Conductometric Gas Sensor Based on MoO3 Nanoribbon Modified with rGO Nanosheets for Ethylenediamine Detection at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MoO3/rGO
2.3. Characterization
2.4. Sensor Fabrication and Measurement
2.5. Electrochemical Measurements
3. Results and Discussion
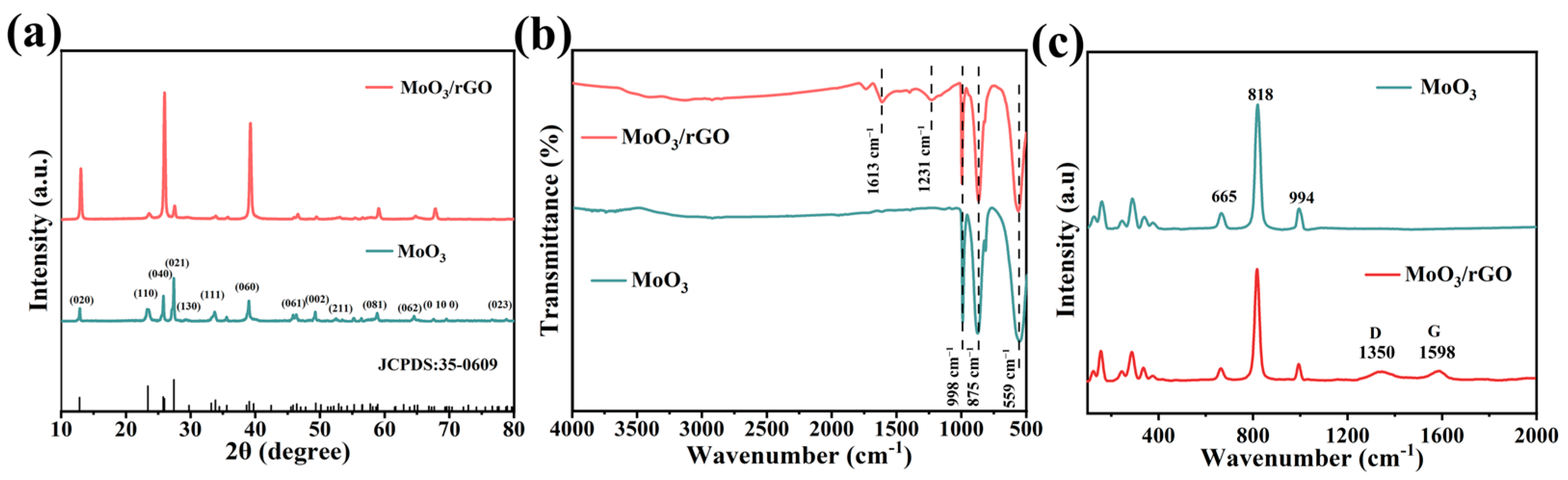
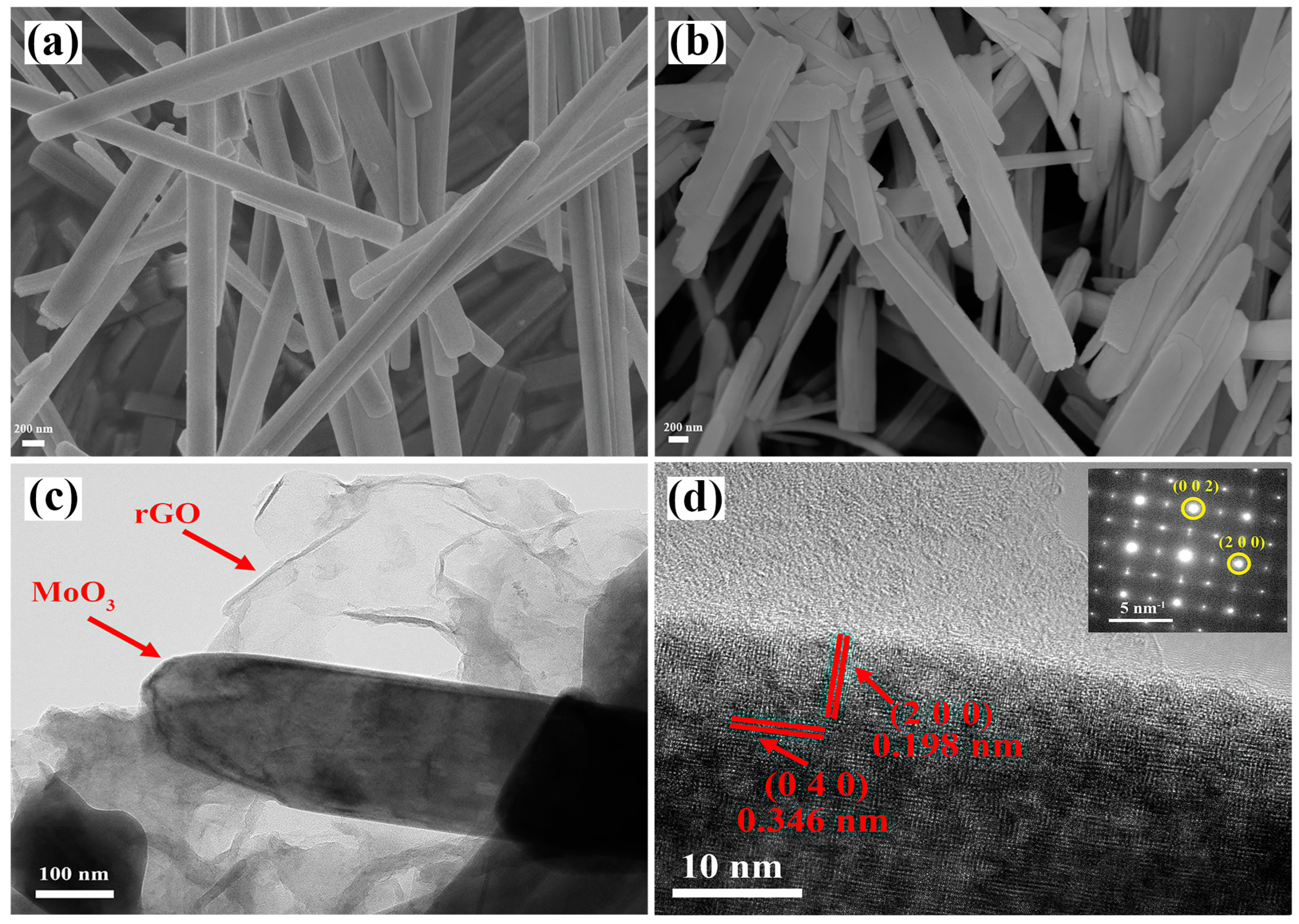
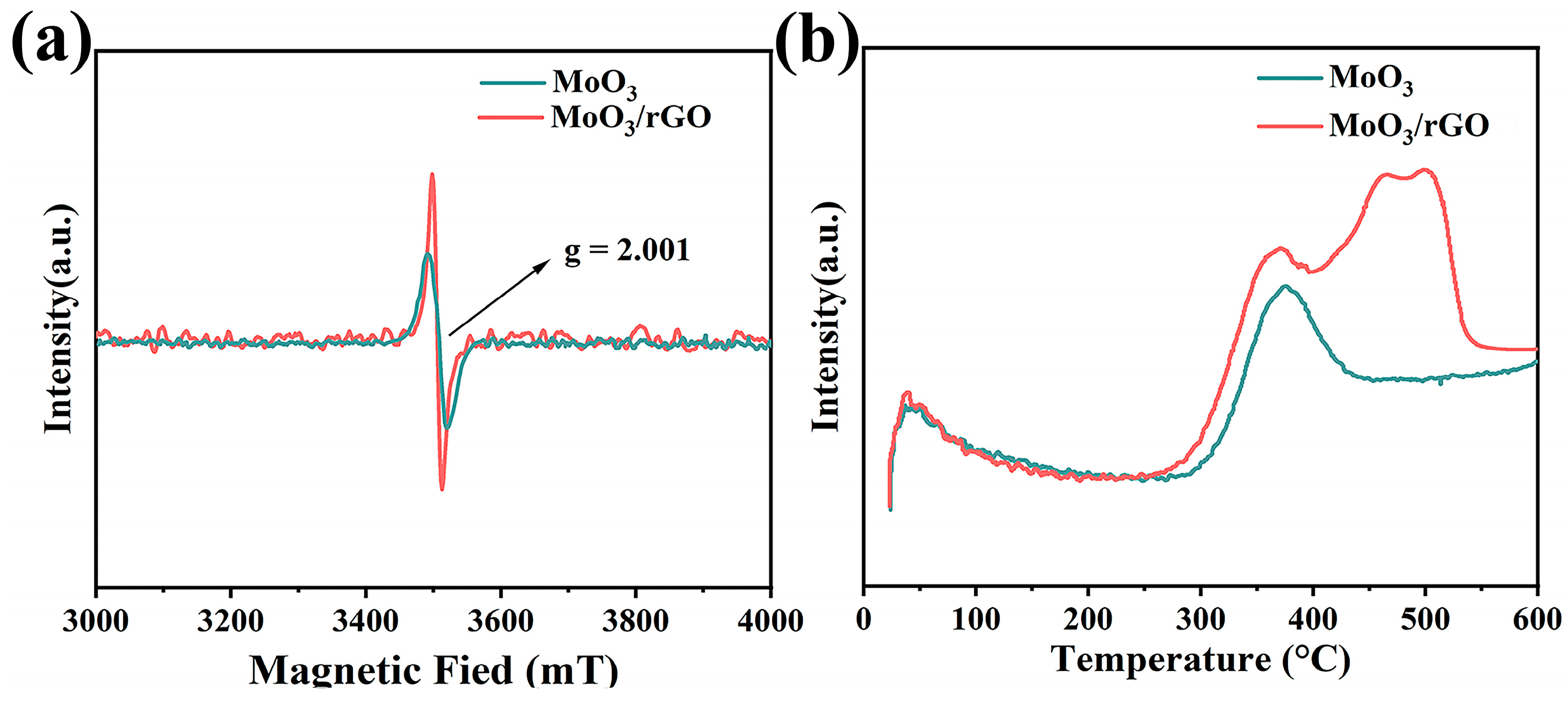
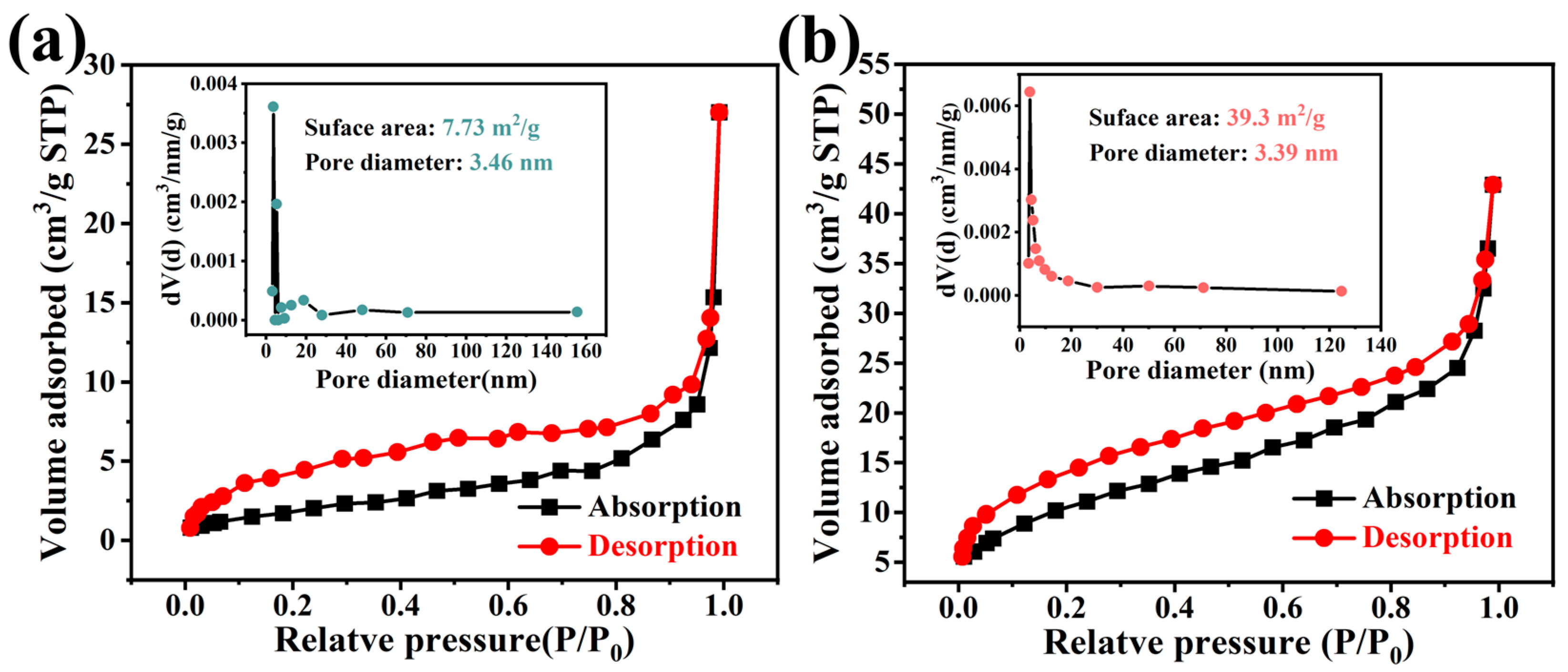
3.1. Structural and Morphological Characterization
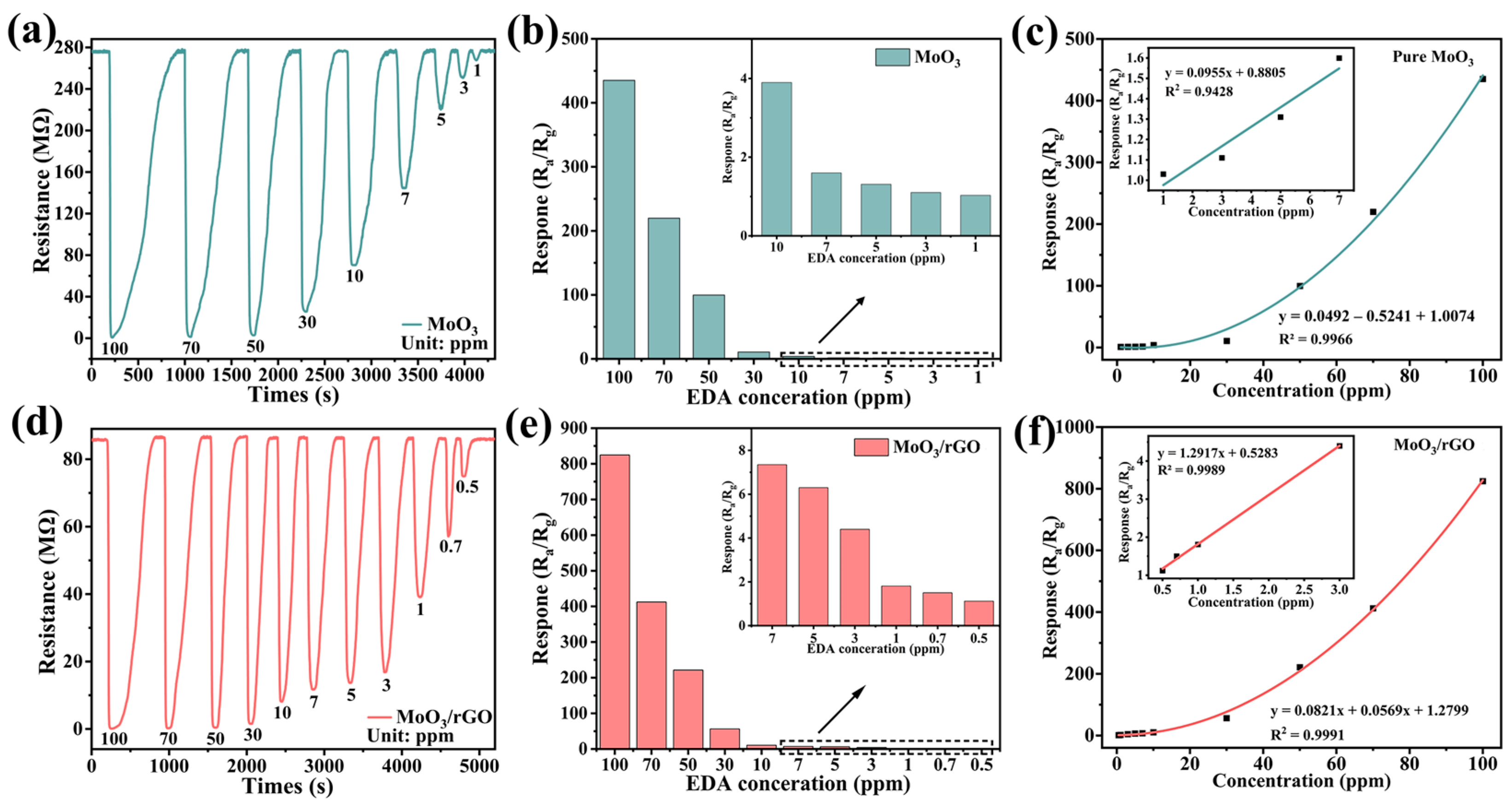
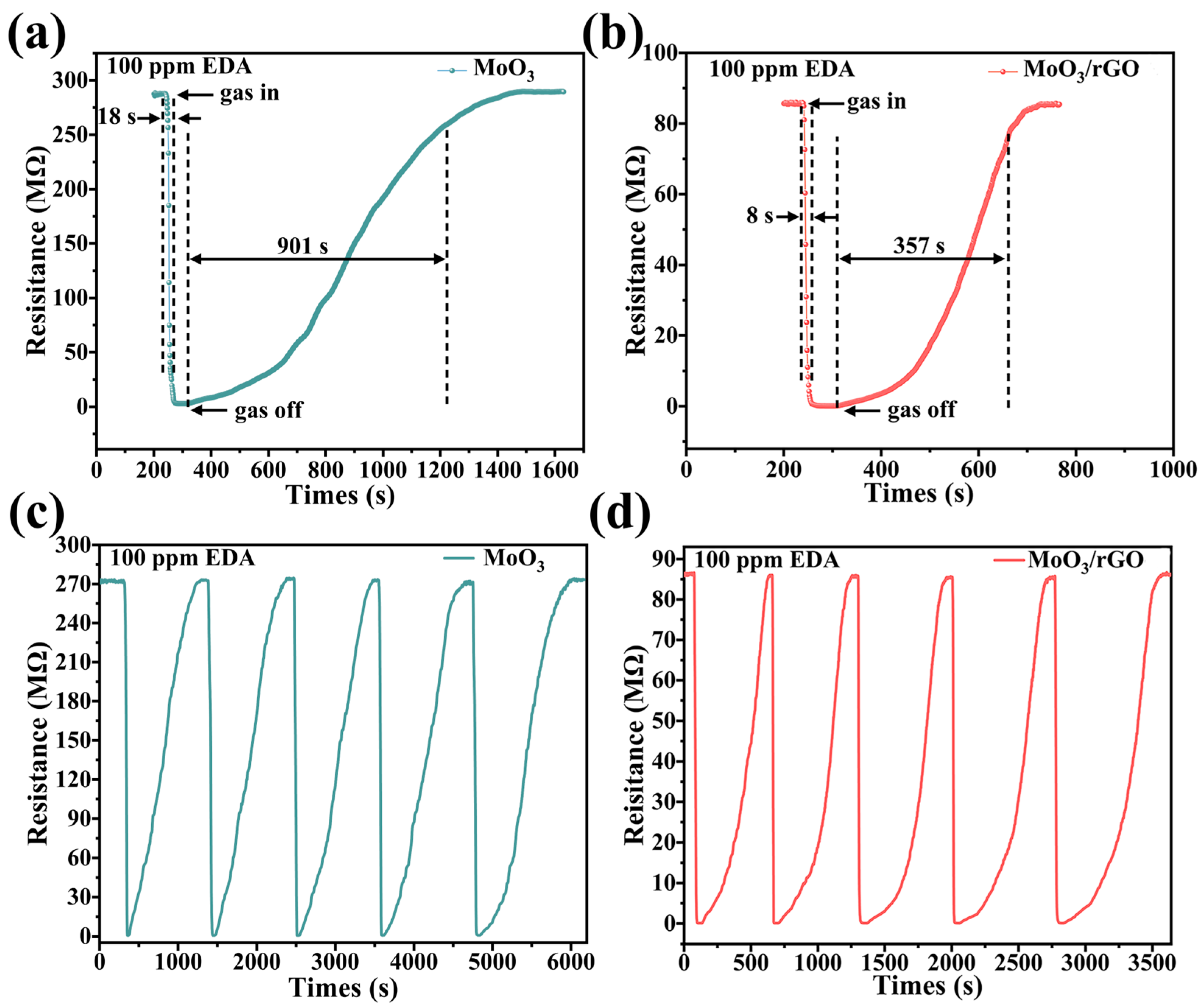
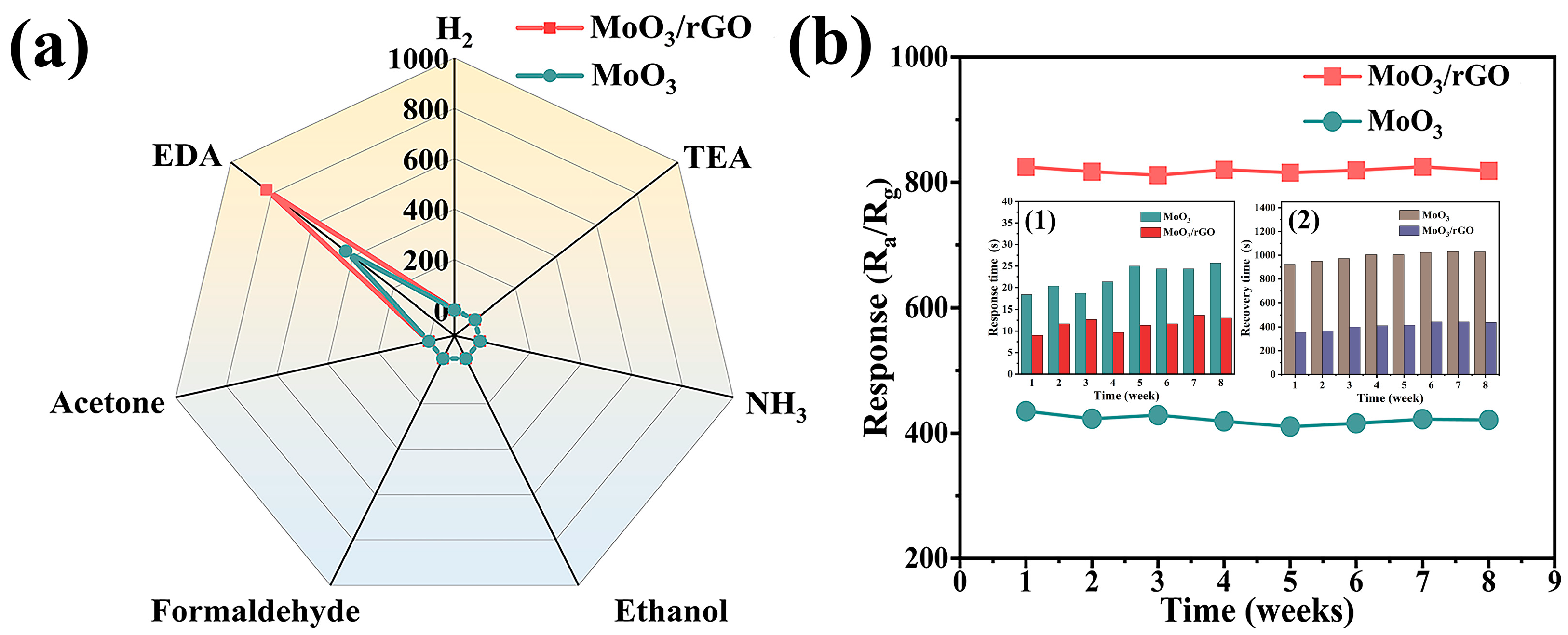
3.2. Gas-Sensing Properties
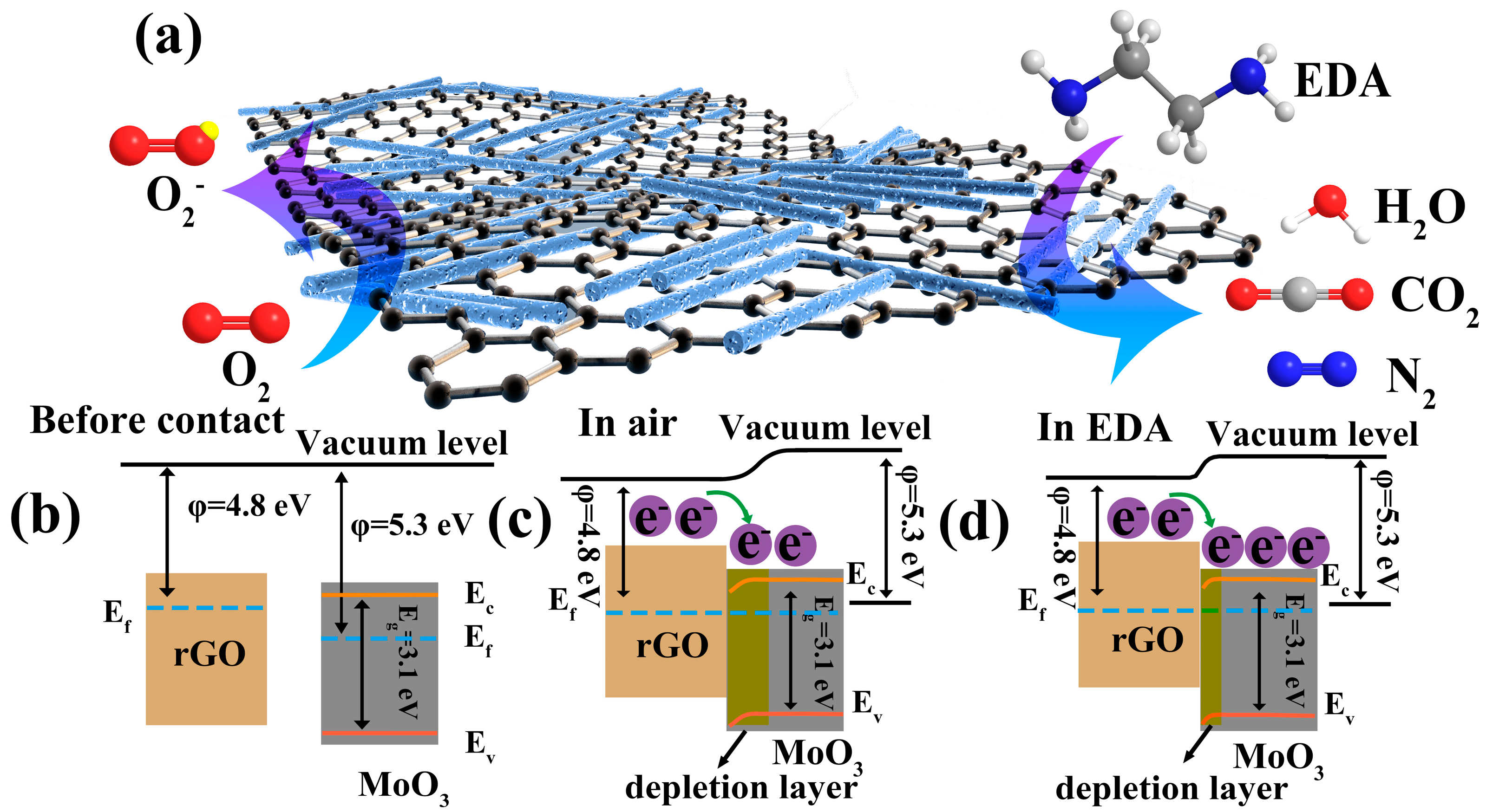
3.3. Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; Hu, J.-P.; Dang, L.-R.; Yao, H.; Shi, B.; Zhang, Y.-M.; Wei, T.-B.; Lin, Q. Rational Tuning of Binding Properties of Pillar[5]arene-Based Crystalline Material by Synergistic Effect and Its Application for Fluorescent Detection and Adsorption of 1,2-Ethylenediamine. ACS Sustain. Chem. Eng. 2021, 9, 16203–16209. [Google Scholar] [CrossRef]
- Li, P.; Yang, D.; Li, H. Luminescence ethylenediamine sensor based on terbium complexes entrapment. Dye. Pigment. 2016, 132, 306–309. [Google Scholar] [CrossRef]
- Chuang, P.-M.; Tu, Y.-J.; Wu, J.-Y. A thiadiazole-functionalized Zn(II)-based luminescent coordination polymer with seven-fold interweaved herringbone nets showing solvent-responsive fluorescence properties and discriminative detection of ethylenediamine. Sens. Actuators B Chem. 2022, 366, 131967. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zhu, W.-J.; Yang, Z.-X.; Feng, Y.; Fan, L.-L.; Gao, G.-G.; Liu, H. Ultrasensitive photochromic and Raman dual response to ethylenediamine gas through polyoxometalate–viologen crystalline hybrid. J. Mater. Chem. C 2022, 10, 15451–15457. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, Y.; Zu, B.; Lei, D.; Wang, G.; Li, J.; Ren, W.; Dou, X. Electronic Tuning in Reaction-Based Fluorescent Sensing for Instantaneous and Ultrasensitive Visualization of Ethylenediamine. Angew. Chem. Int. Ed. Engl. 2022, 61, e202203358. [Google Scholar] [CrossRef]
- Nizamidin, P.; Yimit, A.; Yan, Y.; Kutilike, B.; Kari, N.; Mamtimin, G. Fast fabrication and gas-sensing characteristics of petal-like Co-MOF membrane optical waveguide. Sens. Actuators B Chem. 2021, 346, 130342. [Google Scholar] [CrossRef]
- Ni, J.; Li, M.Y.; Liu, Z.; Zhao, H.; Zhang, J.J.; Liu, S.Q.; Chen, J.; Duan, C.Y.; Chen, L.Y.; Song, X.D. Discrimination of Various Amine Vapors by a Triemissive Metal-Organic Framework Composite via the Combination of a Three-Dimensional Ratiometric Approach and a Confinement-Induced Enhancement Effect. ACS Appl. Mater. Interfaces 2020, 12, 12043–12053. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X.; Wang, Q.; Fu, J.; Zhao, L.; Liu, Z.; Jin, D. Highly responsive ethylenediamine vapor sensor based on a perylenediimide–camphorsulfonic acid complex via ionic self-assembly. J. Mater. Chem. C 2017, 5, 7644–7651. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Cheng, X.; Zhang, X.; Guo, C.; Xu, Y.; Gao, S.; Major, Z.; Zhao, H.; Huo, L. Fast detection of NO2 by porous SnO2 nanotoast sensor at low temperature. J. Hazard. Mater. 2021, 419, 126414. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.; Hu, Y.; Luo, X.; Lei, J.; Zhou, D.; Fei, L.; Wang, Y.; Gu, H. Highly Responsive Room-Temperature Hydrogen Sensing of alpha-MoO3 Nanoribbon Membranes. ACS Appl. Mater. Interfaces 2015, 7, 9247–9253. [Google Scholar] [CrossRef]
- Sui, L.-L.; Xu, Y.-M.; Zhang, X.-F.; Cheng, X.-L.; Gao, S.; Zhao, H.; Cai, Z.; Huo, L.-H. Construction of three-dimensional flower-like α-MoO3 with hierarchical structure for highly selective triethylamine sensor. Sens. Actuators B Chem. 2015, 208, 406–414. [Google Scholar] [CrossRef]
- Fu, H.; Yang, X.; Wu, Z.; He, P.; Xiong, S.; Han, D.; An, X. Gas-sensing performance of In2O3@MoO3 hollow core-shell nanospheres prepared by a two-step hydrothermal method. Sens. Actuators B Chem. 2022, 352, 131007. [Google Scholar] [CrossRef]
- Sui, L.; Zhang, X.; Cheng, X.; Wang, P.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Au-Loaded Hierarchical MoO3 Hollow Spheres with Enhanced Gas-Sensing Performance for the Detection of BTX (Benzene, Toluene, And Xylene) And the Sensing Mechanism. ACS Appl. Mater. Interfaces 2017, 9, 1661–1670. [Google Scholar] [CrossRef]
- Hermawan, A.; Septiani, N.L.W.; Taufik, A.; Yuliarto, B.; Suyatman; Yin, S. Advanced Strategies to Improve Performances of Molybdenum-Based Gas Sensors. Nano-Micro Lett. 2021, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Chen, S.; Chen, L.; Zhang, K.; Luo, R.; Li, D.; Liu, C.C. Ultrasonic synthesis of MoO3 nanorods and their gas sensing properties. Sens. Actuators B Chem. 2012, 174, 51–58. [Google Scholar] [CrossRef]
- Mo, Y.; Tan, Z.; Sun, L.; Lu, Y.; Liu, X. Ethanol-sensing properties of α-MoO3 nanobelts synthesized by hydrothermal method. J. Alloys Compd. 2020, 812, 152166. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Jin, L.; Zhang, B.; Zhang, H.; Zhu, M.; Yang, W. Self-assembly gridding α-MoO3 nanobelts for highly toxic H2S gas sensors. Sens. Actuators B Chem. 2016, 237, 350–357. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; Liu, W.; Lu, Y.; et al. Au@ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem. C 2022, 10, 4295–4305. [Google Scholar] [CrossRef]
- Moon, D.-B.; Bag, A.; Lee, H.-B.; Meeseepong, M.; Lee, D.-H.; Lee, N.-E. A stretchable, room-temperature operable, chemiresistive gas sensor using nanohybrids of reduced graphene oxide and zinc oxide nanorods. Sens. Actuators B Chem. 2021, 345, 130373. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.-L.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D.; et al. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B Chem. 2019, 290, 443–452. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, S.; Yang, Y.; Yu, H.; Dong, X. Highly sensitive H2S sensors based on metal-organic framework driven γ-Fe2O3 on reduced graphene oxide composites at room temperature. Sens. Actuators B Chem. 2020, 325, 128804. [Google Scholar] [CrossRef]
- He, L.; Lv, H.; Ma, L.; Li, W.; Si, J.; Ikram, M.; Ullah, M.; Wu, H.; Wang, R.; Shi, K. Controllable synthesis of intercalated γ-Bi2MoO6/graphene nanosheet composites for high performance NO2 gas sensor at room temperature. Carbon 2020, 157, 22–32. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A.; Sriprachuabwong, C.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Electrolytically exfoliated graphene-loaded flame-made Ni-doped SnO2 composite film for acetone sensing. ACS Appl. Mater. Interfaces 2015, 7, 3077–3092. [Google Scholar] [CrossRef]
- Bai, H.; Guo, H.; Wang, J.; Dong, Y.; Liu, B.; Xie, Z.; Guo, F.; Chen, D.; Zhang, R.; Zheng, Y. A room-temperature NO2 gas sensor based on CuO nanoflakes modified with rGO nanosheets. Sens. Actuators B Chem. 2021, 337, 129783. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Li, N.; Ye, M. Fabrication of γ-MnS/rGO composite by facile one-pot solvothermal approach for supercapacitor applications. J. Power Sources 2015, 282, 194–201. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Pan, Q.; Pan, K.; Zhang, G. Metal-organic framework (MOF) derived In2O3 and g-C3N4 composite for superior NOx gas-sensing performance at room temperature. Sens. Actuators B Chem. 2022, 352, 131001. [Google Scholar] [CrossRef]
- Mane, A.A.; Suryawanshi, M.P.; Kim, J.H.; Moholkar, A.V. Highly selective and sensitive response of 30.5 % of sprayed molybdenum trioxide (MoO3) nanobelts for nitrogen dioxide (NO2) gas detection. J. Colloid Interface Sci. 2016, 483, 220–231. [Google Scholar] [CrossRef]
- Chen, J.; Han, S.; Zhao, H.; Bai, J.; Wang, L.; Sun, G.; Zhang, Z.; Pan, X.; Zhou, J.; Xie, E. Robust wire-based supercapacitors based on hierarchical α-MoO3 nanosheet arrays with well-aligned laminated structure. Chem. Eng. J. 2017, 320, 34–42. [Google Scholar] [CrossRef]
- Bai, S.; Sun, L.; Sun, J.; Han, J.; Zhang, K.; Li, Q.; Luo, R.; Li, D.; Chen, A. Pine dendritic bismuth vanadate loaded on reduced graphene oxide for detection of low concentration triethylamine. J. Colloid Interface Sci. 2021, 587, 183–191. [Google Scholar] [CrossRef]
- Kawase, M.; Akaike, K.; Aoyama, K.; Ito, Y.; Tamura, M.; Kanai, K. Elucidation of the enhanced photoactivity of melon calcined with MoO3. Appl. Catal. B Environ. 2020, 273, 119068. [Google Scholar] [CrossRef]
- Wang, Z.; Han, T.; Fei, T.; Liu, S.; Zhang, T. Investigation of Microstructure Effect on NO2 Sensors Based on SnO2 Nanoparticles/Reduced Graphene Oxide Hybrids. ACS Appl. Mater. Interfaces 2018, 10, 41773–41783. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, P.; Bao, D.; Wang, Y.; Chen, Y.; Chang, C.; Li, G.; Yang, P. Synthesis of Crystalline/Amorphous Core/Shell MoO3 Composites through a Controlled Dehydration Route and Their Enhanced Ethanol Sensing Properties. Cryst. Growth Des. 2014, 14, 569–575. [Google Scholar] [CrossRef]
- Hou, X.; Ma, C.; Ji, H.; Yi, S.; Zhang, L.; Zhang, Z.; Wang, Y.; Yuan, L.; Chen, D.; Zhou, Y. Loading regulation of gold nanoparticles on self-assembled 3D MoO3 hierarchical structure for high triethylamine sensing. Sens. Actuators B Chem. 2023, 393, 134241. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Gao, L.; Zhao, J.; Chen, Z.; Liu, B.; Tang, D.; Yu, B.; Jiang, C.; Cheng, H.-M. Synthesis of Graphene Sheets with High Electrical Conductivity and Good Thermal Stability by Hydrogen Arc Discharge Exfoliation. ACS Nano 2009, 3, 411–417. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, X.; Chen, P.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Creating oxygen-vacancies in MoO3-x nanobelts toward high volumetric energy-density asymmetric supercapacitors with long lifespan. Nano Energy 2019, 58, 455–465. [Google Scholar] [CrossRef]
- Zi, X.; Wan, J.; Yang, X.; Tian, W.; Zhang, H.; Wang, Y. Vacancy-rich 1T-MoS2 monolayer confined to MoO3 matrix: An interface-engineered hybrid for efficiently electrocatalytic conversion of nitrogen to ammonia. Appl. Catal. B Environ. 2021, 286, 119870. [Google Scholar] [CrossRef]
- Fang, L.; Shu, Y.; Wang, A.; Zhang, T. Green Synthesis and Characterization of Anisotropic Uniform Single-Crystal α-MoO3 Nanostructures. J. Phys. Chem. C 2007, 111, 2401–2408. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, M.; Ni, J.; Li, L. Ultrastable Sodium Storage in MoO3 Nanotube Arrays Enabled by Surface Phosphorylation. ACS Appl. Mater. Interfaces 2019, 11, 37761–37767. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wang, Q.; Wang, Q.; Zhu, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J.; Zhang, Q. High-Capacity and Kinetically Accelerated Lithium Storage in MoO3 Enabled by Oxygen Vacancies and Heterostructure. Adv. Energy Mater. 2021, 11, 2101712. [Google Scholar] [CrossRef]
- Ma, J.; Fan, H.; Zhang, W.; Sui, J.; Wang, C.; Zhang, M.; Zhao, N.; Kumar Yadav, A.; Wang, W.; Dong, W.; et al. High sensitivity and ultra-low detection limit of chlorine gas sensor based on In2O3 nanosheets by a simple template method. Sens. Actuators B Chem. 2020, 305, 127456. [Google Scholar] [CrossRef]
- Zhang, B.; Bao, N.; Wang, T.; Xu, Y.; Dong, Y.; Ni, Y.; Yu, P.; Wei, Q.; Wang, J.; Guo, L.; et al. High-performance room temperature NO2 gas sensor based on visible light irradiated In2O3 nanowires. J. Alloys Compd. 2021, 867, 159076. [Google Scholar] [CrossRef]
- Lei, Z.; Cheng, P.; Wang, Y.; Xu, L.; Lv, L.; Li, X.; Sun, S.; Hao, X.; Zhang, Y.; Zhang, Y.; et al. Pt-doped α-Fe2O3 mesoporous microspheres with low-temperature ultra-sensitive properties for gas sensors in diabetes detection. Appl. Surf. Sci. 2023, 607, 154558. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Zhang, Q.; He, X.; Wang, X. Synthesis of MoO3 (1D) @SnO2 (2D) core-shell heterostructures for enhanced ethanol gas sensing performance. Sens. Actuators B Chem. 2023, 382, 133484. [Google Scholar] [CrossRef]
- Okai Amu-Darko, J.N.; Hussain, S.; Zhang, X.; Alothman, A.A.; Ouladsmane, M.; Nazir, M.T.; Qiao, G.; Liu, G. Metal-organic frameworks-derived In2O3/ZnO porous hollow nanocages for highly sensitive H2S gas sensor. Chemosphere 2023, 314, 137670. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, J.; Liu, H.; Pan, Q.; Zhang, G. The development of high-performance room temperature NOX one-dimensional Na0.23TiO2/TiO2 compound gas sensor. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129444. [Google Scholar] [CrossRef]
- Yin, H.; Chen, Z.; Peng, Y.; Xiong, S.; Li, Y.; Yamashita, H.; Li, J. Dual Active Centers Bridged by Oxygen Vacancies of Ruthenium Single-Atom Hybrids Supported on Molybdenum Oxide for Photocatalytic Ammonia Synthesis. Angew. Chem. Int. Ed. 2022, 61, e202114242. [Google Scholar] [CrossRef]
- Jin, Y.-J.; Kwak, G. Detection of biogenic amines using a nitrated conjugated polymer. Sens. Actuators B Chem. 2018, 271, 183–188. [Google Scholar] [CrossRef]
- Shang, C.; Wang, L.; An, Y.; Chen, P.; Chang, X.; Qi, Y.; Kang, R.; Fang, Y. Langmuir-Blodgett films of perylene bisimide derivatives and fluorescent recognition of diamines. Phys. Chem. Chem. Phys. 2017, 19, 23898–23904. [Google Scholar] [CrossRef]
- Ma, J.-X.; Zhou, T.; Ma, T.; Yang, Z.; Yang, J.-H.; Guo, Q.; Liu, W.; Yang, Q.; Liu, W.; Yang, T. Construction of Transition Metal Coordination Polymers with Free Carboxyl Groups and Turn-On Fluorescent Detection for α,β-Diamine. Cryst. Growth Des. 2020, 21, 383–395. [Google Scholar] [CrossRef]
- Caraballo, R.M.; Onna, D.; López Abdala, N.; Soler Illia, G.J.A.A.; Hamer, M. Metalloporphyrins into mesoporous photonic crystals: Towards molecularly-tuned photonic sensing devices. Sens. Actuators B Chem. 2020, 309, 127712. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Zhao, H.; Guo, Z.; Xing, H.; Gao, Y. Electrically Conductive Coordination Polymer for Highly Selective Chemiresistive Sensing of Volatile Amines. Inorg. Chem. 2017, 57, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Tian, Y.; Yao, S.L.; Zhang, J.; Feng, R.Y.; Bian, Y.J.; Liu, S.J. Cd(II) -Organic Frameworks Fabricated with a N-Rich Ligand and Flexible Dicarboxylates: Structural Diversity and Multi-Responsive Luminescent Sensing for Toxic Anions and Ethylenediamine. Chem. Asian J. 2019, 14, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, B. Wearable Eu@HOF luminescent fabric as a highly selective and sensitive optical synapse sensor for identification of six laboratory volatile compounds by neuromorphic computing. J. Mater. Chem. A 2022, 10, 15427–15437. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Han, N.; Liao, D.; Bai, S.; Yang, X.; Luo, R.; Li, D.; Chen, A. rGO decorated W doped BiVO4 novel material for sensing detection of trimethylamine. Sens. Actuators B Chem. 2019, 298, 126749. [Google Scholar] [CrossRef]
- Singh, S.K.; Samanta, U.K.; Dhar, A.; Pal, M.; Paul, M.C. Preparation of Bi-doped ZnO thin film over optical fiber and their application as detection of ethylenediamine in an aqueous medium based on the evanescent field technique. Phys. Status Solidi (a) 2020, 217, 2000537. [Google Scholar] [CrossRef]
- Li, W.; Ou, Q.; Wang, X.; Xing, K.; Tesfamichael, T.; Motta, N.; Qi, D.-C. Large-sized α-MoO3 layered single crystals for superior NO2 gas sensing. Appl. Surf. Sci. 2022, 586, 152793. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Liu, Y.; Yang, S.; Zhou, J.; Chen, W. SnO2 Quantum Dots-Functionalized MoO3 Nanobelts for High-Selectivity Ethylene Sensing. ACS Appl. Nano Mater. 2022, 5, 10485–10494. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, J.; Song, P.; Li, J.; Yang, Z.; Wang, Q. MOF-derived α-Fe2O3 porous spindle combined with reduced graphene oxide for improvement of TEA sensing performance. Sens. Actuators B Chem. 2020, 304, 127306. [Google Scholar] [CrossRef]
- Mo, R.; Han, D.; Yang, C.; Tang, J.; Wang, F.; Li, C. MOF-derived porous Fe2O3 nanocubes combined with reduced graphene oxide for n-butanol room temperature gas sensing. Sens. Actuators B Chem. 2021, 330, 129326. [Google Scholar] [CrossRef]


| Samples | Mo5+ (%) | Mo6+ (%) | OL (%) | OV (%) | OC (%) |
|---|---|---|---|---|---|
| MoO3 | 4.6 | 65.4 | 90.9 | 6.2 | 2.9 |
| MoO3/rGO | 9.7 | 90.3 | 80.3 | 11.9 | 7.8 |
| Materials | Method | EDA (ppm) | Response | LOD (ppm) | Ref. |
|---|---|---|---|---|---|
| Nitrated polythiophen | Colorimetry | 5010 | 1.99 (A/A0) | 5.6 | [47] |
| Perylene bisimide | Fluorescence | 85.2 | 1.39 (I0/I) | 4.0 | [48] |
| [Zn4(HIDCPy)4(DMSO)(DMF)3]n | Fluorescence | 450 | 1.77 (I/I0 − 1) | 3.9 | [49] |
| MP@MOP | Optical | 80 | 0.17 (∆Abs) | 15 | [50] |
| OPTA-MSA | Fluorescence | 50 | 80.3 (∆E) | 0.70 | [5] |
| Zn2(bcpBTD)2(bpBTD)(H2O)2]·DMF(1) | Fluorescence | 59.8 | 3.8 (I/I0) | 0.052 | [3] |
| [Cd(H2L)2]·3H2O·2DMF | Resistance | 900 | 45% | / | [51] |
| {[Cd(L)(glu)]·3H2O}∞ | Fluorescence | 2500 | 3.7 (I0/I) | 64.5 | [52] |
| Eu@IsoMe@Cu/Ni fabric | Optical | 200 | 51.1 | 4.74 | [53] |
| MoO3/rGO | Resistance | 100 | 834.7 | 0.235 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, J.; Liu, Q.; Li, Y.; Zhang, G.; He, C. Conductometric Gas Sensor Based on MoO3 Nanoribbon Modified with rGO Nanosheets for Ethylenediamine Detection at Room Temperature. Nanomaterials 2023, 13, 2220. https://doi.org/10.3390/nano13152220
Liu H, Liu J, Liu Q, Li Y, Zhang G, He C. Conductometric Gas Sensor Based on MoO3 Nanoribbon Modified with rGO Nanosheets for Ethylenediamine Detection at Room Temperature. Nanomaterials. 2023; 13(15):2220. https://doi.org/10.3390/nano13152220
Chicago/Turabian StyleLiu, Hongda, Jiongjiang Liu, Qi Liu, Yinghui Li, Guo Zhang, and Chunying He. 2023. "Conductometric Gas Sensor Based on MoO3 Nanoribbon Modified with rGO Nanosheets for Ethylenediamine Detection at Room Temperature" Nanomaterials 13, no. 15: 2220. https://doi.org/10.3390/nano13152220
APA StyleLiu, H., Liu, J., Liu, Q., Li, Y., Zhang, G., & He, C. (2023). Conductometric Gas Sensor Based on MoO3 Nanoribbon Modified with rGO Nanosheets for Ethylenediamine Detection at Room Temperature. Nanomaterials, 13(15), 2220. https://doi.org/10.3390/nano13152220






