Enhanced Lithium Storage Performance of α-MoO3/CNTs Composite Cathode
Abstract
:1. Introduction
2. Results and Discussion
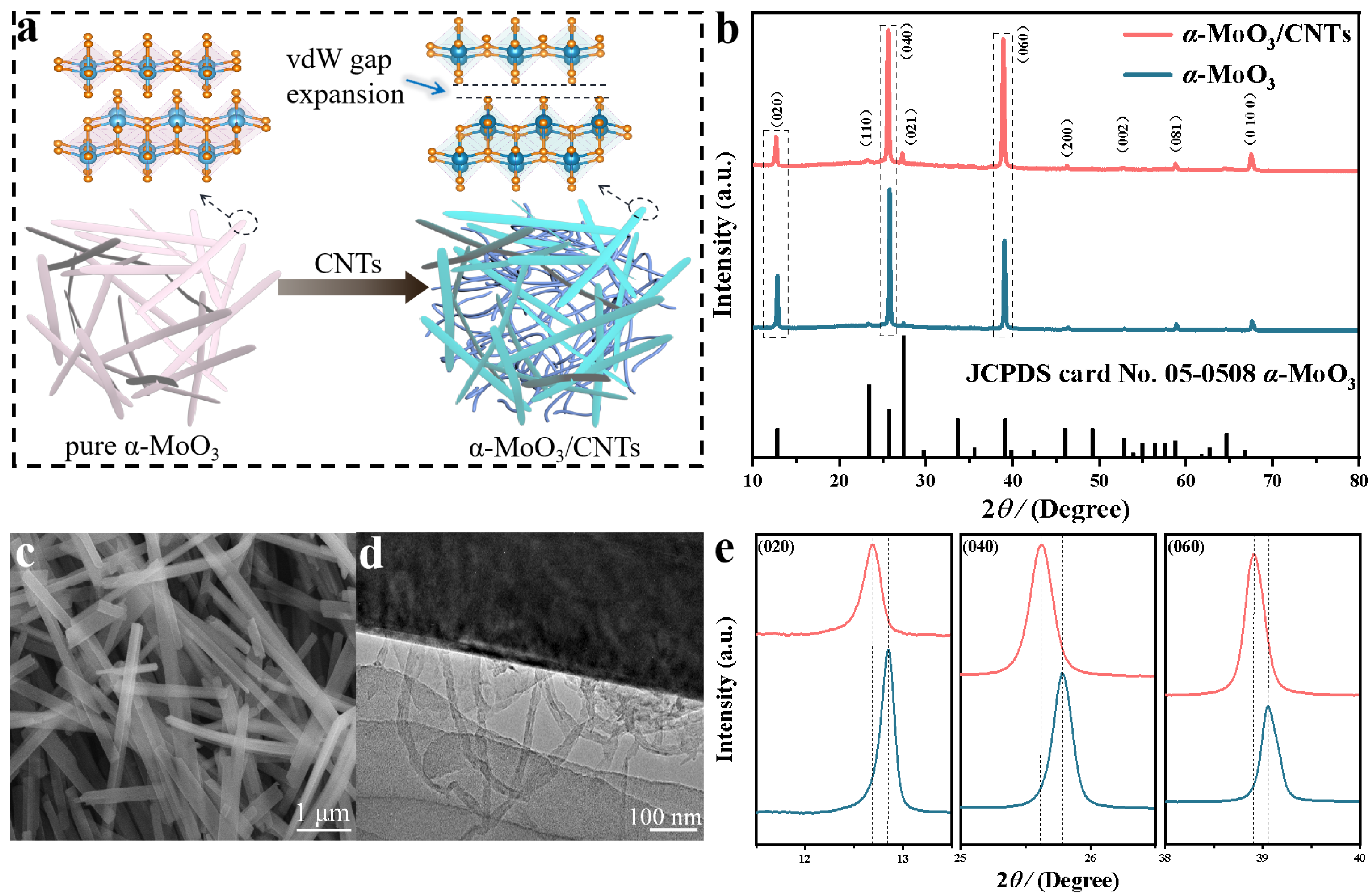
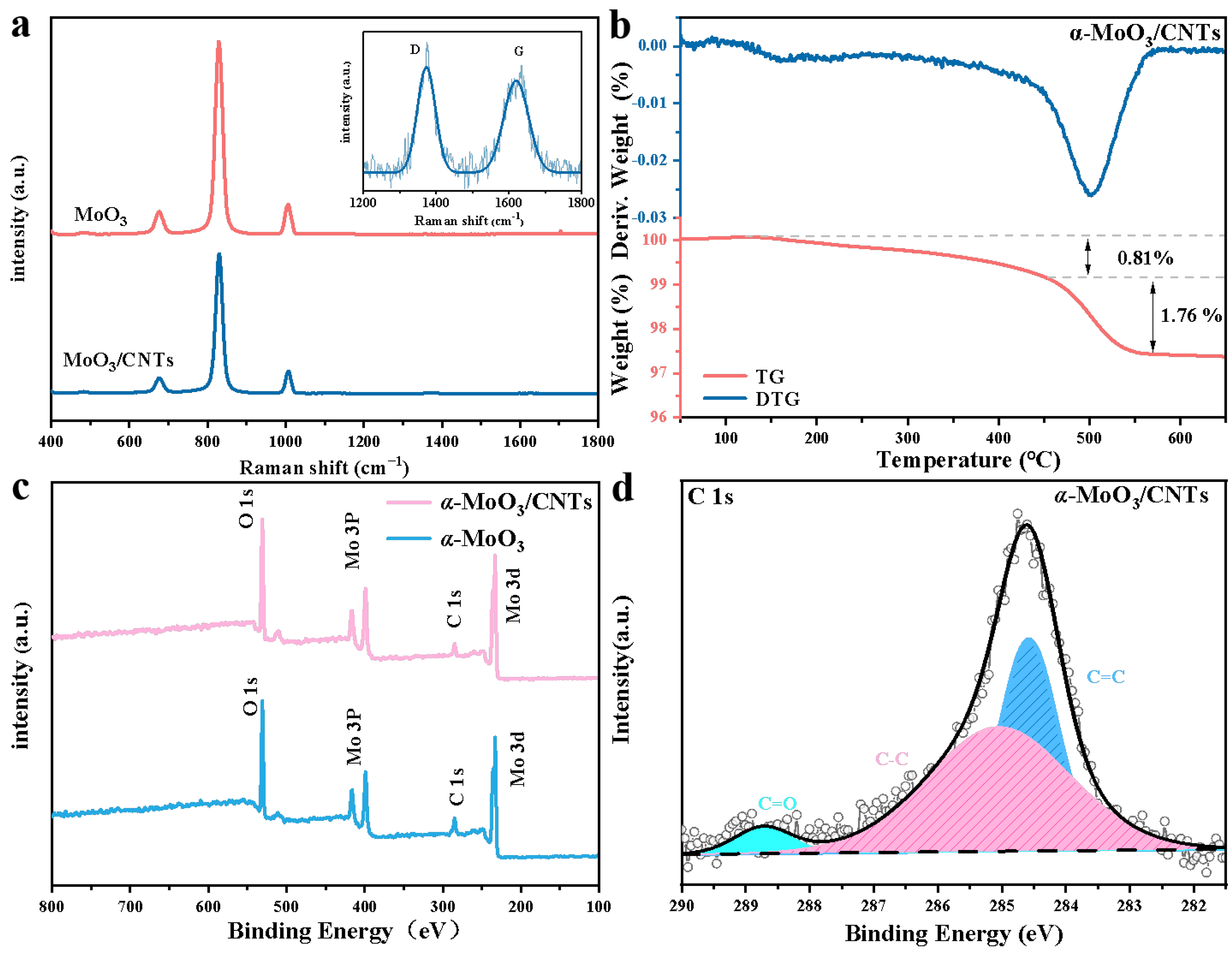
2.1. Structure and Morphology Analysis
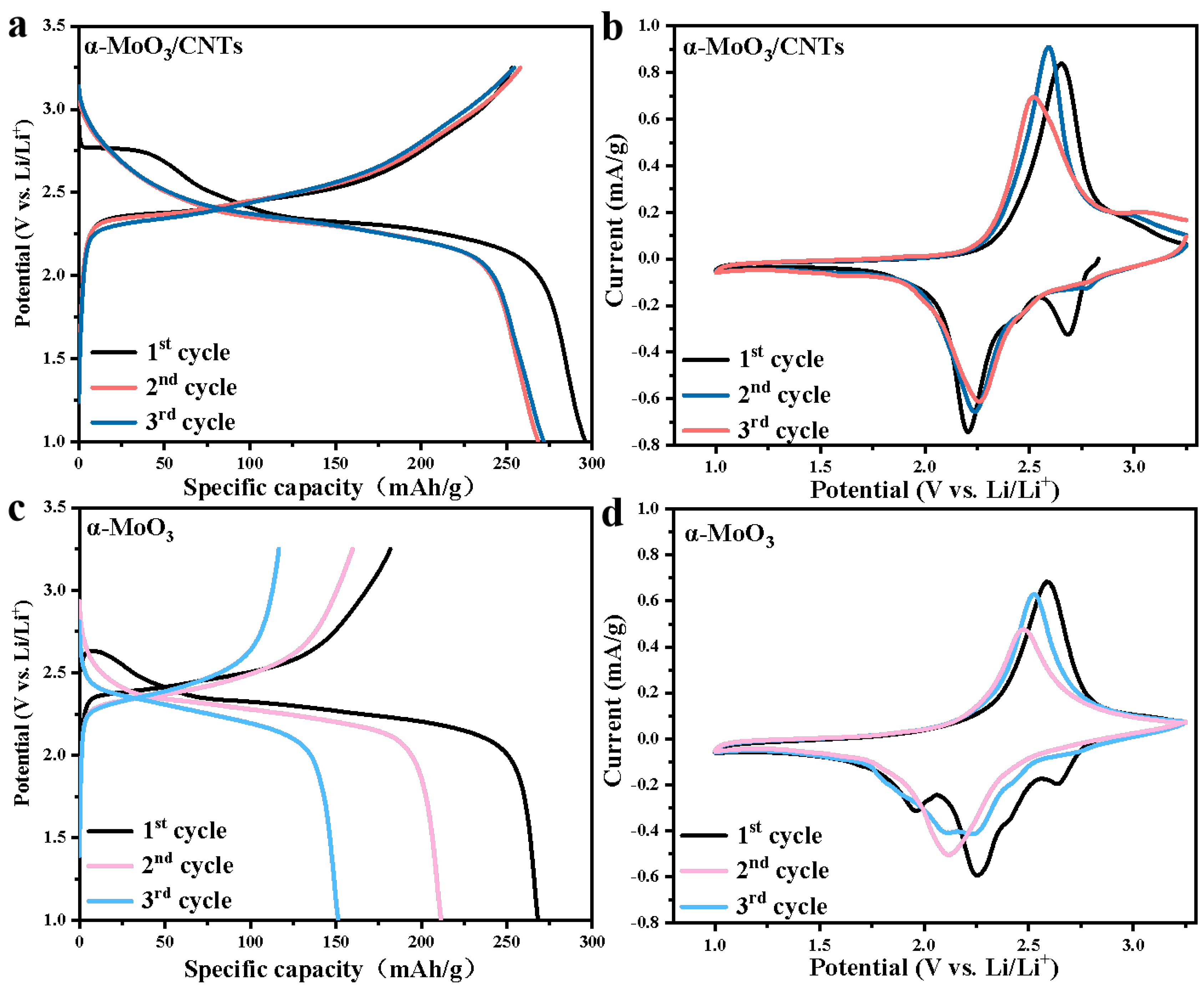
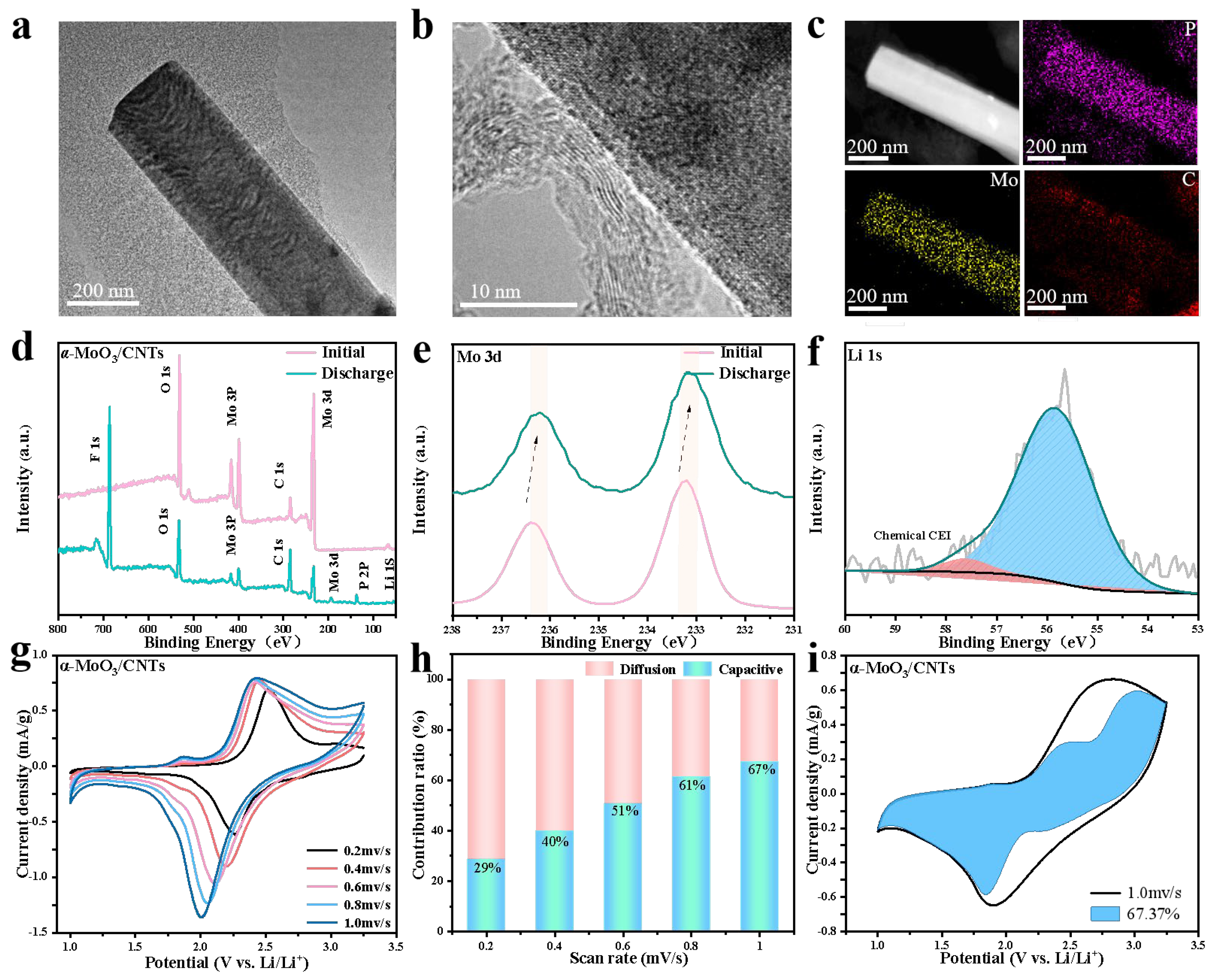
2.2. Electrochemical Analysis
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [PubMed]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Whittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar]
- Mai, L.; Yang, F.; Zhao, Y.; Xu, X.; Xu, L.; Hu, B.; Luo, Y.; Liu, H. Molybdenum oxide nanowires: Synthesis & properties. Mater. Today 2011, 14, 346–353. [Google Scholar]
- Qu, G.; Wang, J.; Liu, G.; Tian, B.; Su, C.; Chen, Z.; Rueff, J.-P.; Wang, Z. Vanadium Doping Enhanced Electrochemical Performance of Molybdenum Oxide in Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 29, 1805227. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, L.; Lou, X.W. Metal oxide hollow nanostructures for lithium-ion batteries. Adv. Mater. 2012, 24, 1903–1911. [Google Scholar] [CrossRef]
- Wang, X.-J.; Nesper, R.; Villevieille, C.; Novák, P. Ammonolyzed MoO3 Nanobelts as Novel Cathode Material of Rechargeable Li-Ion Batteries. Adv. Energy Mater. 2013, 3, 606–614. [Google Scholar] [CrossRef]
- Sheng, D.; Zhang, M.; Wang, X.; Zhou, S.; Fu, S.; Liu, X.; Zhang, Q. Carbon nanotubes embedded in α-MoO3 nanoribbons for enhanced lithium-ion storage. J. Mater. Sci. Mater. Electron. 2022, 33, 11743–11752. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, Q.; Xu, F.; Sun, L. New Insights into Electrochemical Lithiation/Delithiation Mechanism of α-MoO3 Nanobelt by in Situ Transmission Electron Microscopy. ACS Appl. Mater. Interfaces 2016, 8, 9170–9177. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Wang, R.; Mi, R.; Li, S.; Cui, Y.; Deng, Y.; Mei, J.; Liu, H. Coating of a-MoO3 on nitrogen-doped carbon nanotubes by electrodeposition as a high-performance cathode material for lithium-ion batteries. J. Power Sources 2015, 274, 1063–1069. [Google Scholar]
- Kim, H.-S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 2017, 16, 454–460. [Google Scholar]
- Hu, Z.; Zhang, X.; Peng, C.; Lei, G.; Li, Z. Pre-intercalation of potassium to improve the electrochemical performance of carbon-coated MoO3 cathode materials for lithium batteries. J. Alloys Compd. 2020, 826, 154055. [Google Scholar]
- Ji, H.; Liu, X.; Liu, Z.; Yan, B.; Chen, L.; Xie, Y.; Liu, C.; Hou, W.; Yang, G. In Situ Preparation of Sandwich MoO3/C Hybrid Nanostructures for High-Rate and Ultralong-Life Supercapacitors. Adv. Funct. Mater. 2015, 25, 1886–1894. [Google Scholar] [CrossRef]
- Noerochim, L.; Wang, J.-Z.; Wexler, D.; Chao, Z.; Liu, H.-K. Rapid synthesis of free-standing MoO3/Graphene films by the microwave hydrothermal method as cathode for bendable lithium batteries. J. Power Sources 2016, 228, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Zhao, H.; Du, Z.; Zeng, Z.; Gao, C.; Zhang, Z.; Du, X.; Kulka, A.; Świerczek, K. Facile synthesis of MoO3/carbon nanobelts as high-performance anode material for lithium ion batteries. Electrochim. Acta 2015, 180, 947–956. [Google Scholar]
- Dong, Y.; Li, S.; Xu, H.; Yan, M.; Xu, X.; Tian, X.; Liu, Q.; Mai, L. Wrinkled-graphene enriched MoO3 nanobelts with increased conductivity and reduced stress for enhanced electrochemical performance. Phys. Chem. Chem. Phys. 2013, 15, 17165–17170. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.-M.; Lu, Z.-H. Transition metal oxide work functions: The influence of cation oxidation state and oxygen vacancies. Adv. Funct. Mater. 2012, 22, 4557–4568. [Google Scholar]
- Huang, L.; Yao, B.; Sun, J.; Gao, X.; Wu, J.; Wan, J.; Li, T.; Hu, Z.; Zhou, J. Highly conductive and flexible molybdenum oxide nanopaper for high volumetric supercapacitor electrode. J. Mater. Chem. 2017, 5, 2897–2903. [Google Scholar]
- Li, T.; Beidaghi, M.; Xiao, X.; Huang, L.; Hu, Z.; Sun, W.; Chen, X.; Gogotsi, Y.; Zhou, J. Ethanol reduced molybdenum trioxide for Li-ion capacitors. Nano Energy 2016, 26, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Qin, J.; Yin, Z.; Cao, M. Achieving Fully Reversible Conversion in MoO3 for Lithium Ion Batteries by Rational Introduction of CoMoO4. ACS Nano 2017, 16, 454–460. [Google Scholar]
- Mendoza-Sánchez, B.; Grant, P.S. Charge storage properties of a α-MoO3/carboxyl-functionalized single-walled carbon nanotube composite electrode in a Li ion electrolyte. Electrochim. Acta 2013, 98, 294–302. [Google Scholar]
- Zheng, Y.; Liu, Z.; Liu, B.; Wang, S.; Xiong, C. Fabrication of cactus-like CNT/SiO2/MoO3 ternary composites for superior lithium storage. Energy 2020, 11, 19. [Google Scholar]
- Hashem, A.M.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Electrochemical properties of nanofibers α-MoO3 as cathode materials for Li batteries. J. Power Sources 2012, 219, 126–132. [Google Scholar]
- Ramana, C.V.; Mauger, A.; Julien, C.M. Growth, characterization and performance of bulk and nanoengineered molybdenum oxides for electrochemical energy storage and conversion. Prog. Progress. Cryst. Growth Charact. Mater. 2021, 67, 100533. [Google Scholar]
- Zhang, H.; Wu, W.; Liu, Q.; Yang, F.; Shi, X.; Liu, X.; Yu, M.; Lu, X. Interlayer Engineering of α-MoO3 Modulates Selective Hydronium Intercalation in Neutral Aqueous Electrolyte. Angew. Chem. Int. Ed. Engl. 2021, 60, 896–903. [Google Scholar]
- Wang, Z.; Madhavi, S.; Lou, X.W. Ultralong α-MoO3 Nanobelts: Synthesis and Effect of Binder Choice on Their Lithium Storage Properties. J. Phys. Chem. C 2012, 116, 12508–12513. [Google Scholar]
- Yang, C.; Liu, X.; Yang, Z.; Gu, L.; Yu, Y. Improvement of lithium storage performance of molybdenum trioxide by a synergistic effect of surface coating and oxygen vacancies. Adv. Mater. Interfaces 2016, 3, 1600730. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, J.; Ma, Y.; Cui, S.; Zhang, P.; Zhai, P.; Meng, L.; Wang, X.; Wei, Y.; Du, Z.; et al. Tin Intercalated Ultrathin MoO3 Nanoribbons for Advanced Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1803137. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; He, C.; Ren, X.; Zhang, P.; Mi, H. 2D Electrocatalysts: Recent Progress in 2D Catalysts for Photocatalytic and Electrocatalytic Artificial Nitrogen Reduction to Ammonia. Adv. Energy Mater. 2021, 11, 2170043. [Google Scholar]
- Zhao, G.; Zhang, L.; Sun, K. Capacitive contribution to lithium storage capacity in porous MoO3 films. J. Electroanal. Chem. 2013, 694, 61–67. [Google Scholar] [CrossRef]
- Yu, M.; Shao, H.; Wang, G.; Yang, F.; Liang, C.; Rozier, P.; Wang, C.-Z.; Lu, X.; Simon, P.; Feng, X. Interlayer gap widened α-phase molybdenum trioxide as high-rate anodes for dual-ion-intercalation energy storage devices. Nat. Commun. 2020, 11, 1348. [Google Scholar]
- Zhang, G.; Xiong, T.; Yan, M.; He, L.; Liao, X.; He, C.; Yin, C.; Zhang, H.; Mai, L. α-MoO3-x by plasma etching with improved capacity and stabilized structure for lithium storage. Nano Energy 2018, 49, 555–563. [Google Scholar] [CrossRef]
- Nadimicherla, R.; Chen, W.; Guo, X. Synthesis and characterization of α-MoO3 nanobelt composite positive electrode materials for lithium battery application. Mater Res. Bull. 2015, 66, 140–146. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Cheng, X.; Zhang, Y.; Zhao, K. In-situ TEM experiments and first-principles studies on the electrochemical and mechanical behaviors of α-MoO3 in Li-ion batteries. Nano Energy 2016, 27, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Kim, Y.-H.; Deshpande, R.; Parilla, P.A.; Whitney, E.; Gillaspie, D.T.; Jones, K.M.; Mahan, A.H.; Zhang, S.; Dillon, A.C. Reversible lithium-ion insertion in molybdenum oxide nanoparticles. Adv. Mater. 2008, 08, 3627–3632. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Savilov, S.; Wang, G.; Chen, Z.; Chen, Q. Enhanced structurally stable cathodes by surface and grain boundary tailoring of Ni-Rich material with molybdenum trioxide. J. Power Sources 2020, 478, 229051. [Google Scholar] [CrossRef]
- Ette, P.M.; Babu, D.B.; Roy, M.L.; Ramesha, K. Mo3Nb2O14: A high-rate intercalation electrode material for Li-ion batteries with liquid and garnet based hybrid solid electrolytes. J. Power Sources 2019, 436, 226850. [Google Scholar] [CrossRef]
- Villevieille, C.; Gorzkowska-Sobas, A.; Fjellvåg, H.; Novák, P. Freeze-dryed LixMoO3 nanobelts used as cathode materials for lithium-ion batteries: A bulk and interface study. J. Power Sources 2015, 297, 276–282. [Google Scholar]
- Hashem, A.M.; Askar, M.H.; Winter, M.; Albering, J.H.; Besenhard, J.O. Electrical properties of polycrystalline TiO2· Thermoelectric power. Ionics 2007, 13, 155–162. [Google Scholar]

| Cathode | Initial Capacity | Rate: Capability/Current Density | Cycling Life | Ref. |
|---|---|---|---|---|
| carbon-coated MoO3 | 258 mA h g−1 | 118 mAh g−1/3 A g−1 | 125 mAh g−1 at 1.5 A g−1 after 500 cycles | [13] |
| α-MoO3−x plasma etching | 224.2 mA h g−1 | ≈90 mAh g−1/5 A g−1 | 67.3 mAh g−1 at 1 A g−1 after 1000 cycles | [33] |
| α-MoO3/SWCNT-COOH | 193.8 mA h g−1 | -- | 70 mAh g−1 at 0.5 A g−1 after 117 cycles | [22] |
| α-MoO3/N-CNTs | 250 mA h g−1 | 190 mAh g−1/0.3 A g−1 | 250 mAh g−1 at 0.3 A g−1 after 50 cycles | [11] |
| α-MoO3/PEO | 352 mA h g−1 | -- | 124 mAh g−1 at 0.03 A g−1 after 50 cycles | [34] |
| This work | 296 mA h g−1 | 77.2 mAh g−1/1 A g−1 | 93 mAh g−1 at 0.1 A g−1 after 150 cycles | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, D.; Gao, A.; Liu, X.; Zhang, Q. Enhanced Lithium Storage Performance of α-MoO3/CNTs Composite Cathode. Nanomaterials 2023, 13, 2272. https://doi.org/10.3390/nano13152272
Sheng D, Gao A, Liu X, Zhang Q. Enhanced Lithium Storage Performance of α-MoO3/CNTs Composite Cathode. Nanomaterials. 2023; 13(15):2272. https://doi.org/10.3390/nano13152272
Chicago/Turabian StyleSheng, Dawei, Ang Gao, Xiaoxu Liu, and Qiang Zhang. 2023. "Enhanced Lithium Storage Performance of α-MoO3/CNTs Composite Cathode" Nanomaterials 13, no. 15: 2272. https://doi.org/10.3390/nano13152272
APA StyleSheng, D., Gao, A., Liu, X., & Zhang, Q. (2023). Enhanced Lithium Storage Performance of α-MoO3/CNTs Composite Cathode. Nanomaterials, 13(15), 2272. https://doi.org/10.3390/nano13152272






