Temperature-Dependent Optical Properties of Oxidized Graphenes
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
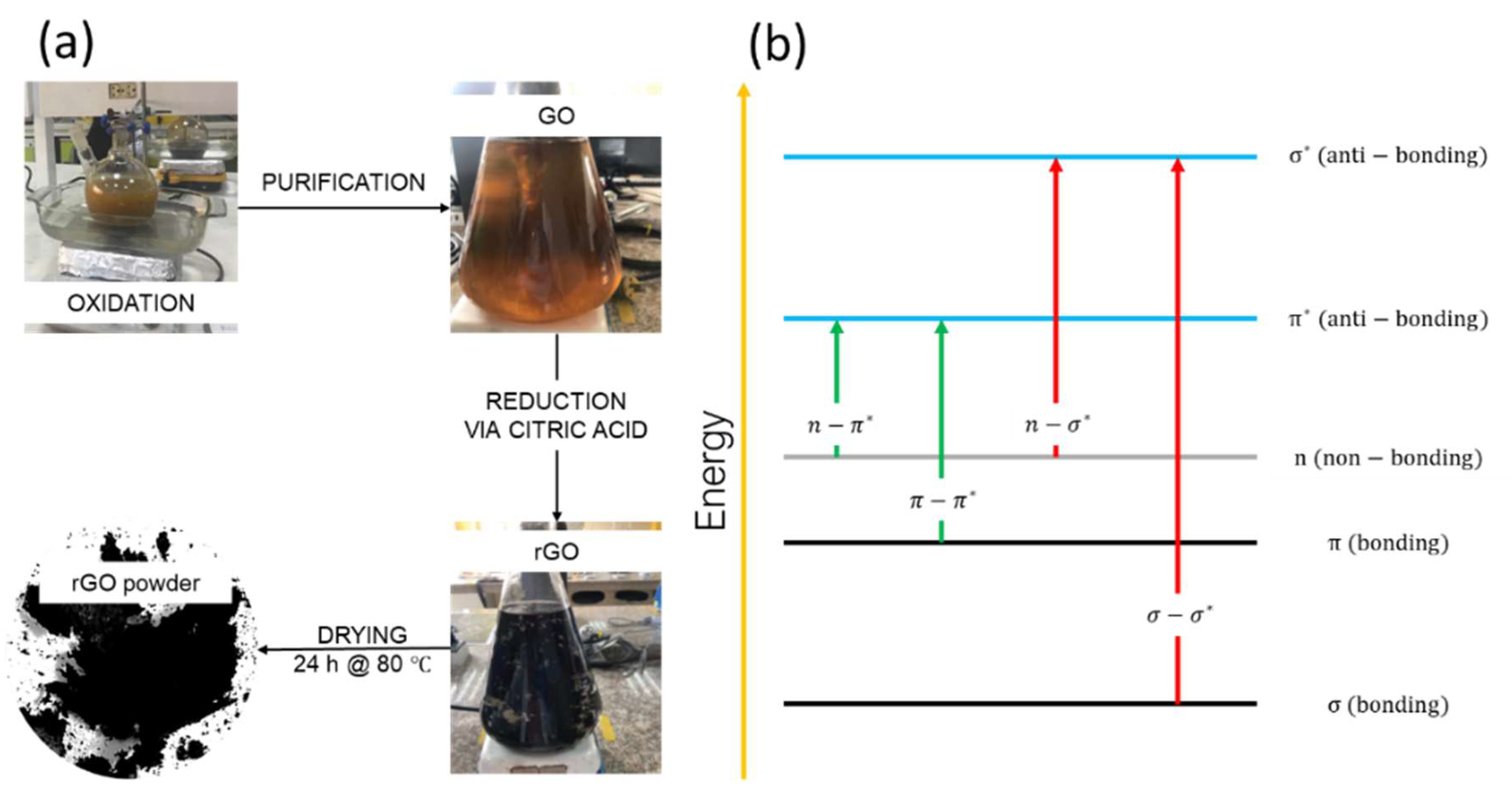
2.2. Synthesis of GO and rGO
2.3. Characterization
3. Results and Discussion
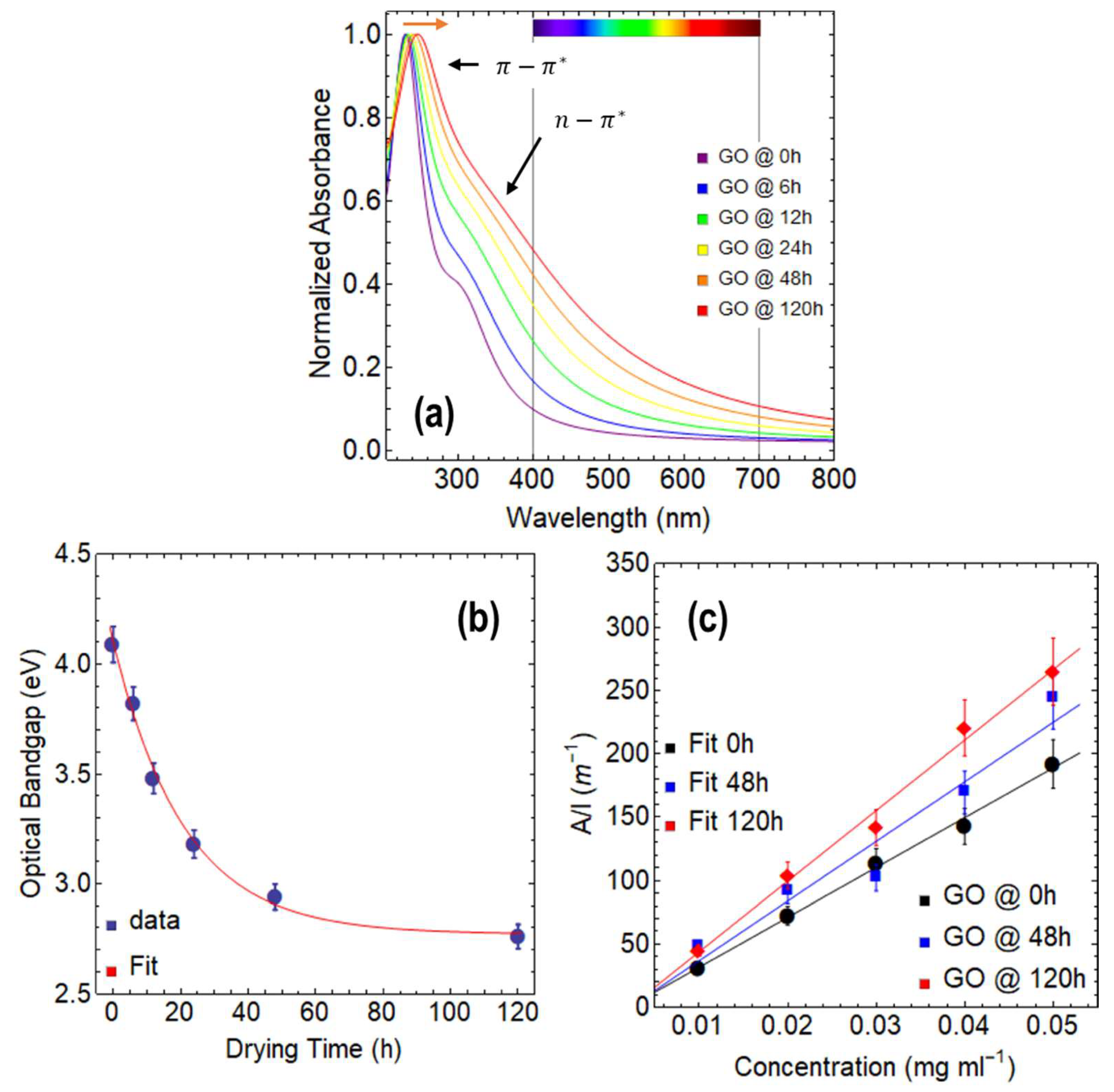
3.1. Optical Properties of GO
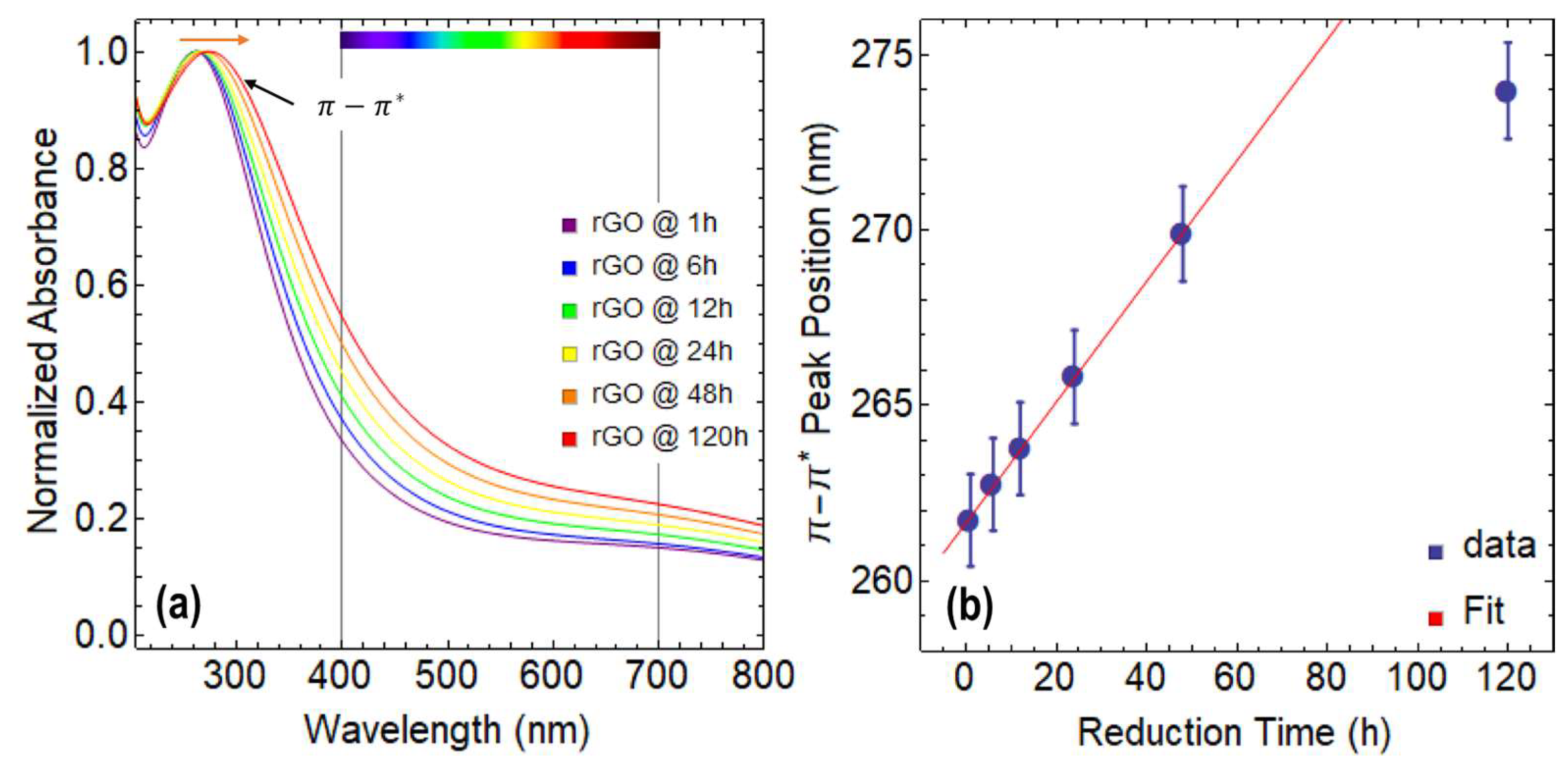
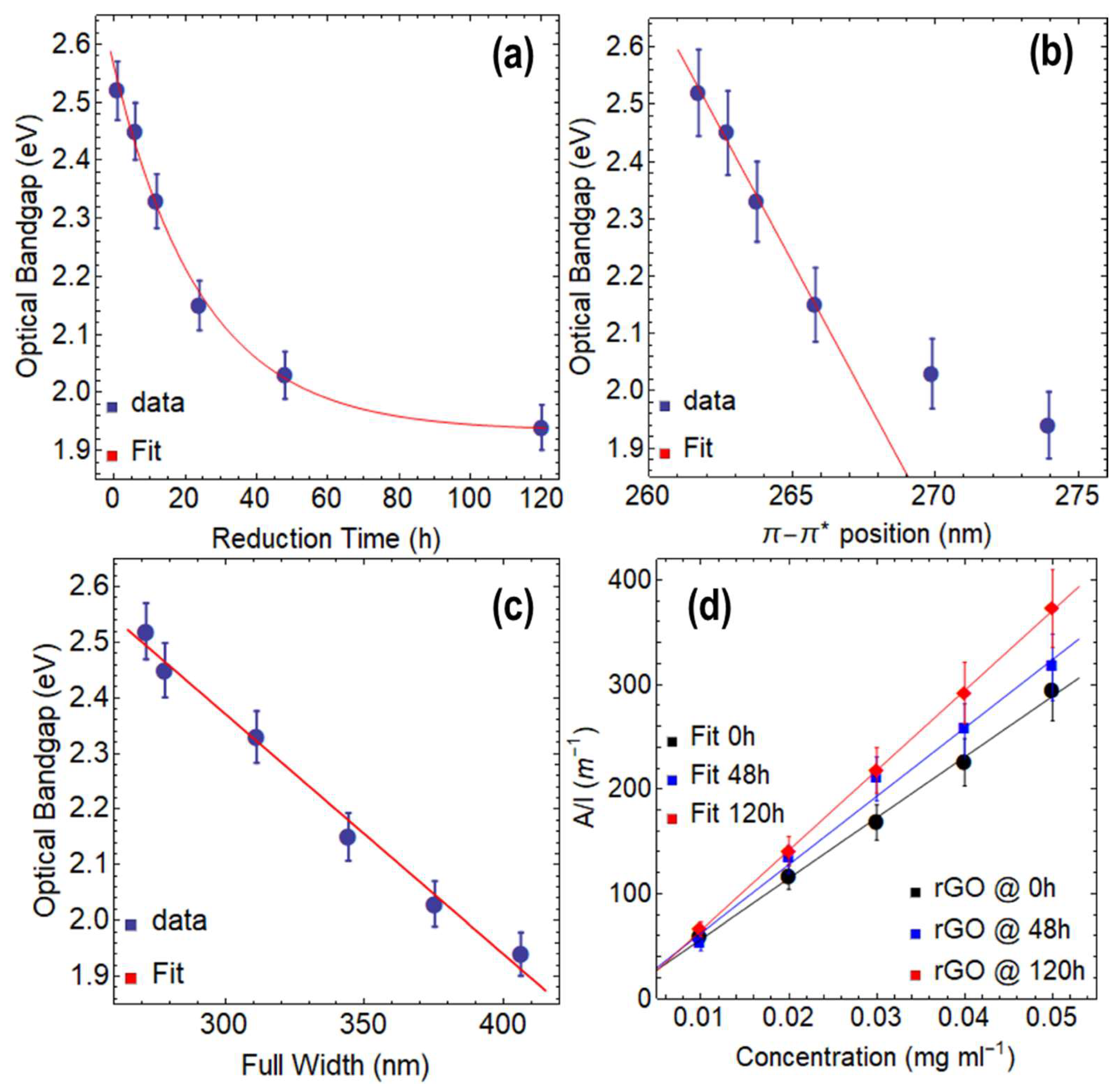
3.2. Optical Properties of rGO Reduced at 80 °C
3.3. Optical Properties of rGO Reduced at 50 °C
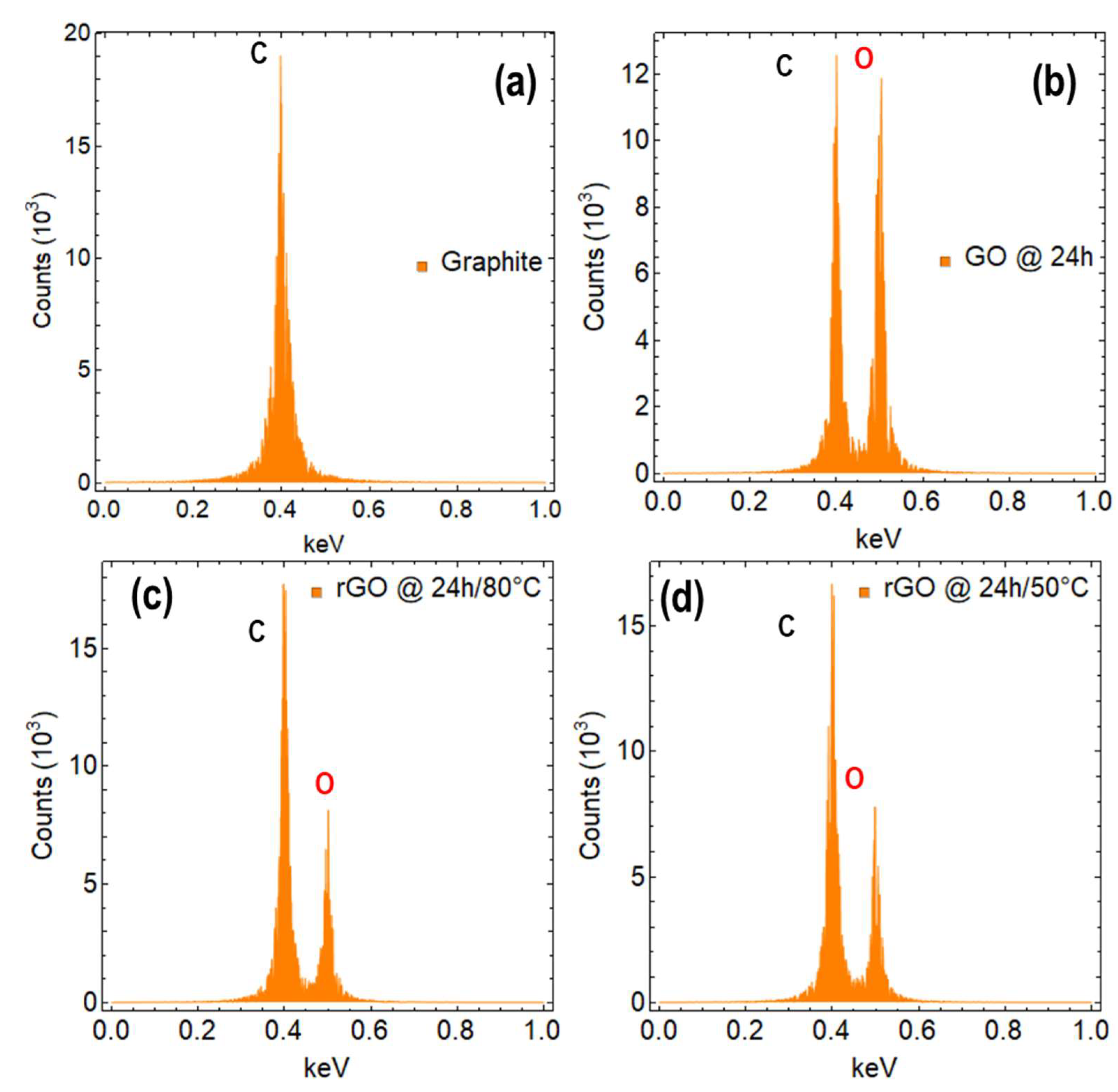
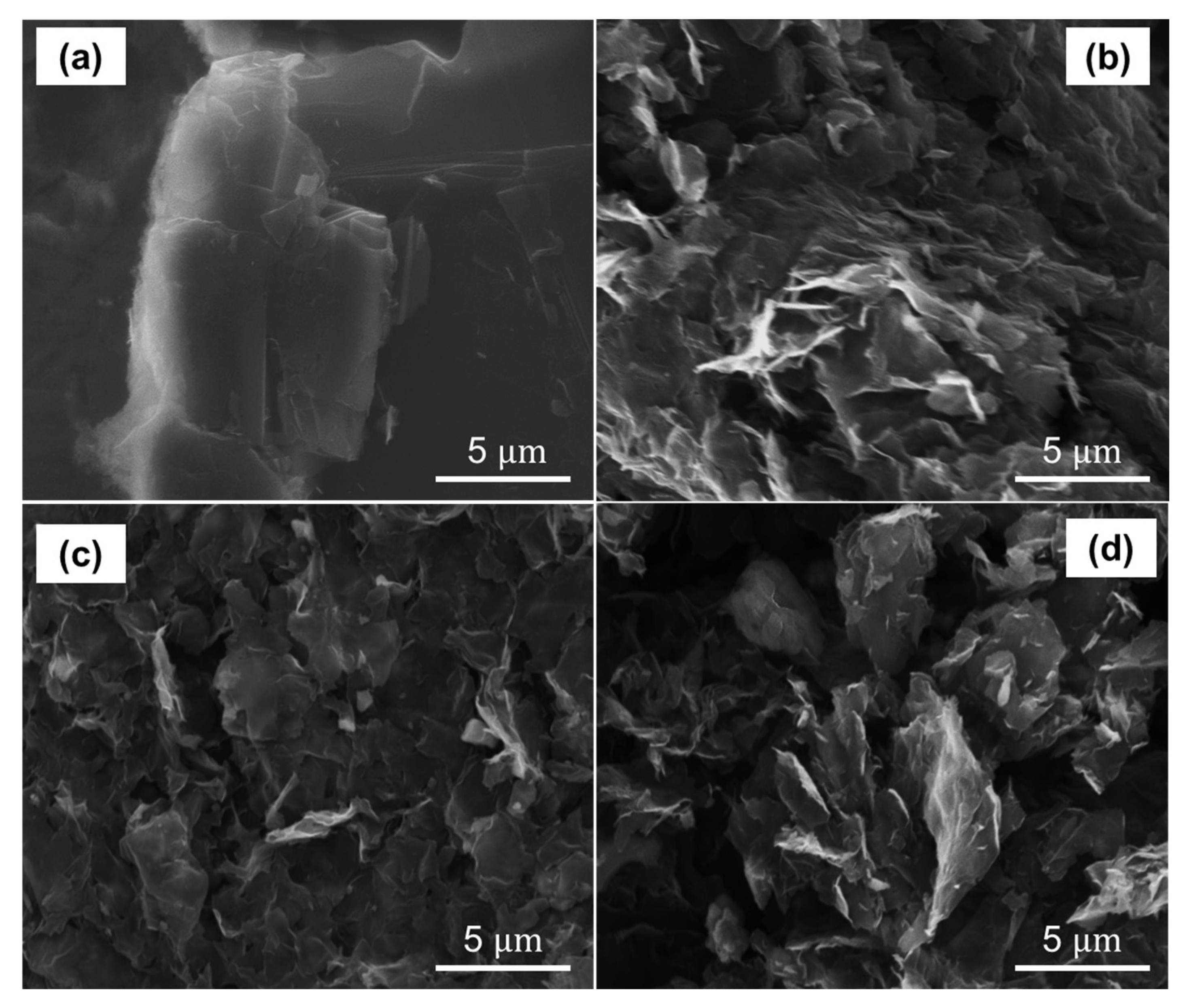
3.4. Spectroscopic and Morphological Measurements
4. Conclusions
- According to our research, GO has an optical bandgap of around 4 eV at 0 h of drying time, which gradually drops to 2.77 eV after 120 h. On the other side, rGO shows a bandgap reduction with longer reduction times. After 120 h of reduction time, the optical bandgaps of rGO at 80 °C and 50 °C were 1.94 eV and 2.03 eV, respectively.
- The transition predominately defines the absorbance spectra of both GO and rGO. Significantly, the aforementioned oxidation–reduction process parameters have a strong influence on this transition. For instance, the transition in GO occurs at about 230 nm at 0 h of drying time. On the other hand, regardless of the temperature at which the reduction is carried out, these transitions occur for rGO with only 1 h of reduction at wavelengths larger than 260 nm.
- Our study further validates that extending the drying time yields a notable enhancement in absorption within the visible region. Specifically, we observed a substantial improvement in photon collection of approximately 40% at 400 nm and 15% at 700 nm when increasing the drying time of GO. Similarly, in the case of rGO reduced at 80 °C, we observed a significant increase in absorption within the visible region, with up to a 22% rise at 400 nm and a 9% increase at 700 nm as the reduction time is extended.
- We measured high absorption coefficients in both GO and rGO, surpassing those reported for exfoliated graphene dispersions by two to three times. These findings confirm the superior optical properties of oxidized graphenes, highlighting their improved capacity for absorbing light.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yee, K.; Ghayesh, M.H. A review on the mechanics of graphene nanoplatelets reinforced structures. Int. J. Eng. Sci. 2023, 186, 103831. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Xu, J.; Wang, C.; Wang, Y.; Yuan, D.; Chew, J.W. Electrosorption performance on graphene-based materials: A review. RSC Adv. 2023, 13, 6518–6529. [Google Scholar] [CrossRef]
- Wu, W.; Du, M.; Shi, H.; Zheng, Q.; Bai, Z. Application of graphene aerogels in oil spill recovery: A review. Sci. Total Environ. 2023, 856, 159107. [Google Scholar] [CrossRef]
- Demirel, E.; Durmaz, Y.Y. PEGylated Reduced Graphene Oxide as nanoplatform for Targeted Gene and Drug Delivery. Eur. Polym. J. 2023, 186, 111841. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, I.; Motta, A.; Zanoni, R.; Scaramuzzo, F.A.; Amato, F.; Dalchiele, E.A.; Marrani, A.G. Understanding the nature of graphene oxide functional groups by modulation of the electrochemical reduction: A combined experimental and theoretical approach. Carbon 2023, 203, 29–38. [Google Scholar] [CrossRef]
- Gomez, C.V.; Robalino, E.; Haro, D.; Tene, T.; Escudero, P.; Haro, A.; Orbe, J. Structural and electronic properties of graphene oxide for different degree of oxidation. Mater. Today Proc. 2016, 3, 796–802. [Google Scholar] [CrossRef]
- Chen, T.; Huang, Y.; Wei, L.; Xu, T.; Xie, Y. Thermal and electrical transport in partly-reduced graphene oxide films: The effect of low temperature and structure domain size. Carbon 2023, 203, 130–140. [Google Scholar] [CrossRef]
- Manikandan, V.; Lee, N.Y. Reduced graphene oxide: Biofabrication and environmental applications. Chemosphere 2023, 311, 136934. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bobade, H.; Sharma, R.; Sharma, S. Graphene derivatives: Properties and potential food applications. J. Ind. Eng. Chem. 2023, 123, 1–18. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Tene, T.; Bellucci, S.; Guevara, M.; Arias Arias, F.; Sáez Paguay, M.Á.; Quispillo Moyota, J.M.; Torres, F.J. Adsorption of Mercury on Oxidized Graphenes. Nanomaterials 2022, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of oxygen functional groups in reduced graphene oxide for lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Cai, C.; Liang, H.; Huang, Y.; Zhang, Y.; Jia, T.; Liu, T. Nonlinear variable band gap of reduced graphene oxide examined by in-situ absorption spectrum and light response. Diam. Relat. Mater. 2022, 122, 108822. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bardhan, N.M.; Tongay, S.; Wu, J.; Belcher, A.M.; Grossman, J.C. Scalable enhancement of graphene oxide properties by thermally driven phase transformation. Nat. Chem. 2014, 6, 151–158. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Tene, T.; Usca, G.T.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Gomez, C.V. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef]
- Tene, T.; Bellucci, S.; Guevara, M.; Viteri, E.; Polanco, M.A.; Salguero, O.; Vera-Guzmán, E.; Valladares, S.; Scarcello, A.; Alessandro, F.; et al. Cationic Pollutant Removal from Aqueous Solution Using Reduced Graphene Oxide. Nanomaterials 2022, 12, 309. [Google Scholar] [CrossRef]
- Tene, T.; Arias, F.A.; Guevara, M.; Nuñez, A.; Villamagua, L.; Tapia, C.; Pisarra, M.; Torres, F.J.; Caputi, L.S.; Gomez, C.V. Removal of mercury(II) from aqueous solution by partially reduced graphene oxide. Sci. Rep. 2022, 12, 6326. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, R.; Andersan, G.; Permatasari Santoso, S.; Irawaty, W. Green reduction of graphene oxide using kaffir lime peel extract (Citrus hystrix) and its application as adsorbent for methylene blue. Sci. Rep. 2020, 10, 667. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Cho, S.; Jeon, S. Simultaneous reduction and nitrogen functionalization of graphene oxide using lemon for metal-free oxygen reduction reaction. J. Power Sources 2017, 372, 116–124. [Google Scholar] [CrossRef]
- Gul, M.M.; Ahmad, K.S.; Thomas, A.G.; Ibrahim, S.M. Remarkable energy storage and photocatalytic remediation potential of novel graphene oxide loaded bi-metal sulphide Ba4Fe2S6-GO nanocomposite thin film. Opt. Mater. 2023, 138, 113682. [Google Scholar] [CrossRef]
- Khan, A.; Kamal, T.; Saad, M.; Ameen, F.; Bhat, S.A.; Khan, M.A.; Rahman, F. Synthesis and antibacterial activity of nanoenhanced conjugate of Ag-doped ZnO nanorods with graphene oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122296. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar]
- Su, R.; Lin, S.F.; Chen, D.Q.; Chen, G.H. Study on the absorption coefficient of reduced graphene oxide dispersion. J. Phys. Chem. C 2014, 118, 12520–12525. [Google Scholar] [CrossRef]
- Gomez, C.V.; Guevara, M.; Tene, T.; Villamagua, L.; Usca, G.T.; Maldonado, F.; Caputi, L.S. The liquid exfoliation of graphene in polar solvents. Appl. Surf. Sci. 2021, 546, 149046. [Google Scholar] [CrossRef]
- Fei, Z.; Goldflam, M.D.; Wu, J.-S.; Dai, S.; Wagner, M.; McLeod, A.S.; Liu, M.K.; Post, K.W.; Zhu, S.; Janssen, G.C.A.M.; et al. Edge and Surface Plasmons in Graphene Nanoribbons. Nano Lett. 2015, 15, 8271–8276. [Google Scholar] [CrossRef] [PubMed]



| Reduction Time (h) | Optical Bandgap (eV) | R2 |
|---|---|---|
| 1 | 2.52 | 0.999 |
| 6 | 2.45 | 0.999 |
| 12 | 2.33 | 0.999 |
| 24 | 2.15 | 0.999 |
| 48 | 2.03 | 0.999 |
| 120 | 1.94 | 0.999 |
| Material | Absorption Coefficient (mL mg−1 m−1) | R2 |
|---|---|---|
| rGO @ 1 h/80 °C | 5803.89 | 0.997 |
| rGO @ 48 h/80 °C | 6534.43 | 0.989 |
| rGO @ 120 h/80 °C | 7638.10 | 0.989 |
| Reduction Time (h) | Optical Bandgap (eV) | R2 |
|---|---|---|
| 1 | 2.40 | 0.999 |
| 6 | 2.34 | 0.999 |
| 12 | 2.27 | 0.999 |
| 24 | 2.19 | 0.999 |
| 48 | 2.11 | 0.999 |
| 120 | 2.03 | 0.999 |
| Material | Absorption Coefficient (mL mg−1 m−1) | R2 |
|---|---|---|
| rGO @ 1 h/50 °C | 5294.11 | 0.993 |
| rGO @ 48 h/50 °C | 5975.30 | 0.983 |
| rGO @ 120 h/50 °C | 6540.53 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Vinueza-Naranjo, P.G.; Cevallos, Y.; Arias Arias, F.; La Pietra, M.; Scarcello, A.; Salazar, Y.C.; Polanco, M.A.; Straface, S.; Vacacela Gomez, C.; et al. Temperature-Dependent Optical Properties of Oxidized Graphenes. Nanomaterials 2023, 13, 2263. https://doi.org/10.3390/nano13152263
Tene T, Vinueza-Naranjo PG, Cevallos Y, Arias Arias F, La Pietra M, Scarcello A, Salazar YC, Polanco MA, Straface S, Vacacela Gomez C, et al. Temperature-Dependent Optical Properties of Oxidized Graphenes. Nanomaterials. 2023; 13(15):2263. https://doi.org/10.3390/nano13152263
Chicago/Turabian StyleTene, Talia, Paola G. Vinueza-Naranjo, Yesenia Cevallos, Fabian Arias Arias, Matteo La Pietra, Andrea Scarcello, Yolenny Cruz Salazar, Melvin Arias Polanco, Salvatore Straface, Cristian Vacacela Gomez, and et al. 2023. "Temperature-Dependent Optical Properties of Oxidized Graphenes" Nanomaterials 13, no. 15: 2263. https://doi.org/10.3390/nano13152263
APA StyleTene, T., Vinueza-Naranjo, P. G., Cevallos, Y., Arias Arias, F., La Pietra, M., Scarcello, A., Salazar, Y. C., Polanco, M. A., Straface, S., Vacacela Gomez, C., Caputi, L. S., & Bellucci, S. (2023). Temperature-Dependent Optical Properties of Oxidized Graphenes. Nanomaterials, 13(15), 2263. https://doi.org/10.3390/nano13152263












