Retention of Phthalates in Wine Using Nanomaterials as Chemically Modified Clays with H20, H30, H40 Boltron Dendrimers
Abstract
:1. Introduction
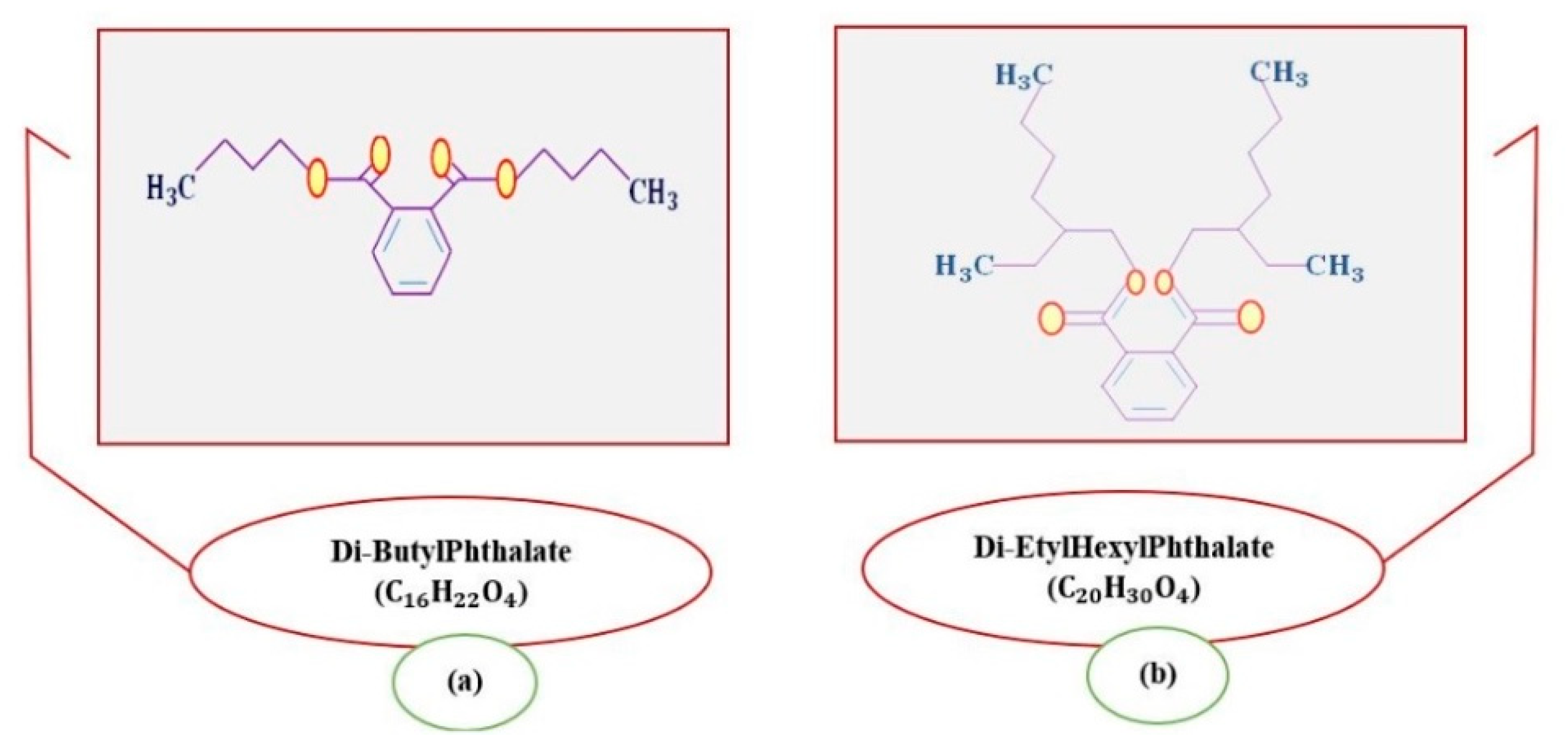
2. Materials and Methods
2.1. Materials
2.2. Modification of Clay-Based Material
2.3. Preparation of Phthalic Solutions and Synthetic Solutions of Pollutants
2.4. Determination of the Degree of Protein Stability
2.5. Characterization of the Prepared Nanomaterials
3. Results and Discussions
3.1. BET Analysis
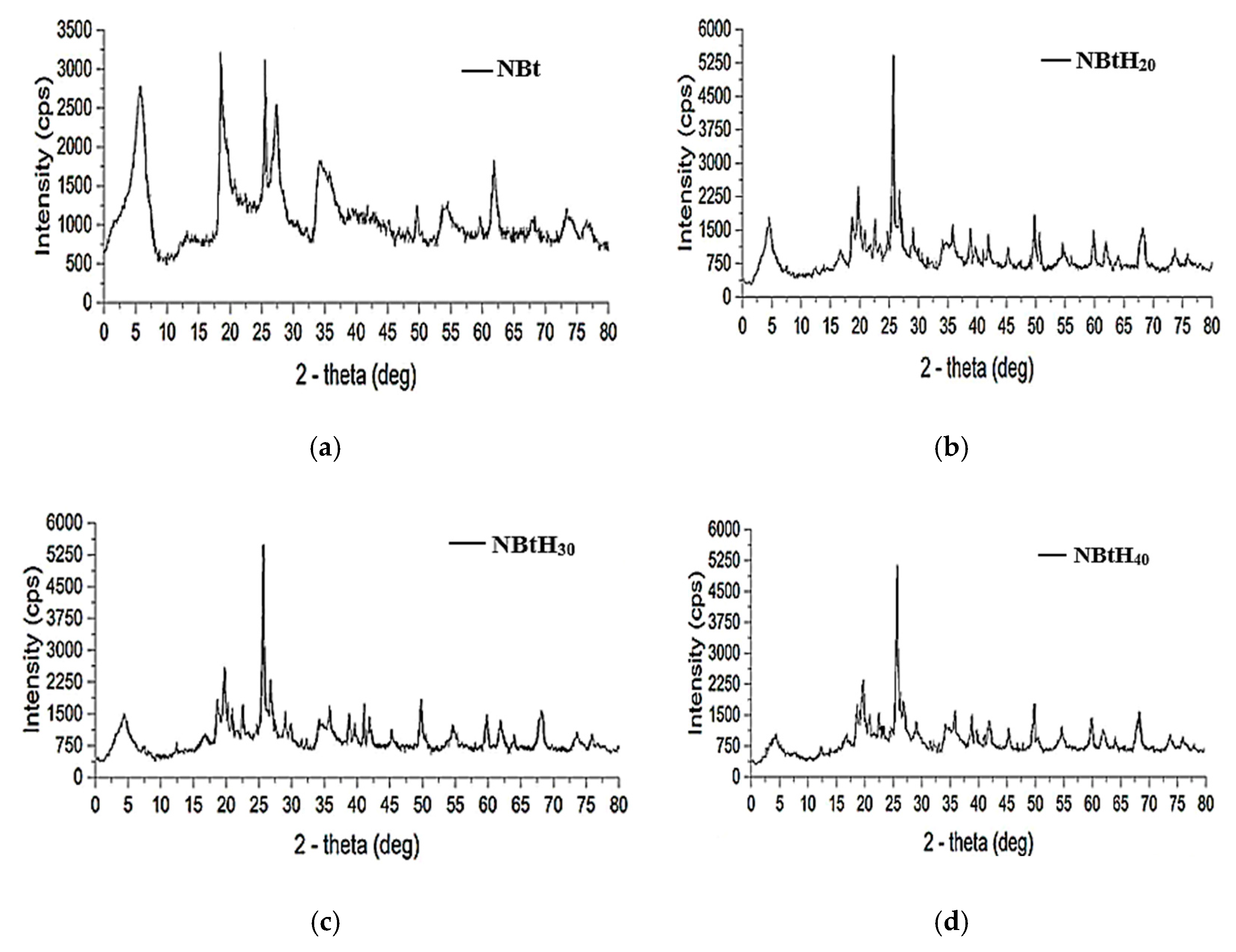
3.2. DRX Analysis
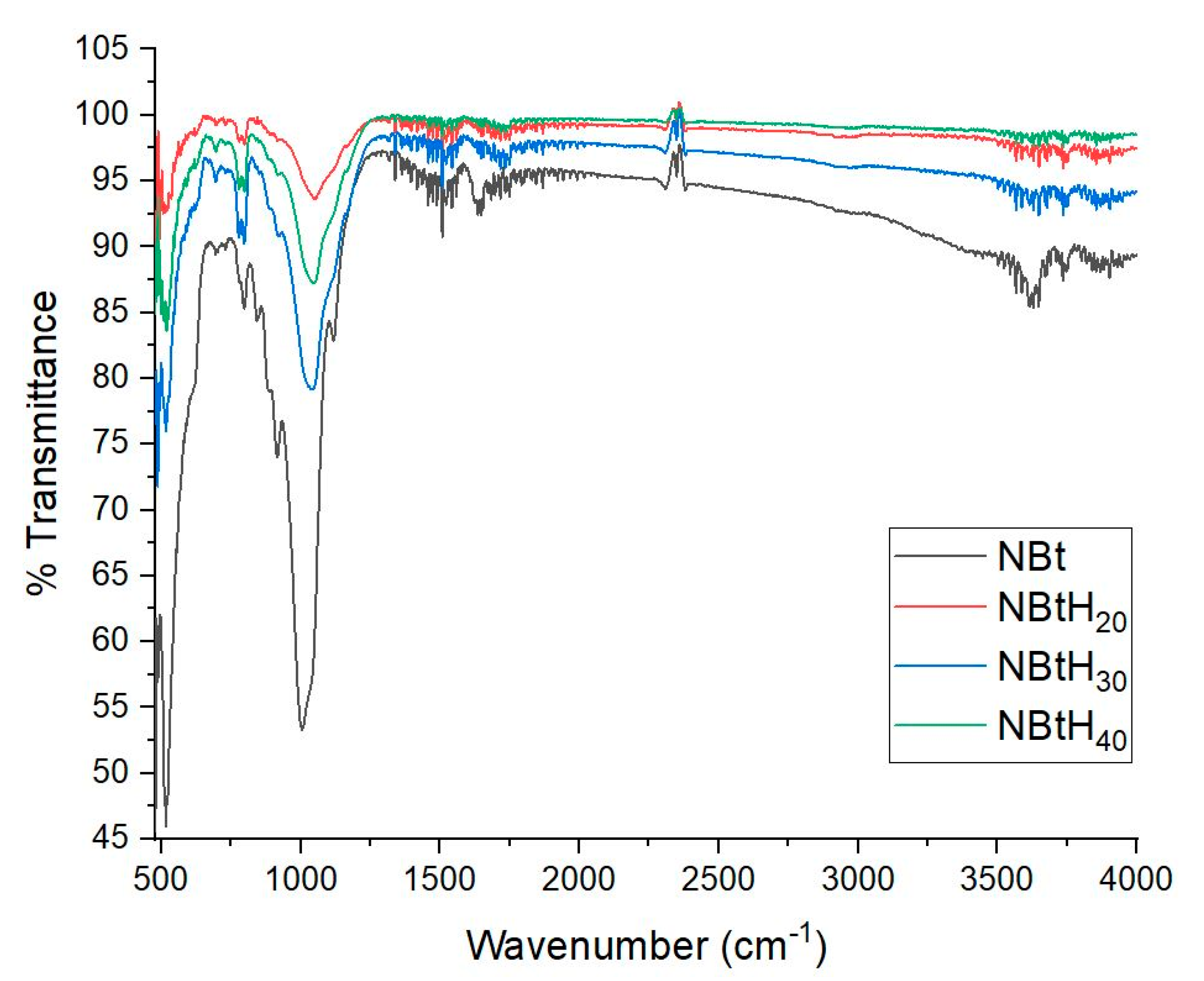
3.3. FTIR Analysis
3.4. Analysis of Synthesized Wine Samples Using the Nephelometric and UV–Vis Spectroscopic Methods
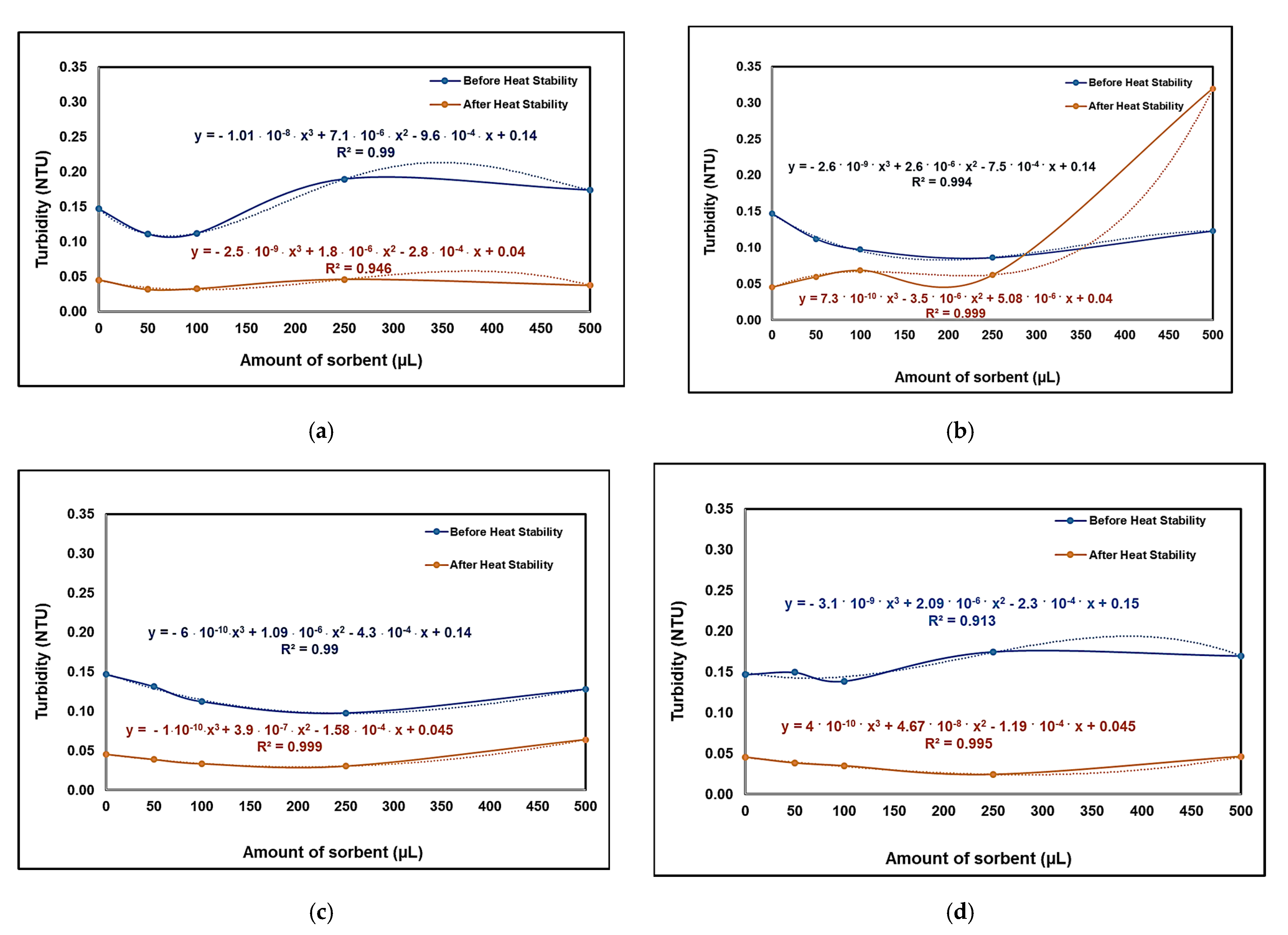
3.4.1. Analysis of the Synthesized Wine Using the Turbidimetric Method
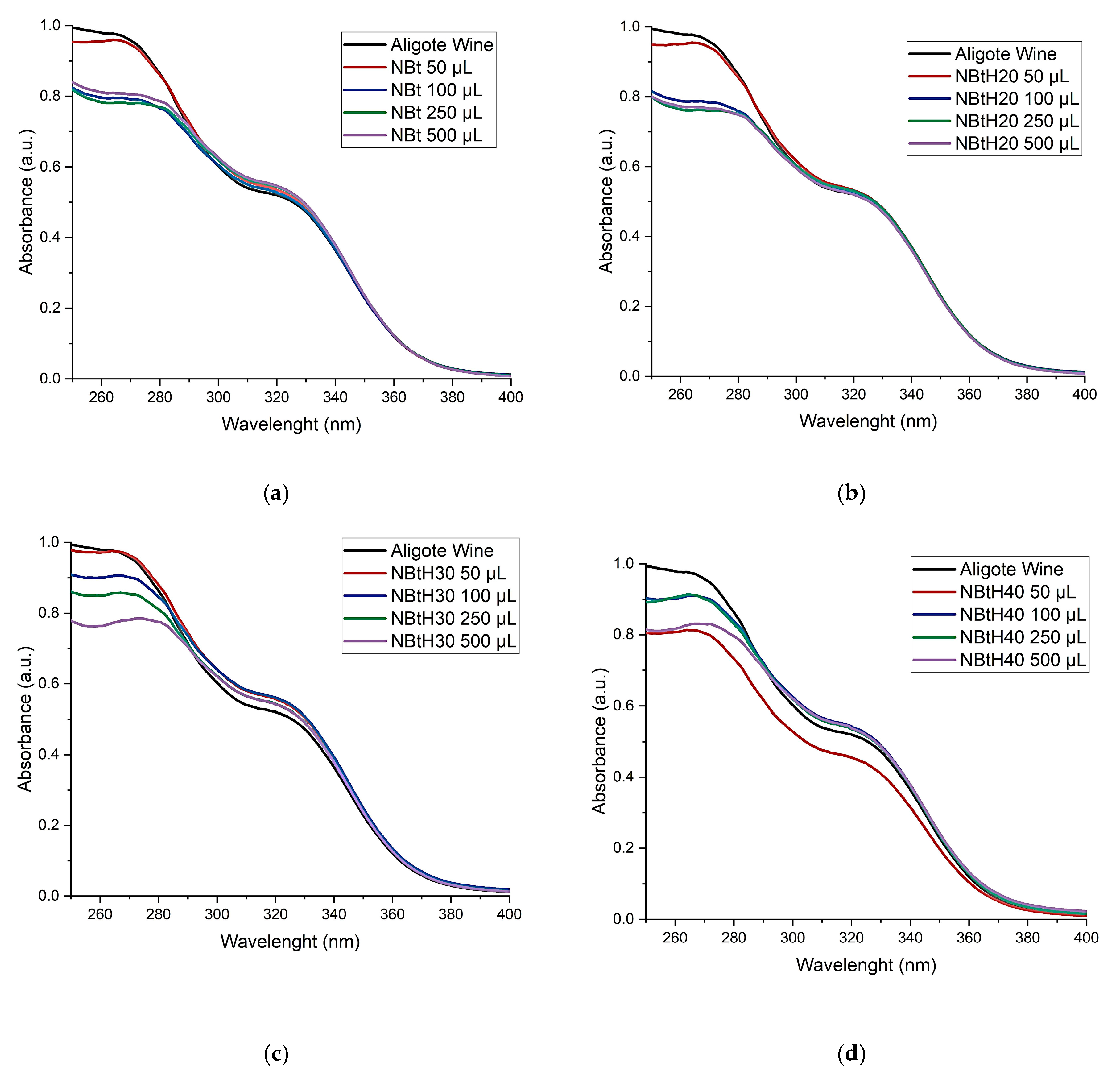
3.4.2. UV–Vis Spectroscopic Analysis
3.5. Determination of Phthalate Concentrations by GC–MS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Revi, M.; Badeka, A.; Kontakos, S.; Kontominas, M.G. Effect of packaging material on enological parameters and volatile compounds of dry white wine. Food Chem. 2014, 152, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Hanyu, N.; Nakada, K.; Suzuki, Y.; Yamamoto, T.; Takahashi, T.; Kawasaki, N.; Kawakami, M.; Matsushima, M.; Urashima, M. Phenolic compounds and total antioxidant potential of commercial wines. Eur. J. Cancer 2003, 39, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Briones-Labarca, V.; Perez-Wom, M.; Habib, G.; Giovagnoli-Vicuña, C.; Cañas-Sarazua, R.; Tabilo-Munizaga, G.; Salazar, F.N. Oenological and Quality Characteristic on Young White Wines (Sauvignon Blanc): Effects of High Hydrostatic Pressure Processing. J. Food Qual. 2017, 2017, 8524073. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Aurand, J.M.; Grinbaum, M.; Camponovo, A.; Desseigne, J.M.; Poupault, P.; Meisterman, E.; Chatelet, B.; Davaux, F.; Lempereur, V. Phthalates: Potential sources and control measures. BIO Web Conf. 2019, 12, 04008. [Google Scholar] [CrossRef] [Green Version]
- -Barciela-Alonso, M.C.; OteroLavandeira, N.; Bermejo-Barrera, P. Solid phase extraction using molecular imprinted polymers for phthalate determination in water and wine samples by HPLC-ESI-MS. Microchem. J. 2017, 132, 233–237. [Google Scholar] [CrossRef]
- Montevecchi, G.; Masino, F.; Di Pascale, N.; Vasile Simone, G.; Antonelli, A. Study of the repartition of phthalate esters during distillation of wine for spirit production. Food Chem. 2017, 237, 46–52. [Google Scholar] [CrossRef]
- Gagliardi, M.; Tori, G.; Agostini, M.; Lunardelli, F.; Mencarelli, F.; Sanmartin, C.; Cecchini, M. Detection of Oenological Polyphenols via QCM-D Measurements. Nanomaterials 2022, 12, 166. [Google Scholar] [CrossRef]
- Sturza, R.; Lazacovici, D. Monitoring of Phthalate Contamination in the Wine Sector of the Republic of Moldova, Ecological Chemistry: History and Achievements; CEP USM: Chisinau, Moldova, 2022; pp. 314–330. [Google Scholar]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid-liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography-ion trap mass spectrometry. Anal. Chim. Acta 2013, 769, 72–78. [Google Scholar] [CrossRef]
- Hayasaka, Y. Analysis of phthalates in wine using liquid chromatography tandem mass spectrometry combined with a hold-back column: Chromatographic strategy to avoid the influence of pre-existing phthalate contamination in a liquid chromatography system. J. Chromatogr. A 2014, 1372, 120–127. [Google Scholar] [CrossRef]
- Guo, J.; Luo, K.; Chen, D.; Tan, X.; Song, Z. A rapid and sensitive method for the determination of dibutyl phthalate in wine by flow-injection chemiluminescence analysis. J. Food Compos. Anal. 2013, 31, 226–231. [Google Scholar] [CrossRef]
- Sorensen, L.K. Determination of phthalates in milk and milk products by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1135–1143. [Google Scholar] [CrossRef]
- Ustun, I.; Sungur, S.; Okur, R.; Sumbul, A.T.; Oktar, S.; Yilmaz, N.; Gokce, C. Determination of Phthalates Migrating from Plastic Containers into Beverages. Food Anal. Methods 2014, 8, 222–228. [Google Scholar] [CrossRef]
- Amanzadeh, H.; Yamini, Y.; Moradi, M.; Asl, Y.A. Determination of phthalate esters in drinking water and edible vegetable oil samples by headspace solid phase microextraction using graphene/polyvinylchloride nanocomposite coated fiber coupled to gas chromatography-flame ionization detector. J. Chromatogr. A 2016, 1465, 38–46. [Google Scholar] [CrossRef]
- Cavaliere, B.; Macchione, B.; Sindona, G.; Tagarelli, A. Tandem mass spectrometry in food safety assessment: The determination of phthalates in olive oil. J. Chromatogr. A 2008, 1205, 137–143. [Google Scholar] [CrossRef]
- Carrillo, J.D.; Salazar, C.; Moreta, C.; Tena, M.T. Determination of phthalates in wine by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry: Fibre comparison and selection. J. Chromatogr. A 2007, 1164, 248–261. [Google Scholar] [CrossRef]
- He, J.; Lv, R.; Zhu, J.; Lu, K. Selective solid-phase extraction of dibutyl phthalate from soybean milk using molecular imprinted polymers. Anal. Chim. Acta 2010, 661, 215–221. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on Food Additives, Processing Aids and Material in Contact with Food (AFC) on a request from the Commission related to Di-Butylphthalate (DBP) for use in food contact materials. EFSA J. 2015, 242, 1–17. [Google Scholar]
- Guo, D.; Huang, S.; Zhu, Y. The Adsorption of Heavy Metal Ions by Poly (Amidoamine) Dendrimer-Functionalized Nanomaterials: A Review. Nanomaterials 2022, 12, 1831. [Google Scholar] [CrossRef]
- Karaconji, I.B.; Jurica, S.A.; Lasic, D.; Jurica, K. Facts about phthalate toxicity in humans and their occurrence in alcoholic beverages. Arh. Hig. Rada Toksikol. 2017, 68, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Chatonnet, P.; Boutou, S.; Plana, A. Contamination of wines and spirits by phthalates: Types of contaminants present, contamination sources and means of prevention. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2014, 31, 1605–1615. [Google Scholar] [CrossRef]
- Putzu, C. The Occurrence of Bisphenol A and Phthalates in Portuguese Wines and the Migration of Selected Substances from Coatings in Contact with a Wine Simulant. Master’s Thesis, Universidade Catolica Portuguesa, Lisboa, Portugal, 2016. [Google Scholar]
- Pellerin, P.; Waters, E.J.; Brillouet, J.; Moutounet, M. Effet de polysaccharides sur la formation de trouble protéique dans un vin blanc. OENO One 1994, 28, 213–225. [Google Scholar] [CrossRef]
- Catarino, S.; Madeira, M.; Monteiro, F.; Rocha, F.; Curvelo-Garcia, A.S.; de Sousa, R.B. Effect of bentonite characteristics on the elemental composition of wine. J. Agric. Food Chem. 2008, 56, 158–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhlack, R.A.; Colby, C.B. Reduced product loss associated with inline bentonite treatment of white wine by simultaneous centrifugation with yeast lees. Food Bioprod. Process. 2018, 108, 51–57. [Google Scholar] [CrossRef]
- Boudissa, F.; Mirila, D.; Arus, V.A.; Terkmani, T.; Semaan, S.; Proulx, M.; Nistor, I.D.; Roy, R.; Azzouz, A. Acid-treated clay catalysts for organic dye ozonation—Thorough mineralization through optimum catalyst basicity and hydrophilic character. J. Hazard. Mater. 2019, 364, 356–366. [Google Scholar] [CrossRef]
- Pateiro-Moure, M.; Novoa-Munoz, J.C.; Arias-Estevez, M.; Lopez-Periago, E.; Martinez-Carballo, E.; Simal-Gandara, J. Quaternary herbicides retention by the amendment of acid soils with a bentonite-based waste from wineries. J. Hazard. Mater. 2009, 164, 769–775. [Google Scholar] [CrossRef]
- Caminade, A.B.; Beraa, A.; Laurent, R.; Delavaux-Nicot, B.; Hajjaji, M. Dendrimers and hyper-branched polymers interacting with clays: Fruitful associations for functional materials. J. Mat. Chem. A 2019, 7, 19634–19650. [Google Scholar] [CrossRef]
- Higashihara, T.; Segawa, Y.; Sinananwanich, W.; Ueda, M. Synthesis of hyperbranched polymers with controlled degree of branching. Polym. J. 2012, 44, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Jang, J.W.; Park, J.W. Carboxymethyl chitosan-modified magnetic-cored dendrimer as an amphoteric adsorbent. J. Hazard. Mater. 2016, 317, 608–616. [Google Scholar] [CrossRef]
- Monge, M.; Moreno-Arribas, M.V. Applications of Nanotechnology in Wine Production and Quality and Safety Control. In Wine Safety, Consumer Preference, and Human Health; Springer: Cham, Switzerland, 2016; pp. 51–69. [Google Scholar]
- Cruz, L.; Correa, J.; Mateus, N.; de Freitas, V.; Tawara, M.H.; Fernandez-Megia, E. Dendrimers as Color-Stabilizers of Pyranoanthocyanins: The Dye Concentration Governs the Host-Guest Interaction Mechanisms. ACS Appl. Polym. Mater. 2021, 3, 1457–1464. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Qin, W. Combination of Acid-Free Open-Vessel Wet Digestion and Poly(amidoamine) Dendrimer-Enhanced Capillary Electrophoresis for Determination of Metal Ions in Wines. Food Anal. Methods 2014, 7, 165–171. [Google Scholar] [CrossRef]
- Schramm, O.G.; Lopez-Cortes, X.; Santos, L.S.; Laurie, V.F.; Gonzalez Nilo, F.D.; Krolik, M.; Fischer, R.; Di Fiore, S. pH-dependent nano-capturing of tartaric acid using dendrimers. Soft Matter. 2014, 10, 600–608. [Google Scholar] [CrossRef]
- Reddy, K.R.; Venkata Reddy, C.; Babu, B.; Ravindranadh, K.; Naveen, S.; Raghu, A.V. Chapter 8—Recent advances in layered clays–intercalated polymer nanohybrids: Synthesis strategies, properties, and their applications. In Modified Clay and Zeolite Nanocomposite Materials; Mercurio, M., Sarkar, B., Langella, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–218. [Google Scholar]
- Arkas, M.; Anastopoulos, I.; Giannakoudakis, D.A.; Pashalidis, I.; Katsika, T.; Nikoli, E.; Panagiotopoulos, R.; Fotopoulou, A.; Vardavoulias, M.; Douloudi, M. Catalytic Neutralization of Water Pollutants Mediated by Dendritic Polymers. Nanomaterials 2022, 12, 445. [Google Scholar] [CrossRef]
- González-García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Lazarian, A.; Cavallaro, A.A.; Morsbach, S.; Mierczynska-Vasilev, A.; Mailänder, V.; Landfester, K.; Vasilev, K. Nanoparticles Surface Chemistry Influence on Protein Corona Composition and Inflammatory Responses. Nanomaterials 2022, 12, 682. [Google Scholar] [CrossRef]
- Azzouz, A.; Ursu, A.-V.; Nistor, D.; Sajin, T.; Assaad, E.; Roy, R. TPD study of the reversible retention of carbon dioxide over montmorillonite intercalated with polyol dendrimers. Thermochim. Acta 2009, 496, 45–49. [Google Scholar] [CrossRef]
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.V.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of acid–base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34. [Google Scholar] [CrossRef]
- Muntianu, G.; Simion, A.-I.; Grigoraș, C.-G.; Platon, N.; Nistor, I.-D.; Jinescu, G. Aluminum pillared bentonite–characterization and synthesis optimization by response surface methodology. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 73–88. [Google Scholar] [CrossRef]
- Sennour, R.; Pricop, D.; Platon, N.; Roy, R.; Azzouz, A. Optimized diffusion-convection compromise for reversible CO2 capture on hydroxylatedorgano-montmorillonite. ComptesRendusChimie 2022, 25, 27–38. [Google Scholar] [CrossRef]
- Boudissa, F.; Arus, V.-A.; Foka-Wembe, E.-N.; Zekkari, M.; Ouargli-Saker, R.; Dewez, D.; Roy, R.; Azzouz, A. Role of Silica on Clay-Catalyzed Ozonation for Total Mineralization of Bisphenol-A. Molecules 2023, 28, 3825. [Google Scholar] [CrossRef]
- Siminel, N. Structure of polymer/clay nanocomposites, a molecular modelling perspective. J. Eng. Sci. 2023, 30, 55–64. [Google Scholar] [CrossRef]
- Mirilă, D.-C.; Boudissa, F.; Beltrao-Nuñes, A.-P.; Platon, N.; Didi, M.-A.; Nistor, I.-D.; Roy, R.; Azzouz, A. Organic Dye Ozonation Catalyzed by Chemically Modified Montmorillonite K10—Role of Surface Basicity and Hydrophilic Character. Ozone Sci. Eng. 2020, 42, 517–530. [Google Scholar] [CrossRef]
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-enriched organo-montmorillonites for potential applications in carbon dioxide separation and concentration. Sep. Purif. Technol. 2013, 108, 181–188. [Google Scholar] [CrossRef]
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Shiao, T.C.; Hersant, G.; Bergeron, J.-Y.; Roy, R. Truly reversible capture of CO2 by montmorillonite intercalated with soya oil-derived polyglycerols. Int. J. Greenh. Gas. Con. 2013, 17, 140–147. [Google Scholar] [CrossRef]
- Müllerová, M.; Šabata, S.; Matoušek, J.; Kormunda, M.; Holubová, J.; Bálková, R.; Petričkovič, R.; Koštejn, M.; Kupčík, J.; Fajgar, R.; et al. Organoclays with carbosilane dendrimers containing ammonium or phosphonium groups. New J. Chem. 2018, 42, 1187–1196. [Google Scholar] [CrossRef]
- Mirila, D.C.; Pîrvan, M.-Ș.; Platon, N.; Georgescu, A.-M.; Zichil, V.; Nistor, I.D. Total Mineralization of Malachite Green Dye by Advanced Oxidation Processes. Acta Chem. 2018, 26, 263–280. [Google Scholar] [CrossRef] [Green Version]
- Kirbay, F.O.; Yalcinkaya, E.E.; Atik, G.; Evren, G.; Unal, B.; Demirkol, D.O.; Timur, S. Biofunctionalization of PAMAM-montmorillonite decorated poly (E-caprolactone)-chitosan electrospun nanofibers for cell adhesion and electrochemical cytosensing. Biosens. Bioelectron. 2018, 109, 286–294. [Google Scholar] [CrossRef]
- Esteruelas, M.; Poinsaut, P.; Sieczkowski, N.; Manteau, S.; Fort, M.F.; Canals, J.M.; Zamora, F. Characterization of natural hazeprotein in sauvignon white wine. Food Chem. 2009, 113, 28–35. [Google Scholar] [CrossRef]
- Scutaru, I.; Balanuta, A.; Zgardan, D. The determination of oxidation behavior of white wines produced from local and european grape varietes using spectrophotometric method. J. Eng. Sci. 2018, 4, 82–93. [Google Scholar] [CrossRef]
- Ye, H.; Zhao, B.; Zhou, Y.; Du, J.; Huang, M. Recent advances in adsorbents for the removal of phthalate esters from water: Material, modification, and application. Chem. Eng. J. 2021, 409, 128127. [Google Scholar] [CrossRef]
- Li, J.; Su, Q.; Li, K.Y.; Sun, C.F.; Zhang, W.B. Rapid analysis of phthalates in beverage and alcoholic samples by multi-walled carbon nanotubes/silica reinforced hollow fibre-solid phase microextraction. Food Chem. 2013, 141, 3714–3720. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiao, J.; Wang, W.; Liu, G.; Shi, Q.; Wang, J. Determination of atrazine and simazine in environmental water samples using multiwalled carbon nanotubes as the adsorbents for preconcentration prior to high performance liquid chromatography with diode array detector. Talanta 2006, 68, 1309–1315. [Google Scholar] [CrossRef]
- Fang, Z.Q.; Huang, H.J. Adsorption of Di-N-butyl Phthalate onto Nutshell-Based Activated Carbon. Equilibrium, Kinetics and Thermodynamics. Adsorpt. Sci. Technol. 2009, 27, 685–700. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Shailaja, S.; Rama Krishna, M.; Sarma, P.N. Adsorptive removal of phthalate ester (Di-ethyl phthalate) from aqueous phase by activated carbon: A. kinetic study. J. Hazard. Mater. 2007, 146, 278–282. [Google Scholar] [CrossRef]
- Muscalu Plescan, O.M.; Nedeff, F.M.; Partal, E.; Mosnegutu, E.; PanainteLehadus, M.; Irimia, O.; Tomozei, C. Influence of soil fertilization systems on soil characteristics for a monoculture of sunflower. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2019, 20, 585–595. [Google Scholar]
- Tirtoaca Irimia, O.; Panainte-Lehadus, M.; Tomozei, C.; Mosnegutu, E.; Chitimus, A.D.; Barsan, N. Study regarding the measuring of Particulate matter PM2.5 and PM10 in industrial working environments. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2020, 21, 271–278. [Google Scholar]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Study of XAD-2 adsorbent for the enrichment of trace levels of phthalate esters in hydroalcoholic food beverages and analysis by gas chromatography coupled with flame ionization and ion-trap mass spectrometry detectors. Food Chem. 2014, 146, 181–187. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Cheng, Y.; Cai, D. Functionalized UIO-66@Ag nanoparticles substrate for rapid and ultrasensitive SERS detection of di-(2-ethylhexyl) phthalate in plastics. Sens. Actuators B Chem. 2021, 349, 130793. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, M.; Wu, S.; Fornara, D.; Sarkar, B.; Sun, L.; Wang, H. Electrochemical sensor based on corncob biochar layer supported chitosan-MIPs for determination of dibutyl phthalate (DBP). J. Electroanal. Chem. 2021, 897, 115549. [Google Scholar] [CrossRef]



| Sample Name | ||||
|---|---|---|---|---|
| NBt | NBtH20 | NBtH30 | NBtH40 | |
| Specific Surface Area (m2·g−1) | 18.74 | 8.68 | 7.84 | 8.58 |
| Total Pore Volume (cm3·g−1) | 0.034 | 0.024 | 0.020 | 0.021 |
| Average Pore Diameter (nm) | 7.18 | 11.06 | 10.17 | 9.67 |
| NBt (cm−1) | NBtH20 (cm−1) | NBtH30 (cm−1) | NBtH40 (cm−1) | Assignements |
|---|---|---|---|---|
| 517 | 557 | 518 | 521 | Al–O–Si stretching |
| 648 | 627 | OH bending | ||
| 694 | 696 | 698 | Quartz | |
| 712 | Free or Amorphous Silica | |||
| 785 | (Al, Mg)–O–H | |||
| 790 | Si–O stretching Si–O–Al stretching | |||
| 801 | 810 | Al–Mg–OH bending | ||
| 843 | ||||
| 881 | 895 | 885 | 879 | Al–Fe–OH bending |
| 921 | 922 | 912 | Al–Al–OH bending | |
| 1005 | 1059 | 1028 | 1047 | Si–O–Si, Si–O stretching of silica and quartz Si–O stretching, in plane |
| 1119 | 1196 | 1165 | Si–O stretching, out of plane | |
| 1339 | 1362 | 1360 | CO3 stretching of calcite and dolomite | |
| 1638 | 1655 | 1656 | 1647 | OH bending, hydration |
| 3353 | OH stretching, hydration | |||
| 3611 | 3736 3856 | 3674 3854 | 3678 3747 | OH stretching |
| 3676 | ||||
| 3736 |
| Clays Samples | Index of Total Polyphenols—ITP | Total Phenolic Substances—TPS (Galic Acid Eq., mg·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 50 µL | 100 µL | 250 µL | 500 µL | 50 µL | 100 µL | 250 µL | 500 µL | |
| Aligoté | 8.65 ± 0.0121 | 136.77 ± 0.040 | ||||||
| NBt | 8.58 ± 0.025 | 7.63 ± 0.044 | 7.65 ± 0.069 | 7.84 ± 0.042 | 135.64 ± 0.055 | 107.92 ± 0.045 | 108.82 ± 0.043 | 113.87 ± 0.014 |
| NBtH20 | 8.57 ± 0.023 | 7.61 ± 0.045 | 7.52 ± 0.026 | 7.70 ± 0.046 | 134.38 ± 0.041 | 105.64 ± 0.057 | 103.37 ± 0.069 | 113.28 ± 0.085 |
| NBtH30 | 8.79 ± 0.031 | 8.28 ± 0.018 | 8.00 ± 0.092 | 7.33 ± 0.071 | 141.19 ± 0.088 | 130.95 ± 0.055 | 121.25 ± 0.099 | 99.8 ± 0.073 |
| NBtH40 | 7.31 ± 0.038 | 8.32 ± 0.055 | 8.09 ± 0.050 | 7.85 ± 0.049 | 98.87 ± 0.042 | 129.13 ± 0.091 | 127.05 ± 0.040 | 116.71 ± 0.075 |
| Cinnamic Phenolic Substances—CPS (Caffeic Acid Eq., mg·L−1) | Phenolic Flavonoid Substances—PFS (Catechin Eq., mg·L−1) | |||||||
| Aligoté | 38.06 ± 0.050 | 2.13 ± 0.027 | ||||||
| NBt | 39.41 ± 0.030 | 38.73 ± 0.025 | 40.45 ± 0.072 | 40.64 ± 0.063 | 1.99 ± 0.016 | 1.09 ± 0.024 | 0.99 ± 0.058 | 1.12 ± 0.098 |
| NBtH20 | 39.27 ± 0.085 | 38.19 ± 0.057 | 39.14 ± 0.070 | 38.05 ± 0.061 | 1.94 ± 0.067 | 1.04 ± 0.063 | 0.94 ± 0.068 | 1.02 ± 0.057 |
| NBtH30 | 41.69 ± 0.046 | 42.02 ± 0.040 | 40.21 ± 0.062 | 40.06 ± 0.093 | 2.00 ± 0.054 | 1.64 ± 0.044 | 1.37 ± 0.082 | 1.00 ± 0.085 |
| NBtH40 | 31.45 ± 0.087 | 40.21 ± 0.075 | 39.45 ± 0.092 | 36.74 ± 0.031 | 1.20 ± 0.075 | 1.71 ± 0.035 | 1.61 ± 0.077 | 1.29 ± 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hortolomeu, A.; Mirila, D.-C.; Georgescu, A.-M.; Rosu, A.-M.; Scutaru, Y.; Nedeff, F.-M.; Sturza, R.; Nistor, I.D. Retention of Phthalates in Wine Using Nanomaterials as Chemically Modified Clays with H20, H30, H40 Boltron Dendrimers. Nanomaterials 2023, 13, 2301. https://doi.org/10.3390/nano13162301
Hortolomeu A, Mirila D-C, Georgescu A-M, Rosu A-M, Scutaru Y, Nedeff F-M, Sturza R, Nistor ID. Retention of Phthalates in Wine Using Nanomaterials as Chemically Modified Clays with H20, H30, H40 Boltron Dendrimers. Nanomaterials. 2023; 13(16):2301. https://doi.org/10.3390/nano13162301
Chicago/Turabian StyleHortolomeu, Andreea, Diana-Carmen Mirila, Ana-Maria Georgescu, Ana-Maria Rosu, Yuri Scutaru, Florin-Marian Nedeff, Rodica Sturza, and Ileana Denisa Nistor. 2023. "Retention of Phthalates in Wine Using Nanomaterials as Chemically Modified Clays with H20, H30, H40 Boltron Dendrimers" Nanomaterials 13, no. 16: 2301. https://doi.org/10.3390/nano13162301





