira, M.; Órfão, J.; Figueiredo, J. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Boreave, A.; Giroir-Fendler, A. Catalytic Removal of Toluene over Co3O4–CeO2 Mixed Oxide Catalysts: Comparison with Pt/Al2O3. Catal. Lett. 2009, 127, 270–276. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Gilbank, A.; Puertolas, B.; Garcia, T.; Solsona, B.; Chadwick, D. Shape-dependency activity of nanostructured CeO2 in the total oxidation of polycyclic aromatic hydrocarbons. Appl. Catal. B Environ. 2013, 132–133, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Konsolakis, M.; Carabineiro, S.A.; Tavares, P.B.; Figueiredo, J.L. Redox properties and VOC oxidation activity of Cu catalysts supported on Ce1−xSmxOδ mixed oxides. J. Hazard. Mater. 2013, 261, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Lamonier, J.-F.; Giraudon, J.-M.; Molina, R.; Moreno, S. Catalytic activity of Co–Mg mixed oxides in the VOC oxidation: Effects of ultrasonic assisted in the synthesis. Catal. Today 2011, 176, 286–291. [Google Scholar] [CrossRef]
- Heidinger, B.; Royer, S.; Alamdari, H.; Giraudon, J.-M.; Lamonier, J.-F. Reactive Grinding Synthesis of LaBO3 (B: Mn, Fe) Perovskite; Properties for Toluene Total Oxidation. Catalysts 2019, 9, 633. [Google Scholar] [CrossRef] [Green Version]
- Kupková, K.; Topka, P.; Balabánová, J.; Koštejn, M.; Jirátová, K.; Giraudon, J.-M.; Lamonier, J.-F.; Maixner, J.; Kovanda, F. Cobalt-Copper Oxide Catalysts for VOC Abatement: Effect of Co:Cu Ratio on Performance in Ethanol Oxidation. Catalysts 2023, 13, 107. [Google Scholar] [CrossRef]
- Bai, L.; Wyrwalski, F.; Safariamin, M.; Bleta, R.; Lamonier, J.-F.; Przybylski, C.; Monflier, E.; Ponchel, A. Cyclodextrin-cobalt (II) molecule-ion pairs as precursors to active Co3O4/ZrO2 catalysts for the complete oxidation of formaldehyde: Influence of the cobalt source. J. Catal. 2016, 341, 191–204. [Google Scholar] [CrossRef]
- Nanba, T.; Chino, T.; Masukawa, S.; Uchisawa, J.; Obuchi, A. Total Oxidation of Toluene over Cu/TiO2/SiO2. Bull. Chem. Soc. Jpn. 2013, 86, 534–539. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Monti, D.A.; Baiker, A. Temperature-programmed reduction. Parametric sensitivity and estimation of kinetic parameters. J. Catal. 1983, 83, 323–335. [Google Scholar] [CrossRef]
- Alexander, M.R.; Short, R.D.; Jones, F.R.; Stollenwerk, M.; Zabold, J.; Michaeli, W. An X-ray photoelectron spectroscopic investigation into the chemical structure of deposits formed from hexamethyldisiloxane/oxygen plasmas. J. Mater. Sci. 1996, 31, 1879–1885. [Google Scholar] [CrossRef]






| Specific Surface Area /m2.g−1 | Pore Volume /cm3.g−1 | Crystallite Size of CuO 1 /nm | |
|---|---|---|---|
| Cu/SiO2-Aerosil | 39 | 0.1 | 44 |
| Cu/SiO2-D | 830 | 2.3 | 13 |
| Cu/SiO2 | 719 | 1.8 | 43 |
| Cu-SiO2-SG | 732 | 2.8 | 46 |
| SiO2 | 1014 | 2.2 | - |
| SiO2-D | 399 | 1.9 | - |
| Sample | Tmax 1/°C | H2/Cu | Cu/wt% 2 | O1s/eV 3 | Si2s/eV 3 | Isat/Imain (Cu2p3/2) 3 | Atomic Ratio 3 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Analysis | 2nd Analysis | 3rd Analysis | Cu/Si | O/Si | ||||||
| Cu/SiO2-Aerosil | 315 | 0.97 | 7.9 | 532.6 | 154.3 | nd * | nd * | nd * | 0.003 | 2.09 |
| Cu/SiO2-D | 274 | 0.94 | 10.1 | 532.6 | 154.3 | 0.35 | 0.22 | 0.12 | 0.038 | 2.29 |
| Cu/SiO2 | 286 | 0.88 | 9.8 | 532.6 | 154.3 | 0.41 | 0.17 | 0.22 | 0.028 | 2.26 |
| Cu-SiO2-SG | 341 | 0.97 | 8.2 | 532.6 | 154.2 | 0.37 | 0.18 | 0.17 | 0.012 | 2.22 |
| Sample | T20/°C | T50/°C | T100/°C | r287/μmol.h−1.g−1 |
|---|---|---|---|---|
| Cu/SiO2-Aerosil | 320 | 345 | 377 | 54 |
| Cu/SiO2-D | 294 | 306 | 333 | 147 |
| Cu/SiO2 | 308 | 323 | 361 | 65 |
| Cu-SiO2-SG | 336 | 363 | 431 | 25 |
| Catalyst | Reaction Mixture Composition | GHSV/h−1 | T100/°C | Ref. |
|---|---|---|---|---|
| Cu/SiO2-Aerosil Cu/SiO2-D Cu/SiO2 Cu-SiO2-SG | 0.2 g 100 mL.min−1 1000 ppm of toluene | 26 | 377 333 361 431 | This work |
| 9 wt% Cu/SBA-16 9 wt% Cu/SBA-15 | 30 mg 30 mL/min−1 Ptoluene = 0.9 kPa | 1.2 | 447 a 447 b | [13] |
| 5 wt% Cu/SiO2 | 0.1 g 160 mL.min−1 230 ppm of toluene | 19 | >500 | [49] |
| 5 wt% Cu/SiO2 | 0.5 g 60 mL.min−1 900 ppm of toluene | 6 | >350 | [10] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deboos, V.; Calabrese, C.; Giraudon, J.-M.; Morent, R.; De Geyter, N.; Liotta, L.F.; Lamonier, J.-F. Copper-Based Silica Nanotubes as Novel Catalysts for the Total Oxidation of Toluene. Nanomaterials 2023, 13, 2202. https://doi.org/10.3390/nano13152202
Deboos V, Calabrese C, Giraudon J-M, Morent R, De Geyter N, Liotta LF, Lamonier J-F. Copper-Based Silica Nanotubes as Novel Catalysts for the Total Oxidation of Toluene. Nanomaterials. 2023; 13(15):2202. https://doi.org/10.3390/nano13152202
Chicago/Turabian StyleDeboos, Victor, Carla Calabrese, Jean-Marc Giraudon, Rino Morent, Nathalie De Geyter, Leonarda Francesca Liotta, and Jean-François Lamonier. 2023. "Copper-Based Silica Nanotubes as Novel Catalysts for the Total Oxidation of Toluene" Nanomaterials 13, no. 15: 2202. https://doi.org/10.3390/nano13152202
APA StyleDeboos, V., Calabrese, C., Giraudon, J.-M., Morent, R., De Geyter, N., Liotta, L. F., & Lamonier, J.-F. (2023). Copper-Based Silica Nanotubes as Novel Catalysts for the Total Oxidation of Toluene. Nanomaterials, 13(15), 2202. https://doi.org/10.3390/nano13152202










