Two-Dimensional Hetero- to Homochiral Phase Transition from Dynamic Adsorption of Barbituric Acid Derivatives
Abstract
:1. Introduction
2. Materials and Methods
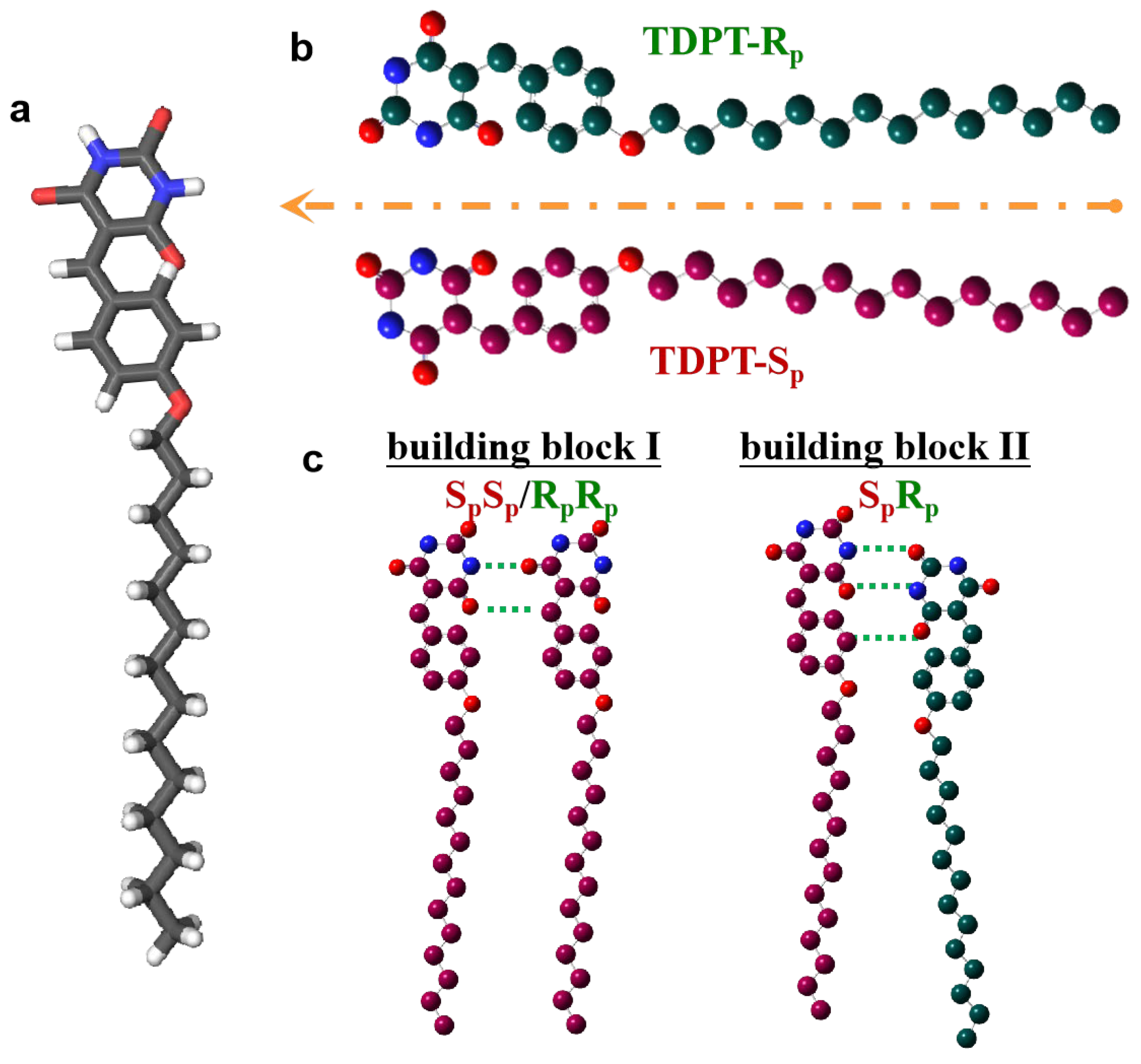
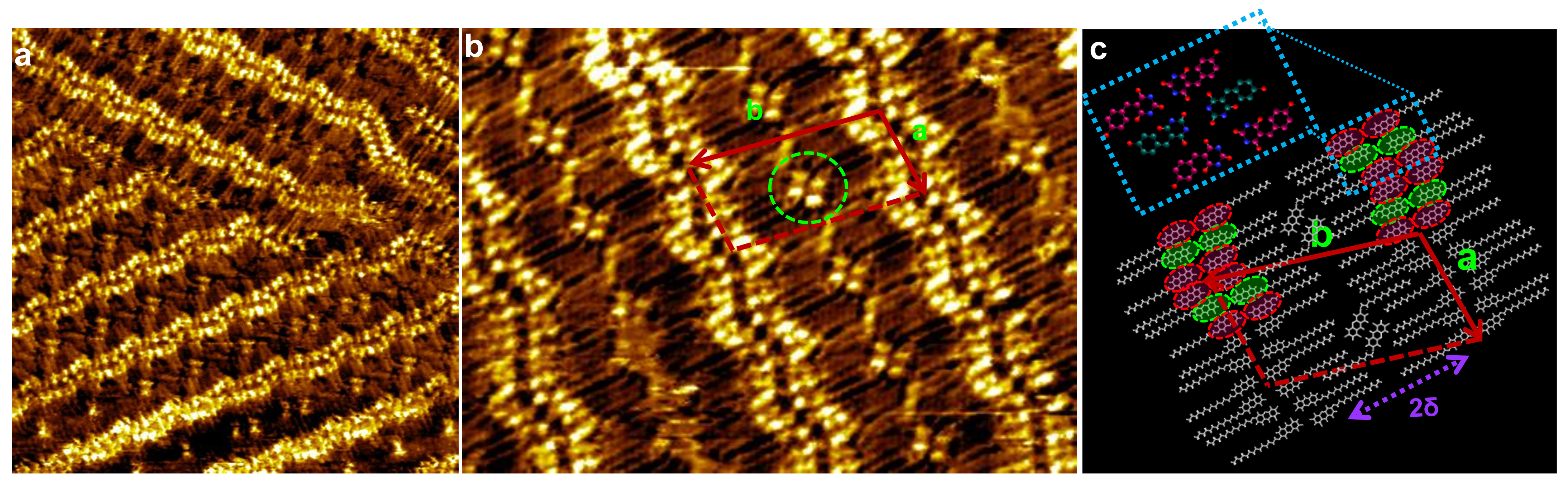
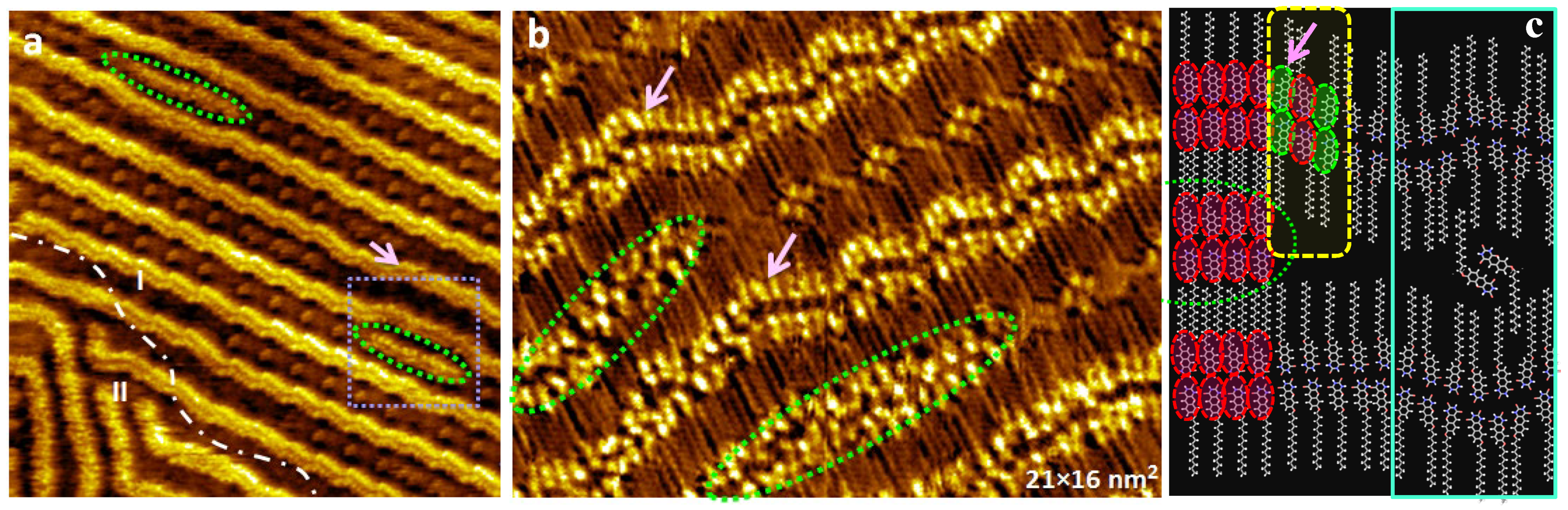
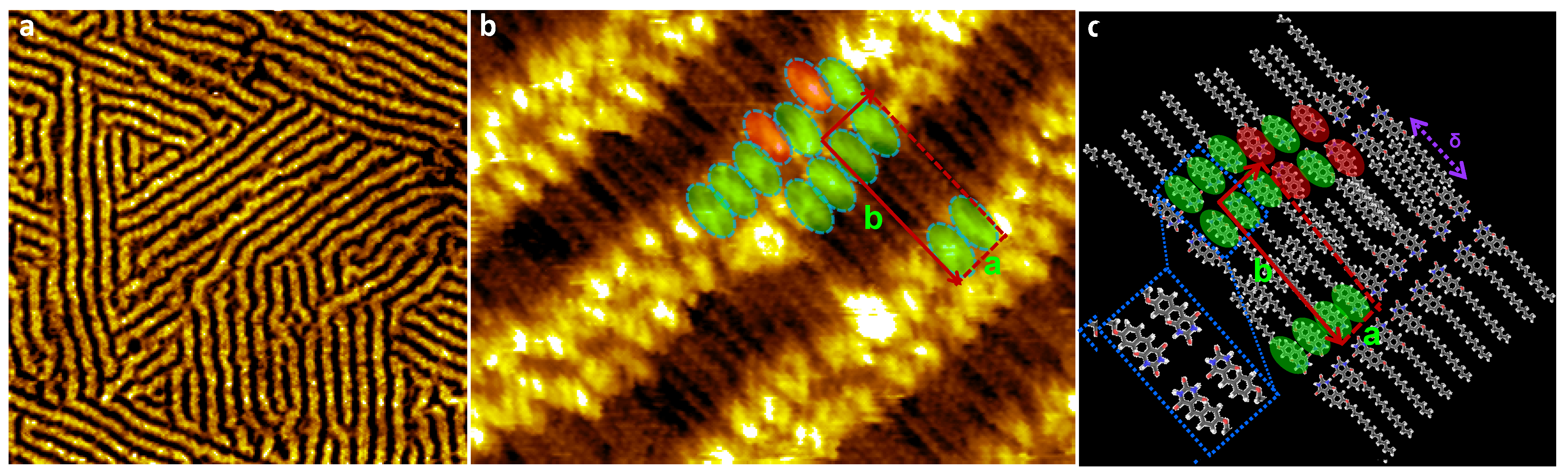
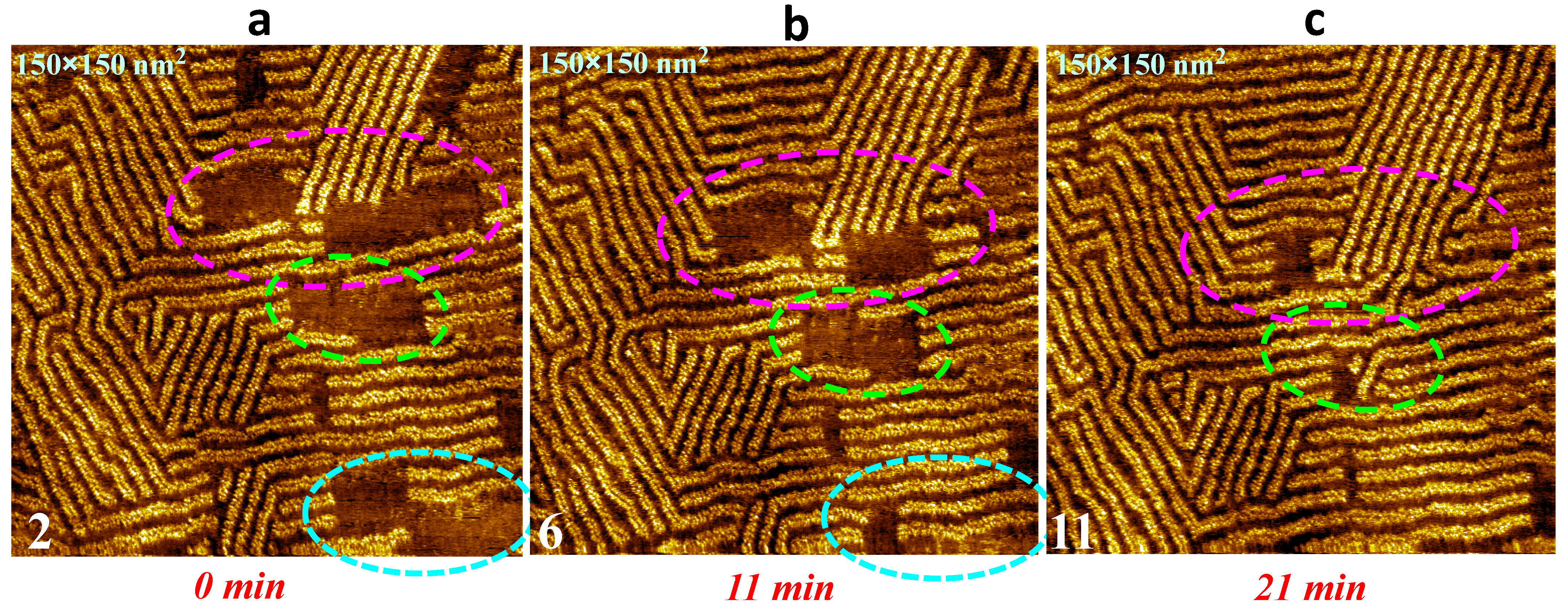
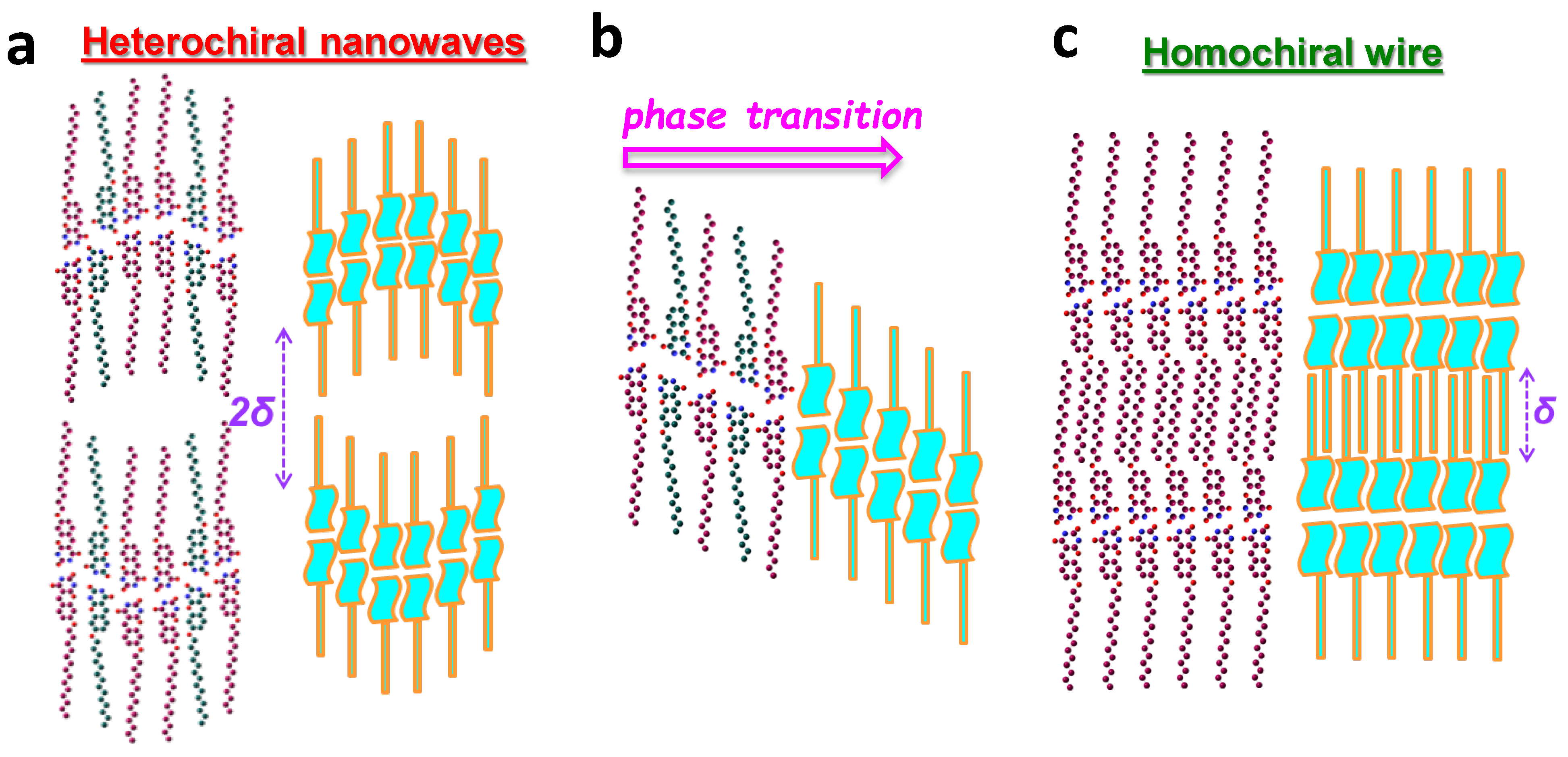
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlow, S.M.; Raval, R. Complex organic molecules at metal surfaces: Bonding, organisation and chirality. Surf. Sci. Rep. 2003, 50, 201–341. [Google Scholar] [CrossRef]
- Suarez, M.; Branda, N.; Lehn, J.M. Supramolecular chirality: Chiral hydrogen-bonded supermolecules from achiral molecular Components. Helv. Chem. Acta 1998, 8, 1–13. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W.; De Cat, I.; Xu, H.; De Feyter, S. Two-dimensional chirality at liquid–solid interfaces. Chem. Soc. Rev. 2009, 38, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Ernst, K.H. Molecular chirality at surfaces. Phys. Status Solidi B 2012, 249, 2057–2088. [Google Scholar] [CrossRef]
- Chen, T.; Wang, D.; Wan, L.J. Two-dimensional chiral molecular assembly on solid surfaces: Formation and regulation. Natl. Sci. Rev. 2015, 2, 205–216. [Google Scholar] [CrossRef]
- Xu, H.; Wolffs, M.; Tomovic, Z.; Meijer, E.W.; Schenning, A.P.H.J.; De Feyter, S. A multivalent hexapod having stereogenic centers: Chirality and conformational dynamics in homochiral and heterochiral systems. Cryst. Eng. Comm. 2011, 13, 5584–5590. [Google Scholar] [CrossRef]
- Haq, S.; Liu, N.; Humblot, V.; Jansen, A.P.J.; Raval, R. Drastic symmetry breaking in supramolecular organization of enantiomerically unbalanced monolayers at surfaces. Nat. Chem. 2009, 1, 409–414. [Google Scholar] [CrossRef]
- Gutzler, R.; Ivasenko, O.; Fu, C.; Brusso, J.L.; Rosei, F.; Perepichka, D.F. Halogen bonds as stabilizing interactions in a chiral self-assembled molecular monolayer. Chem. Commun. 2011, 47, 9453–9455. [Google Scholar] [CrossRef]
- Weckesser, J.; De Vita, A.; Barth, J.V.; Cai, C.; Kern, K. Mesoscopic correlation of supramolecular chirality in one-dimensional hydrogen-bonded assemblies. Phys. Rev. Lett. 2001, 87, 096101. [Google Scholar] [CrossRef] [Green Version]
- Sleczkowski, P.; Katsonis, N.; Kapitanchuk, O.; Marchenko, A.; Mathevet, F.; Croset, B.; Lacaze, E. Emergence of chirality in hexagonally packed monolayers of hexapentyloxytriphenylene on Au(111): A joint experimental and theoretical study. Langmuir 2014, 30, 13275–13282. [Google Scholar] [CrossRef]
- Silly, F.; Ausset, S.; Sun, X. Coexisting chiral two-dimensional self-assembled structures of 1,2,3,4-tetrahydronaphthalene molecules: Porous pinwheel nanoarchitecture and close-packed herringbone arrangement. J. Phys. Chem. C 2017, 121, 15288–15293. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Mura, M.; Jonkman, H.T.; Kantorovich, L.N.; Silly, F. Fabrication of a complex two-dimensional adenine–perylene-3, 4, 9, 10-tetracarboxylic dianhydride chiral nanoarchitecture through molecular self-assembly. J. Phys. Chem. C 2012, 116, 2493–2499. [Google Scholar] [CrossRef] [Green Version]
- Rajwar, D.; Sun, X.; Cho, S.J.; Grimsdale, A.C.; Fichou, D. Synthesis and 2D self-assembly at the liquid-solid interface of end-substituted star-shaped oligophenylenes. Cryst. Eng. Comm. 2012, 14, 5182–5187. [Google Scholar] [CrossRef]
- Sun, X.; Silly, F.; Maurel, F.; Dong, C. Supramolecular chiral host–guest nanoarchitecture induced by the selective assembly of barbituric acid derivative enantiomers. Nanotechnology 2016, 27, 42LT01. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karan, S.; Wang, Y.; Robles, R.; Lorente, N.; Berndt, R. Surface-supported supramolecular pentamers. J. Am. Chem. Soc. 2013, 135, 14004–14007. [Google Scholar] [CrossRef] [PubMed]
- Fasel, R.; Parschau1, M.; Ernst, K.H. Amplification of chirality in two-dimensional enantiomorphous lattices. Nature 2006, 439, 449–452. [Google Scholar] [CrossRef]
- Karageorgakia, C.; Ernst, K.H. A metal surface with chiral memory. Chem. Commun. 2014, 50, 1814–1816. [Google Scholar] [CrossRef]
- Barth, J.V. Molecular Architectonic on Metal Surfaces. Annu. Rev. Phys. Chem. 2007, 58, 375–407. [Google Scholar] [CrossRef] [Green Version]
- Raval, R. Chiral expression from molecular assemblies at metal surfaces: Insights from surface science techniques. Chem. Soc. Rev. 2009, 38, 707–721. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, H.; Shen, C.; Gan, F.; Su, X.; Qiu, H.; Yang, B.; Yu, P. Chiral recognition of hexahelicene on a surface via the forming of asymmetric heterochiral trimers. Int. J. Mol. Sci. 2019, 20, 2018. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; De Cat, I.; Van Averbeke, B.; Lin, J.; Wang, G.; Xu, H.; Lazzaroni, R.; Beljonne, D.; Meijer, E.W.; Schenning, A.P.H.J.; et al. Nucleoside-assisted self-assembly of oligo(p-phenylenevinylene)s at liquid/solid interface: Chirality and nanostructures. J. Am. Chem. Soc. 2011, 133, 17764–17771. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Yamaga, H.; Ghijsens, E.; Inukai, K.; Adisoejoso, J.; Blunt, M.O.; De Feyter, S.; Tobe, Y. Control and induction of surface-confined homochiral porous molecular networks. Nat. Chem. 2011, 3, 714. [Google Scholar] [CrossRef]
- Chen, T.; Yang, W.H.; Wang, D.; Wan, L.J. Globally homochiral assembly of two-dimensional molecular networks triggered by co-absorbers. Nat. Chem. 2013, 4, 1389. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yao, X.; Lafolet, F.; Lemercier, G.; Lacroix, J.C. One-Dimensional Double Wires and Two-Dimensional Mobile Grids: Cobalt/Bipyridine Coordination Networks at the Solid/Liquid Interface. J. Phys. Chem. Lett. 2019, 10, 4164–4169. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, L.; Zou, Y.; Yang, Y.; Wang, C. Steric dependence of chirality effect in surface-mediated peptide assemblies identified with scanning tunneling microscopy. Nano Lett. 2019, 19, 5403–5409. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Craig, S.L.; Martin, T.; Rebek, J., Jr. Chiral guests and their ghosts in reversibly assembled hosts. Angew. Chem. Int. Ed. 2000, 39, 2130–2132. [Google Scholar] [CrossRef]
- Sun, X.; Frath, D.; Lafolet, F.; Lacroix, J.C. Supramolecular Networks and Wires Dominated by Intermolecular BiEDOT Interactions. J. Phys. Chem. C 2018, 122, 22760–22766. [Google Scholar] [CrossRef]
- Yang, B.; Cao, N.; Ju, H.; Lin, H.; Li, Y.; Ding, H.; Ding, J.; Zhang, J.; Peng, C.; Zhang, H.; et al. Intermediate States Directed Chiral Transfer on a Silver Surface. J. Am. Chem. Soc. 2019, 141, 168–174. [Google Scholar] [CrossRef]
- Vidal, F.; Delvigne, E.; Stepanow, S.; Lin, N.; Barth, J.V.; Kern, K. Chiral phase transition in two-dimensional supramolecular assemblies of prochiral molecules. J. Am. Chem. Soc. 2005, 127, 10101–10106. [Google Scholar] [CrossRef]
- Mugarza, A.; Lorente, N.; Ordejon, P.; Krull, C.; Stepanow, S.; Bocquet, M.L.; Fraxedas, J.; Ceballos, G.; Gambardella, P. Orbital specific chirality and homochiral self-assembly of achiral molecules induced by charge transfer and spontaneous symmetry breaking. Phys. Rev. Lett. 2010, 105, 115702. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wang, Y.; Cun, H.; Du, S.; Xu, M.; Wang, Y.; Ernst, K.H.; Gao, H.J. Direct observation of enantiospecific substitution in a two-dimensional chiral phase transition. J. Am. Chem. Soc. 2010, 132, 10440–10444. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Schneider, M.A. Coverage-induced chiral transition of Co(II)-5,15-diphenylporphyrin self-assemblies on Cu(111). J. Phys. Chem. C 2022, 126, 6745–6752. [Google Scholar] [CrossRef]
- Han, D.; Wang, T.; Huang, J.; Li, X.; Zeng, Z.; Zhu, J. Chiral nanoporous networks featuring various chiral vertices from an achiral molecule on Ag(100). Nano Res. 2022, 15, 3753–3762. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, Z.; Sun, K.; Duan, R.; Ji, P.; Li, L.; Li, C.; Müllen, K.; Chi, L. Chirality transfer via on-surface reaction. J. Am. Chem. Soc. 2016, 138, 11743–11748. [Google Scholar] [CrossRef]
- Stolz, S.; Gröning, O.; Prinz, J.; Brune, H.; Widmer, R. Molecular motor crossing the frontier of classical to quantum tunneling motion. Proc. Natl. Acad. Sci. USA 2020, 117, 14838–14842. [Google Scholar] [CrossRef]
- Schied, M.; Prezzi, D.; Liu, D.; Kowarik, S.; Jacobson, P.A.; Corni, S.; Tour, J.M.; Grill, L. Chirality-specific unidirectional rotation of molecular motors on Cu(111). ACS Nano 2023, 17, 3958–3965. [Google Scholar] [CrossRef]
- De Cat, I.; Guo, Z.; George, S.J.; Meijer, E.W.; Schenning, A.P.H.J.; De Feyter, S. Induction of chirality in an achiral monolayer at the liquid/solid interface by a supramolecular chiral auxiliary. J. Am. Chem. Soc. 2012, 134, 3171–3177. [Google Scholar] [CrossRef]
- Cucinotta, A.; Kahlfuss, C.; Minoia, A.; Eyley, S.; Zwaenepoel, K.; Velpula, G.; Thielemans, W.; Lazzaroni, R.; Bulach, V.; Hosseini, M.W.; et al. Metal ion and guest-mediated spontaneous resolution and solvent-induced chiral Symmetry breaking in guanine-based metallosupramolecular networks. J. Am. Chem. Soc. 2023, 145, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Maeda, M.; Tessari, Z.; Mali, K.S.; Tobe, Y.; De Feyter, S.; Tahara, K. Solvent mediated nanoscale quasi-periodic chirality reversal in self-assembled molecular networks featuring mirror twin boundaries. Small 2023, 19, 2207209. [Google Scholar] [CrossRef]
- Prins, L.J.; De Jong, F.; Timmerman, P.; Reinhoudt, D.N. An enantiomerically pure hydrogen-bonded assembly. Nature 2000, 408, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Iski, E.V.; Tierney, H.L.; Jewell, A.D.; Sykes, E.C.H. Spontaneous transmission of chirality through multiple length scales. Chem. Eur. J. 2011, 17, 7205–7212. [Google Scholar] [CrossRef]
- Suarez, M.; De Armas, M.; Ramirez, O.; Alvarez, A.; Martınez-Alvarez, R.; Molero, D.; Seoane, C.; Liz, R.; De Armas, H.N.; Blaton, N.M.; et al. Synthesis and structural study of new highly lipophilic 1,4-dihydropyridines. New J. Chem. 2005, 29, 1567–1576. [Google Scholar] [CrossRef]
- Silly, F. A robust method for processing scanning probe microscopy images and determining nanoobject position and dimensions. J. Microsc. 2009, 236, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Moss, G.P. Basic Terminology of Stereochemistry. Pure Appl. Chem. 1996, 68, 2193–2222. [Google Scholar] [CrossRef]
- Dong, M.; Miao, K.; Hu, Y.; Wu, J.; Li, J.; Pang, P.; Miao, X.; Deng, W. Cooperating dipole–dipole and van der Waals interactions driven 2D self-assembly of fluorenone derivatives: Ester chain length effect. Phys. Chem. Chem. Phys. 2017, 19, 31113–31120. [Google Scholar] [CrossRef] [PubMed]
- Colle, R.; Grosso, G.; Ronzani, A.; Zicovich-Wilson, C.M. Structure and X-ray spectrum of crystalline poly(3-hexylthiophene) from DFT-van der Waals calculations. Phys. Status Solidi B 2011, 248, 1360–1368. [Google Scholar] [CrossRef]
- Ciesielski, A.; Haar, S.; Bényei, A.; Paragi, G.; Guerra, C.F.; Bickelhaupt, F.M.; Masiero, S.; Szolomájer, J.; Samorì, P.; Spada, G.P.; et al. Self-Assembly of N3-Substituted Xanthines in the Solid State and at the Solid–Liquid Interface. Langmuir 2013, 29, 7283–7290. [Google Scholar] [CrossRef]
- Pierro, A.D.; Saracco, G.; Fina, A. Molecular junctions for thermal transport between graphene nanoribbons: Covalent bonding vs. interdigitated chains. Comput. Mater. Sci. 2018, 142, 255–260. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silly, F.; Dong, C.; Maurel, F.; Sun, X. Two-Dimensional Hetero- to Homochiral Phase Transition from Dynamic Adsorption of Barbituric Acid Derivatives. Nanomaterials 2023, 13, 2304. https://doi.org/10.3390/nano13162304
Silly F, Dong C, Maurel F, Sun X. Two-Dimensional Hetero- to Homochiral Phase Transition from Dynamic Adsorption of Barbituric Acid Derivatives. Nanomaterials. 2023; 13(16):2304. https://doi.org/10.3390/nano13162304
Chicago/Turabian StyleSilly, Fabien, Changzhi Dong, François Maurel, and Xiaonan Sun. 2023. "Two-Dimensional Hetero- to Homochiral Phase Transition from Dynamic Adsorption of Barbituric Acid Derivatives" Nanomaterials 13, no. 16: 2304. https://doi.org/10.3390/nano13162304
APA StyleSilly, F., Dong, C., Maurel, F., & Sun, X. (2023). Two-Dimensional Hetero- to Homochiral Phase Transition from Dynamic Adsorption of Barbituric Acid Derivatives. Nanomaterials, 13(16), 2304. https://doi.org/10.3390/nano13162304





