Photoelectrocatalytic Detection and Degradation Studies of a Hazardous Textile Dye Safranin T
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instrumentation
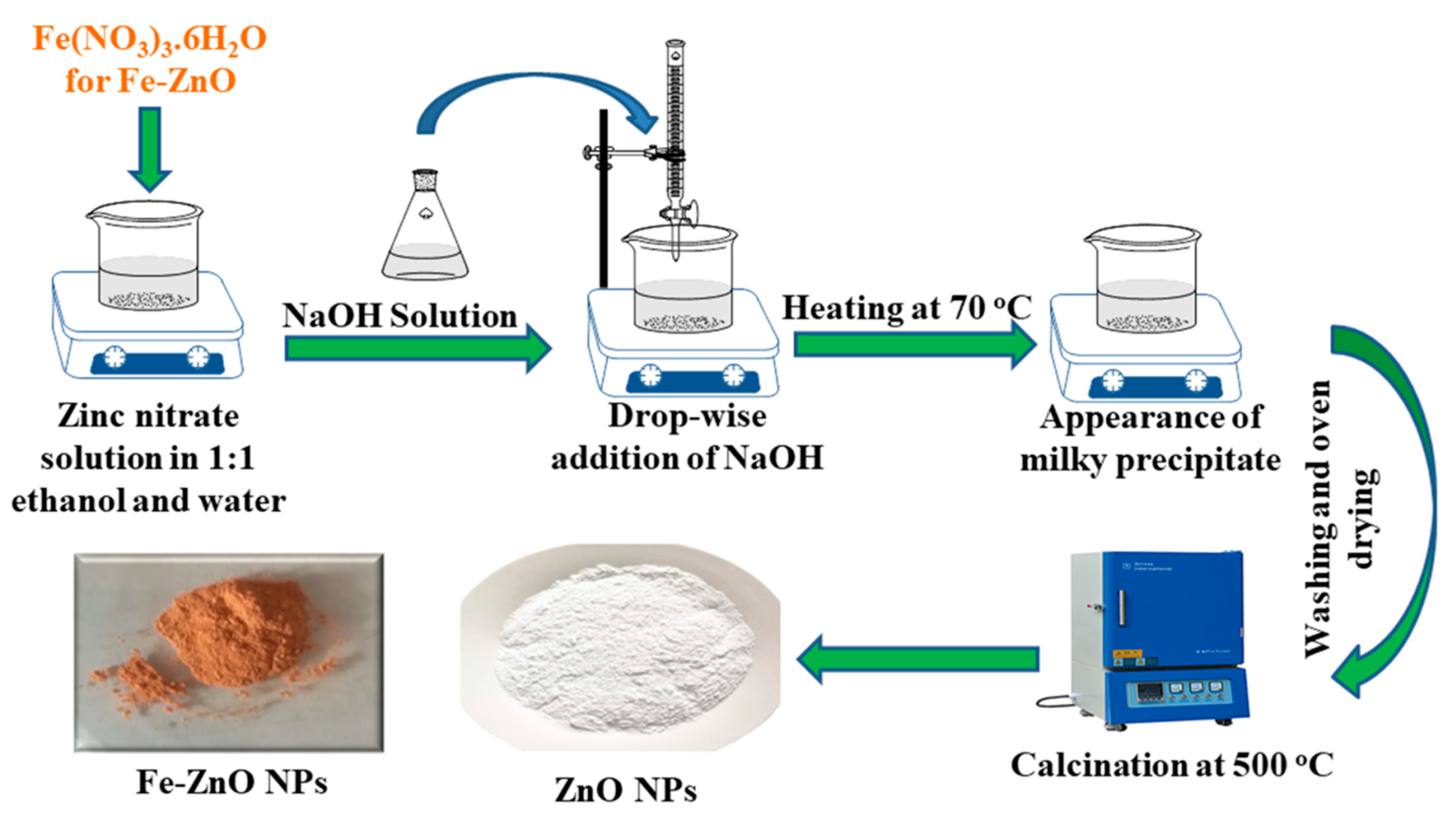
2.3. Synthesis Route of ZnO and Fe-ZnO NPs
2.4. Fabrication of Modified Electrode
2.5. Dye Degradation Procedure
3. Results and Discussion
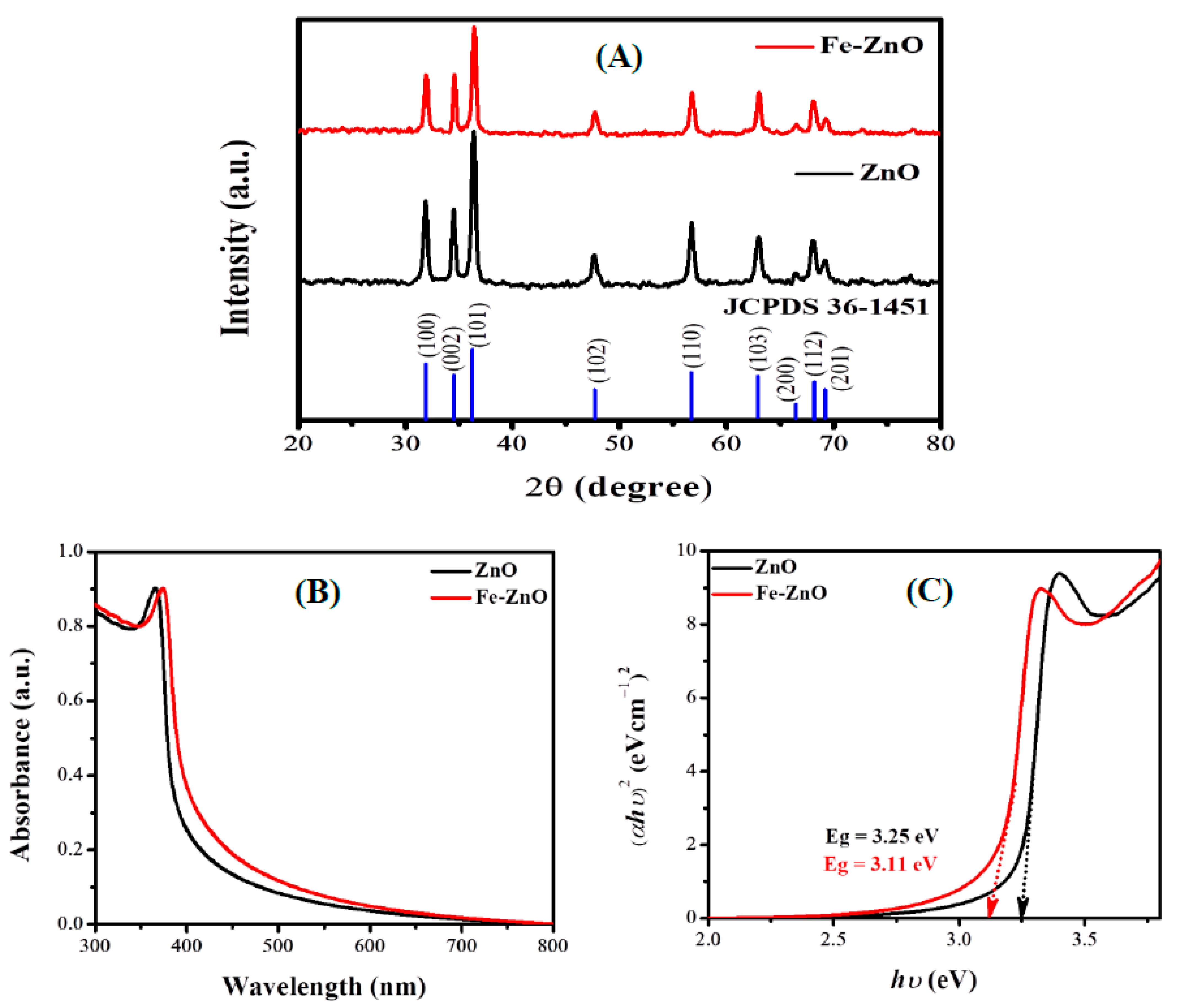
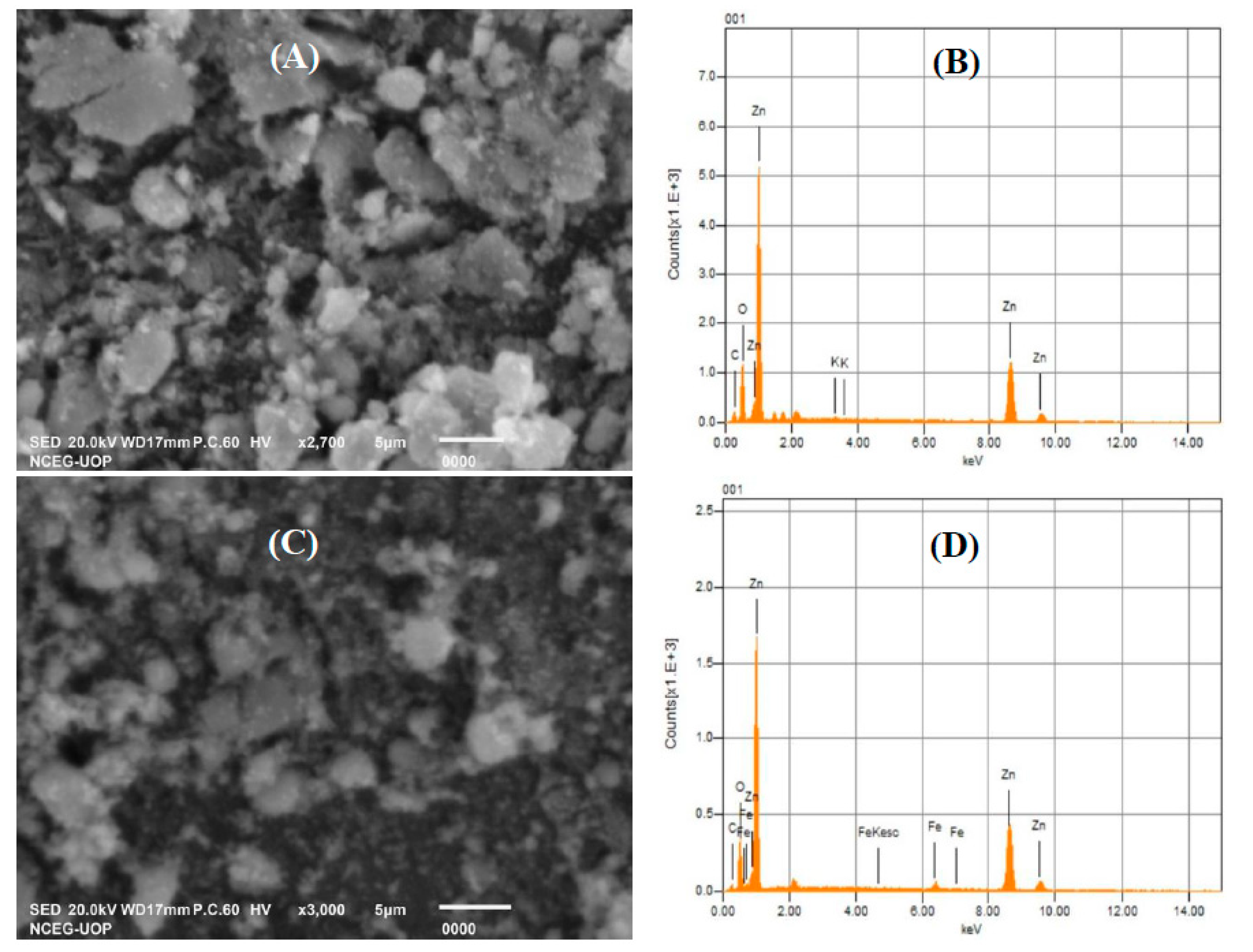
3.1. Characterization of Pure ZnO and Fe-ZnO NPs
3.2. Electrochemical Characterizations
3.3. Voltammetric Behaviour of Safranin T
3.4. Effect of Scan Rates
3.5. Optimization of Experimental Parameters
3.6. Analytical Application of Developed Sensing Scaffold
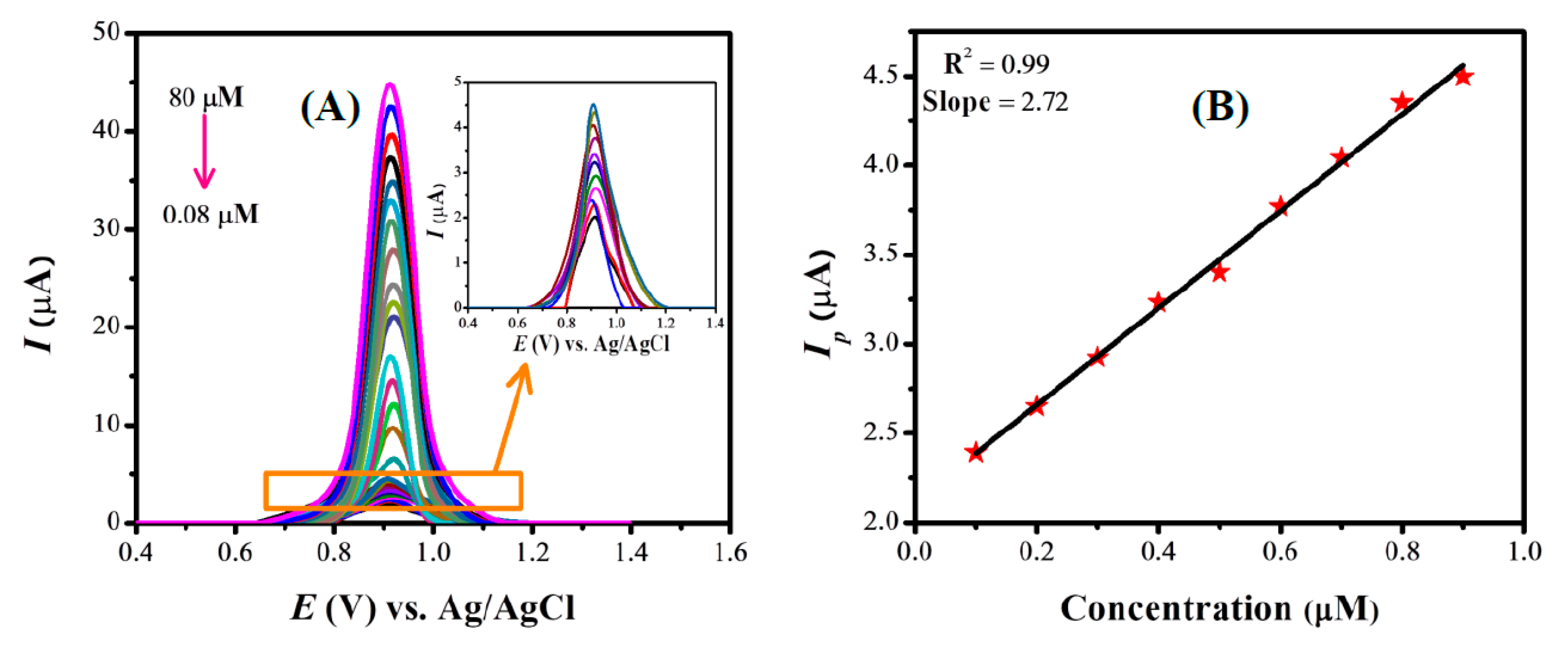
3.6.1. Evaluation of Quantification and Detection Limits of ST
3.6.2. Evaluation of the Stability of the Designed Sensing Scaffold
3.7. Photodegradation of ST
3.7.1. Influence of pH on Photocatalytic Degradation
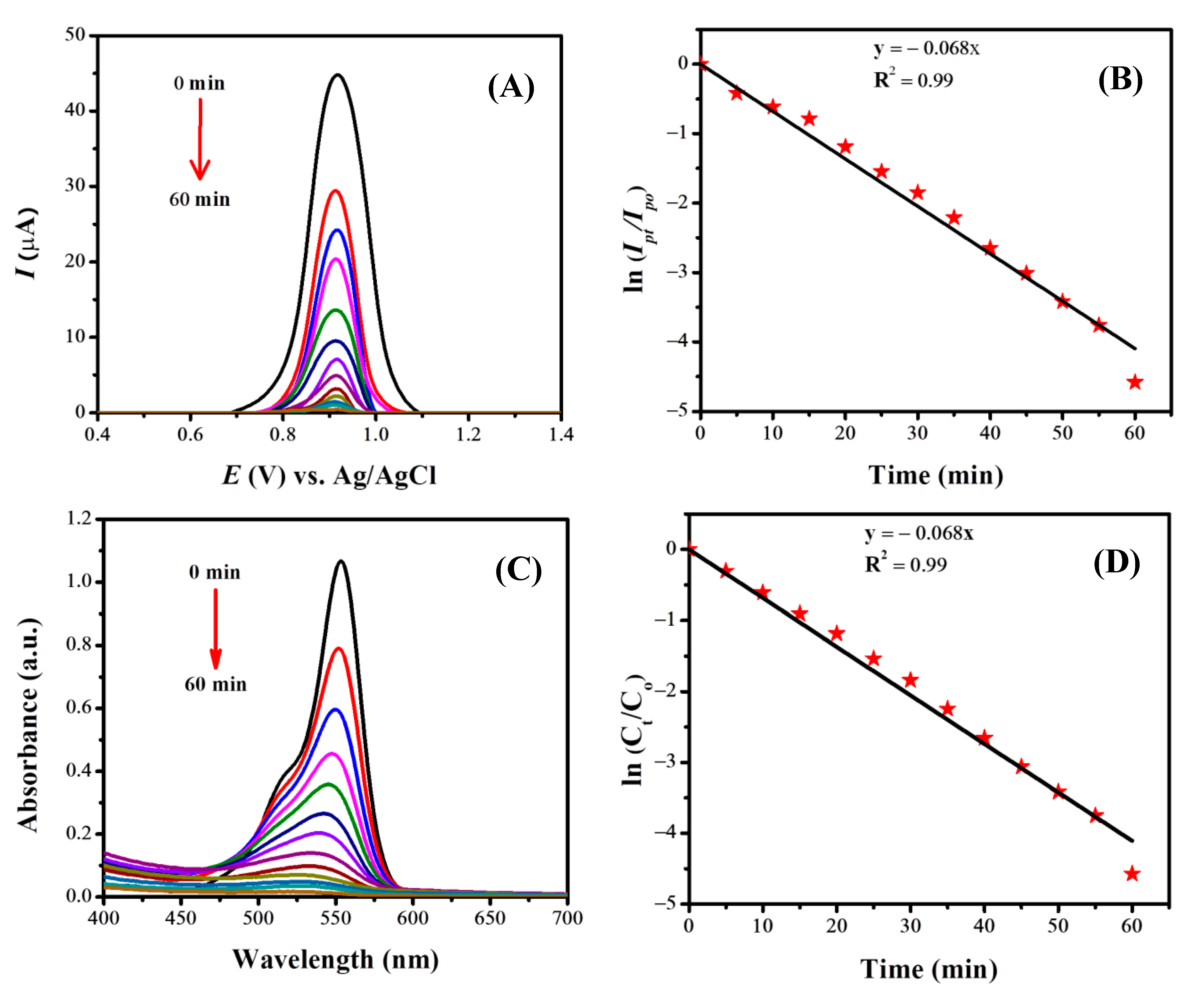
3.7.2. Electrochemical and Spectroscopic Monitoring of the Photocatalytic Degradation of ST
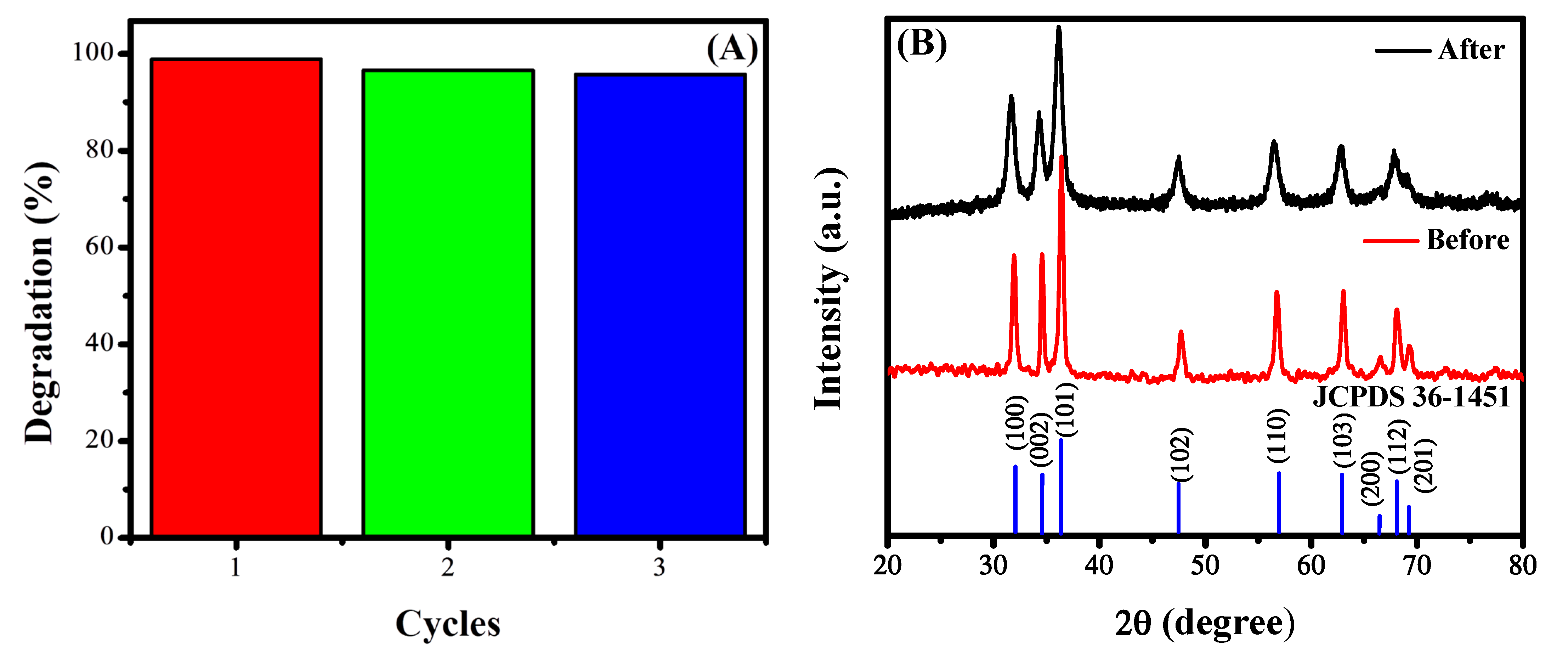
3.7.3. Stability of the Photocatalyst
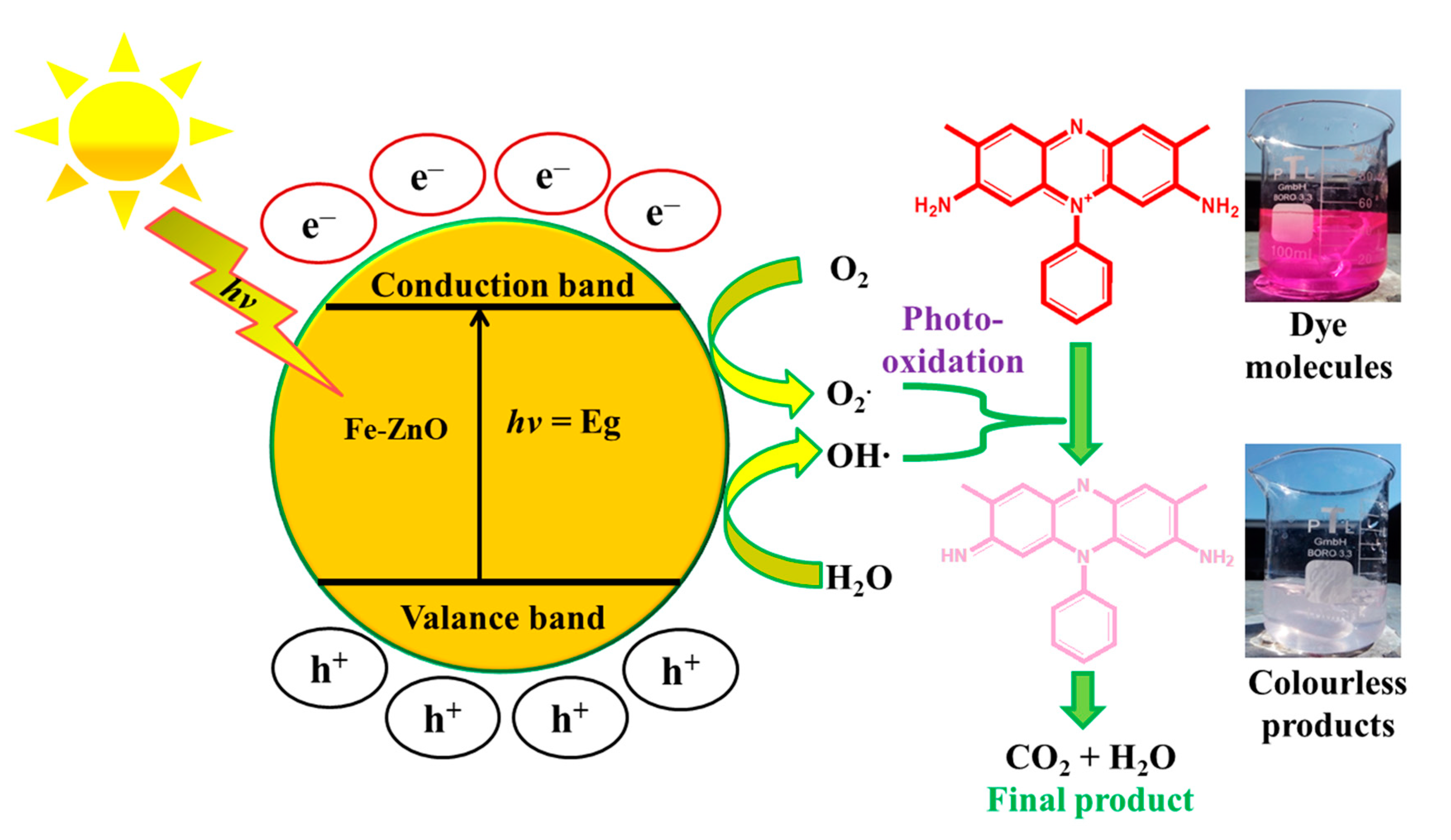
3.7.4. Proposed Mechanism of ST Photodegradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly efficient visible light active doped ZnO photocatalysts for the treatment of wastewater contaminated with dyes and pathogens of emerging concern. Nanomaterials 2022, 12, 486. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Nisar, J.; Ashiq, M.N.; Khan, M.A.; Khan, S.B.; Bakhsh, E.M. Phenolic water toxins: Redox mechanism and method of their detection in water and wastewater. RSC Adv. 2021, 11, 35783–35795. [Google Scholar] [CrossRef]
- Fei, L.; Bilal, M.; Qamar, S.A.; Imran, H.M.; Riasat, A.; Jahangeer, M.; Ghafoor, M.; Ali, N.; Iqbal, H.M. Nano-remediation technologies for the sustainable mitigation of persistent organic pollutants. Environ. Res. 2022, 211, 113060. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Akhtar, M.; Aftab, S.; Shah, A.H.; Kraatz, H.-B. Gold copper alloy nanoparticles (Au-Cu NPs) modified electrode as an enhanced electrochemical sensing platform for the detection of persistent toxic organic pollutants. Electrochim. Acta 2017, 241, 281–290. [Google Scholar] [CrossRef]
- Munir, S.; Dionysiou, D.D.; Khan, S.B.; Shah, S.M.; Adhikari, B.; Shah, A. Development of photocatalysts for selective and efficient organic transformations. J. Photochem. Photobiol. B 2015, 148, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, V.; Kim, K.-H.; Rawat, M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 2019, 177, 108569. [Google Scholar] [CrossRef]
- Khan, K.A.; Shah, A.; Nisar, J.; Haleem, A.; Shah, I. Photocatalytic Degradation of Food and Juices Dyes via Photocatalytic Nanomaterials Synthesized through Green Synthetic Route: A Systematic Review. Molecules 2023, 28, 4600. [Google Scholar] [CrossRef]
- Saleem, M.N.; Shah, A.; Ullah, N.; Nisar, J.; Iftikhar, F.J. Detection and Degradation Studies of Nile Blue Sulphate Using Electrochemical and UV-Vis Spectroscopic Techniques. Catalysts 2023, 13, 141. [Google Scholar] [CrossRef]
- Imtiaz, T.; Shah, A.; Ullah, N.; Iftikhar, F.J.; Shah, I.; Shah, S.M.; Shah, S.S. Electrochemical nanosensor for ultrasensitive detection of malachite green and monitoring of its photocatalytic degradation. Npj Clean Water 2022, 5, 69. [Google Scholar] [CrossRef]
- Yahya, R.; Shah, A.; Kokab, T.; Ullah, N.; Hakeem, M.K.; Hayat, M.; Haleem, A.; Shah, I. Electrochemical Sensor for detection and degradation studies of ethyl violet dye. ACS Omega 2022, 7, 34154–34165. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Irfan, M.; Shah, A.; Iftikhar, F.J.; Hayat, M.; Ashiq, M.N.; Shah, I. Electrochemical sensing platform based on functionalized multi-walled carbon nanotubes and metal oxide for the detection and degradation studies of orange II dye. ACS Omega 2022, 7, 32302–32312. [Google Scholar] [CrossRef] [PubMed]
- Hayat, M.; Shah, A.; Hakeem, M.K.; Irfan, M.; Haleem, A.; Khan, S.B.; Shah, I. A designed miniature sensor for the trace level detection and degradation studies of the toxic dye Rhodamine B. RSC Adv. 2022, 12, 15658–15669. [Google Scholar] [CrossRef]
- Hayat, M.; Shah, A.; Nisar, J.; Shah, I.; Haleem, A.; Ashiq, M.N. A novel electrochemical sensing platform for the sensitive detection and degradation monitoring of methylene blue. Catalysts 2022, 12, 306. [Google Scholar] [CrossRef]
- Shah, A. A novel electrochemical nanosensor for the simultaneous sensing of two toxic food dyes. ACS Omega 2020, 5, 6187–6193. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Bashir, S.; Hamid, S.; Zia, A.; Abbas, Y.; Umbreen, K.; Ashiq, M.N.; Shah, A. One-pot facile synthesis of the ZnO/ZnSe heterostructures for efficient photocatalytic degradation of azo dye. Appl. Surf. Sci. 2018, 459, 194–200. [Google Scholar] [CrossRef]
- Sieren, B.; Baker, J.; Wang, X.; Rozzoni, S.J.; Carlson, K.; McBain, A.; Kerstan, D.; Allen, L.; Liao, L.; Li, Z. Sorptive removal of color dye safranin o by fibrous clay minerals and zeolites. Adv. Mater. Sci. Eng. 2020, 2020, 8845366. [Google Scholar] [CrossRef]
- Baldacci-Cresp, F.; Spriet, C.; Twyffels, L.; Blervacq, A.S.; Neutelings, G.; Baucher, M.; Hawkins, S. A rapid and quantitative safranin-based fluorescent microscopy method to evaluate cell wall lignification. Plant J. 2020, 102, 1074–1089. [Google Scholar] [CrossRef]
- Sonker, E.; Tiwari, R.; Singh, S.; Kumar, K.; Srivastava, P.; Krishnamoorthi, S. Vat Dye Safranin O-and Perylene-Based Conjugated Donor–Acceptor Polyimide as Sensitizer for Dye-Sensitized Solar Cells. Energy Fuels 2020, 34, 14527–14534. [Google Scholar] [CrossRef]
- Nenavathu, B.P.; Kandula, S.; Verma, S. Visible-light-driven photocatalytic degradation of safranin-T dye using functionalized graphene oxide nanosheet (FGS)/ZnO nanocomposites. RSC Adv. 2018, 8, 19659–19667. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Liu, Z.; Gao, N.; Du, L.; Fu, Y. Ionic liquid-magnetic nanoparticle microextraction of safranin T in food samples. Food Anal. Methods 2015, 8, 541–548. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, T.; Liu, X.; Zhang, H. A novel molecularly imprinted polymer for simultaneous extraction and determination of sudan dyes by on-line solid phase extraction and high performance liquid chromatography. J. Chromatogr. A 2010, 1217, 6995–7002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X. Determination of safranine T in foods by ultra performance liquid chromatography-tandem mass spectrometry. J. Fuzhou Univ. 2009, 37, 752–755. [Google Scholar]
- Wang, W.; Zhu, X.; Yan, C. Determination of safranine T in food samples by CTAB sensitised fluorescence quenching method of the derivatives of calix [4] arene. Food Chem. 2013, 141, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, A.A.A.A.; Zhu, X.S. Magnetic Solid Phase Extraction Using Ionic Liquid Coated Magnetic Core Fe3O4@ SiO2 Nanoparticles Followed by UV Spectrophotometry for Separation/Analysis of Safranine T in Food. Am. J. Heterocycl. Chem. 2017, 3, 67–73. [Google Scholar] [CrossRef]
- Ali, M.; Usman, M.; Shah, A.; Rehman, A. Encapsulation of ethyl violet by anionic-cationic mixed micellar solution: Spectroscopic and conductometric studies. Colloids Surf. A 2021, 629, 127423. [Google Scholar] [CrossRef]
- Gohar, R.S.; Ehsan, M.F.; Karamat, N.; Najam-Ul-Haq, M.; Shah, A.; Nisar, J.; Qureshi, A.M.; Ashiq, M.N. Photomineralization of untreated wastewater by a novel LaCeZr2O7–SnSe nanocomposite as a visible light driven heterogeneous photocatalyst. Solid State Sci. 2020, 106, 106305. [Google Scholar] [CrossRef]
- Gohar, R.S.; Karamat, N.; Mumtaz, S.; Ahmad, B.; Shah, A.; Ashiq, M.N. Facile synthesis of LaDySn2O7 SnSe nanocomposite with excellent photocatalytic Activity under visible light. Mater. Chem. Phys. 2019, 229, 362–371. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Mehrabi, F.; Bazrafshan, A.A.; Ghaedi, A.M. Trace determination of safranin O dye using ultrasound assisted dispersive solid-phase micro extraction: Artificial neural network-genetic algorithm and response surface methodology. Ultrason. Sonochem. 2016, 33, 129–140. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Z.; Chen, Y.; Zhang, L.; Bu, X.; Zhang, H.; Tian, Y. Simple and rapid surface-enhanced Raman Spectroscopy assay for safranine T and its application in highly sensitive determination of mercury (II). Int. J. Environ. Anal. Chem. 2017, 97, 1178–1191. [Google Scholar] [CrossRef]
- Shah, A.; Malik, M.S.; Zahid, A.; Iftikhar, F.J.; Anwar, A.; Akhter, M.S.; Shah, M.R.; Zia, M.A.; Ashiq, M.N.; Shah, A.H. Carbamazepine coated silver nanoparticles for the simultaneous electrochemical sensing of specific food toxins. Electrochim. Acta 2018, 274, 131–142. [Google Scholar] [CrossRef]
- Kokab, T.; Manzoor, A.; Shah, A.; Siddiqi, H.M.; Nisar, J.; Ashiq, M.N.; Shah, A.H. Development of tribenzamide functionalized electrochemical sensor for femtomolar level sensing of multiple inorganic water pollutants. Electrochim. Acta 2020, 353, 136569. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Kokab, T.; Shah, A.; Khan, M.A.; Arshad, M.; Nisar, J.; Ashiq, M.N.; Zia, M.A. Simultaneous femtomolar detection of paracetamol, diclofenac, and orphenadrine using a carbon nanotube/zinc oxide nanoparticle-based electrochemical sensor. ACS Appl. Nano Mater. 2021, 4, 4699–4712. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar]
- Naqvi, S.T.R.; Rasheed, T.; Hussain, D.; ul Haq, M.N.; Majeed, S.; Ahmed, N.; Nawaz, R. Modification strategies for improving the solubility/dispersion of carbon nanotubes. J. Mol. Liq. 2020, 297, 111919. [Google Scholar] [CrossRef]
- Munir, A.; Bozal-Palabiyik, B.; Eren, G.; Shah, A.; Ali, S.; Uddin, N.; Uslu, B. Electrochemical Biosensor Design with Multi-walled Carbon Nanotube to Display DNA-Schiff Base Interaction. Electroanalysis 2021, 33, 1761–1770. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Khan, M.A.; Nisar, J.; Ashiq, M.N. Electrochemical sensing platform for the simultaneous femtomolar detection of amlodipine and atorvastatin drugs. RSC Adv. 2021, 11, 27135–27151. [Google Scholar] [CrossRef] [PubMed]
- Aslam, F.; Shah, A.; Ullah, N.; Munir, S. Multiwalled Carbon Nanotube/Fe-Doped ZnO-Based Sensors for Droplet Electrochemical Detection and Degradation Monitoring of Brilliant Green. ACS Appl. Nano Mater. 2023, 6, 6172–6185. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K. Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorg. Chem. Commun. 2020, 120, 108140. [Google Scholar] [CrossRef]
- Sadiq, M.U.; Shah, A.; Haleem, A.; Shah, S.M.; Shah, I. Eucalyptus globulus Mediated Green Synthesis of Environmentally Benign Metal Based Nanostructures: A Review. Nanomaterials 2023, 13, 2019. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Umar, A.; Al-Hajry, A.; Ahmad, R.; Ansari, S.; Al-Assiri, M.S.; Algarni, H. Fabrication and characterization of a highly sensitive hydroquinone chemical sensor based on iron-doped ZnO nanorods. Dalton Trans. 2015, 44, 21081–21087. [Google Scholar] [CrossRef] [PubMed]
- Khayatian, A.; Kashi, M.A.; Azimirad, R.; Safa, S. Enhanced gas-sensing properties of ZnO nanorods encapsulated in an Fe-doped ZnO shell. J. Phys. D Appl. Phys. 2014, 47, 075003. [Google Scholar] [CrossRef]
- Nurfani, E.; Lailani, A.; Kesuma, W.; Anrokhi, M.; Kadja, G.; Rozana, M. UV sensitivity enhancement in Fe-doped ZnO films grown by ultrafast spray pyrolysis. Opt. Mater. 2021, 112, 110768. [Google Scholar] [CrossRef]
- Ullah, R.; Iftikhar, F.J.; Ajmal, M.; Shah, A.; Akhter, M.S.; Ullah, H.; Waseem, A. Modified clays as an efficient adsorbent for brilliant green, ethyl violet and allura red dyes: Kinetic and thermodynamic studies. Pol. J. Environ. Stud. 2020, 29, 3831. [Google Scholar] [CrossRef]
- Liu, D.; Tian, R.; Wang, J.; Nie, E.; Piao, X.; Li, X.; Sun, Z. Photoelectrocatalytic degradation of methylene blue using F doped TiO2 photoelectrode under visible light irradiation. Chemosphere 2017, 185, 574–581. [Google Scholar] [CrossRef]
- Sarkar, S.; Ponce, N.T.; Banerjee, A.; Bandopadhyay, R.; Rajendran, S.; Lichtfouse, E. Green polymeric nanomaterials for the photocatalytic degradation of dyes: A review. Environ. Chem. Lett. 2020, 18, 1569–1580. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review of solar and visible light active oxo-bridged materials for energy and environment. Catal. Sci. Technol. 2017, 7, 2153–2164. [Google Scholar] [CrossRef]
- El-Kemary, M.; Abdel-Moneam, Y.; Madkour, M.; El-Mehasseb, I. Enhanced photocatalytic degradation of Safranin-O by heterogeneous nanoparticles for environmental applications. J. Lumin. 2011, 131, 570–576. [Google Scholar] [CrossRef]
- Janaki, V.; Oh, B.-T.; Shanthi, K.; Lee, K.-J.; Ramasamy, A.; Kamala-Kannan, S. Efficiency of various semiconductor catalysts for photodegradation of Safranin-T. Res. Chem. Intermed. 2012, 38, 1431–1442. [Google Scholar] [CrossRef]
- Hammad, E.N.; Salem, S.S.; Zohair, M.M.; Mohamed, A.A.; El-Dougdoug, W. Purpureocillium lilacinum mediated biosynthesis copper oxide nanoparticles with promising removal of dyes. Biointerface Res. Appl. Chem. 2022, 12, 1397–1404. [Google Scholar]
- Chandrasekar, L.P.; Sethuraman, B.D.; Subramani, M.; Mohandos, S. Green synthesised ZnO nanoparticles from Plectranthus amboinicus plant extract: Removal of Safranin-O and Malachite green dyes & anti-bacterial activity. Int. J. Environ. Anal. Chem. 2023, 1–18. [Google Scholar] [CrossRef]
- Kamal, A.; Saleem, M.H.; Alshaya, H.; Okla, M.K.; Chaudhary, H.J.; Munis, M.F.H. Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalytic ability against different organic and inorganic pollutants. J. Saudi Chem. Soc. 2022, 26, 101445. [Google Scholar] [CrossRef]
- Saraffin, R.S.; Kapuria, A.; Saha, S.K.; Dutta, K. Eco-friendly hetero-system silver decorated nanocomposite zinc oxide/reduced graphene oxide: Improved broad-ranged absorptivity and enhanced photocatalytic performance under white-light irradiation. React. Kinet. Mech. Catal. 2022, 135, 2827–2850. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef]
- Ahmad, K.S.; Jaffri, S.B. Phytosynthetic Ag doped ZnO nanoparticles: Semiconducting green remediators: Photocatalytic and antimicrobial potential of green nanoparticles. Open Chem. 2018, 16, 556–570. [Google Scholar] [CrossRef]



| S. No. | Photocatalyst | Catalyst Dose (mg) | % Removal | Irradiation Time (min) | Light Source | References |
|---|---|---|---|---|---|---|
| 1 | Maize biochar-ZnO nanocomposite | 5 | 87 | 60 | 500 W Xe lamp | [56] |
| 2 | Zn0 | 100 | 85.29 | 90 | UV light | [55] |
| 3 | Ag decorated ZnO/rGO | - | 100 | 80 | Philips white LED | [57] |
| 4 | ZnS | - | 51 | 40 | Xe lamp | [52] |
| 5 | Zn0.98Mn0.02S, Zn0.94Ni0.06S and Zn0.90Cu0.10S | - | 85.4–93.2 | - | 100 W mercury lamp | [58] |
| 6 | Ag-doped ZnO | 50 | 74.11 | 20 | Sunlight | [59] |
| 7 | Fe-ZnO | 2 | ≈99 | 60 | Sunlight | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, M.U.; Shah, A.; Nisar, J.; Shah, I. Photoelectrocatalytic Detection and Degradation Studies of a Hazardous Textile Dye Safranin T. Nanomaterials 2023, 13, 2218. https://doi.org/10.3390/nano13152218
Sadiq MU, Shah A, Nisar J, Shah I. Photoelectrocatalytic Detection and Degradation Studies of a Hazardous Textile Dye Safranin T. Nanomaterials. 2023; 13(15):2218. https://doi.org/10.3390/nano13152218
Chicago/Turabian StyleSadiq, Muhammad Usman, Afzal Shah, Jan Nisar, and Iltaf Shah. 2023. "Photoelectrocatalytic Detection and Degradation Studies of a Hazardous Textile Dye Safranin T" Nanomaterials 13, no. 15: 2218. https://doi.org/10.3390/nano13152218
APA StyleSadiq, M. U., Shah, A., Nisar, J., & Shah, I. (2023). Photoelectrocatalytic Detection and Degradation Studies of a Hazardous Textile Dye Safranin T. Nanomaterials, 13(15), 2218. https://doi.org/10.3390/nano13152218








