High-Power-Density Thermoelectrochemical Cell Based on Ni/NiO Nanostructured Microsphere Electrodes with Alkaline Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasan, S.W.; Said, S.M.; Sabri, M.F.M.; Bakar, A.S.A.; Hashim, N.A.; Hasnan, M.M.I.M.; Pringle, J.M.; MacFarlane, D.R. High thermal gradient in thermo-electrochemical cells by insertion of a poly (vinylidene fluoride) membrane. Sci. Rep. 2016, 6, 29328. [Google Scholar] [CrossRef] [Green Version]
- Gunawan, A.; Lin, C.-H.; Buttry, D.A.; Mujica, V.; Taylor, R.A.; Prasher, R.S.; Phelan, P.E. Liquid thermoelectrics: Review of recent and limited new data of thermogalvanic cell experiments. Nanoscale Microscale Thermophys. Eng. 2013, 17, 304–323. [Google Scholar] [CrossRef]
- Duan, J.; Yu, B.; Liu, K.; Li, J.; Yang, P.; Xie, W.; Xue, G.; Liu, R.; Wang, H.; Zhou, J. P-N Conversion in thermogalvanic cells induced by thermo-sensitive nanogels for body heat harvesting. Energy 2019, 57, 473–479. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, X.; Wang, C.; Hang, S.; Li, M.; Liu, Y. Exploring Material Properties and Device Output Performance of a Miniaturized Flexible Thermoelectric Generator Using Scalable Synthesis of Bi2Se3. Nanomaterials 2023, 13, 1937. [Google Scholar] [CrossRef]
- Burmistrov, I.; Khanna, R.; Gorshkov, N.; Kiselev, N.; Artyukhov, D.; Boychenko, E.; Yudin, A.; Konyukhov, Y.; Kravchenko, M.; Gorokhovsky, A.; et al. Advances in thermo-electrochemical (TEC) cell performances for harvesting low-grade heat energy: A Review. Sustainability 2022, 14, 9483. [Google Scholar] [CrossRef]
- Qian, W.; Cao, M.; Xie, F.; Dong, C. Thermo-Electrochemical Cells Based on Carbon Nanotube Electrodes by Electrophoretic Deposition. Nano-Micro Lett. 2016, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Cao, M.; Xie, F.; Dong, C.; Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar]
- Artyukhov, D.; Gorshkov, N.; Vikulova, M.; Kiselev, N.; Zemtsov, A.; Artyukhov, I. Power Supply of Wireless Sensors Based on Energy Conversion of Separated Gas Flows by Thermoelectrochemical Cells. Energies 2022, 15, 1256. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Sherrell, P.C.; Liu, L.; Wang, Y.; Chen, J. Potentially Wearable Thermo-Electrochemical Cells for Body Heat Harvesting: From Mechanism, Materials, Strategies to Applications. Adv. Sci. 2021, 8, 2100669. [Google Scholar] [CrossRef]
- Liang, L.; Lv, H.; Shi, X.L.; Liu, Z.; Chen, G.; Chen, Z.G.; Sun, G. A flexible quasi-solid-state thermoelectrochemical cell with high stretchability as an energy-autonomous strain sensor. Mater. Horiz. 2021, 8, 2750–2760. [Google Scholar] [CrossRef]
- Kazim, A.H.; Booeshaghi, A.S.; Stephens, S.T.; Cola, B.A. Thermo-electrochemical generator: Energy harvesting & thermoregulation for liquid cooling applications. Sustain. Energy Fuels 2017, 1, 1381–1389. [Google Scholar]
- Jiang, L.; Kirihara, K.; Nandal, V.; Seki, K.; Mukaida, M.; Horike, S.; Wei, Q. Thermoelectrochemical Cells Based on Ferricyanide/Ferrocyanide/Guanidinium: Application and Challenges. ACS Appl. Mater. Interfaces 2022, 14, 22921–22928. [Google Scholar] [CrossRef]
- Li, M.; Hong, M.; Dargusch, M.; Zou, J.; Chen, Z.G. High-efficiency thermocells driven by thermo-electrochemical processes. Trends Chem. 2021, 3, 561–574. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, S.; Buckingham, M.A.; Aldous, L.; Beirne, S.; Wu, C.; Liu, Y.; Wallace, G.; Chen, J. Novel porous thermosensitive gel electrolytes for wearable thermo-electrochemical cells. Chem. Eng. J. 2022, 449, 137775. [Google Scholar] [CrossRef]
- deBethune, A.J.; Licht, T.S.; Swendeman, N. The temperature coefficients of electrode potentials: The isothermal and thermal coefficients—The standard ionic entropy of electrochemical transport of the hydrogen ion. J. Electrochem. Soc. 1959, 106, 616. [Google Scholar] [CrossRef]
- Burmistrov, I.; Gorshkov, N.; Kovyneva, N.; Kolesnikov, E.; Khaidarov, B.; Karunakaran, G.; Cho, E.-B.; Kiselev, N.; Artyukhov, D.; Kuznetsov, D.; et al. High seebeck coefficient thermo-electrochemical cell using nickel hollow microspheres electrodes. Renew. Energy 2020, 157, 1–8. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, P. High Seebeck Coefficient Electrochemical Thermocells for Efficient Waste Heat Recovery. ACS Appl. Energy Mater. 2018, 1, 1424–1428. [Google Scholar] [CrossRef]
- Dupont, M.F.; MacFarlane, D.R.; Pringle, J.M. Thermo-electrochemical cells for waste heat harvesting—Progress and perspectives. Chem. Commun. 2017, 53, 6288–6302. [Google Scholar] [CrossRef]
- Artyukhov, D.; Kiselev, N.; Gorshkov, N.; Kovyneva, N.; Ganzha, O.; Vikulova, M.; Gorokhovsky, A.; Offor, P.; Boychenko, E.; Burmistrov, I. Harvesting waste thermal energy using a surface-modified carbon fiber-based thermo-electrochemical cell. Sustainability 2021, 13, 1377. [Google Scholar] [CrossRef]
- Alzahrani, H.A.; Buckingham, M.A.; Marken, F.; Aldous, L. Success and failure in the incorporation of gold nanoparticles inside ferri/ferrocyanide thermogalvanic cells. Electrochem. Commun. 2019, 102, 41–45. [Google Scholar] [CrossRef]
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renew. Energy Focus 2019, 29, 42–48. [Google Scholar] [CrossRef]
- Gunawan, A.; Tarakeshwar, P.; Mujica, V.; Buttry, D.A.; Phelan, P.E. Improving Seebeck coefficient of thermoelectrochemical cells by controlling ligand complexation at metal redox centers. Appl. Phys. Lett. 2021, 118, 253901. [Google Scholar] [CrossRef]
- Salazar, P.F.; Kumar, S.; Cola, B.A. Nitrogen- and Boron-Doped Carbon Nanotube Electrodes in a Thermo-Electrochemical Cell. J. Electrochem. Soc. 2012, 159, B483. [Google Scholar] [CrossRef] [Green Version]
- Buckingham, M.A.; Hammoud, S.; Li, H.; Beale, C.J.; Sengel, J.T.; Aldous, L. A fundamental study of the thermoelectrochemistry of ferricyanide/ferrocyanide: Cation, concentration, ratio, and heterogeneous and homogeneous electrocatalysis effects in thermogalvanic cells. Sustain. Energy 2020, 4, 3388–3399. [Google Scholar] [CrossRef]
- Buckingham, M.A.; Laws, K.; Sengel, J.T.; Aldous, L. Using iron sulphate to form both n-type and p-type pseudo-thermoelectrics: Non-hazardous and ‘second life’ thermogalvanic cells. Green Chem. 2020, 22, 6062–6074. [Google Scholar] [CrossRef]
- Buckingham, M.A.; Laws, K.; Cross, E.; Surman, A.J.; Aldous, L. Developing iron-based anionic redox couples for thermogalvanic cells: Towards the replacement of the ferricyanide/ferrocyanide redox couple. Green Chem. 2021, 23, 8901–8915. [Google Scholar] [CrossRef]
- Deng, W.; Xu, Y.; Zhang, X.; Li, C.; Liu, Y.; Xiang, K.; Chen, H. (NH4)2Co2V10O28·16H2O/(NH4)2V10O25·8H2O heterostructure as cathode for high-performance aqueous Zn-ion batteries. J. Alloys Compd. 2022, 903, 163824. [Google Scholar] [CrossRef]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Wen, X.; Luo, J.; Xiang, K.; Zhou, W.; Zhang, C.; Chen, H. High-performance monoclinic WO3 nanospheres with the novel NH4+ diffusion behaviors for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Li, D.; Guo, H.; Jiang, S.; Zeng, G.; Zhou, W.; Li, Z. Microstructures and electrochemical performances of TiO2-coated Mg–Zr co-doped NCM as a cathode material for lithium-ion batteries with high power and long circular life. New J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Jung, S.-M.; Kwon, J.; Lee, J.; Han, I.K.; Kim, K.-S.; Kim, Y.S.; Kim, Y.-T. Cost-efficient nickel-based thermo-electrochemical cells for utilizing low-grade thermal energy. J. Power Sources 2021, 494, 229705. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. 2017, 5, 874–901. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Kang, S.H.; Zhu, K.; Kim, J.Y.; Neale, N.R.; Frank, A.J. Ni–NiO core–shell inverse opal electrodes for supercapacitors. Chem. Commun. 2011, 47, 5214–5216. [Google Scholar] [CrossRef]
- Sajjad, M.; Amin, M.; Javed, M.S.; Imran, M.; Hu, W.; Mao, Z.; Lu, W. Recent trends in transition metal diselenides (XSe2: X=Ni, Mn, Co) and their composites for high energy faradic supercapacitors. J. Energy Storage 2021, 43, 103176. [Google Scholar] [CrossRef]
- Chen, G.F.; Su, Y.Z.; Kuang, P.Y.; Liu, Z.Q.; Chen, D.Y.; Wu, X.; Li, N.; Qiao, S.Z. Polypyrrole Shell@3D-Ni Metal Core Structured Electrodes for High-Performance Supercapacitors. Chem.—A Eur. J. 2015, 21, 103176. [Google Scholar] [CrossRef]
- Yudin, A.; Shatrova, N.; Khaydarov, B.; Kuznetsov, D.; Dzidziguri, E.; Issi, J.-P. Synthesis of hollow nanostructured nickel oxide microspheres by ultrasonic spray atomization. J. Aerosol Sci. 2016, 98, 30–40. [Google Scholar] [CrossRef]
- Qi, X.; Zheng, W.; Li, X.; He, G. Multishelled NiO hollow microspheres for highperformance supercapacitors with ultrahigh energy density and robust cycle life. Sci. Rep. 2016, 6, 33241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorkipli, N.N.M.; Kaus, N.H.M.; Mohamad, A.A. Synthesis of NiO nanoparticles through sol-gel method. Procedia Chem. 2016, 19, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, G.; Kundu, M.; Kumari, S.; Kolesnikov, E.; Gorshenkov, M.V.; Maduraiveeran, G.; Sasidharan, M.; Kuznetsov, D. ZnO/Cu2MgO3 hollow porous nanocage: A new class of hybrid anode material for advanced lithium-ion batteries. J. Alloys Compd. 2018, 763, 94–101. [Google Scholar] [CrossRef]
- Taganova, A.A.; Boychenko, E.A.; Kiselev, N.V.; Khaidarov, B.B.; Kolesnikov, E.A.; Yudin, A.G.; Vikulova, M.A.; Gorshkov, N.V.; Kuznetsov, D.V.; Burmistrov, I.N. Synthesis and study of the composition of hollow microspheres of NiO and NiO/Ni composition for thermoelectrochemical energy converters of low-potential temperature gradients of thermal units into electricity. Refract. Ind. Ceram. 2021, 61, 715–719. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Zidi, N.; Chaouchi, A.; Rguiti, M.; Lorgouilloux, Y.; Courtois, C. Dielectric, ferroelectric, piezoelectric properties, and impedance spectroscopy of (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3−x% (K0.5Bi0.5)TiO3 leadfree ceramics. Ferroelectrics 2019, 511, 152–177. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Hasnan, M.M.I.M.; Said, S.M.; Sabri, M.F.M.; Hussin, S.A.M.; Abdullah, N.; Ibrahim, N.M.J.N.; Miyazaki, Y.; Salleh, M.F.M.; Shah, N.M. Optimised thermally driven molecular stability of an SCO metal complex for TEC Seebeck generation enhancement. RSC Adv. 2019, 9, 10626–10634. [Google Scholar] [CrossRef]
- Hassan, H.C.; Said, S.M.; Ibrahim, N.M.J.N.; Hasnan, M.M.I.M.; Noor, I.S.M.; Zakaria, R.; Salleh, M.F.M.; Noor, N.L.M.; Abdullah, N. Ultra-high Seebeck coefficient of a thermal sensor through entropic optimisation of ligand length of Fe(ii) spin-crossover (SCO) materials. RSC Adv. 2021, 11, 20970–20982. [Google Scholar] [CrossRef] [PubMed]

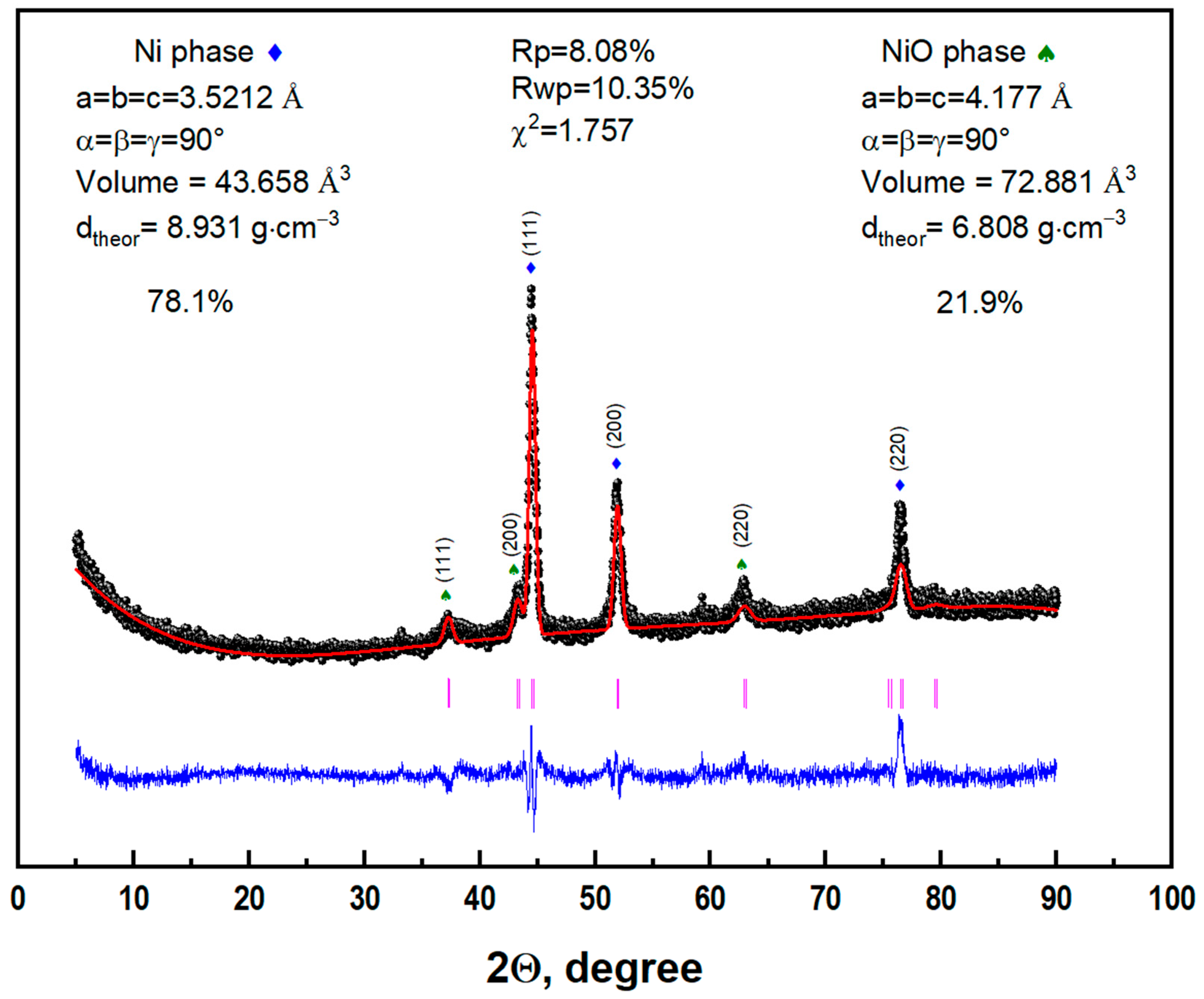
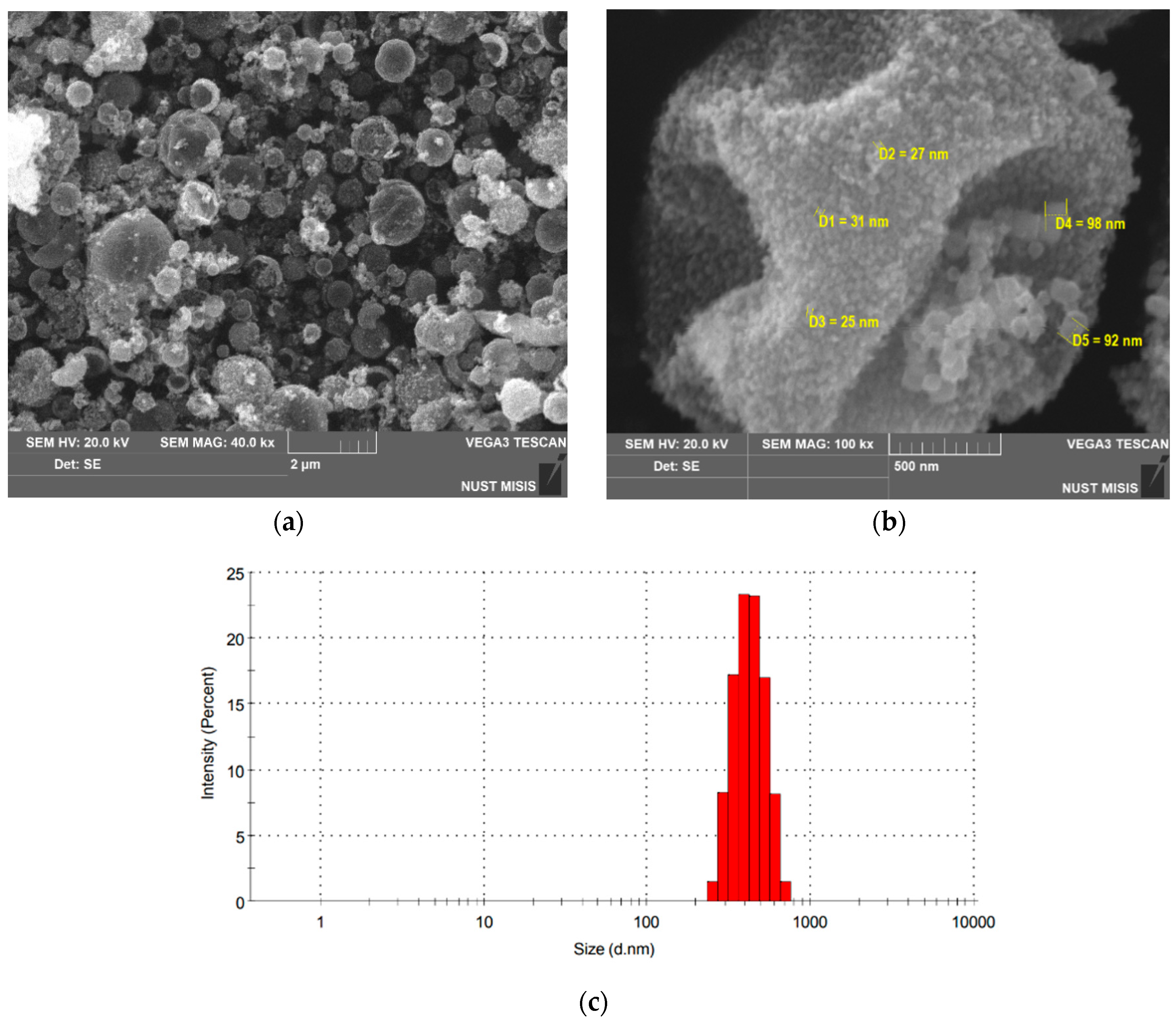
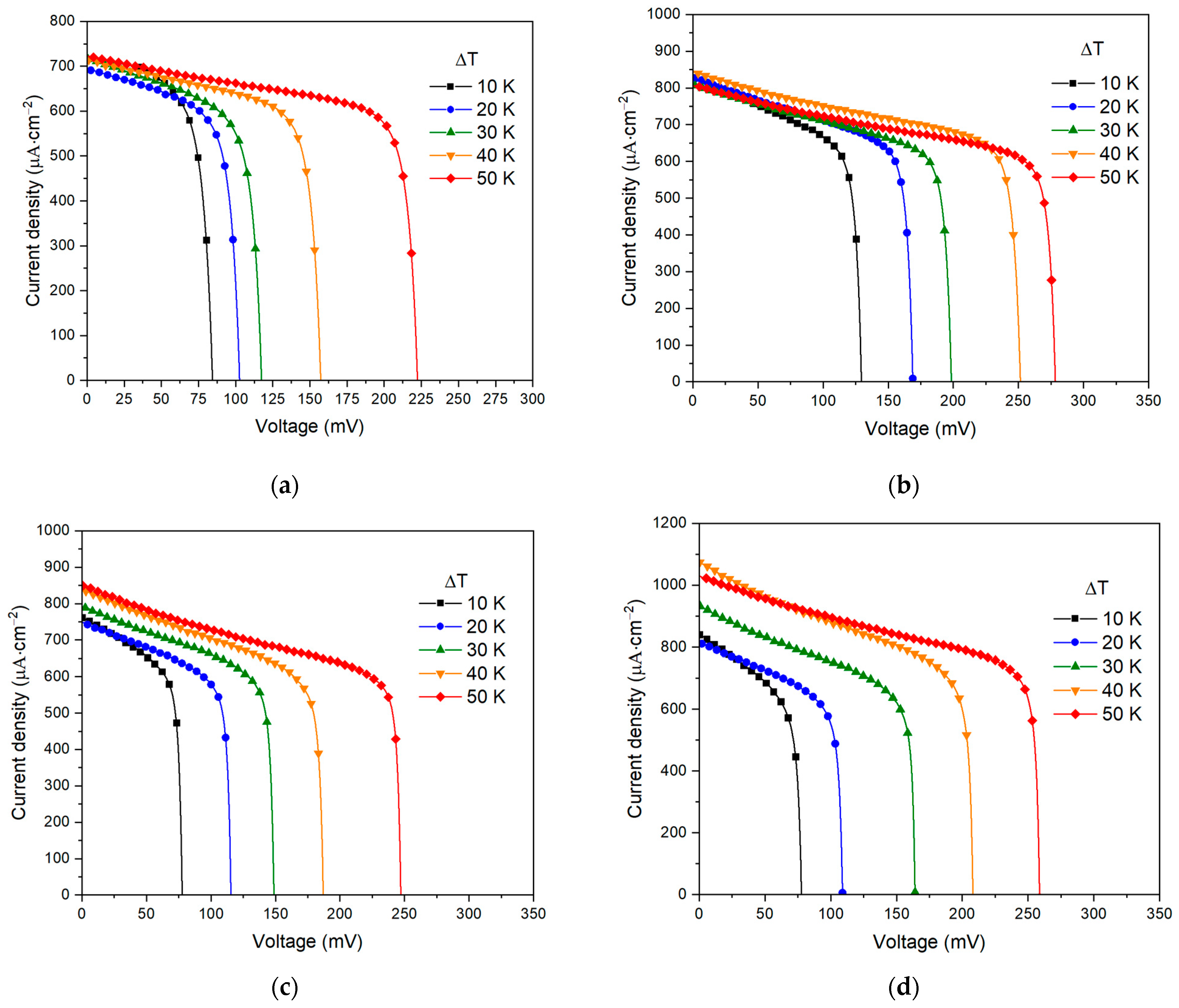


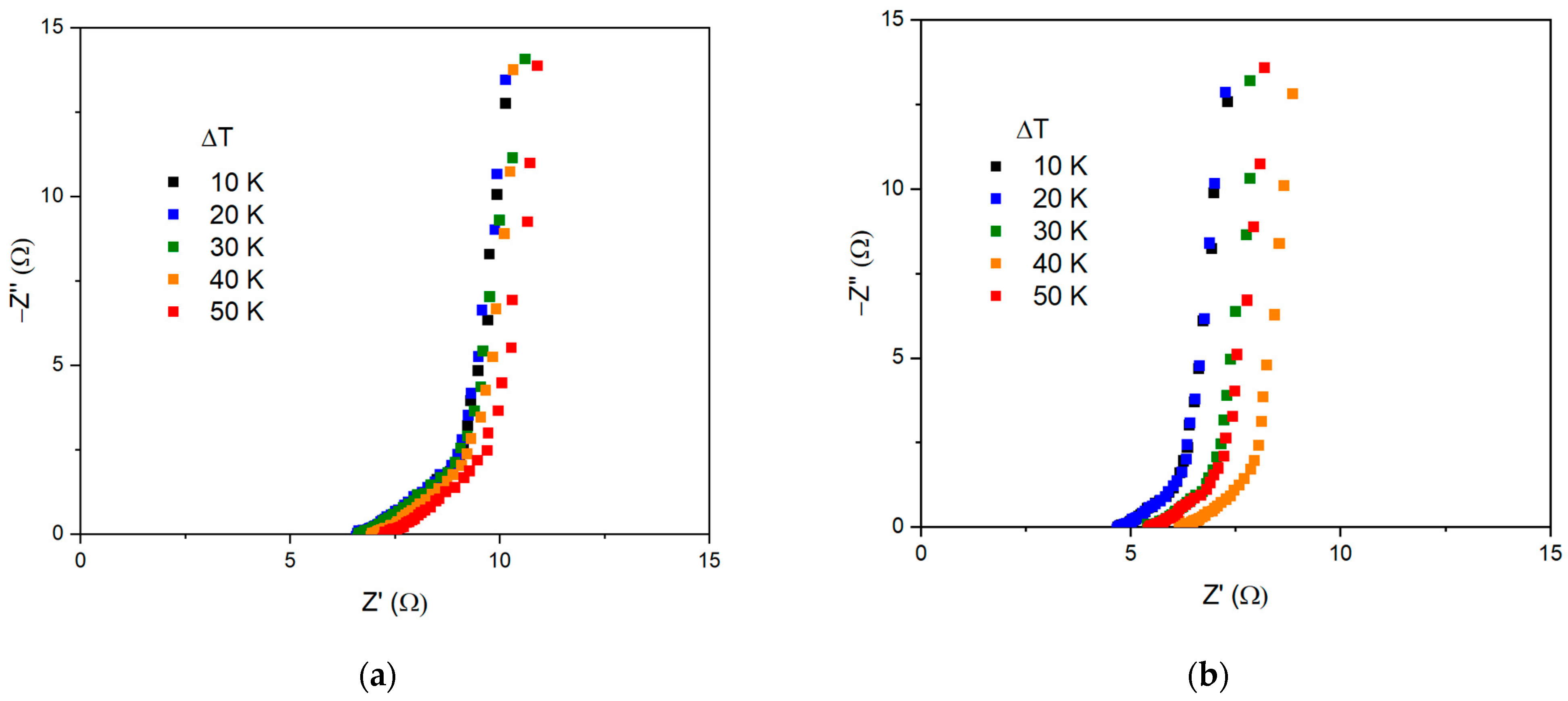
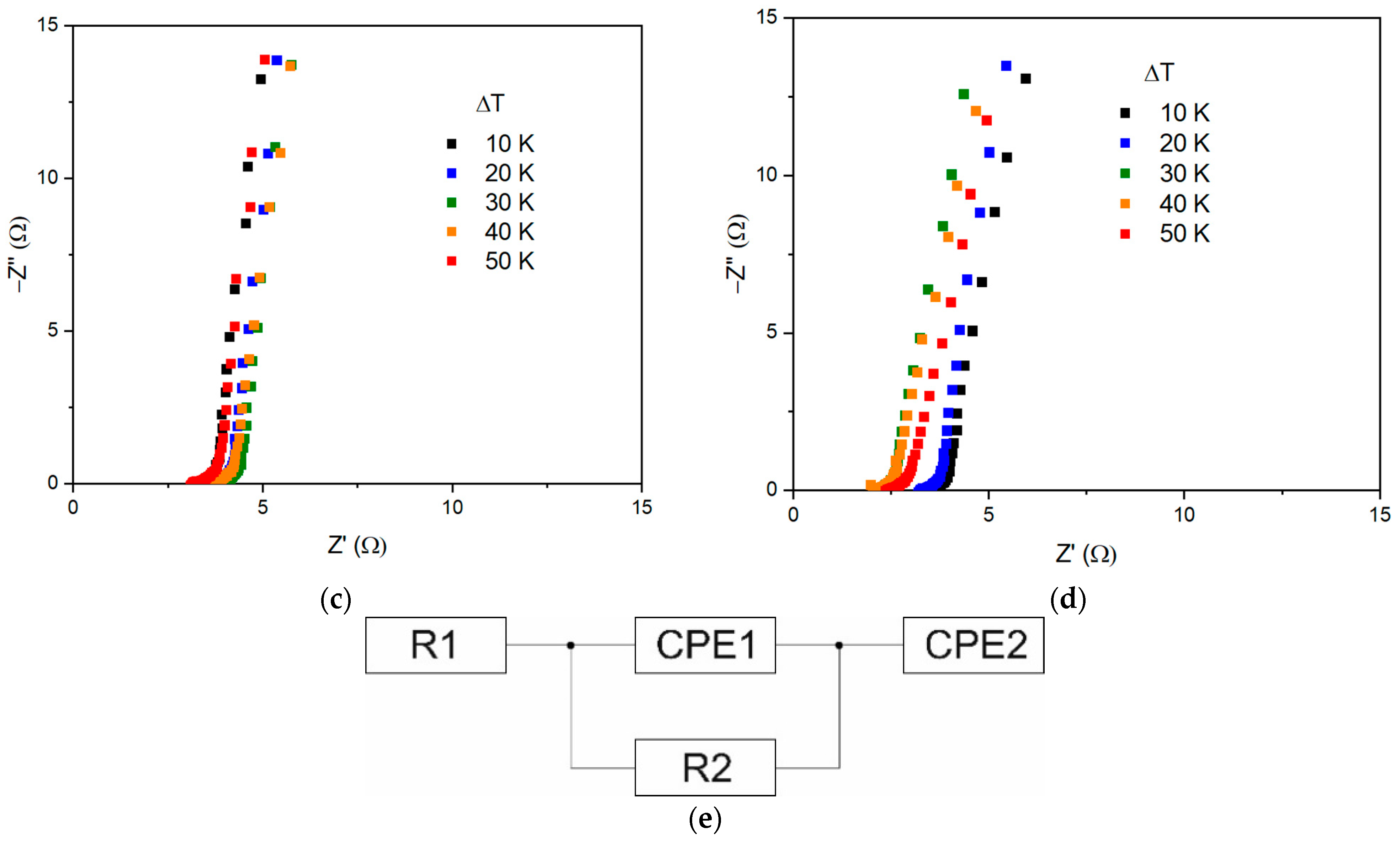
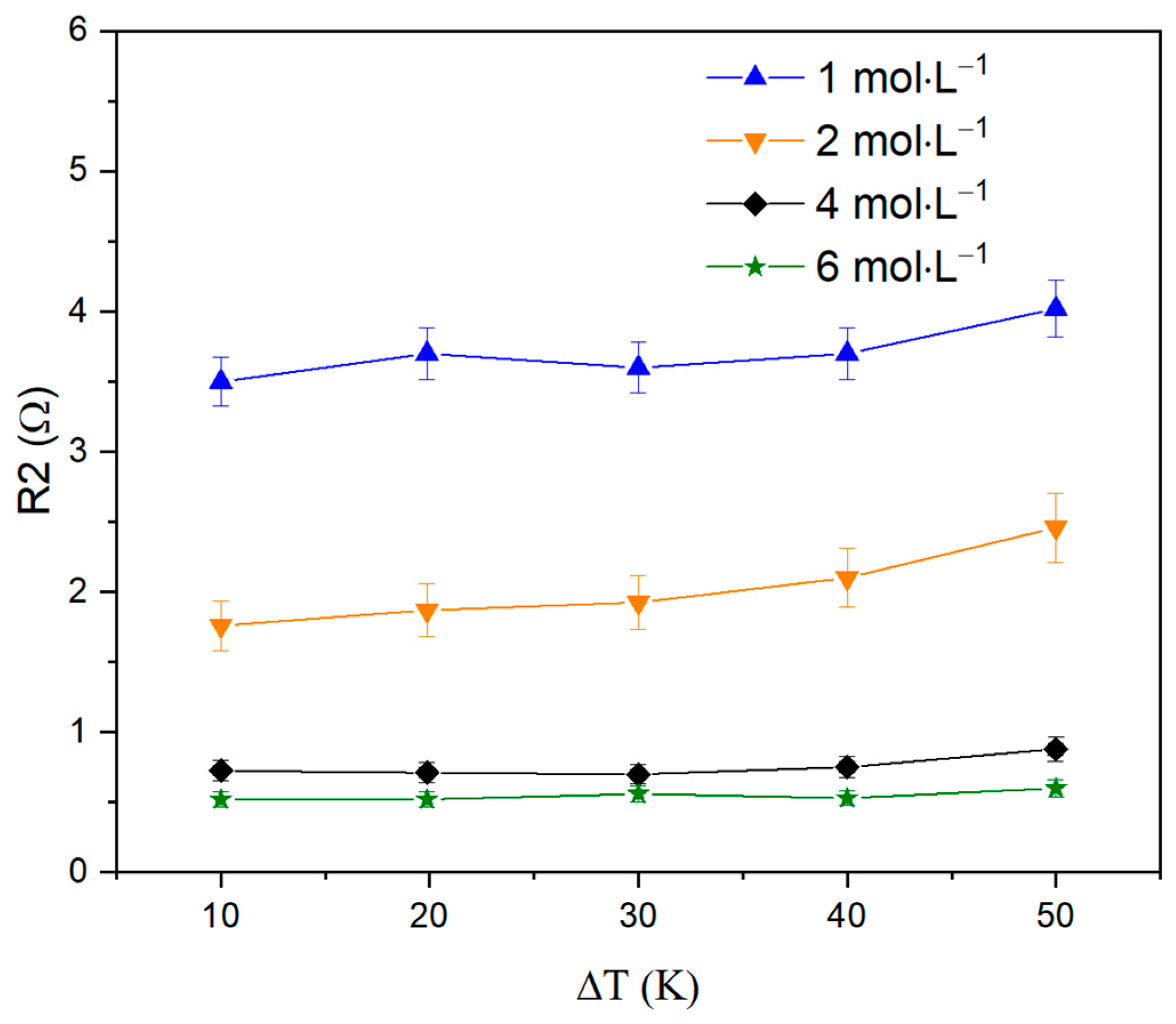
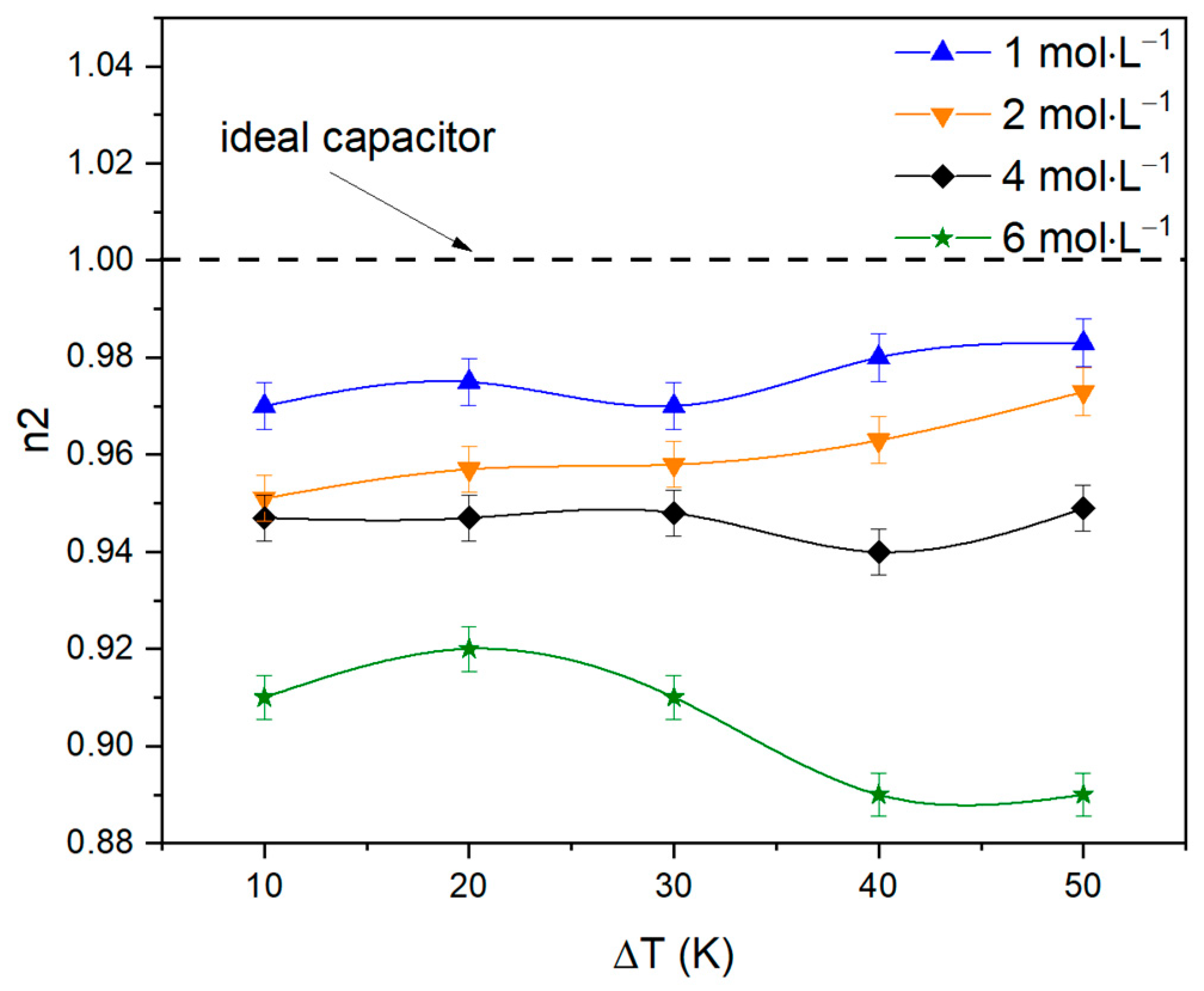
| ΔT, °C | R1, Ω | R2, Ω | P1 | n1 | P2 | n2 |
|---|---|---|---|---|---|---|
| 1 mol·L−1 | ||||||
| 10 | 6.7 | 3.5 | 0.09 | 0.47 | 0.124 | 0.97 |
| 20 | 6.6 | 3.7 | 0.09 | 0.47 | 0.115 | 0.975 |
| 30 | 6.63 | 3.6 | 0.09 | 0.45 | 0.11 | 0.970 |
| 40 | 7 | 3.7 | 0.09 | 0.49 | 0.115 | 0.98 |
| 50 | 7.2 | 4.02 | 0.09 | 0.488 | 0.112 | 0.983 |
| 2 mol·L−1 | ||||||
| 10 | 4.7 | 1.76 | 0.09 | 0.52 | 0.12 | 0.951 |
| 20 | 4.69 | 1.87 | 0.09 | 0.496 | 0.118 | 0.957 |
| 30 | 5.43 | 1.926 | 0.09 | 0.5 | 0.116 | 0.958 |
| 40 | 6.22 | 2.1 | 0.09 | 0.5 | 0.117 | 0.963 |
| 50 | 5.476 | 2.458 | 0.09 | 0.528 | 0.114 | 0.973 |
| 4 mol·L−1 | ||||||
| 10 | 3.1 | 0.725 | 0.12 | 0.366 | 0.11 | 0.947 |
| 20 | 3.5 | 0.71 | 0.12 | 0.41 | 0.11 | 0.947 |
| 30 | 3.7 | 0.7 | 0.12 | 0.474 | 0.108 | 0.948 |
| 40 | 3.58 | 0.75 | 0.12 | 0.47 | 0.1 | 0.941 |
| 50 | 3.08 | 0.88 | 0.12 | 0.41 | 0.1 | 0.949 |
| 6 mol·L−1 | ||||||
| 10 | 3.4 | 0.52 | 0.09 | 0.46 | 0.11 | 0.91 |
| 20 | 3.22 | 0.52 | 0.09 | 0.5 | 0.1 | 0.92 |
| 30 | 2.05 | 0.56 | 0.09 | 0.5 | 0.11 | 0.91 |
| 40 | 2.01 | 0.53 | 0.09 | 0.47 | 0.12 | 0.89 |
| 50 | 2.36 | 0.6 | 0.09 | 0.49 | 0.12 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artyukhov, D.; Kiselev, N.; Boychenko, E.; Asmolova, A.; Zheleznov, D.; Artyukhov, I.; Burmistrov, I.; Gorshkov, N. High-Power-Density Thermoelectrochemical Cell Based on Ni/NiO Nanostructured Microsphere Electrodes with Alkaline Electrolyte. Nanomaterials 2023, 13, 2290. https://doi.org/10.3390/nano13162290
Artyukhov D, Kiselev N, Boychenko E, Asmolova A, Zheleznov D, Artyukhov I, Burmistrov I, Gorshkov N. High-Power-Density Thermoelectrochemical Cell Based on Ni/NiO Nanostructured Microsphere Electrodes with Alkaline Electrolyte. Nanomaterials. 2023; 13(16):2290. https://doi.org/10.3390/nano13162290
Chicago/Turabian StyleArtyukhov, Denis, Nikolay Kiselev, Elena Boychenko, Aleksandra Asmolova, Denis Zheleznov, Ivan Artyukhov, Igor Burmistrov, and Nikolay Gorshkov. 2023. "High-Power-Density Thermoelectrochemical Cell Based on Ni/NiO Nanostructured Microsphere Electrodes with Alkaline Electrolyte" Nanomaterials 13, no. 16: 2290. https://doi.org/10.3390/nano13162290











