Facile Synthesis of Chromium-Doped Fe1.1Mn1.9O4 Nanoparticles and the Effect of Cr Content on Their Magnetic and Structural Properties
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Cr-Doped Fe1.1Mn1.9O4 Nanoparticles
2.2. Characterizations
3. Results and Discussion
3.1. Structural Analysis
3.2. Magnetic Measurements
3.3. Temperature-Dependent Magnetization Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penoyer, R.F.; Shafer, M.W. On the Magnetic Anisotropy in Manganese-Iron Spinels. J. Appl. Phys. 1959, 30, 315S–316S. [Google Scholar] [CrossRef]
- Crapo, W.A. Time Decrease of Initial Permeability in MnxFe3−xO4+y. J. Appl. Phys. 1960, 31, 267S–268S. [Google Scholar] [CrossRef]
- Amoli-Diva, M.; Asli, M.D.; Karimi, S. FeMn2O4 nanoparticles coated dual responsive temperature and pH-responsive polymer as a magnetic nano-carrier for controlled delivery of letrozole anti-cancer. Nanomed. J. 2017, 4, 218–223. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, C.; Luo, Z.; Zhang, T.; Liu, J.; Li, J.; Zuo, Y.; Biendicho, J.J.; Llorca, J.; Arbiol, J.; et al. A low temperature solid state reaction to produce hollow MnxFe3−xO4 nanoparticles as anode for lithium-ion batteries. Nano Energy 2019, 66, 104199. [Google Scholar] [CrossRef]
- Yamaguchi, N.U.; Bergamasco, R.; Hamoudi, S. Magnetic MnFe2O4—Graphene hybrid composite for efficient removal of glyphosate from water. Chem. Eng. J. 2016, 295, 391–402. [Google Scholar] [CrossRef]
- Li, M.; Gao, Q.; Wang, T.; Gong, Y.-S.; Han, B.; Xia, K.-S.; Zhou, C.-G. Solvothermal synthesis of MnxFe3−xO4 nanoparticles with interesting physicochemical characteristics and good catalytic degradation activity. Mater. Des. 2016, 97, 341–348. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Vijayakumar, S.; Lee, S.-H.; Ryu, K.-S. Hybrid supercapacitor devices based on MnCo2O4 as the positive electrode and FeMn2O4 as the negative electrode. Appl. Surf. Sci. 2016, 390, 202–208. [Google Scholar] [CrossRef]
- Sunaryono, M.F.; Hidayat, M.N.; Kholifah, S.; Hidayat, N.; Mufti, A. Taufiq Magneto-thermal behavior of MnxFe3−xO4-PVA/PVP magnetic hydrogel and its potential application. AIP Confer. Proc. 2020, 2228, 030018. [Google Scholar] [CrossRef]
- Brabers, V.A.M. Infrared Spectra of Cubic and Tetragonal Manganese Ferrites. Phys. Status Solidi 1969, 33, 563. [Google Scholar] [CrossRef]
- Brabers, V.A.M. Cation Migration, Cation Valencies and the Cubic-Tetragonal Transition in MnxFe3−xO4. J. Phys. Chem. Solids 1971, 32, 2181–2191. [Google Scholar] [CrossRef]
- Van Landuyt, J.; De Ridder, R.; Brabers, V.A.M.; Amelinckx, S. Jahn-Teller domains in MnxFe3−xO4 as observed by electron microscopy. Mater. Res. Bull. 1972, 7, 327–337. [Google Scholar] [CrossRef]
- Wojtowicz, P.J. Theoretical Model for Tetragonal-to-Cubic Phase Transformations in Transition Metal Spinels. Phys. Rev. 1959, 116, 32–45. [Google Scholar] [CrossRef]
- Wickham, D.G. The chemical composition of spinels in the system Fe3O4-Mn3O4. J. Inorg. Nucl. Chem. 1969, 31, 313–320. [Google Scholar] [CrossRef]
- Noda, Y.; Naito, K. The Thermal Conductivity and Diffusivity of MnxFe3-xO4 (0≤ x ≤ 1.5) from 200 to 700 K. Netsu Sokutei 1978, 5, 11–18. [Google Scholar] [CrossRef]
- Gillot, B.; Laarj, M.; Kacim, S. Reactivity towards oxygen and cation distribution of manganese iron spinel Mn3−xFexO4 (0 ≤ x ≤ 3) fine powders studied by thermogravimetry and IR spectroscopy. J. Mater. Chem. 1997, 7, 827–831. [Google Scholar] [CrossRef]
- Desai, I.; Nadagouda, M.N.; Elovitz, M.; Mills, M.; Boulanger, B. Synthesis and characterization of magnetic manganese ferrites. Mater. Sci. Energy Technol. 2019, 2, 150–160. [Google Scholar] [CrossRef]
- Kour, S.; Jasrotia, R.; Kumari, N.; Singh, V.P.; Singh, P.; Kumar, R. A Current Review on Synthesis and Magnetic Properties of Pure and Doped Manganese Ferrites. AIP Conf. Proc. 2022, 2357, 050007. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yoon, H.Y.; Lee, J.S.; Wu, J.H.; Kim, Y.K. Synthesis and magnetic properties of size-tunable MnxFe3−xO4 ferrite nanoclusters. J. Appl. Phys. 2014, 115, 17B517. [Google Scholar] [CrossRef]
- Nakano, A.; Nakano, J.; Seetharaman, S. Synthesis of nano-manganese ferrite by an oxalate method and characterization of its magnetic properties. Int. J. Mater. Res. 2015, 106, 1264–1268. [Google Scholar] [CrossRef]
- Han, A.; Liao, J.; Ye, M.; Li, Y.; Peng, X. Preparation of Nano-MnFe2O4 and Its Catalytic Performance of Thermal Decomposition of Ammonium Perchlorate. Chin. J. Chem. Eng. 2011, 19, 1047–1051. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Goga, F.; Cadar, O. Influence of Mn2+ substitution with Co2+ on structural, morphological and coloristic properties of MnFe2O4/SiO2 nanocomposites. Mater. Charact. 2021, 172, 110835. [Google Scholar]
- Sam, S.; Nesaraj, A.S. Preparation of MnFe2O4 Nanoceramic Particles by Soft Chemical Routes. Int. J. Appl. Sci. Eng. 2011, 9, 223–239. [Google Scholar] [CrossRef]
- Baig, M.M.; Zulfiqar, S.; Yousuf, M.A.; Touqeer, M.; Ullah, S.; Agboola, P.; Warsi, M.F.; Shakir, I. Structural and photocatalytic properties of new rare earth La3+ substituted MnFe2O4 ferrite nanoparticles. Ceram. Int. 2020, 46, 23208–23217. [Google Scholar] [CrossRef]
- Rashmi, S.K.; Naik, H.S.B.; Jayadevappa, H.; Sudhamani, C.N.; Patil, S.B.; Naik, M.M. Influence of Sm3+ ions on structural, optical and solar light driven photocatalytic activity of spinel MnFe2O4 nanoparticles. J. Solid State Chem. 2017, 255, 178–192. [Google Scholar] [CrossRef]
- Al-Mokdad, F.; Hassan, R.S.; Awad, R. Physical and dielectric properties of MnFe2O4 doped by Mo. Curr. Nanomater. 2019, 4, 125–136. [Google Scholar] [CrossRef]
- Yousuf, M.A.; Baig, M.M.; Waseem, M.; Haider, S.; Shakir, I.; Khan, S.U.D.; Warsi, M.F. Low cost micro-emulsion route synthesis of Cr-substituted MnFe2O4 nanoparticles. Ceram. Int. 2019, 45, 22316–22323. [Google Scholar] [CrossRef]
- Patra, K.P.; Ravi, S. Effect of chromium in magnetic and dielectric properties of inverse spinel FeMn2O4. Appl. Phys. A 2022, 128, 289. [Google Scholar] [CrossRef]
- Patil, K.; Phadke, S.; Mishra, A. A study of structural and dielectric properties of Zn2+ doped MnFe2O4 and NiFe2O4 spinel ferrites. Mater. Today Proc. 2021, 46, 2226–2228. [Google Scholar] [CrossRef]
- Vijaya, J.J.; Sekaran, G.; Bououdina, M. Effect of Cu2+ doping on structural, morphological, optical and magnetic properties of MnFe2O4 particles/sheets/flakes-like nanostructures. Ceram. Int. 2015, 41, 15–26. [Google Scholar] [CrossRef]
- Spivakov, A.A.; Lin, C.-R.; Lin, E.-S.; Chen, Y.-Z.; Tseng, Y.-T. Preparation and Magnetic Properties of Cobalt-Doped FeMn2O4 Spinel Nanoparticles. Nanoscale Res. Lett. 2021, 16, 162. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Salamati, H.; Concas, G.; Fernandez, M.S.; Talone, A.; Muscas, G.; Peddis, D. Co-doped MnFe2O4 nanoparticles: Magnetic anisotropy and interparticle interactions. Beilstein J. Nanotechnol. 2019, 10, 856–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayestefar, M.; Mashreghi, A.; Hasani, S.; Rezvan, M.T. Optimization of the structural and magnetic properties of MnFe2O4 doped by Zn and Dy using Taguchi method. J. Magn. Magn. Mater. 2022, 541, 168390. [Google Scholar] [CrossRef]
- Ouyahia, S.; Rais, A.; Bozzo, B.; Taibi, K.; Addou, A. Cations distribution by Rietveld refinement and magnetic properties of MgCrxFe2−xO4 spinel ferrites. Appl. Phys. A 2020, 126, 666. [Google Scholar] [CrossRef]
- Lenin, N.; Karthik, A.; Srither, S.R.; Sridharpanday, M.; Surendhiran, S.; Balasubramanian, M. Synthesis, structural and microwave absorption properties of Cr-doped zinc lanthanum nanoferrites Zn1-xCrxLa0.1Fe1.9O4 (x = 0.09, 0.18, 0.27 and 0.36). Ceram. Int. 2021, 47, 34891–34898. [Google Scholar] [CrossRef]
- Sankpal, A.M.; Kakatkar, S.V.; Chaudhari, N.D.; Patil, R.S.; Sawant, S.R.; Suryavanshi, S.S. Initial permeability studies on Al3+ and Cr3+ substituted Ni–Zn ferrites. J. Mater. Sci. Mater. Electron. 1998, 9, 173–179. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Khan, K.; Tareen, A.K.; Wu, D.; Gao, J. Room and low-temperature magnetic characterization of Cr doped CoFe2O4 nanostructures. Solid State Sci. 2022, 133, 107001. [Google Scholar] [CrossRef]
- Nepal, R.; Zhang, Q.; Dai, S.; Tian, W.; Nagler, S.E.; Jin, R. Structural and magnetic transitions in spinel FeMn2O4 single crystals. Phys. Rev. B 2018, 97, 024410. [Google Scholar] [CrossRef] [Green Version]
- Agusu, L.; Alimin; Ahmad, L.O.; Firihu, M.Z.; Mitsudo, S.; Kikuchi, H. Crystal and microstructure of MnFe2O4 synthesized by ceramic method using manganese ore and iron sand as raw materials. J. Phys. Conf. Ser. 2019, 1153, 012056. [Google Scholar] [CrossRef]
- Zhandun, V.S.; Nemtsev, A.V. Ab initio comparative study of the magnetic, electronic and optical properties of AB2O4 (A, B = Mn, Fe) spinels. Mater. Chem. Phys. 2021, 259, 124065. [Google Scholar] [CrossRef]
- Del Sol Fernandez, S.; Odio, O.F.; Crespo, P.M.; Perez, E.O.; Salas, G.; Gutierrez, L.; Morales, M.D.P.; Reguera, E. Tunable Control of the Structural Features and Related Physical Properties of MnxFe3–xO4 Nanoparticles: Implication on Their Heating Performance by Magnetic Hyperthermia. J. Phys. Chem. C 2022, 126, 10110–10128. [Google Scholar] [CrossRef]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, S.; Akbar, A.; Naseem, S. Ferromagnetic Effects in Cr-Doped Fe2O3 Thin Films. IEEE Trans. Magn. 2014, 50, 2200704. [Google Scholar] [CrossRef]
- Werth, J.H.; Linsenbuhler, M.; Dammer, S.M.; Farkas, Z.; Hinrichsen, H.; Wirth, K.-E.; Wolf, D.E. Agglomeration of charged nanopowders in suspensions. Powder Technol. 2003, 133, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Thakur, S.S.; Pathania, A.; Thakur, P.; Thakur, A.; Hsu, J.-H. Improved structural, electrical and magnetic properties of Mn–Zn–Cd nanoferrites. Ceram. Int. 2015, 41, 5072–5078. [Google Scholar] [CrossRef]
- Haralkar, S.J.; Kadam, R.H.; More, S.S.; Shirsath, S.E.; Mane, M.L.; Patil, S.; Mane, D.R. Substitutional effect of Cr3+ ions on the properties of Mg–Zn ferrite nanoparticles. Phys. B Condens. Matter 2012, 407, 4338–4346. [Google Scholar] [CrossRef]
- Singhal, S.; Jauhar, S.; Lakshmi, N.; Bansal, S. Mn3+ substituted Co–Cd ferrites, CoCd0.4MnxFe1.6−xO4 (0.1 ≤ x ≤ 0.6): Cation distribution, structural, magnetic and electrical properties. J. Mol. Struct. 2013, 1038, 45–51. [Google Scholar] [CrossRef]
- Avazpour, L.; Shokrollahi, H.; Toroghinejad, M.R.; Khajeh, M.Z. Effect of rare earth substitution on magnetic and structural properties of Co1-xRExFe2O4 (RE: Nd, Eu) nanoparticles prepared via EDTA/EG assisted sol–gel synthesis. J. Alloys Compd. 2016, 662, 441–447. [Google Scholar] [CrossRef]
- Chakraverty, S.; Bandyopadhyay, M. Coercivity of magnetic nanoparticles: A stochastic model. J. Phys. Condens. Matter 2007, 19, 216201. [Google Scholar] [CrossRef]
- Chiu, W.S.; Radiman, S.; Abd-Shukor, R.; Abdullah, M.H.; Khiew, P.S. Tunable coercivity of CoFe2O4 nanoparticles via thermal annealing treatment. J. Alloys Compd. 2008, 459, 291–297. [Google Scholar] [CrossRef]
- Moskowitz, B.M. Micromagnetic study of the influence of crystal defects on coercivity in magnetite. J. Geophys. Res. Solid Earth 1993, 98, 18011–18026. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, X.; Zhang, D.; Wu, C.; Silva, S.R.P. Cr3+ substituted spinel ferrite nanoparticles with high coercivity. Nanotechnology 2016, 27, 245707. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wu, X.C.; Zou, B.S.; Wang, Y.J. Magnetic properties of nanosized MnFe2O4 particles. J. Magn. Magn. Mater. 1998, 183, 152–156. [Google Scholar] [CrossRef]
- Gajbhiye, N.S.; Balaji, G.; Ghafari, M. Magnetic Properties of Nanostructured MnFe2O4 Synthesized by Precursor Technique. Phys. Status Solidi 2002, 189, 357–361. [Google Scholar]
- Cao, L.F.; Dan, X.I.E.; Guo, M.X.; Park, H.S.; Fujita, T. Size and shape effects on Curie temperature of ferromagnetic nanoparticles. Trans. Nonferrous Met. Soc. China 2007, 17, 1451–1455. [Google Scholar] [CrossRef]
- Chandramohan, P.; Srinivasan, M.P.; Velmurugan, S.; Narasimhan, S.V. Cation distribution and particle size effect on Raman spectrum of CoFe2O4. J. Solid State Chem. 2011, 184, 89–96. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Hnatko, M.; Šajgalík, P.; Alexander, C.; Palou, M.; Bartoníčková, E.; Boháč, M.; Frajkorová, F.; Masilko, J.; et al. Magnetic properties of Co1-xZnxFe2O4 spinel ferrite nanoparticles synthesized by starch-assisted sol–gel autocombustion method and its ball milling. J. Magn. Magn. Mater. 2015, 378, 190–199. [Google Scholar] [CrossRef]
- Kremenovic, A.; Antic, B.; Nikolic, A.S.; Blanusa, J.; Jancar, B.; Meden, A.; Mentus, S. The dependence of cation distribution, microstrain and magnetic susceptibility on particle size in nanocrystalline Gd2O3/Y2O3. Scr. Mater. 2007, 57, 1061–1064. [Google Scholar] [CrossRef]
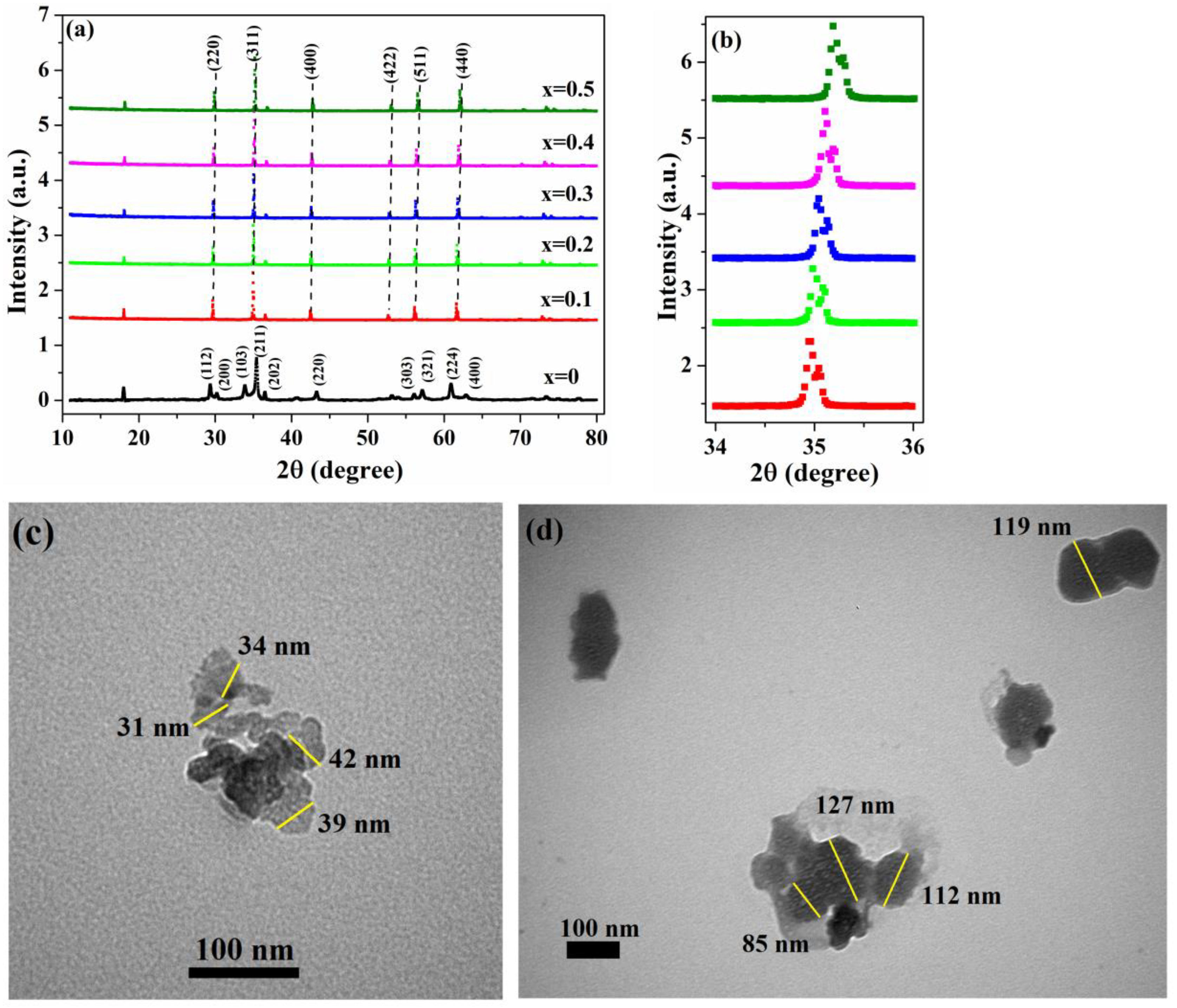

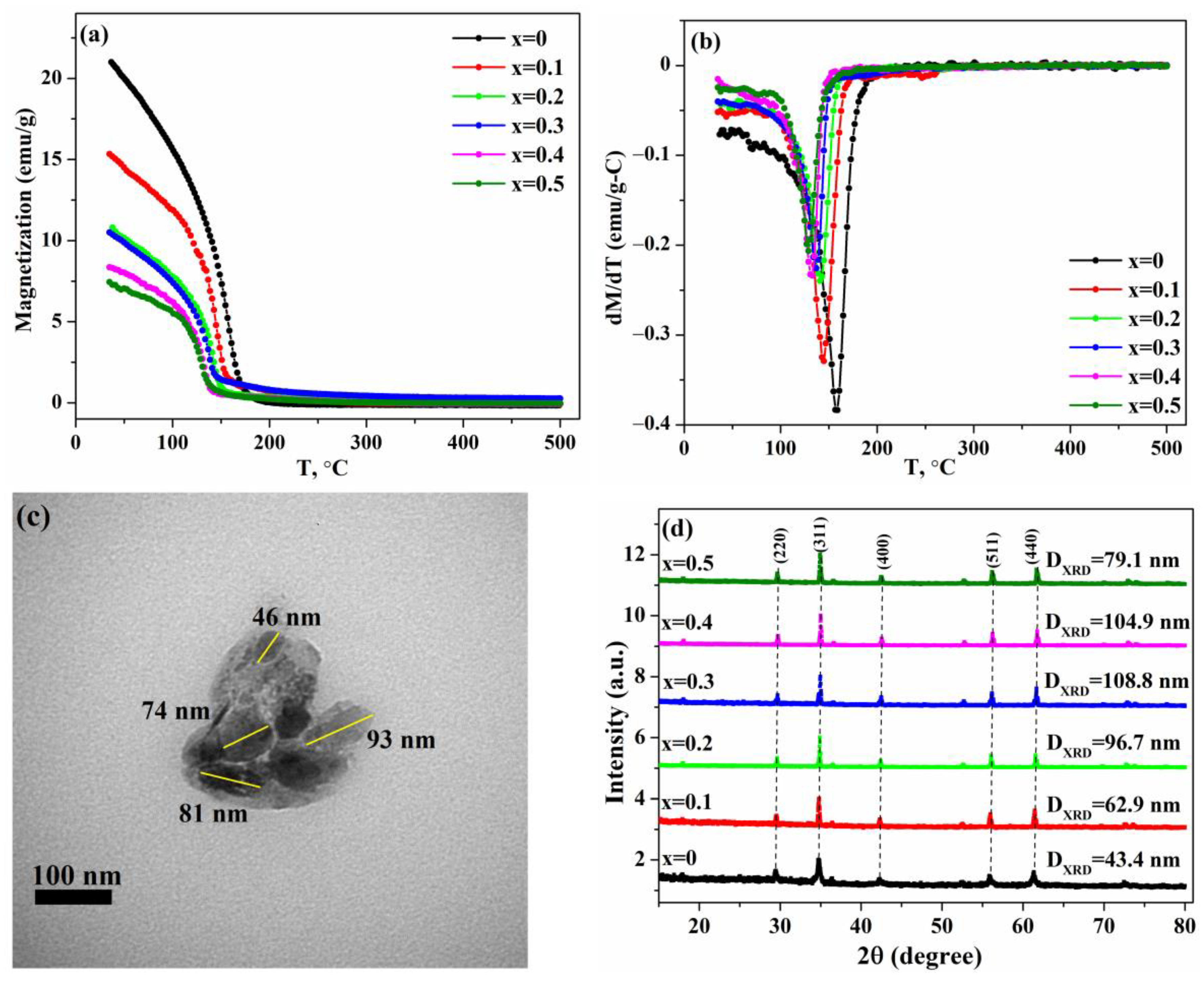
| Concentration of Chromium, x | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
|---|---|---|---|---|---|---|
| DXRD, nm | 26.7 | 97.4 | 121.5 | 119.2 | 116.7 | 102.4 |
| a, Å | 5.93 | 8.51 | 8.5 | 8.48 | 8.46 | 8.43 |
| c, Å | 8.67 | – | – | – | – | – |
| Cr concentration | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
|---|---|---|---|---|---|---|
| MS, emu/g | 20.2 | 15.1 | 10.9 | 9.8 | 8.7 | 7.5 |
| MR, emu/g | 3.4 | 2.3 | 1.7 | 1.9 | 2.2 | 0.9 |
| MR/MS | 0.17 | 0.15 | 0.16 | 0.19 | 0.25 | 0.12 |
| HC, Oe | 58.9 | 32.2 | 25.8 | 42.6 | 57.7 | 20.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spivakov, A.A.; Huang, L.-H.; Chen, Y.-Z.; Lin, C.-R. Facile Synthesis of Chromium-Doped Fe1.1Mn1.9O4 Nanoparticles and the Effect of Cr Content on Their Magnetic and Structural Properties. Nanomaterials 2023, 13, 2203. https://doi.org/10.3390/nano13152203
Spivakov AA, Huang L-H, Chen Y-Z, Lin C-R. Facile Synthesis of Chromium-Doped Fe1.1Mn1.9O4 Nanoparticles and the Effect of Cr Content on Their Magnetic and Structural Properties. Nanomaterials. 2023; 13(15):2203. https://doi.org/10.3390/nano13152203
Chicago/Turabian StyleSpivakov, Aleksandr A., Li-Huai Huang, Ying-Zhen Chen, and Chun-Rong Lin. 2023. "Facile Synthesis of Chromium-Doped Fe1.1Mn1.9O4 Nanoparticles and the Effect of Cr Content on Their Magnetic and Structural Properties" Nanomaterials 13, no. 15: 2203. https://doi.org/10.3390/nano13152203





