Pillared Carbon Membranes Derived from Cardo Polymers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Polymer Synthesis
2.3. Polymer Precursor Preparation
2.4. CMSM Fabrication
- 20 to 250 °C at a rate of 13 °C/min;
- 250 to 535 °C at a rate of 3.85 °C/min;
- 535 to 550 °C at a rate of 0.25 °C/min;
- 0 h soak time at 550 °C.
3. Characterization
3.1. Polymer
3.2. Membranes
3.3. CMSM
3.4. Gas Permeability Testing
3.5. Aging Experiments
4. Result and Discussion
4.1. Thermal Analysis (TGA, TGA-MS, DSC) of Membranes
4.2. Spectroscopic Characterization of Mixed Matrix and Carbon Membranes
4.3. Transmission Electron Microscopy (TEM)
4.4. XRD, Raman, and X-ray Photoelectron Spectroscopic Analysis
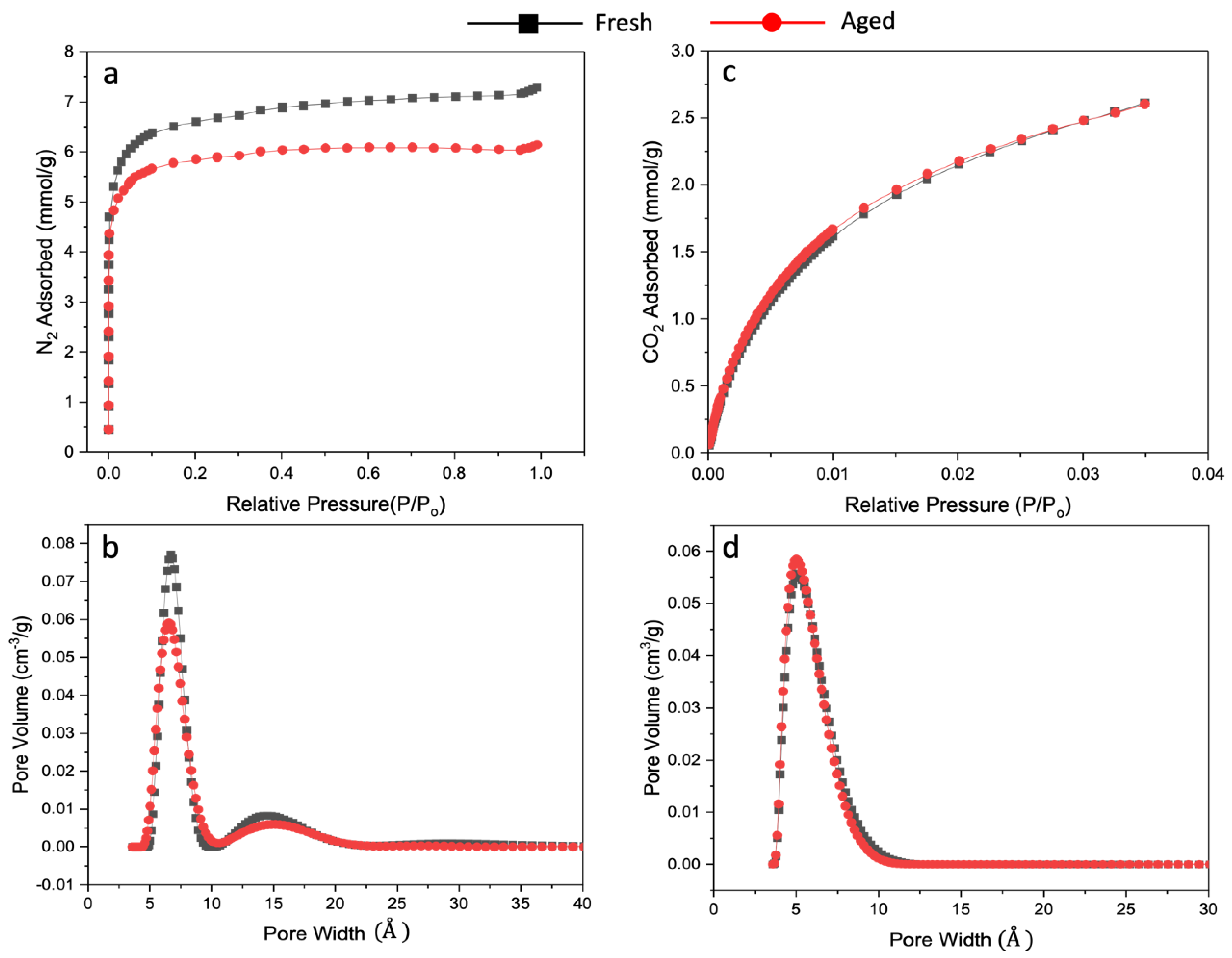
4.5. Gas Adsorption and Pore Size Distribution Analysis
4.6. Gas Permeability
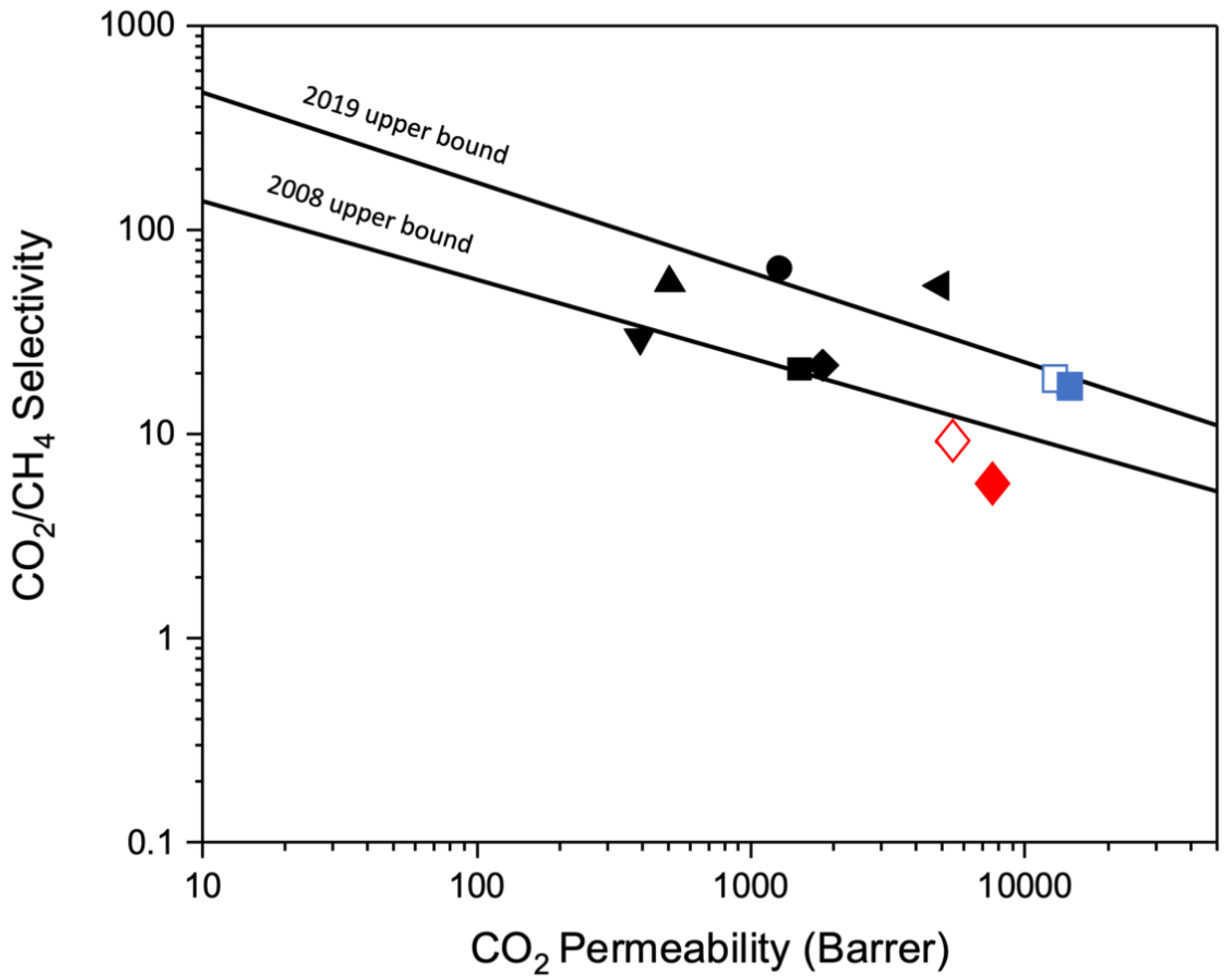
4.6.1. Pure Gas Permselectivity of CMSM
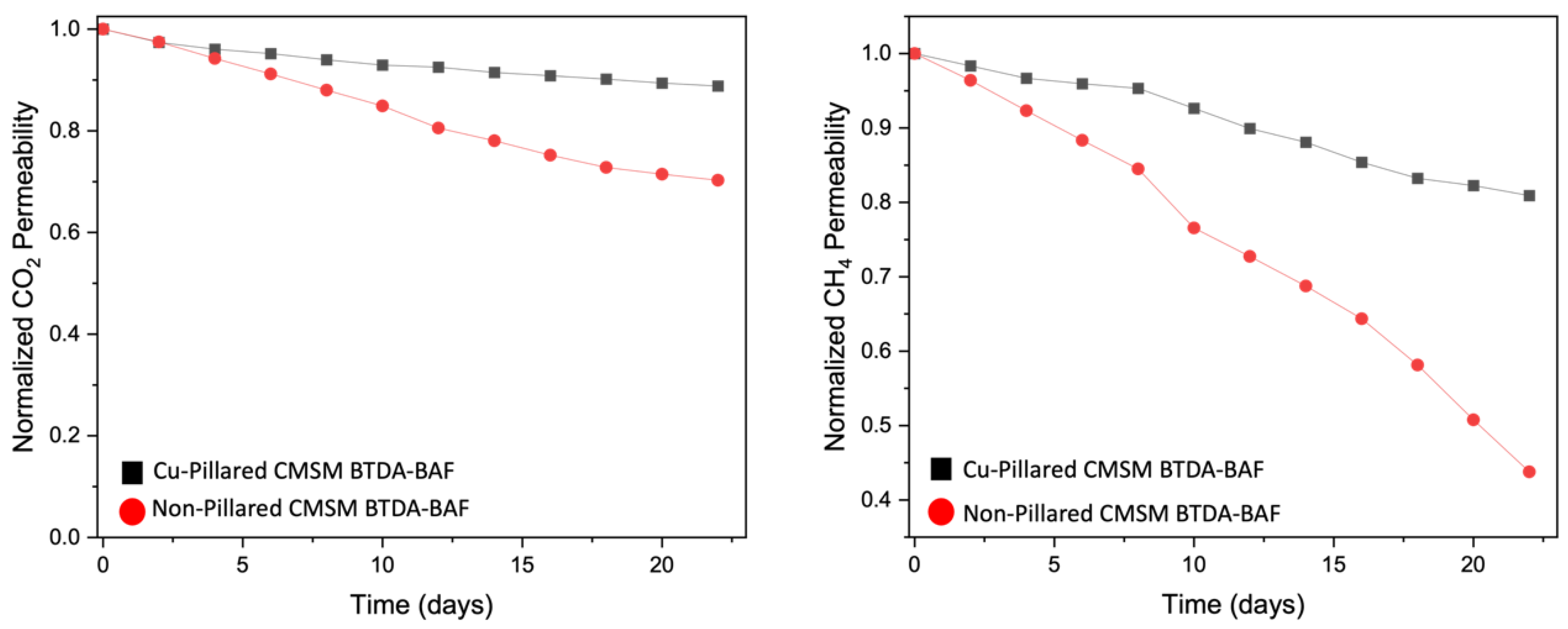
4.6.2. Stability of CMSMs to Aging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon Molecular Sieve Structure Development and Membrane Performance Relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Qiu, W.; Paul, D.R.; Koros, W.J. Responses of 6FDA-Based Polyimide Thin Membranes to CO2 Exposure and Physical Aging as Monitored by Gas Permeability. Polymer 2011, 52, 5528–5537. [Google Scholar] [CrossRef]
- Zhang, C. Synthesis and Characterization of Bis (Phenyl) Fluorene- Based Cardo Polyimide Membranes for H2/CH4 Separation. J. Mater. Sci. 2019, 54, 10560–10569. [Google Scholar] [CrossRef]
- Xu, R.; Li, L.; Jin, X.; Hou, M.; He, L.; Lu, Y.; Song, C.; Wang, T. Thermal Crosslinking of a Novel Membrane Derived from Phenolphthalein- Based Cardo Poly (Arylene Ether Ketone) to Enhance CO2/CH4 Separation Performance and Plasticization Resistance. J. Memb. Sci. 2019, 586, 306–317. [Google Scholar] [CrossRef]
- Korshak, V.V.; Vinogradova, S.V.; Vygodskii, Y.S. Cardo Polymers. J. Macromol. Sci. Part C 1974, 11, 45–142. [Google Scholar] [CrossRef]
- Tokuda, Y.; Fujisawa, E.; Okabayashi, N.; Matsumiya, N.; Takagi, K.; Mano, H.; Sato, M. Development of Hollow Fiber Membranes for CO2 Separation. Energy Convers. Manag. 1997, 38, S111–S116. [Google Scholar] [CrossRef]
- Chenar, M.P.; Savoji, H.; Soltanieh, M. Removal of Hydrogen Sulfide from Methane Using Commercial Polyphenylene Oxide and Cardo-Type Polyimide Hollow Fiber Membranes. Korean J. Chem. Eng. 2011, 28, 902–913. [Google Scholar] [CrossRef]
- Xu, Z.; Dannenberg, C. Gas Separation Properties of Polymers Containing Fluorene Moieties. Chem. Mater. 2002, 14, 3271–3276. [Google Scholar] [CrossRef]
- Camacho-Zuniga, C.; Ruiz-Trevino, F.A.; Zolotukhin, M.G.; Castillo, L.F.; Guzman, J.; Chavez, J.; Torres, G.; Gileva, N.G.; Sedova, E.A. Gas Transport Properties of New Aromatic Cardo Poly (Aryl Ether Ketone) S. J. Memb. Sci. 2006, 283, 393–398. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S. Fluorinated Poly(Arylene Ether)s with Aliphatic Chain Appended Cardo Moiety: Synthesis and Gas Transport Properties. J. Memb. Sci. 2014, 470, 535–546. [Google Scholar] [CrossRef]
- Kazama, S.; Teramoto, T.; Haraya, K. Carbon Dioxide and Nitrogen Transport Properties of Bis (Phenyl) Fluorene-Based Cardo Polymer Membranes. J. Memb. Sci. 2002, 207, 91–104. [Google Scholar] [CrossRef]
- Chenar, M.P.; Soltanieh, M.; Matsuura, T.; Tabe-mohammadi, A.; Feng, C. Gas Permeation Properties of Commercial Polyphenylene Oxide and Cardo-Type Polyimide Hollow Fiber Membranes. Sep. Purif. Tech. 2006, 51, 359–366. [Google Scholar] [CrossRef]
- Kazama, S.; Morimoto, S.; Tanaka, S.; Mano, H.; Yashima, T.; Yamada, K.; Haraya, K. Cardo polyimide membranes for CO2 capture from flue gases. Greenh. Gas Control Technol. 7 2005, 1, 75–82. [Google Scholar]
- Chenar, M.P.; Soltanieh, M.; Matsuura, T.; Tabe-mohammadi, A.; Khulbe, K.C. The Effect of Water Vapor on the Performance of Commercial Polyphenylene Oxide and Cardo-Type Polyimide Hollow Fiber Membranes in CO2/CH4 Separation Applications. J. Memb. Sci. 2006, 285, 265–271. [Google Scholar] [CrossRef]
- Yeong, Y.F.; Wang, H.; Pramoda, K.P.; Chung, T.S. Thermal Induced Structural Rearrangement of Cardo-Copolybenzoxazole Membranes for Enhanced Gas Transport Properties. J. Memb. Sci. 2012, 397, 51–65. [Google Scholar] [CrossRef]
- Lu, Y.; Hao, J.; Li, L.; Song, J.; Xiao, G.; Zhao, H.; Hu, Z.; Wang, T. Preparation and Gas Transport Properties of Thermally Induced Rigid Membranes of Copolyimide Containing Cardo Moieties. React. Funct. Polym. 2017, 119, 134–144. [Google Scholar] [CrossRef]
- Yahaya, G.O.; Mokhtari, I.; Alghannam, A.A.; Choi, S.; Maab, H.; Bahamdan, A.A. Cardo-Type Random Co-Polyimide Membranes for High Pressure Pure and Mixed Sour Gas Feed Separations. J. Memb. Sci. 2018, 550, 526–535. [Google Scholar] [CrossRef]
- Hu, C.; Polintan, C.K.; Tayo, L.L.; Chou, S.; Tsai, H.; Hung, W.; Hu, C.; Lee, K.; Lai, J. The Gas Separation Performance Adjustment of Carbon Molecular Sieve Membrane Depending on the Chain Rigidity and Free Volume Characteristic of the Polymeric Precursor. Carbon 2019, 143, 343–351. [Google Scholar] [CrossRef]
- Sun, H.; Gao, W.; Zhang, Y.; Cao, X.; Bao, S.; Li, P.; Kang, Z.; Niu, Q.J. Bis (Phenyl) Fluorene-Based Polymer of Intrinsic Microporosity/Functionalized Multi-Walled Carbon Nanotubes Mixed Matrix Membranes for Enhanced CO2 Separation Performance. React. Funct. Polym. 2020, 147, 104465. [Google Scholar] [CrossRef]
- Rahmani, M.; Kazemi, A.; Talebnia, F.; Gamali, P.A. Fabrication and Characterization of Brominated Separation: Application of Response Surface Methodology (RSM). E-Polymers 2016, 16, 481–492. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Koros, W.J. Matrimid® Derived Carbon Molecular Sieve Hollow Fiber Membranes for Ethylene/Ethane Separation. J. Memb. Sci. 2011, 380, 138–147. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. An Investigation of the Effects of Pyrolysis Parameters on Gas Separation Properties of Carbon Materials. Carbon 2005, 43, 1843–1856. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. Investigation of Porosity of Carbon Materials and Related Effects on Gas Separation Properties. Carbon 2003, 41, 253–266. [Google Scholar] [CrossRef]
- Hägg, M.; Lie, J.O.N.A.; Lindbråthen, A. A Promising Alternative for Selected Industrial Applications. Ann. N. Y. Acad. Sci. 2003, 984, 329–345. [Google Scholar] [CrossRef]
- Llosa Tanco, M.; Pacheco Tanaka, D. Recent Advances on Carbon Molecular Sieve Membranes (CMSMs) and Reactors. Processes 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.S.; Itta, A.K.; Zhang, C.; Wenz, G.B.; Sanyal, O.; Koros, W.J. New Insights into Structural Evolution in Carbon Molecular Sieve Membranes during Pyrolysis. Carbon 2019, 141, 238–246. [Google Scholar] [CrossRef]
- Lagorsse, S.; Magalh, F.D.; Mendes, A. Aging Study of Carbon Molecular Sieve Membranes. J. Memb. Sci. 2008, 310, 494–502. [Google Scholar] [CrossRef]
- Centeno, T.A.; Vilas, J.L.; Fuertes, A.B. Effects of Phenolic Resin Pyrolysis Conditions on Carbon Membrane Performance for Gas Separation. J. Memb. Sci. 2004, 228, 45–54. [Google Scholar] [CrossRef]
- Geiszler, V.C.; Koros, W.J. Effects of Polyimide Pyrolysis Conditions on Carbon Molecular Sieve Membrane Properties. Ind. Eng. Chem. Res. 1996, 5885, 2999–3003. [Google Scholar] [CrossRef]
- Shao, L.; Chung, T.; Goh, S.H.; Pramoda, K.P. The Effects of 1, 3-Cyclohexanebis (Methylamine) Modification on Gas Transport and Plasticization Resistance of Polyimide Membranes. J. Memb. Sci. 2005, 267, 78–89. [Google Scholar] [CrossRef]
- Shao, L.; Liu, L.; Cheng, S.; Huang, Y.; Ma, J. Comparison of Diamino Cross-Linking in Different Polyimide Solutions and Membranes by Precipitation Observation and Gas Transport. J. Memb. Sci. 2008, 312, 174–185. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural Gas Permeation in Polyimide Membranes. J. Memb. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Nguyen, C.N.; Huang, Y.; Balkus, K.J., Jr.; Musselman, I.H.; Ferraris, J.P. Composite Membranes with a Highly Selective Polymer Skin for Hydrogen Separation. Sep. Purif. Technol. 2014, 135, 190–198. [Google Scholar] [CrossRef]
- Wijenayake, S.N.; Panapitiya, N.P.; Versteeg, S.H.; Nguyen, C.N.; Goel, S.; Balkus, K.J., Jr.; Musselman, I.H.; Ferraris, J.P. Surface Cross-Linking of ZIF-8/Polyimide Mixed Matrix Membranes (MMMs) for Gas Separation. Ind. Eng. Chem. Res. 2013, 52, 6991–7001. [Google Scholar] [CrossRef]
- Lin, M.; Xiao, Y.; Chung, T.; Toriida, M.; Tamai, S. Enhanced Propylene/Propane Separation by Carbonaceous Membrane Derived from Poly (Aryl Ether Ketone)/Interpenetrating Network. Carbon 2009, 47, 1857–1866. [Google Scholar]
- Vaughn, J.T.; Qiu, W.; Koros, W.J.; Xu, L.; Brayden, M.K. Cross-Linked Polyimide Membranes and Carbon Molecular Sieve Hollow Fiber Membranes Made Therefrom. U.S. Patent Application No. 17/266,778, 21 October 2021. [Google Scholar]
- Karunaweera, C.; Musselman, I.H.; Balkus, K.J., Jr.; Ferraris, J.P. Fabrication and Characterization of Aging Resistant Carbon Molecular Sieve Membranes for C3 Separation Using High Molecular Weight Crosslinkable. J. Memb. Sci. 2019, 581, 430–438. [Google Scholar] [CrossRef]
- Ma, C.; Koros, W.J. Physical Aging of Ester-Cross-Linked Hollow Fi Ber Membranes for Natural Gas Separations and Mitigation Thereof. J. Memb. Sci. 2018, 551, 214–221. [Google Scholar] [CrossRef]
- Tamaddondar, M.; Foster, A.B.; Carta, M.; Gorgojo, P.; Mckeown, N.B.; Budd, P.M. Mitigation of Physical Aging with Mixed Matrix Membranes Based on Cross-Linked PIM-1 Fillers and PIM-1. ACS Appl. Mater. Interfaces 2020, 12, 46756–46766. [Google Scholar] [CrossRef]
- Hernandez-Martinez, H.; Ruiz-Trevino, F.A.; Aguilar-vega, M.J.; Zolotukhin, M.G.; Marcial-hernandez, R.; Olvera, L.I. Simultaneous Thermal Cross-Linking and Decomposition of Side Groups to Mitigate Physical Aging in Poly (Oxyindole Biphenylylene) Gas Separation Membranes. Ind. Eng. Chem. Res. 2018, 57, 4640–4650. [Google Scholar] [CrossRef]
- Nakagawa, H.; Watanabe, K.; Harada, Y.; Miura, K. Control of Micropore Formation in the Carbonized Ion Exchange Resin by Utilizing Pillar Effect. Carbon 1999, 37, 1455–1461. [Google Scholar] [CrossRef]
- Cosey, W.K.; Balkus Jr, K.J.; Ferraris, J.P.; Musselman, I.H. Reduced aging in carbon molecular sieve membranes derived from PIM-1 and MOP-18. Ind. Eng. Chem. Res. 2021, 60, 9962–9970. [Google Scholar] [CrossRef]
- Furukawa, H.; Kim, J.; Plass, K.E.; Yaghi, O.M.; Arbor, A. Crystal Structure, dissolution, and deposition of a 5 nm Functionalized metal-organic great rhombicuboctahedron. J. Am Chem. Soc. 2006, 128, 8398–8399. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.V.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Metal-Organic Polyhedra 18 Mixed-Matrix Membranes for Gas Separation. J. Memb. Sci. 2014, 463, 82–93. [Google Scholar] [CrossRef]
- Reid, B.D.; Ruiz-trevino, F.A.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Gas Permeability Properties of Polysulfone Membranes Containing the Mesoporous Molecular Sieve MCM-41. Chem. Mater. 2001, 13, 2366–2373. [Google Scholar] [CrossRef]
- Riley, S.J. Gas Permeability and Selectivity Studies of Pure Poly (3-[2, 5, 8-Trioxynonyl] Thiophene), PTONT, and PTONT/Poly (Ethylene Oxide) Polymer Blend Membranes; The University of Texas at Dallas: Richardson, TX, USA, 2000. [Google Scholar]
- Cosey, W.K.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Age Reduction in Highly Porous Carbon Molecular Sieve Membranes; The University of Texas at Dallas: Richardson, TX, USA, 2020. [Google Scholar]
- Yeshchenko, O.A.; Dmitruk, I.M.; Alexeenko, A.A. Size-Dependent Melting of Spherical Copper Nanoparticles Embedded in a Silica Matrix. Phys. Rev. B 2007, 75, 085434. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Vallerot, J.; Bourrat, X.; Mouchon, A.; Chollon, G. Quantitative Structural and Textural Assessment of Laminar Pyrocarbons through Raman Spectroscopy, Electron Diffraction and Few Other Techniques. Carbon 2006, 44, 1833–1844. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Qiu, W.; Vaughn, J.; Liu, G.; Xu, L.; Brayden, M.; Martinez, M.; Koros, W.J. Hyperaging Tuning of a Carbon Molecular-Sieve Hollow Fiber Membrane with Extraordinary Gas-Separation Performance and Stability. Angew. Chem. Int. Ed. 2019, 58, 11700–11703. [Google Scholar] [CrossRef]
- Parent, P.; Laffon, C.; Marhaba, I.; Ferry, D.; Regier, T.Z.; Ortega, I.K.; Chazallon, B.; Carpentier, Y.; Focsa, C. Nanoscale Characterization of Aircraft Soot: A High-Resolution Transmission Electron Microscopy, Raman Spectroscopy, X-Ray Photoelectron and near-Edge X-Ray Absorption Spectroscopy Study. Carbon 2016, 101, 86–100. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Poschl, U. Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Hays, S.S.; Sanyal, O.; Leon, N.E.; Arab, P.; Koros, W.J. Envisioned role of slit bypass pores in physical aging of carbon molecular sieve membranes. Carbon 2020, 157, 385–394. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Koros, W.J. New Insights into Physical Aging-Induced Structure Evolution in Carbon Molecular Sieve Membranes. Angew. Chem. Int. Ed. 2022, 61, e202210831. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Perez-Francisco, J.M.; Santiago-García, J.L.; Loria-Bastarrachea, M.I.; Paul, D.R.; Freeman, B.D.; Aguilar-Vega, M. CMS Membranes from PBI/PI Blends: Temperature Effect on Gas Transport and Separation Performance. J. Memb. Sci. 2020, 597, 117703. [Google Scholar] [CrossRef]
- Hazazi, K.; Ma, X.; Wang, Y.; Ogieglo, W.; Alhazmi, A.; Han, Y.; Pinnau, I. Ultra-Selective Carbon Molecular Sieve Membranes for Natural Gas Separations Based on a Carbon-Rich Intrinsically Microporous Polyimide Precursor. J. Memb. Sci. 2019, 585, 1–9. [Google Scholar] [CrossRef]
- Tin, P.S.; Chung, T.S.; Liu, Y.; Wang, R. Separation of CO2/CH4 through Carbon Molecular Sieve Membranes Derived from P84 Polyimide. Carbon 2004, 42, 3123–3131. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Quan, W.; Koros, W.J. Advanced Carbon Molecular Sieve Membranes Derived from Molecularly Engineered Cross-Linkable Copolyimide for Gas Separations. Nat. Mater. 2023, 22, 1–8. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef] [Green Version]


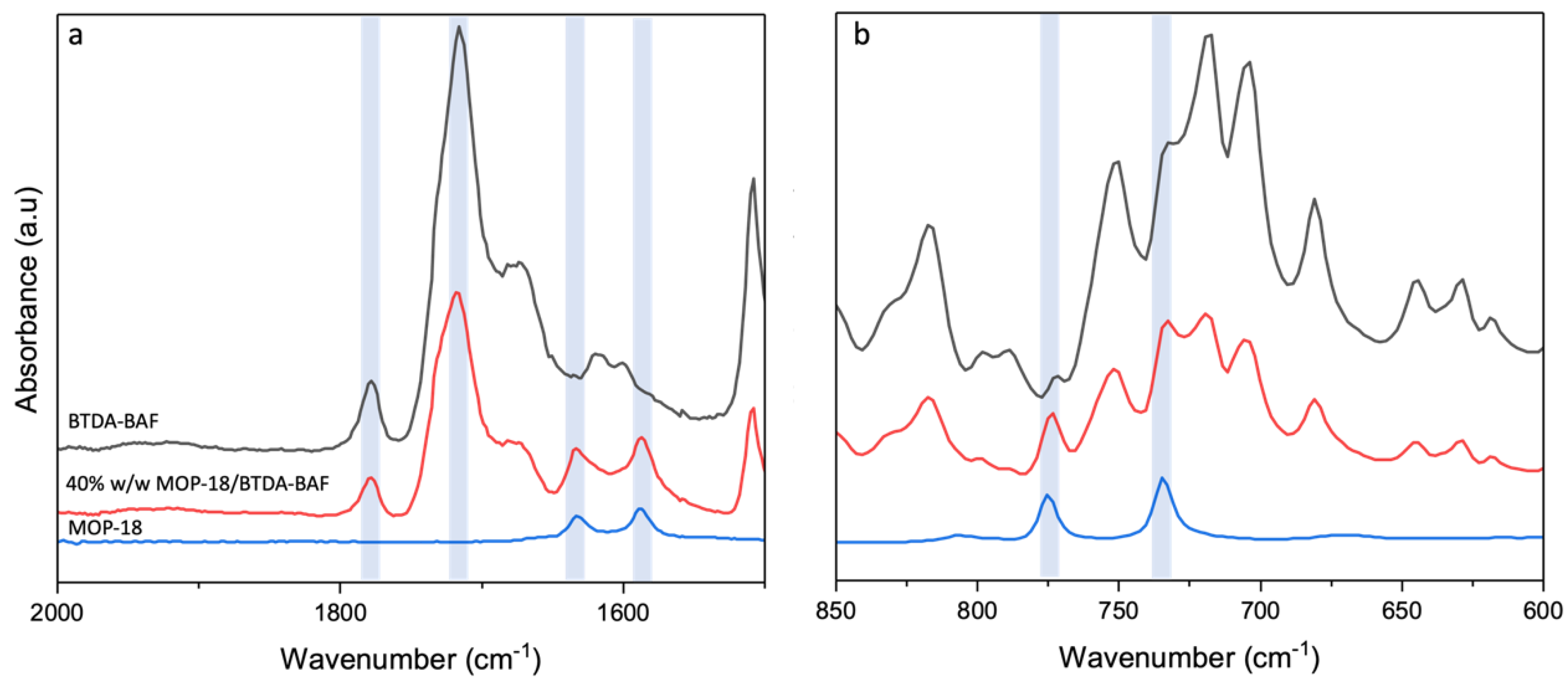
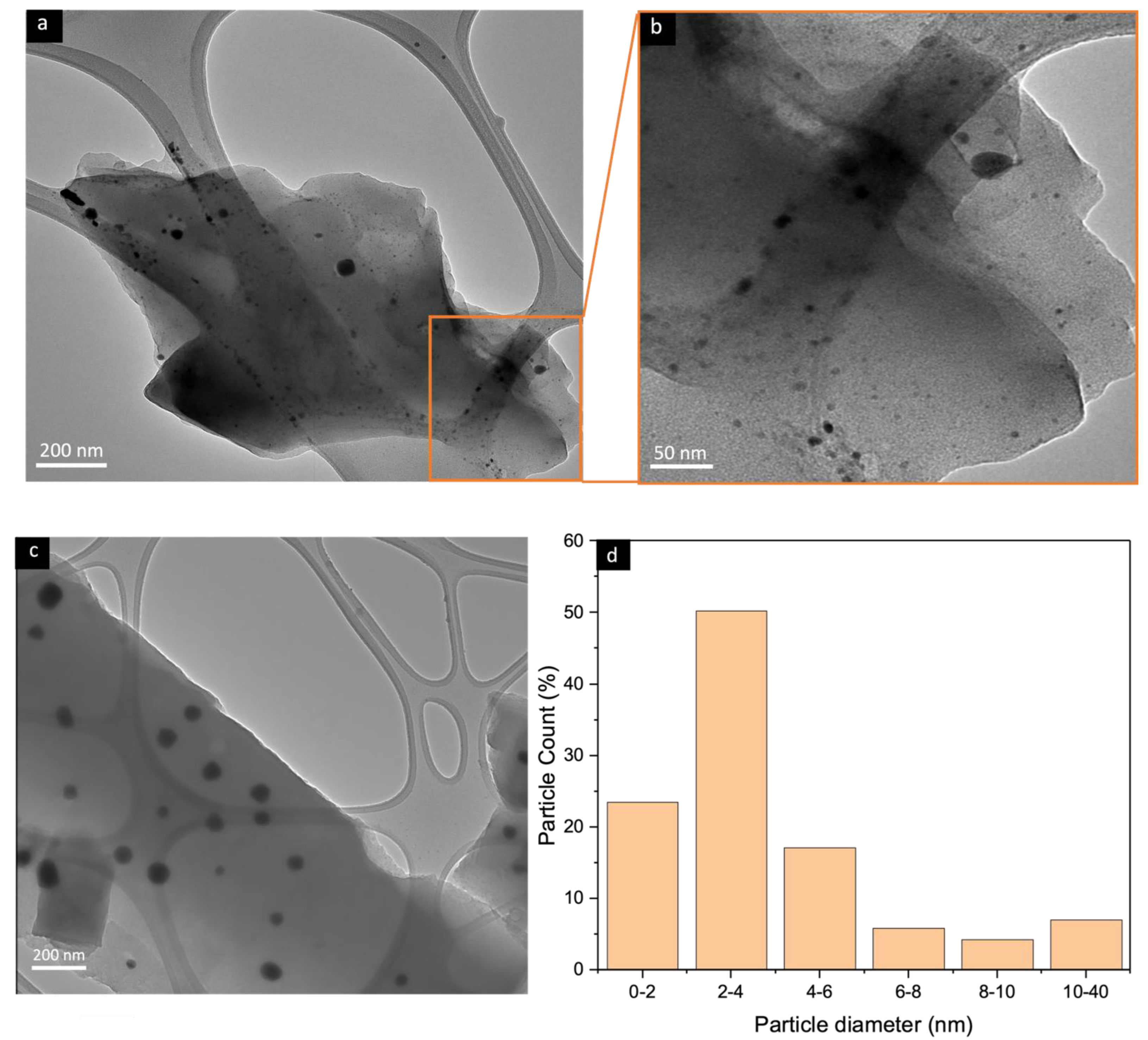


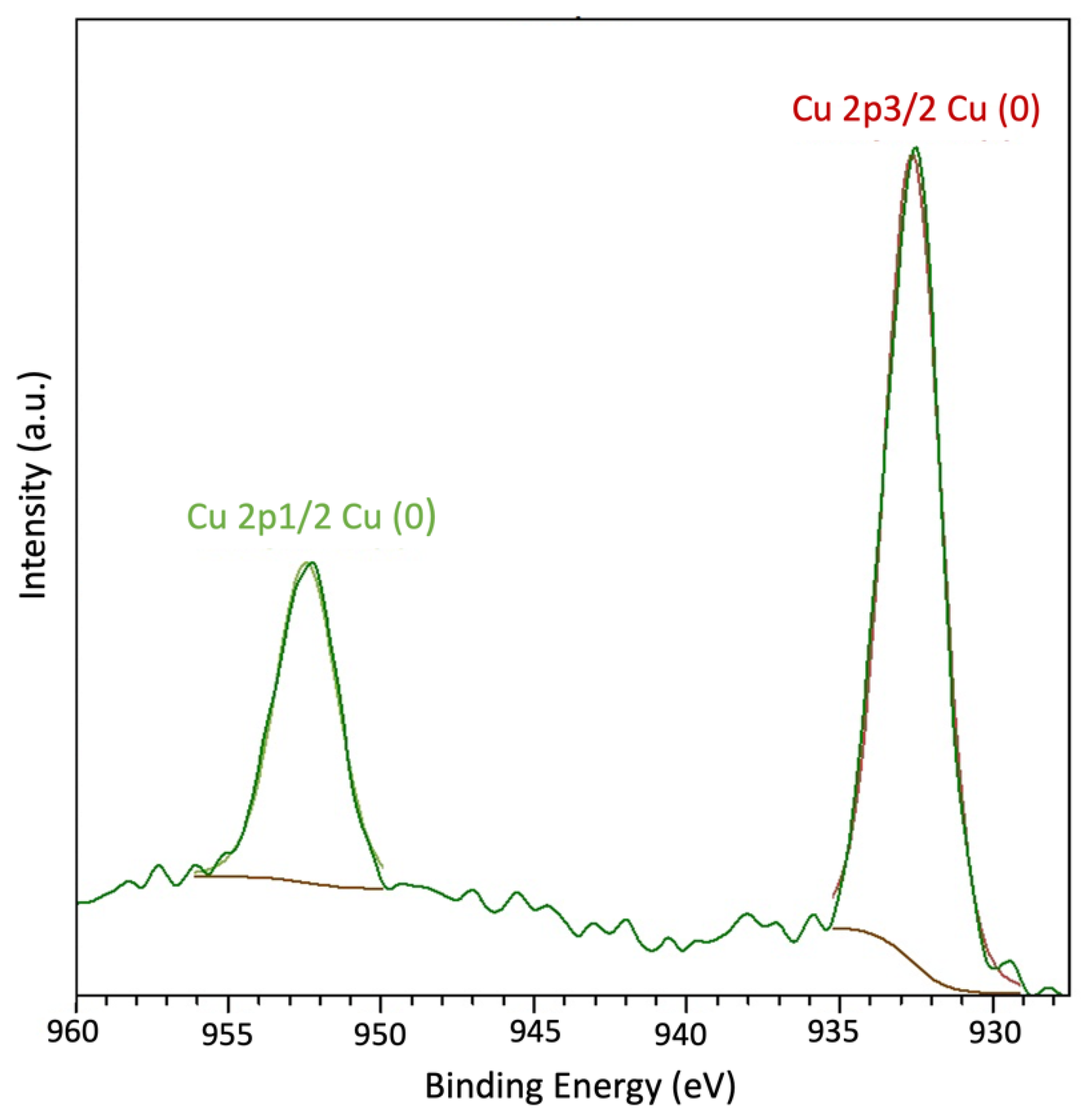
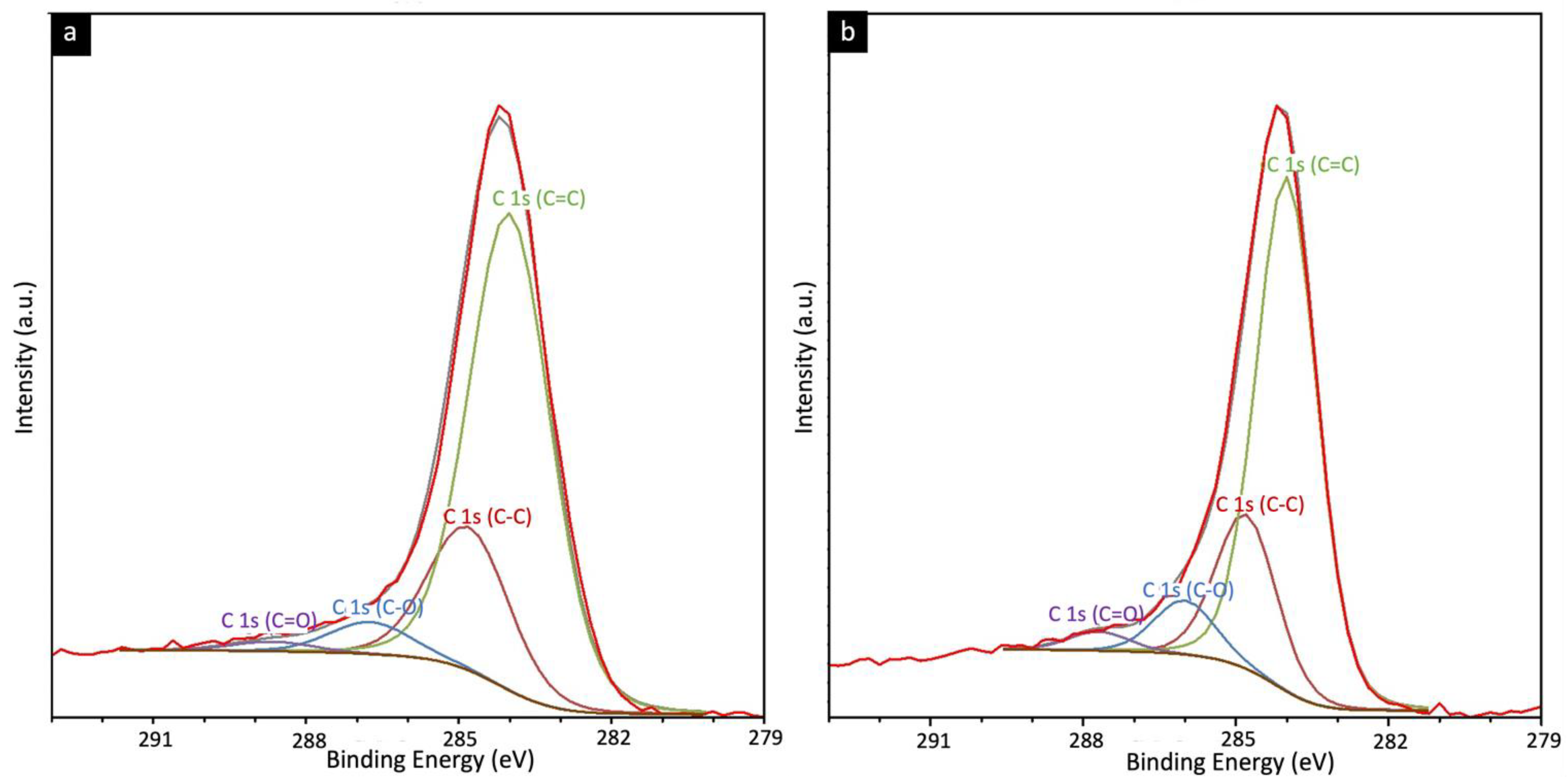



| CMSM | ID/IG | La (Å) | IG/ID2 |
|---|---|---|---|
| BTDA-BAF | 1.02 | 43.13 | 2.57 |
| 40% (w/w) MOP-18/BTDA-BAF | 0.92 | 47.82 | 4.03 |
| CMSM | D (cm2 s−1) 10−8 CO2 | D (cm2 s−1) 10−8 CH4 | D |
|---|---|---|---|
| BTDA-BAF | 10.8 | 7.3 | 1.48 |
| 40% w/w BTDA-BAF | 12.6 | 4.8 | 2.62 |
| CMS Membrane | P-CO2 | PCH4 | α (CO2/CH4) |
|---|---|---|---|
| Fresh BTDA-BAF | 7593 ± 339 | 1309 ± 90 | 5.8 ± 0.5 |
| Aged BTDA-BAF | 5337 ± 263 | 573 ± 42 | 9.3 ± 1 |
| Fresh 40% (w/w) MOP-18/BTDA-BAF | 14,332 ± 608 | 814 ± 55 | 17.6 ± 1 |
| Aged 40% (w/w) MOP-18/BTDA-BAF | 12,729 ± 743 | 659 ± 39 | 19.3 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajik, M.; Bin Haque, S.F.; Perez, E.V.; Vizuet, J.P.; Firouzi, H.R.; Balkus, K.J., Jr.; Musselman, I.H.; Ferraris, J.P. Pillared Carbon Membranes Derived from Cardo Polymers. Nanomaterials 2023, 13, 2291. https://doi.org/10.3390/nano13162291
Tajik M, Bin Haque SF, Perez EV, Vizuet JP, Firouzi HR, Balkus KJ Jr., Musselman IH, Ferraris JP. Pillared Carbon Membranes Derived from Cardo Polymers. Nanomaterials. 2023; 13(16):2291. https://doi.org/10.3390/nano13162291
Chicago/Turabian StyleTajik, Masoumeh, Syed Fahad Bin Haque, Edson V. Perez, Juan P. Vizuet, Hamid Reza Firouzi, Kenneth J. Balkus, Jr., Inga H. Musselman, and John P. Ferraris. 2023. "Pillared Carbon Membranes Derived from Cardo Polymers" Nanomaterials 13, no. 16: 2291. https://doi.org/10.3390/nano13162291





