High Sensitivity Electrochemical As (III) Sensor Based on Fe3O4/MoS2 Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials/Chemicals
2.2. Instrumentation
2.3. Synthesis of Fe3O4/MoS2 Nanocomposites
2.4. Preparation of the Fe3O4/MoS2 Nanocomposite Modified Electrode
2.5. Electrochemical Measurements
3. Results
3.1. Characterization of Fe3O4/MoS2 Nanocomposites
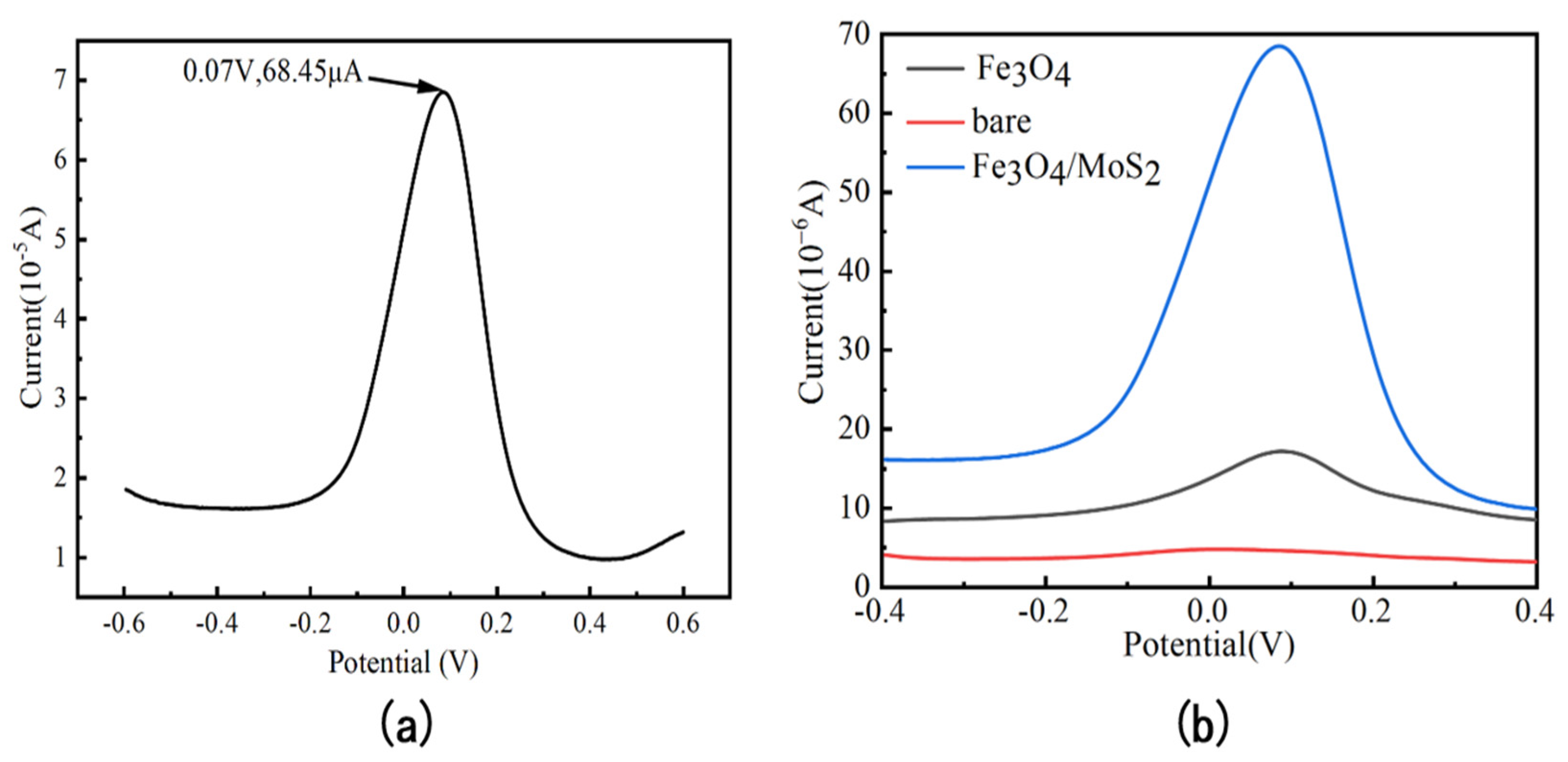
3.2. Electrochemical Characterization of Fe3O4/MoS2 Electrodes
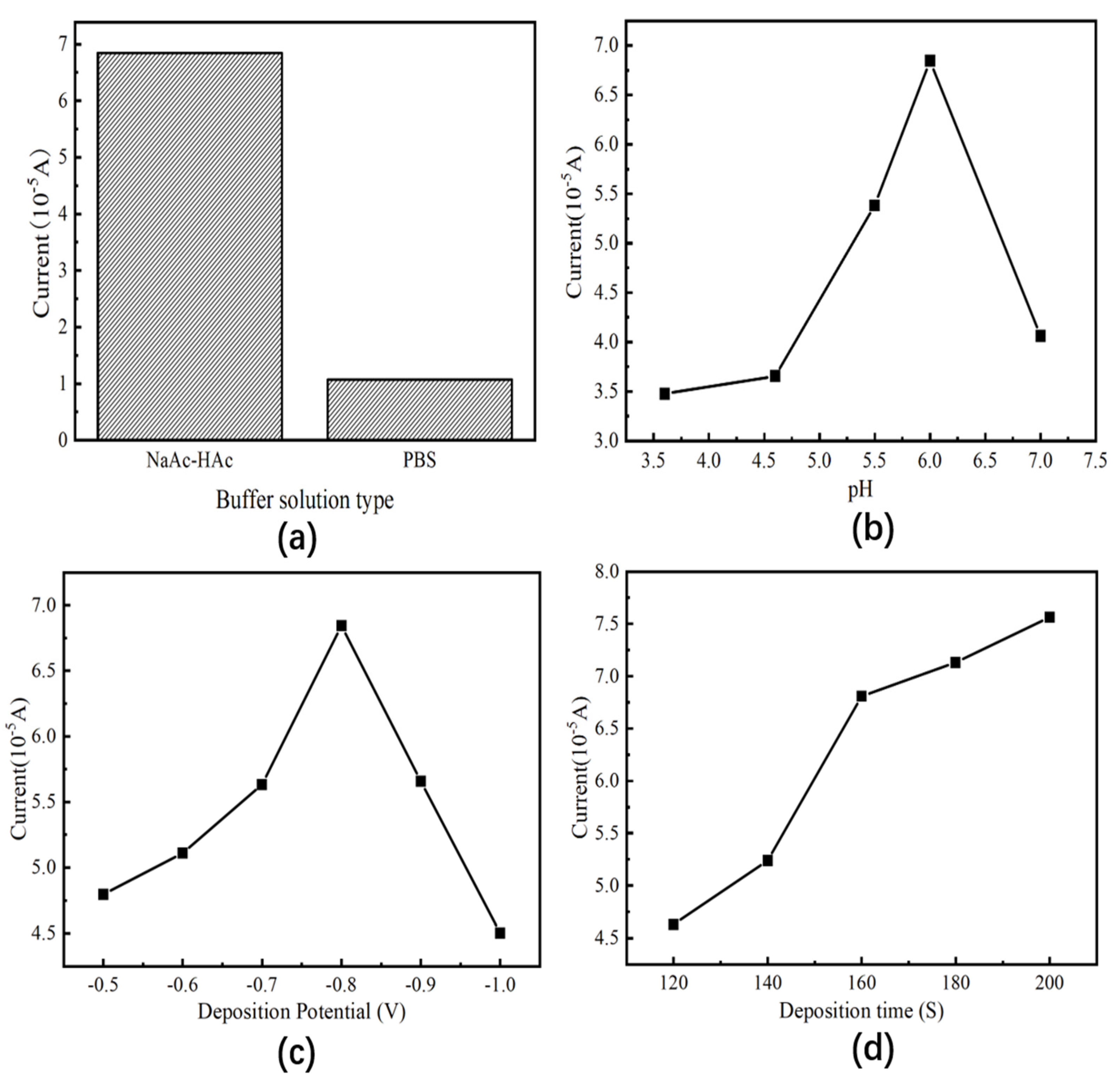
3.3. Optimization of Analytical Conditions
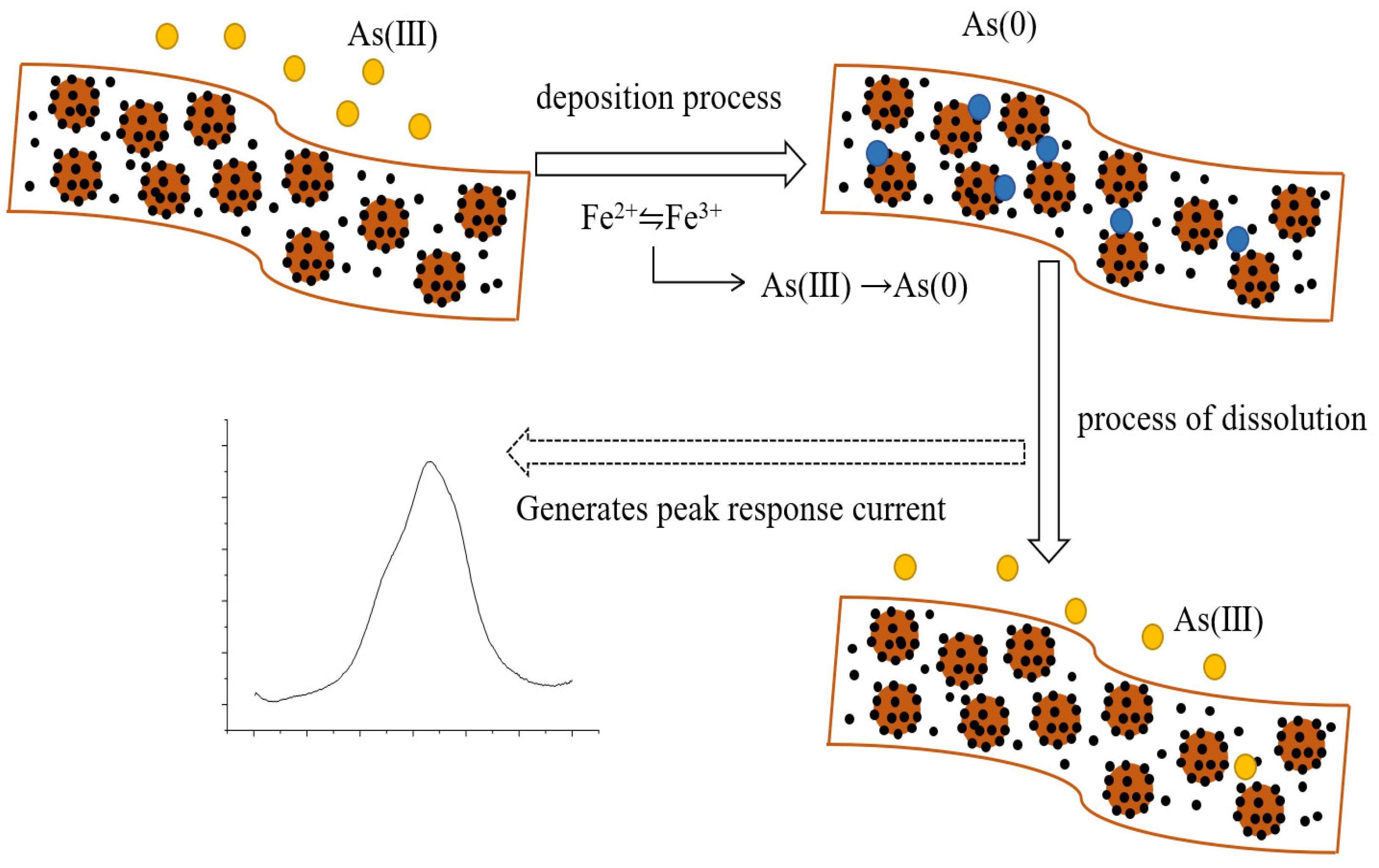
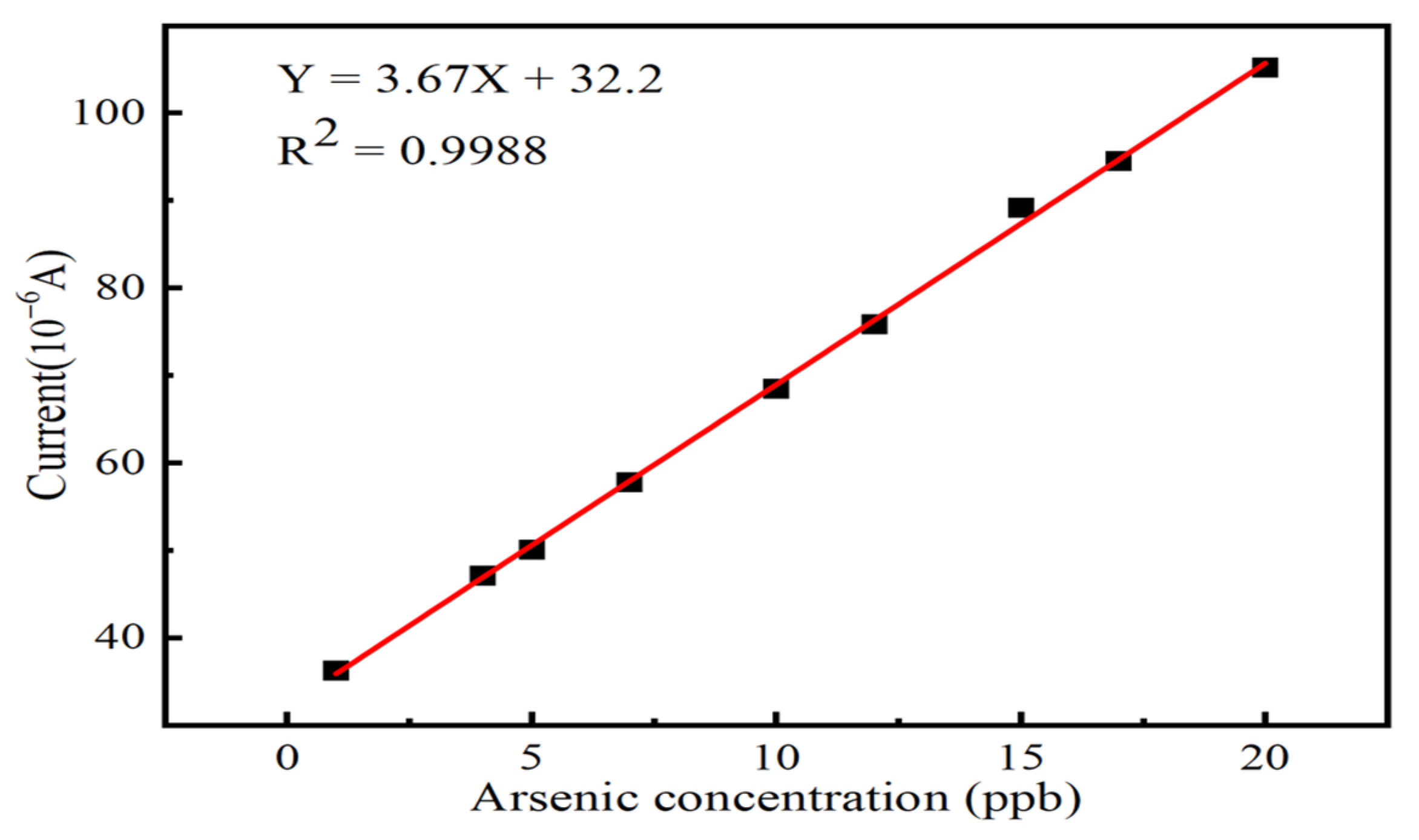
3.4. Electrochemical Detection of As (III)
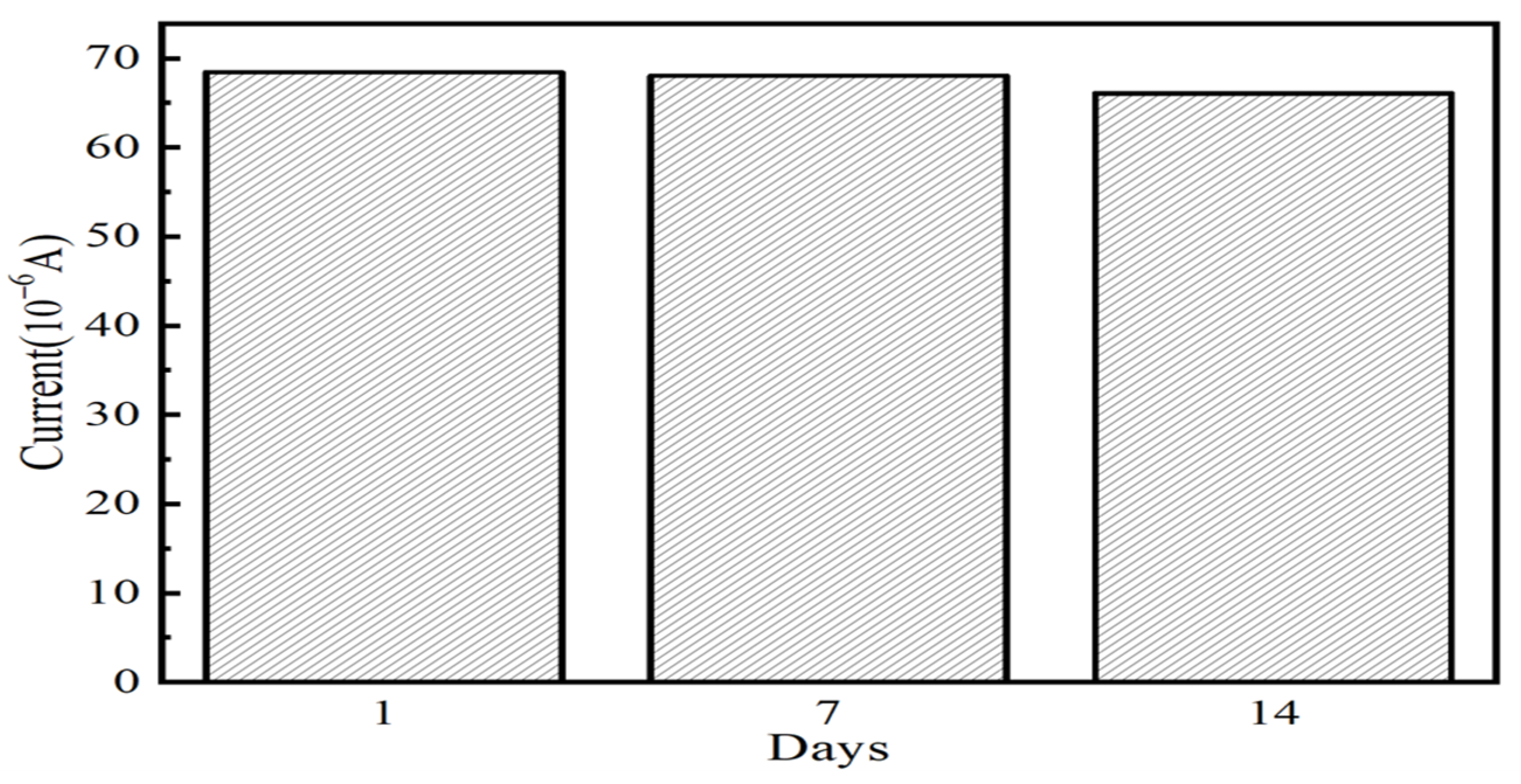
3.5. Interference Resistance, Reproducibility, and Reusability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waalkes, M.P.; Coogan, T.P.; Barter, R.A. Toxicological principles of metal carcinogenesis with special emphasis on cadmium. Crit. Rev. Toxicol. 1992, 22, 175–201. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.F.; Berger, B.L.; Bean, J.R. Mercury, arsenic, lead, cadmium, and selenium residues in fish, 1971-73—National Pesticide Monitoring Program. Pestic. Monit. J. 1977, 11, 5–34. [Google Scholar] [PubMed]
- Shimizu, F.M.; Braunger, M.L.; Riul, A., Jr. Heavy Metal/Toxins Detection Using Electronic Tongues. Chemosensors 2019, 7, 36. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-G.; Huang, X.-J. Voltammetric determination of inorganic arsenic. TrAC Trends Anal. Chem. 2014, 60, 25–35. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Liu, C.; Zhang, R.; Gu, X.; Guan, G.; Jiang, C.; Zhang, L.; Du, S.; Liu, B.; et al. Color-Multiplexing-Based Fluorescent Test Paper: Dosage-Sensitive Visualization of Arsenic(III) with Discernable Scale as Low as 5 ppb. Anal. Chem. 2016, 88, 6105–6109. [Google Scholar] [CrossRef] [Green Version]
- Mirceski, V.; Gulaboski, R. Recent achievements in square-wave voltammetry (a review). Maced. J. Chem. Chem. Eng. 2014, 33, 1–12. [Google Scholar]
- Prakash, R.; Srivastava, R.C.; Seth, P.K. Direct estimation of total arsenic using a novel metal side disk rotating electrode. Electroanalysis 2003, 15, 1410–1414. [Google Scholar] [CrossRef]
- Simm, A.O.; Banks, C.E.; Compton, R.G. Sonically assisted electroanalytical detection of ultratrace arsenic. Anal. Chem. 2004, 76, 5051–5055. [Google Scholar] [CrossRef]
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, Z.-G.; Zhao, Z.-Q.; Liu, J.-H.; Huang, X.-J. SnO2 Tube-in-Tube Nanostructures: Cu@C Nanocable Templated Synthesis and Their Mutual Interferences between Heavy Metal Ions Revealed by Stripping Voltammetry. Small 2013, 9, 2233–2239. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.-Y.; Xiong, S.-Q.; Liu, J.-H.; Huang, X.-J. Electrochemical Detection of Arsenic(III) Completely Free from Noble Metal: Fe3O4 Microspheres-Room Temperature Ionic Liquid Composite Showing Better Performance than Gold. Anal. Chem. 2013, 85, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Manikhezri, H.; Hallaj, R.; Soltanian, S. Electrochemical detection of trace amount of arsenic(III) at glassy carbon electrode modified with cobalt oxide nanoparticles. Sens. Actuators B Chem. 2008, 129, 246–254. [Google Scholar] [CrossRef]
- Ratinac, K.R.; Yang, W.; Gooding, J.J.; Thordarson, P.; Braet, F. Graphene and Related Materials in Electrochemical Sensing. Electroanalysis 2011, 23, 803–826. [Google Scholar] [CrossRef]
- Aragay, G.; Merkoci, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, N.; Wang, H.; Liang, Q.; Bian, Z. Pd-Ag/Graphene Electrochemical Sensor for Chlorophenol Contaminant Determination. J. Electrochem. Soc. 2019, 166, B266–B275. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Li, H.; Wang, G.; Long, Y.; Li, A.; Yang, B. In Situ Detection of Melatonin and Pyridoxine in Plants Using a CuO-Poly(L-lysine)/Graphene-Based Electrochemical Sensor. ACS Sustain. Chem. Eng. 2019, 7, 19537–19545. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lai, G.; Su, W.; Malherbe, F.; Yu, J.; Lin, C.-T.; Yu, A. Defects regulating of graphene ink for electrochemical determination of ascorbic acid, dopamine and uric acid. Talanta 2018, 180, 248–253. [Google Scholar] [CrossRef]
- Xiang, G.; Chen, M.; Ni, Z.; Shen, Y.; Xu, L. Synthesis of a hollow-structured flower-like Fe3O4@MoS2 composite and its microwave-absorption properties. RSC Adv. 2021, 11, 20180–20190. [Google Scholar] [CrossRef]
- Chao, Y.; Zhu, W.; Wu, X.; Hou, F.; Xun, S.; Wu, P.; Ji, H.; Xu, H.; Li, H. Application of graphene-like layered molybdenum disulfide and its excellent adsorption behavior for doxycycline antibiotic. Chem. Eng. J. 2014, 243, 60–67. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Xie, B.; Liu, X.; Hu, H. Highly Sensitive Electrochemical Pb(II) Sensors Based on MoS2/rGO Nanocomposites by Square Wave Voltammetry. J. Electrochem. Soc. 2022, 169, 077509. [Google Scholar] [CrossRef]
- Hu, H.; Lu, W.; Liu, X.; Meng, F.; Zhu, J. A High-Response Electrochemical As(III) Sensor Using Fe3O4-rGO Nanocomposite Materials. Chemosensors 2021, 9, 150. [Google Scholar] [CrossRef]
- Orazem, M.E. Electrochemical impedance spectroscopy: The journey to physical understanding. J. Solid State Electrochem. 2020, 24, 2151–2153. [Google Scholar] [CrossRef]
- Touomo-Wouafo, M.; Donkeng-Dazie, J.; Btatkeu-K, B.D.; Tchatchueng, J.B.; Noubactep, C.; Ludvik, J. Role of pre-corrosion of Fe-0 on its efficiency in remediation systems: An electrochemical study. Chemosphere 2018, 209, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yang, W.; Li, X.; Zhao, H.; Yuan, Z. An electrochemical sensor based on a magnetic Fe3O4 nanoparticles and gold nanoparticles modified electrode for sensitive determination of trace amounts of arsenic(III). Anal. Methods 2012, 4, 4176–4181. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Han, X.-J.; Fan, H.-L.; Zhang, Q.-X.; Liu, Y.-Q. Electrochemical detection of As(III) through mesoporous MnFe2O4 nanocrystal clusters by square wave stripping voltammetry. Electrochim. Acta 2015, 174, 1160–1166. [Google Scholar] [CrossRef]
- Sanllorente-Mendez, S.; Dominguez-Renedo, O.; Arcos-Martinez, M.J. Determination of Arsenic(III) Using Platinum Nanoparticle-Modified Screen-Printed Carbon-Based Electrodes. Electroanalysis 2009, 21, 635–639. [Google Scholar] [CrossRef]
- Wang, L.; Xu, W.-H.; Yang, R.; Zhou, T.; Hou, D.; Zheng, X.; Liu, J.-H.; Huang, X.-J. Electrochemical and Density Functional Theory Investigation on High Selectivity and Sensitivity of Exfoliated Nano-Zirconium Phosphate toward Lead(II). Anal. Chem. 2013, 85, 3984–3990. [Google Scholar] [CrossRef]
- Simm, A.O.; Banks, C.E.; Wilkins, S.J.; Karousos, N.G.; Davis, J.; Compton, R.G. A comparison of different types of gold-carbon composite electrode for detection of arsenic(III). Anal. Bioanal. Chem. 2005, 381, 979–985. [Google Scholar] [CrossRef]
- Huang, J.-F.; Chen, H.-H. Gold-nanoparticle-embedded nafion composite modified on glassy carbon electrode for highly selective detection of arsenic(III). Talanta 2013, 116, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Z.; Xie, Q.; Sun, L.; Gu, T.; Li, Z.; Bu, L.; Yao, S.; Tu, X.; Luo, X.; et al. Electrodeposition of electroreduced graphene oxide-Au nanoparticles composite film at glassy carbon electrode for anodic stripping voltammetric analysis of trace arsenic(III). Sens. Actuators B Chem. 2013, 188, 894–901. [Google Scholar] [CrossRef]
- Kato, D.; Kamata, T.; Kato, D.; Yanagisawa, H.; Niwa, O. Au Nanoparticle-Embedded Carbon Films for Electrochemical As3+ Detection with High Sensitivity and Stability. Anal. Chem. 2016, 88, 2944–2951. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, X.; Liu, J.-H.; Huang, X.-J. Enhanced anti-interference on electrochemical detection of arsenite with nanoporous gold in mild condition. Sens. Actuators B Chem. 2016, 234, 404–411. [Google Scholar] [CrossRef]
- Li, P.-H.; Yang, M.; Song, Z.-Y.; Chen, S.-H.; Xiao, X.-Y.; Lin, C.-H.; Huang, X.-J. Highly Sensitive and Stable Determination of As(III) under Near-Neutral Conditions: Benefit from the Synergetic Catalysis of Pt Single Atoms and Active S Atoms over Pt1/MoS2. Anal. Chem. 2021, 93, 15115–15123. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Jiang, T.-J.; Guo, Z.; Liu, J.-H.; Huang, X.-J. Electrochemical Detection of Trace Arsenic(III) by Nanocomposite of Nanorod-Like alpha-MnO2 Decorated with similar to 5 nm Au Nanoparticles: Considering the Change of Arsenic Speciation. Anal. Chem. 2016, 88, 9720–9728. [Google Scholar] [CrossRef]
- Devi, P.; Bansod, B.; Kaur, M.; Bagchi, S.; Nayak, M.K. Co-electrodeposited rGO/MnO2 nanohybrid for arsenite detection in water by stripping voltammetry. Sens. Actuators B Chem. 2016, 237, 652–659. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Periakaruppan, P.; Selvam, M.; Muthupandian, S.; Jeyaprabha, B.; Selvanathan, R. Clinically Pertinent Manganese Oxide/Polyoxytyramine/Reduced Graphene Oxide Nanocomposite for Voltammetric Detection of Salivary and Urinary Arsenic. J. Clust. Sci. 2020, 31, 877–885. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, T.; Hu, C.; Hu, S.; Tian, Q. Studies on fabrication and application of arsenic electrochemical sensors based on titanium dioxide nanoparticle modified gold strip electrodes. Anal. Methods 2016, 8, 1162–1169. [Google Scholar] [CrossRef]
- Kozu, Y.; Kawashima, S.; Kitamura, F. One-step electrochemical synthesis of Pt-CeO2 composite thin films on a glassy carbon electrode. J. Solid State Electrochem. 2013, 17, 761–765. [Google Scholar] [CrossRef]
- Huang, H.-Q.; Li, Y.-Y.; Chen, S.-H.; Liu, Z.-G.; Cui, Y.-M.; Li, H.-Q.; Guo, Z.; Huang, X.-J. Noble-metal-free Fe3O4/Co3S4 nanosheets with oxygen vacancies as an efficient electrocatalyst for highly sensitive electrochemical detection of As(III). Anal. Chim. Acta 2022, 1189, 339208. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xie, B.; Lu, Y.; Zhu, J. Advances in Electrochemical Detection Electrodes for As(III). Nanomaterials 2022, 12, 781. [Google Scholar] [CrossRef] [PubMed]
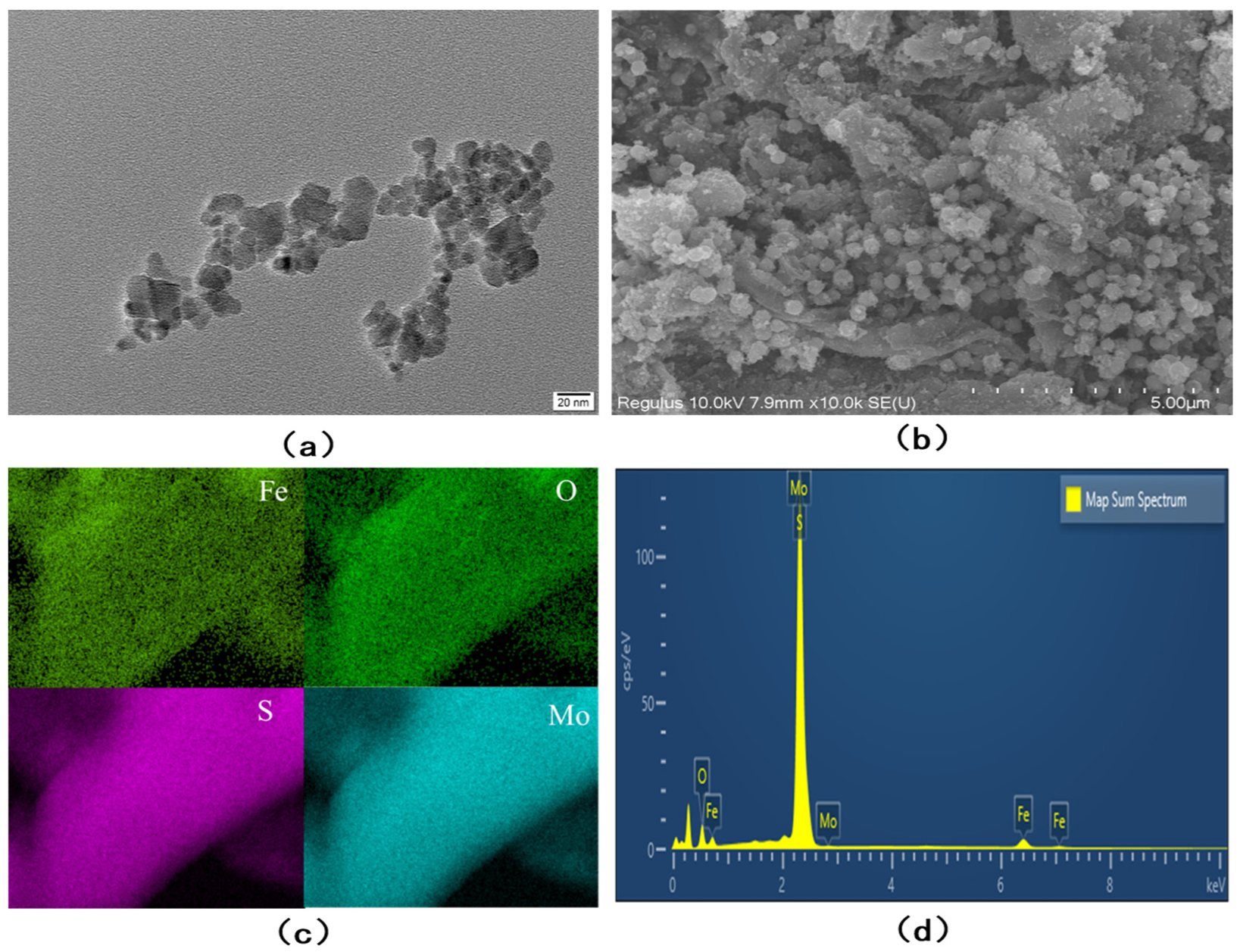
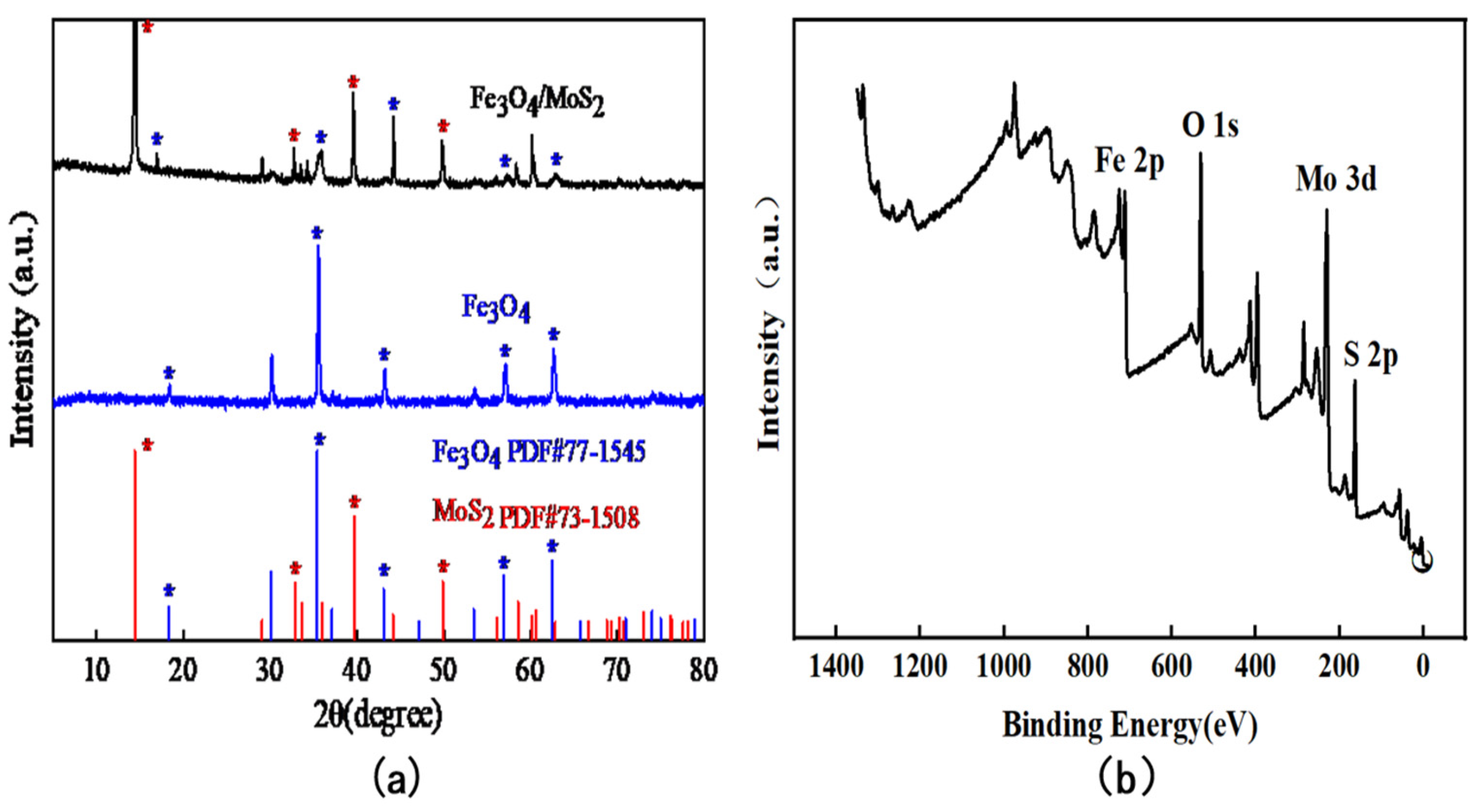
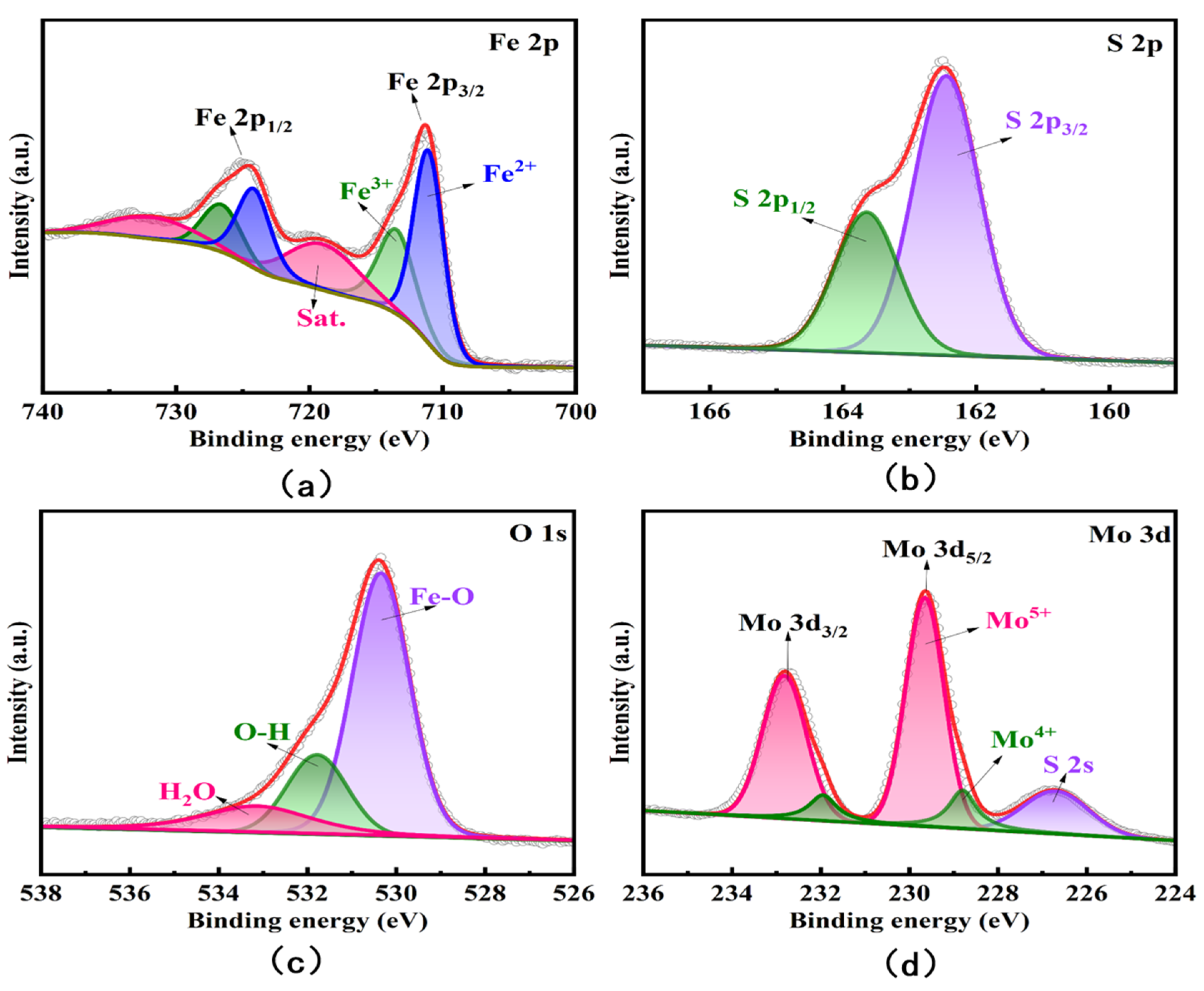
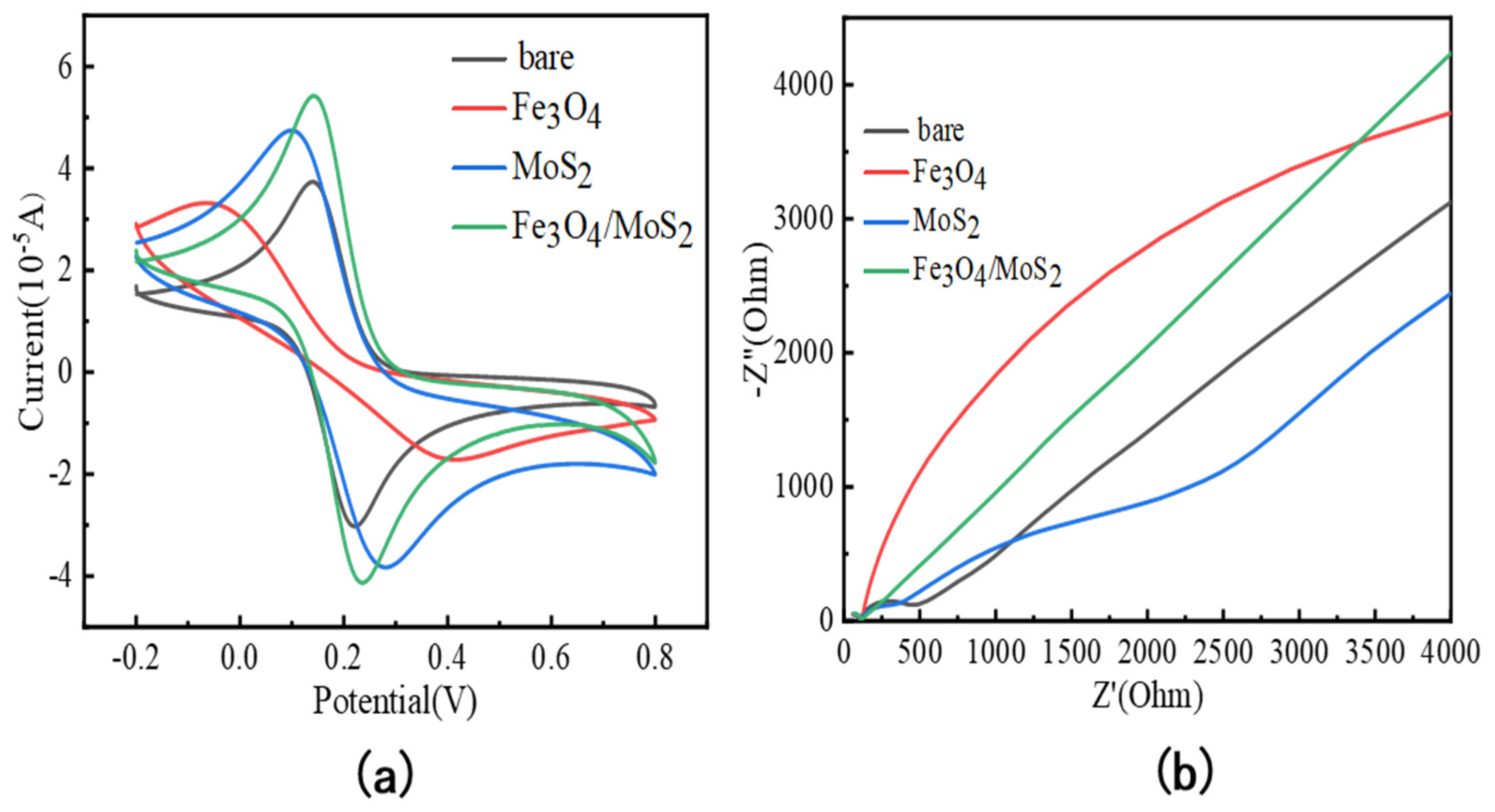



| Electrode | Method | Sensitivity (µA/ppb) | Linear Range (ppb) | LOD (ppb) | Reference |
|---|---|---|---|---|---|
| NF(Au nano) | SWV | 0.32 | 0.1–12.0 | 0.047 | [31] |
| ERGO-AuNPs | ASV | 0.16 | 0.75–374.6 | 0.20 | [32] |
| AuNPs-Cfilms | ASV | 0.026 | 1–100 | 0.55 | [33] |
| np-Au | SWASV | 0.60 | 0.5–15 | 0.0315 | [34] |
| Pt1/MoS2 | SWASV | 3.31 | 0.5–8 | 0.05 | [35] |
| AuNPs/α-MnO2 | SWASV | 0.828 | 1–10 | 0.019 | [36] |
| rGO/MnO2 | SWASV | 0.175 | 0.1–50 | 0.05 | [37] |
| MnO2/POT/rGO | DPV | 0.00163 | 0.01–0.9 | 0.042 | [38] |
| TiO2-GSE | LSV | 1.10 | 10–80 | 10 | [39] |
| Fe3O4/MoS2 | SWV | 3.67 | 1–20 | 0.70 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Hu, Y.; Xie, B.; Zhu, J. High Sensitivity Electrochemical As (III) Sensor Based on Fe3O4/MoS2 Nanocomposites. Nanomaterials 2023, 13, 2288. https://doi.org/10.3390/nano13162288
Hu H, Hu Y, Xie B, Zhu J. High Sensitivity Electrochemical As (III) Sensor Based on Fe3O4/MoS2 Nanocomposites. Nanomaterials. 2023; 13(16):2288. https://doi.org/10.3390/nano13162288
Chicago/Turabian StyleHu, Haibing, Yunhu Hu, Baozhu Xie, and Jianxiong Zhu. 2023. "High Sensitivity Electrochemical As (III) Sensor Based on Fe3O4/MoS2 Nanocomposites" Nanomaterials 13, no. 16: 2288. https://doi.org/10.3390/nano13162288






