Recent Advancements in Metal and Non-Metal Mixed-Doped Carbon Quantum Dots: Synthesis and Emerging Potential Applications
Abstract
:1. Introduction
2. Classification of CQDs
2.1. Undoped Carbon Quantum Dots (CQDs)
2.2. Doped/Co-Doped CQDs
2.3. Mixed-Doped CQDs
3. Synthesis Techniques of Mixed-Doped Carbon Quantum Dots
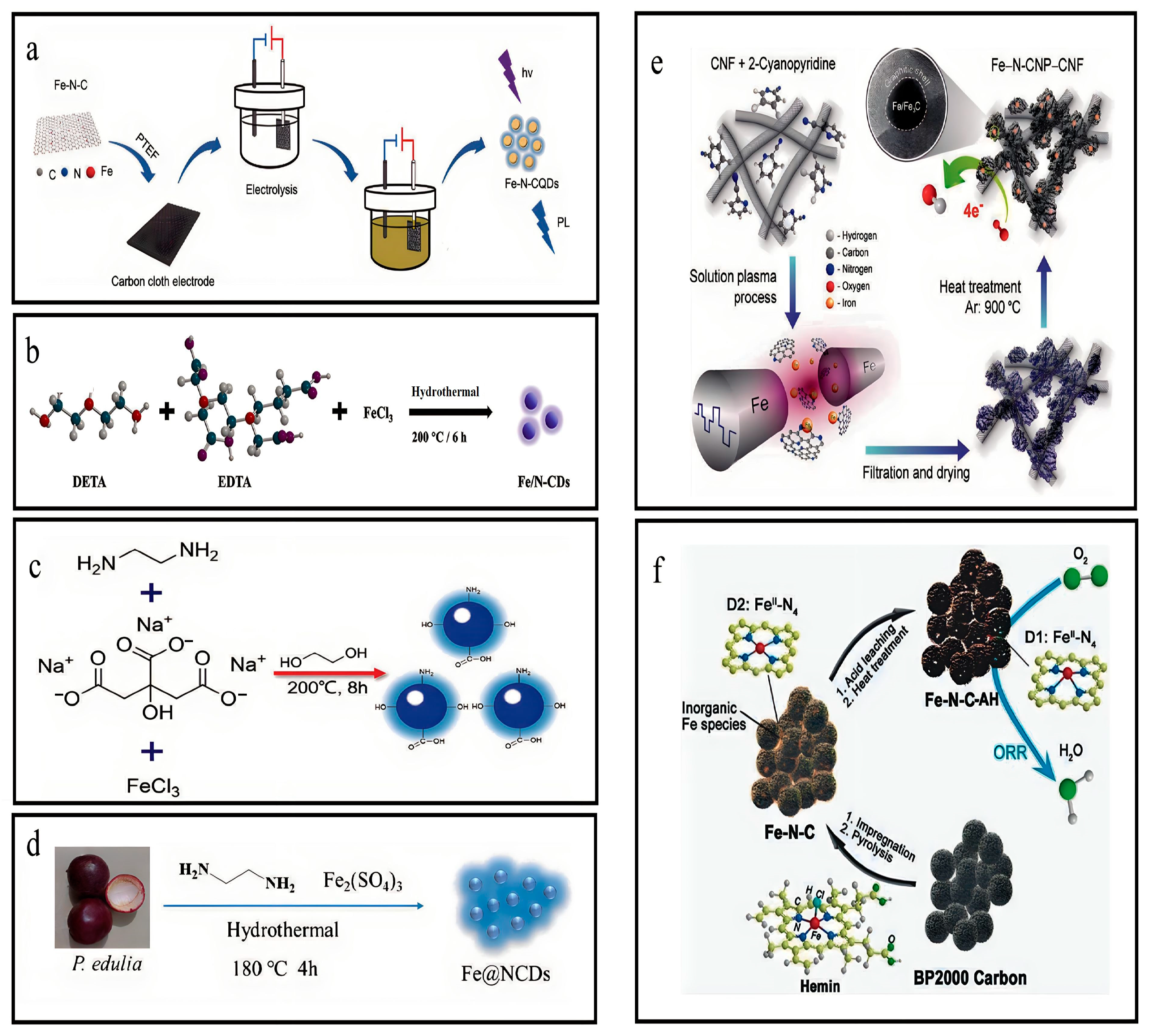
3.1. Synthesis of Fe-N-CQDs
3.2. Synthesis of Fe-N-CNPs
3.3. Synthesis of the Fe,N-C Electrocatalysts
3.4. Synthesis of Pristine Fe-N-GQDs
3.5. Synthesis of Fe/N-CDs
3.6. Synthesis of Fe,N-CDs
3.7. Synthesis of Fe@N-CDs
3.8. Synthesis of N,Cu-CQDs
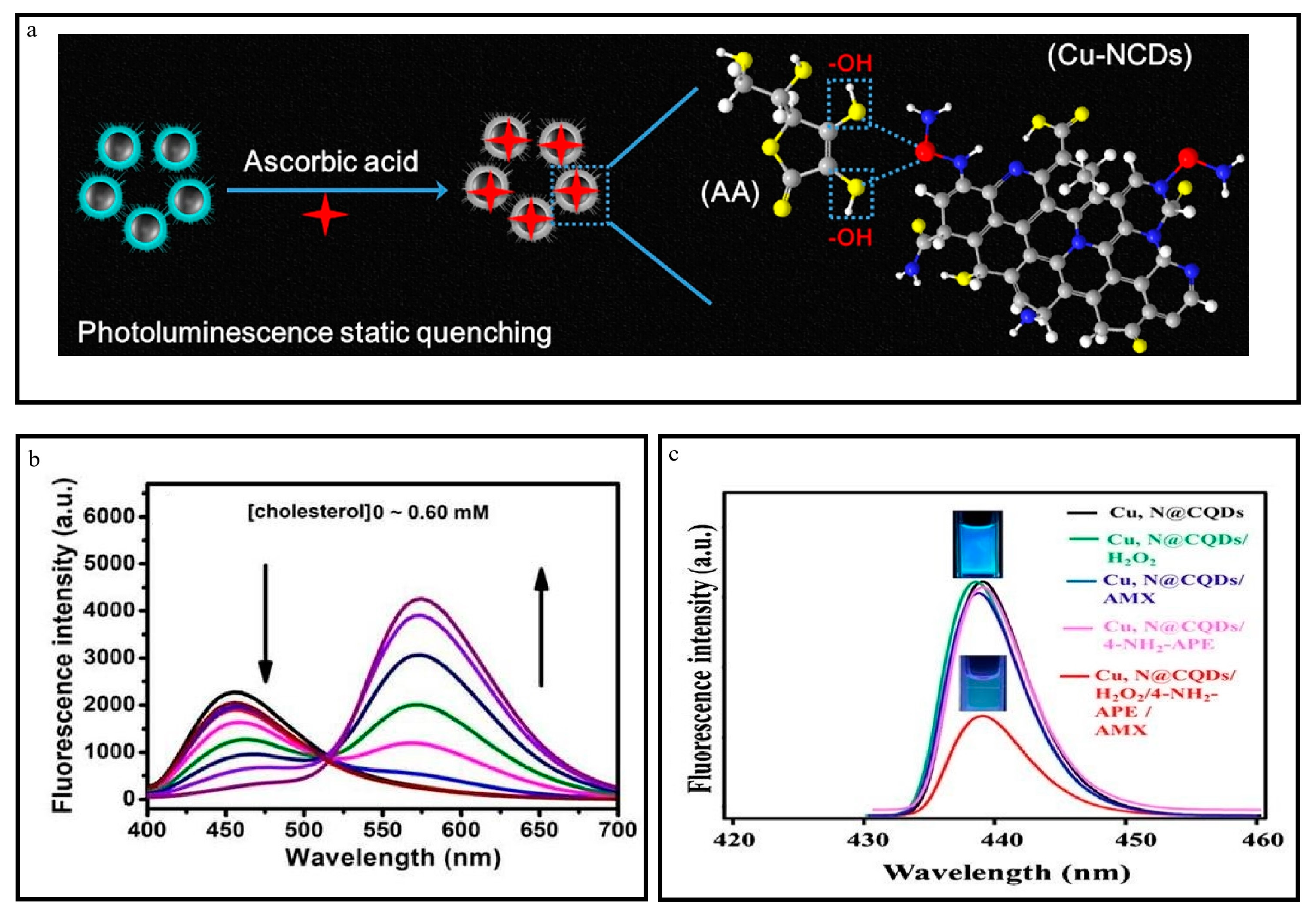
3.9. Synthesis of Cu-N@CDs
3.10. Synthesis of N/CuCDs
3.11. Synthesis of Cu-N-CDs
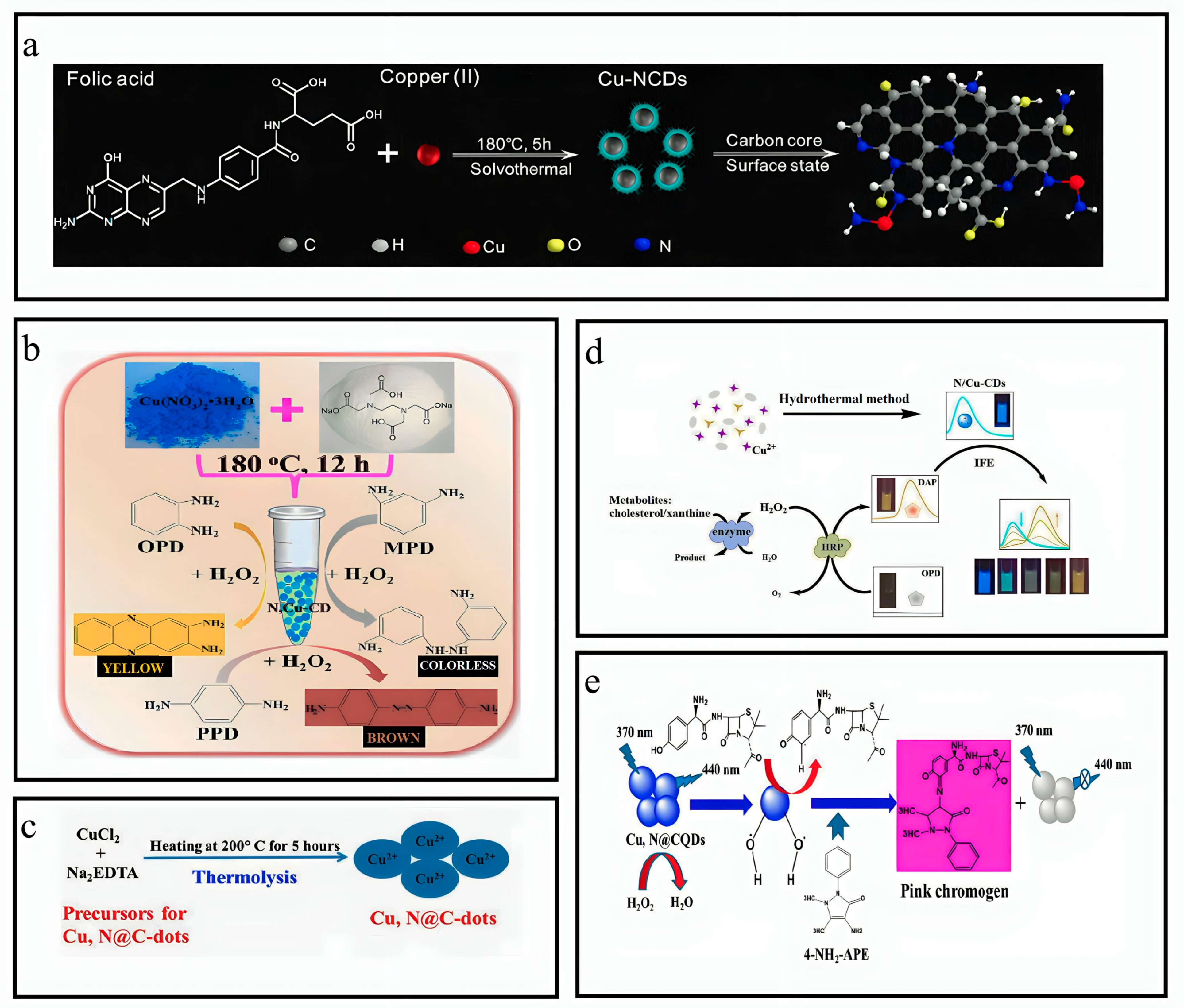
3.12. Synthesis of Cu,N@CQDs
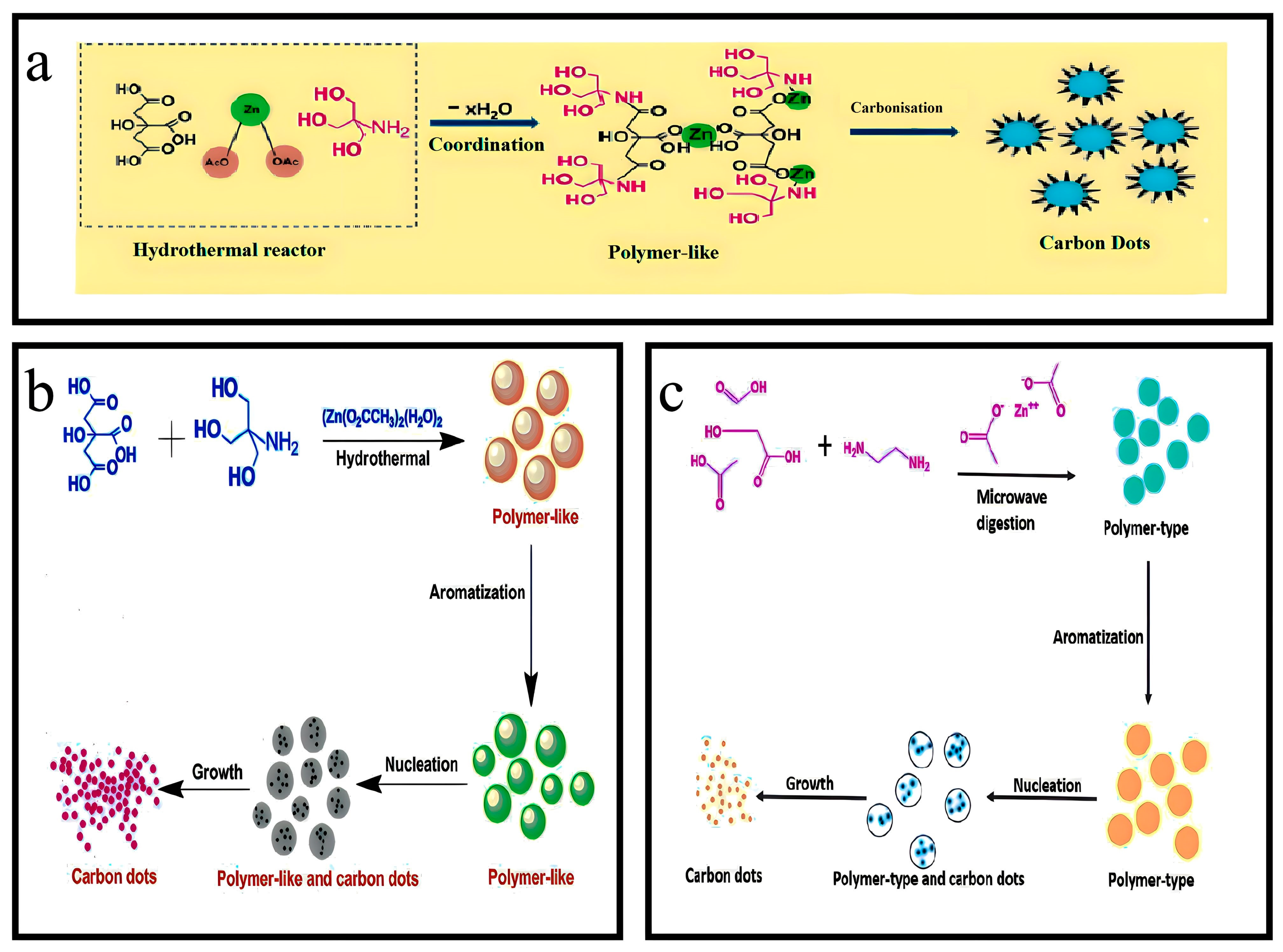
3.13. Synthesis of Zn-N-CQDs
3.14. Synthesis of N,Zn-CDs
3.15. Synthesis of Zn/Co-N-CQDs
3.16. Synthesis of Ce-N-CQDs
3.17. Synthesis of Ni-N-C Materials
3.18. Synthesis of Au/N-CQDs
3.19. Synthesis of Co-N-CDs
3.20. Synthesis of Bi-N-CQDs
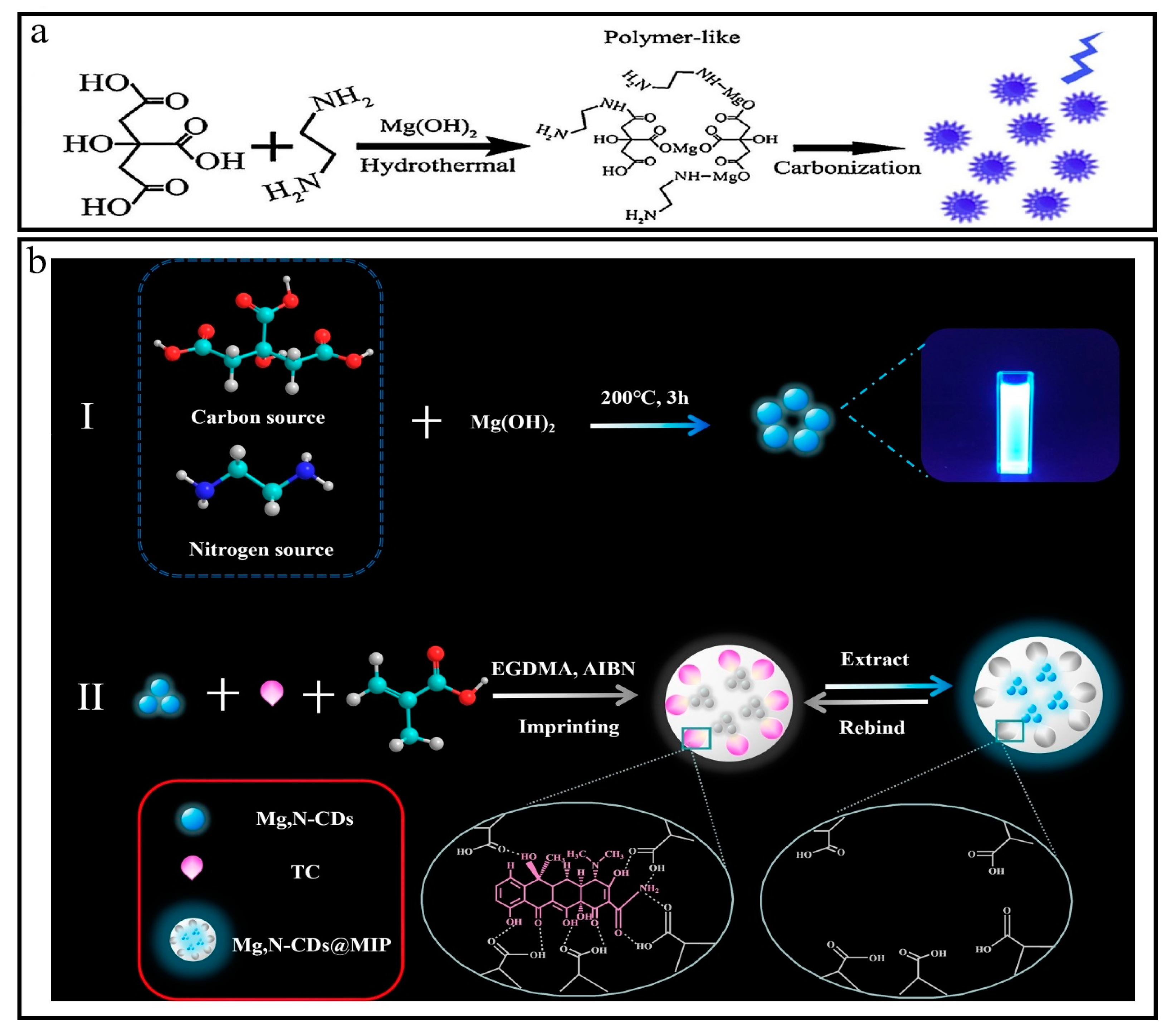
3.21. Synthesis of Mg-N-CQDs
3.22. Synthesis of Ag-N-CQDs
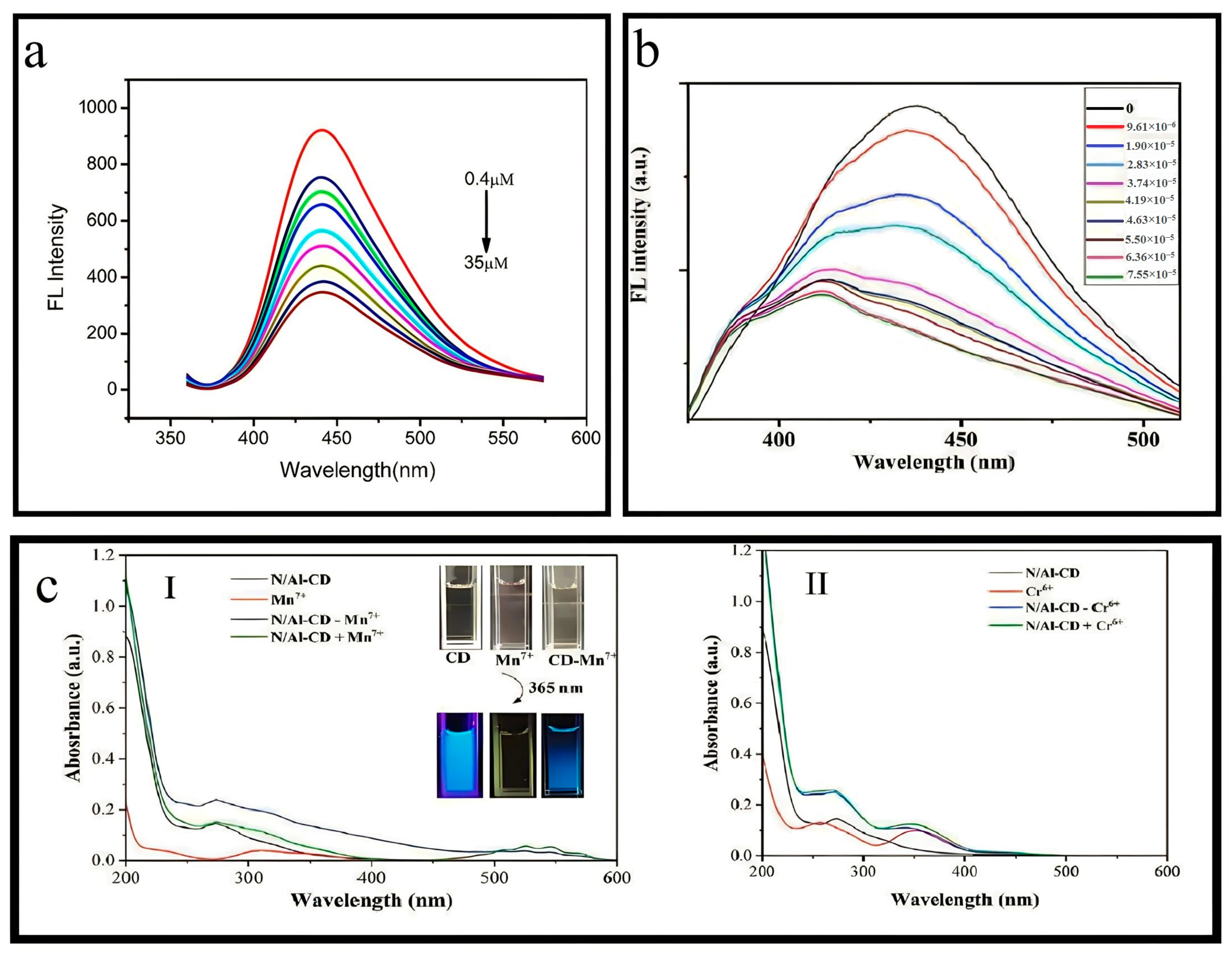
3.23. Synthesis of N/Al-CDs
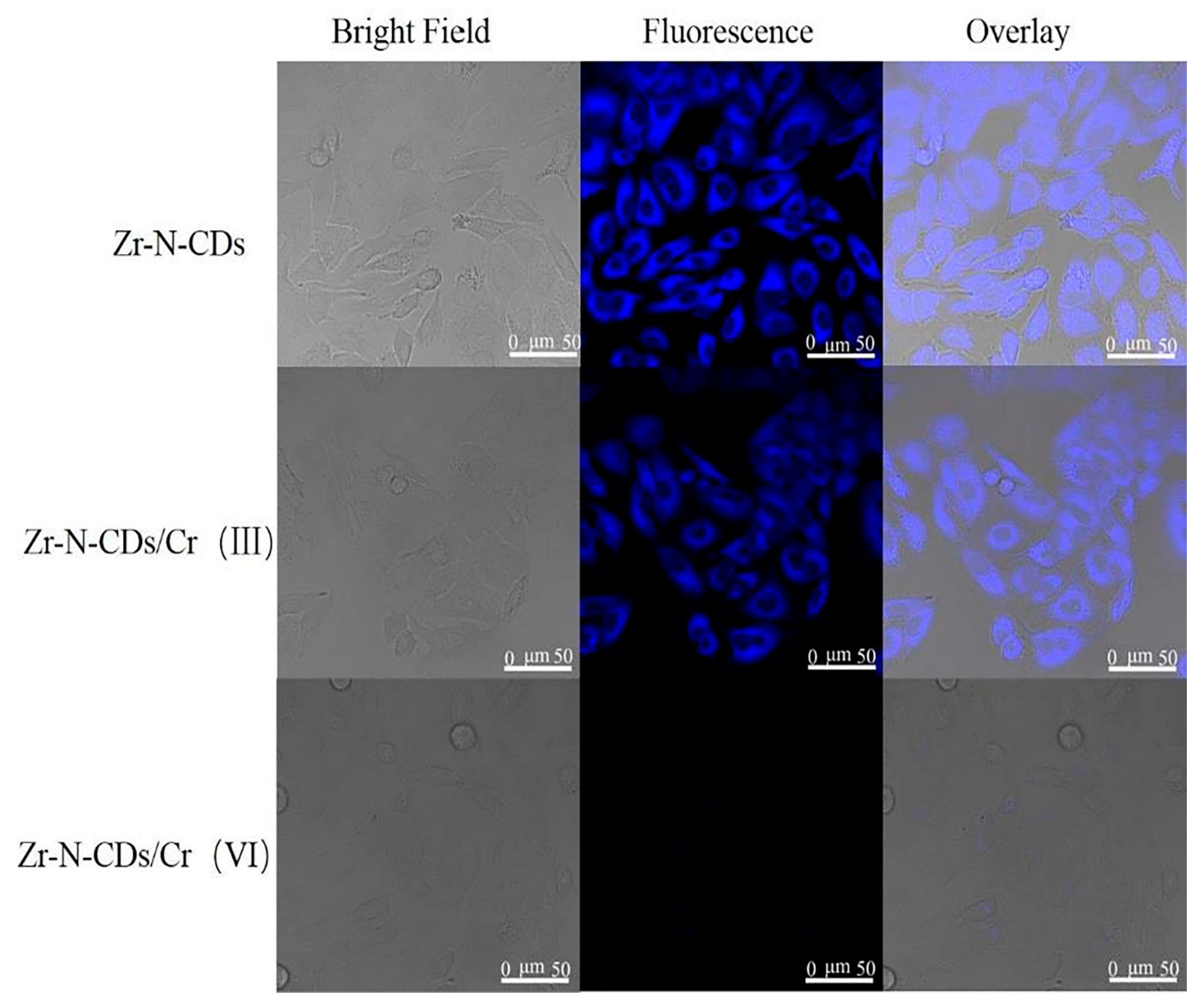
3.24. Synthesis of Zr-N-CDs
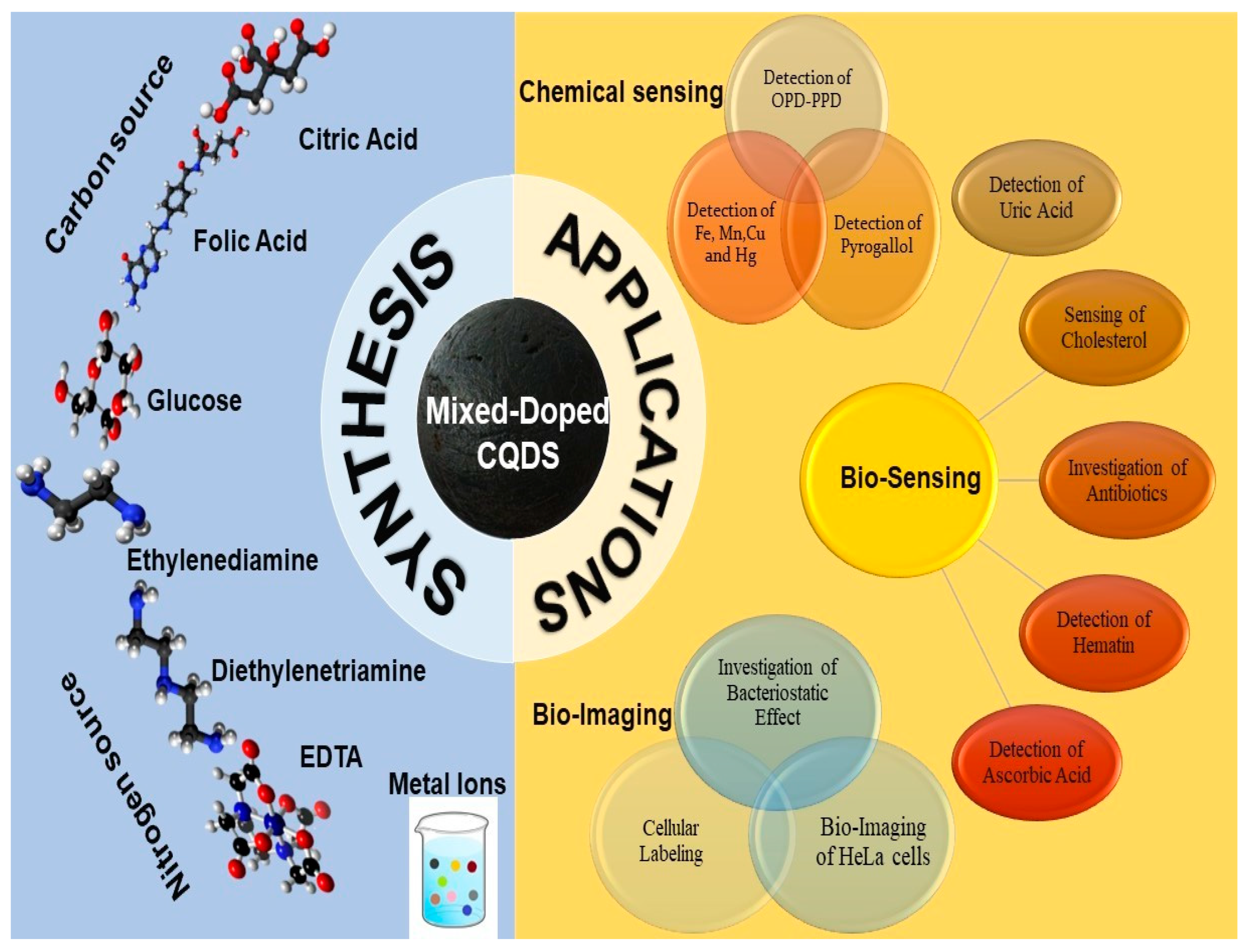
4. Applications of Mixed-Doped CQDs
4.1. Applications of Mixed-Doped CQDs in Chemical Sensing
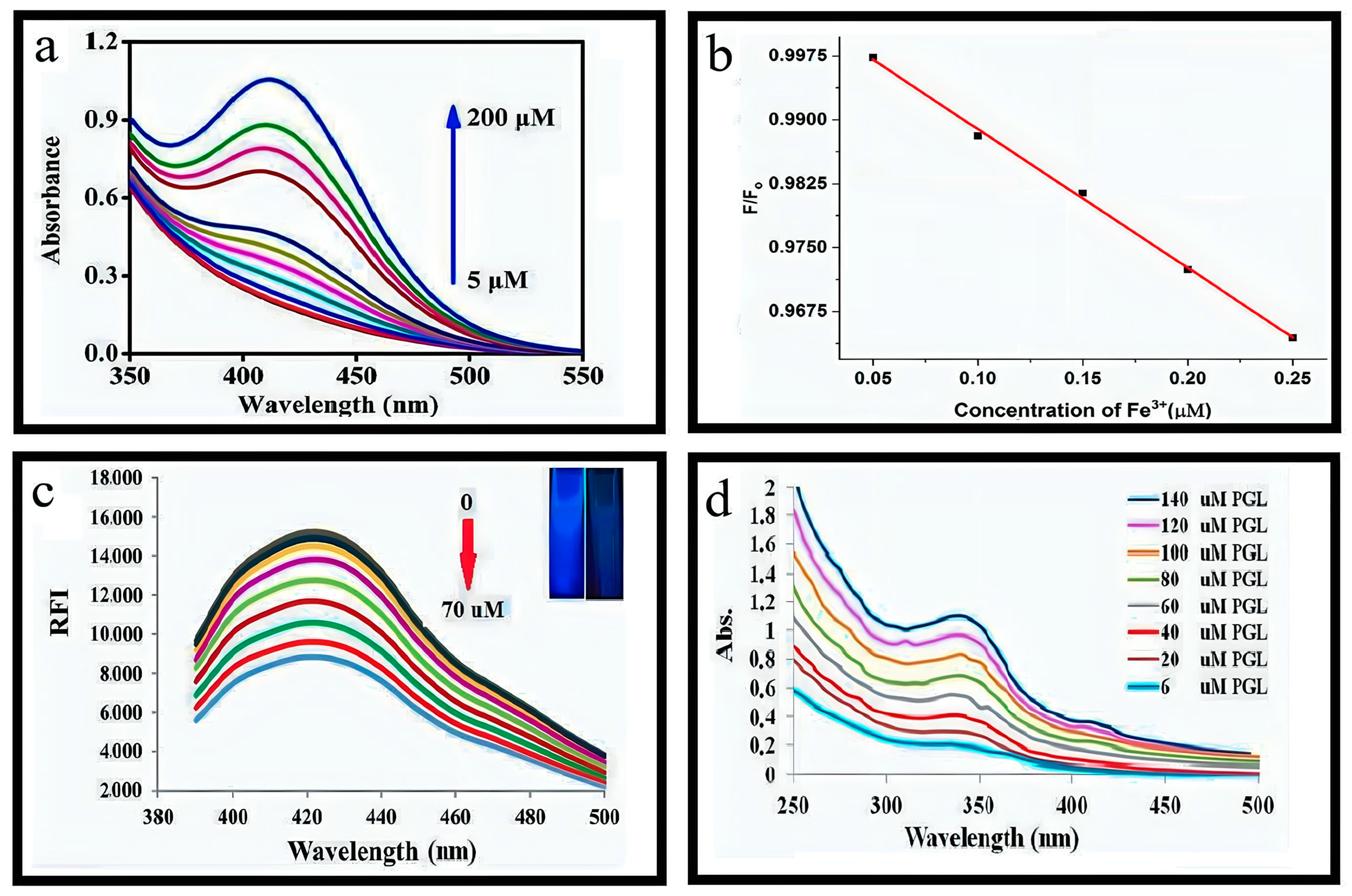
4.1.1. Sensitivity of Fe3+
4.1.2. Determination of Mn(VII)
4.1.3. Detection of Hg(II)
4.1.4. Detection of Hg(II)
4.1.5. Selective and Sensitive Detection of Cu2+
4.1.6. Discrimination between the Isomers of Phenylenediamine
4.1.7. Estimation of Pyrogallol
4.2. Applications of Mixed-Doped CQDs in Bio-Sensing
4.2.1. Investigation of Amoxicillin
4.2.2. Determination of Uric Acid (UA)
4.2.3. Detection of Tetracyclines (TCs)
4.2.4. Determination of Oxytetracycline (OTC)
4.2.5. Determination of Ofloxacin (OFL)
4.2.6. Sensing of Cholesterol
4.2.7. Detection of Ascorbic Acid
4.2.8. Detection of Cholesterol and Uric Acid
4.2.9. Detection of Hematin
4.3. Bio-Imaging
4.3.1. Bio-Imaging of Zr-N-CDs
4.3.2. Cell Imaging
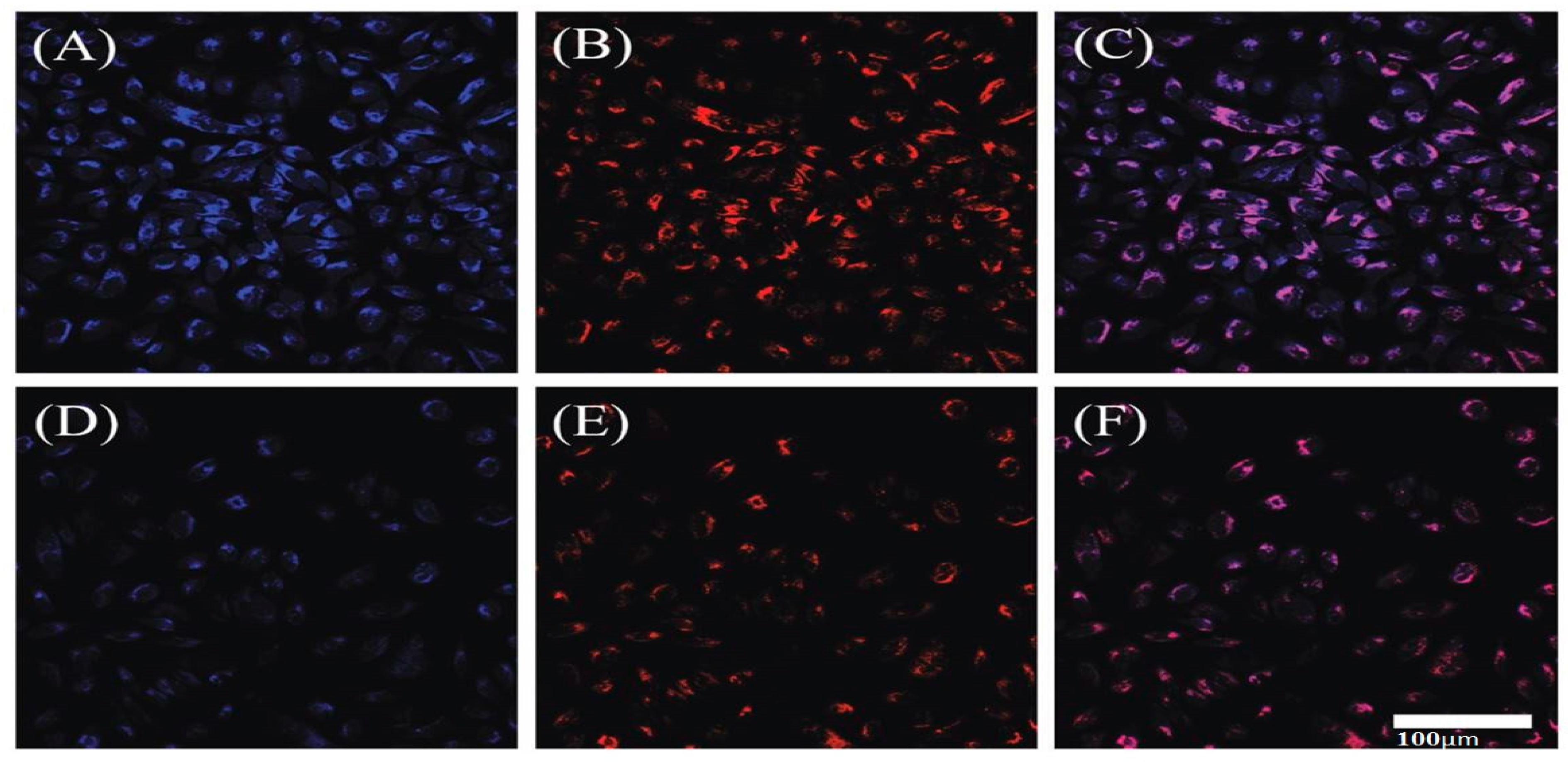
4.3.3. Applications in Cellular Imaging
4.3.4. Investigation of the Bacteriostatic Effect of Zn-N-CQDs on Gram-Negative E. coli Cells
4.3.5. Cell Labeling and Cytotoxicity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nammahachak, N.; Aup-Ngoen, K.K.; Asanithi, P.; Horpratum, M.; Chuangchote, S.; Ratanaphan, S.; Surareungchai, W. Hydrothermal synthesis of carbon quantum dots with size tunability via heterogeneous nucleation. RSC Adv. 2022, 12, 31729–31733. [Google Scholar] [CrossRef] [PubMed]
- Azam, N.; Najabat Ali, M.; Javaid Khan, T. Carbon quantum dots for biomedical applications: Review and analysis. Front. Mater. 2021, 8, 700403. [Google Scholar] [CrossRef]
- Wang, C.; Bi, L.; Liu, J.; Huang, B.; Wang, F.; Zhang, Y.; Yao, C.; Pan, G.; Song, M. Microalgae-derived carbon quantum dots mediated formation of metal sulfide nano-adsorbents with exceptional cadmium removal performance. J. Colloid Interface Sci. 2023, 629, 994–1002. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, P.; Yang, L.; Quimby, J.L.; Sun, Y.-P. Carbon “quantum” dots for bioapplications. Exp. Biol. Med. 2022, 247, 300–309. [Google Scholar] [CrossRef]
- De Boëver, R.; Town, J.R.; Li, X.; Claverie, J.P. Carbon dots for carbon dummies: The quantum and the molecular questions among some others. Chem. Eur. J. 2022, 28, e202200748. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Dimos, K. Carbon quantum dots: Surface passivation and functionalization. Curr. Org. Chem. 2016, 20, 682–695. [Google Scholar] [CrossRef]
- Malavika, J.P.; Shobana, C.; Sundarraj, S.; Ganeshbabu, M.; Kumar, P.; Selvan, R.K. Green synthesis of multifunctional carbon quantum dots: An approach in cancer theranostics. Biomater. Adv. 2022, 136, 212756. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Y.-H.; Liu, C.-Y.; Ma, D.-G. White light-emitting devices based on carbon dots’ electroluminescence. Chem. Commun. 2011, 47, 3502–3504. [Google Scholar] [CrossRef]
- Gao, R.; Wu, Z.; Wang, L.; Liu, J.; Deng, Y.; Xiao, Z.; Fang, J.; Liang, Y. Green preparation of fluorescent nitrogen-doped carbon quantum dots for sensitive detection of oxytetracycline in environmental samples. Nanomaterials 2020, 10, 1561. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yao, Z.; Zhang, B.; Liu, E.; Hu, X.; Xue, W.; Fan, J. Biocompatible sulfur nitrogen co-doped carbon quantum dots for highly sensitive and selective detection of dopamine. Colloids Surf. B Biointerfaces 2021, 205, 111874. [Google Scholar] [CrossRef]
- Zhang, D.; Chao, D.; Yu, C.; Zhu, Q.; Zhou, S.; Tian, L.; Zhou, L. One-step green solvothermal synthesis of full-color carbon quantum dots based on a doping strategy. J. Phys. Chem. Lett. 2021, 12, 8939–8946. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shen, D.; Wang, Q.; Luo, K.H. Interfaces. Green synthesis of tunable fluorescent carbon quantum dots from lignin and their application in anti-counterfeit printing. ACS Appl. Mater. Interfaces 2021, 13, 56465–56475. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, X.; Ding, H.; Li, S.; Li, M.; Wang, D.; Xia, J. Synthesis of green fluorescent carbon materials using byproducts of the sulfite-pulping procedure residue for live cell imaging and Ag+ ion determination. Mater. Sci. Eng. C 2019, 102, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Riaz, N.; Shaikh, A.J.; Shah, J.A.; Hussain, J.; Irshad, M.; Awan, M.S.; Syed, A.; Kallerhoff, J.; Arshad, M.; et al. Graphene quantum dot and iron co-doped TiO2 photocatalysts: Synthesis, performance evaluation and phytotoxicity studies. Ecotoxicol. Environ. Saf. 2021, 226, 112855. [Google Scholar] [CrossRef]
- Yang, X.; Xu, J.; Luo, N.; Tang, F.; Zhang, M.; Zhao, B. N, Cl co-doped fluorescent carbon dots as nanoprobe for detection of tartrazine in beverages. Food Chem. 2020, 310, 125832. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Osipov, V.Y.; Hogan, B.T.; Baldycheva, A. A comprehensive model of nitrogen-free ordered carbon quantum dots. Discov. Nano 2023, 18, 1. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon quantum dots from natural resource: A review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Carbon quantum dots derived from different carbon sources for antibacterial applications. Antibiotics 2021, 10, 623. [Google Scholar] [CrossRef]
- Zhao, A.; Chen, Z.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Recent advances in bioapplications of C-dots. Carbon 2015, 85, 309–327. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Sun, S.; Bao, W.; Yang, F.; Yan, X.; Sun, Y.; Zhang, G.; Yang, W.; Li, Y. Electrochemical synthesis of FeNx doped carbon quantum dots for sensitive detection of Cu2+ ion. Green Energy Environ. 2023, 8, 141–150. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Qian, Z.; Hu, Z.; Zhang, Z.; Li, Z.; Dou, M.; Wang, F. Out-of-plane Fe II–N 4 moiety modified Fe–N co-doped porous carbons as high-performance electrocatalysts for the oxygen reduction reaction. Catal. Sci. Technol. 2017, 7, 4017–4023. [Google Scholar] [CrossRef]
- Faraji, M.; Derakhshi, P.; Tahvildari, K.; Yousefian, Z. High performance Fe and N-codoped graphene quantum dot supported Pd3Co catalyst with synergistically improved oxygen reduction activity and great methanol tolerance. Solid State Sci. 2018, 83, 152–160. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Liu, X.; Liu, Y.; Zhang, Y.; Wang, W.; Mu, X.; Su, R.; Sun, Y.; Song, D.; et al. A universal sensing platform based on iron and nitrogen co-doped carbon dots for detecting hydrogen peroxide and related metabolites in human fluid by ratiometric fluorometry and colorimetry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 272, 121003. [Google Scholar] [CrossRef]
- Zhu, D.; Zhuo, S.; Zhu, C.; Zhang, P.; Shen, W. Synthesis of catalytically active peroxidase-like Fe-doped carbon dots and application in ratiometric fluorescence detection of hydrogen peroxide and glucose. Anal. Methods 2019, 11, 2663–2668. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, L.; Zan, M.; Hou, Z.; Ge, M.; Dong, W.-F.; Li, L. Iron and nitrogen-co-doped carbon quantum dots for the sensitive and selective detection of hematin and ferric ions and cell imaging. Analyst 2021, 146, 4954–4963. [Google Scholar] [CrossRef]
- Liang, C.; Lan, Y.; Sun, Z.; Zhou, L.; Li, Y.; Liang, X.; Qin, X. Synthesis of carbon quantum dots with iron and nitrogen from Passiflora edulis and their peroxidase-mimicking activity for colorimetric determination of uric acid. Microchim. Acta 2020, 187, 405. [Google Scholar] [CrossRef]
- Lin, L.; Xiao, Y.; Wang, Y.; Zeng, Y.; Lin, Z.; Chen, X. Hydrothermal synthesis of nitrogen and copper co-doped carbon dots with intrinsic peroxidase-like activity for colorimetric discrimination of phenylenediamine isomers. Microchim. Acta 2019, 186, 288. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.H.; Hassan, A.I.; Hassan, Y.F.; El-Wekil, M.M. Colorimetric and fluorimetric (dual-mode) nanoprobe for the determination of pyrogallol based on the complexation with copper (II)-and nitrogen-doped carbon dots. Microchim. Acta 2019, 186, 850. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cen, Y.; Sohail, M.; Xu, G.; Wei, F.; Shi, M.; Xu, X.; Song, Y.; Ma, Y.; Hu, Q. A ratiometric fluorescence universal platform based on N, Cu codoped carbon dots to detect metabolites participating in H2O2-generation reactions. ACS Appl. Mater. Interfaces 2017, 9, 33011–33019. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, P.; Wu, X.; Ma, C.; Luo, S.; Xu, M.; Li, W.; Liu, S. Nitrogen and copper (II) co-doped carbon dots for applications in ascorbic acid determination by non-oxidation reduction strategy and cellular imaging. Talanta 2020, 210, 120649. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; El-Wekil, M.M.; Ali, R.; Batakoushy, H.A.; Shahin, R.Y. Double-signal quantification of amoxicillin based on interaction with 4-aminoantipyrine at copper and nitrogen co-doped carbon quantum dots as an artificial nanozyme. Microchim. Acta 2022, 189, 183. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Y.; Shi, L.; Yang, Y. β-Cyclodextrin functionalized N, Zn codoped carbon dots for specific fluorescence detection of fluoroquinolones in milk samples. Microchem. J. 2020, 153, 104517. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Radmanesh, F.; Hosseini, E.S.; Hashemzadeh, I.; Kiani, J.; Nourizadeh, H.; Naseri, M.; Fatahi, Y.; Chegini, F.; Madjd, Z.; et al. Highly photoluminescent nitrogen-and zinc-doped carbon dots for efficient delivery of CRISPR/Cas9 and mRNA. Bioconjug. Chem. 2021, 32, 1875–1887. [Google Scholar] [CrossRef]
- Tammina, S.K.; Yang, D.; Li, X.; Koppala, S.; Yang, Y. High photoluminescent nitrogen and zinc doped carbon dots for sensing Fe3+ ions and temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117141. [Google Scholar] [CrossRef]
- Xu, O.; Yang, J.; Song, H.; Dong, L.; Xia, J.; Zhu, X. Novel Zn/Co–N co-doped carbon quantum dot-based “on-off-on” fluorescent sensor for Fe (III) and ascorbic acid. Talanta Open 2023, 7, 100162. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z. Application of cerium–nitrogen co-doped carbon quantum dots to the detection of tetracyclines residues and bioimaging. Microchem. J. 2021, 165, 106139. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Hu, X.; Cai, L.; Wang, H.; Fang, G.; Wang, S. A novel fluorescent biomimetic sensor based on cerium, nitrogen co-doped carbon quantum dots embedded in cobalt-based metal organic framework@ molecularly imprinted polymer for selective and sensitive detection of oxytetracycline. Microchem. J. 2023, 190, 108606. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Gao, J.; Jiang, J.; Jiang, X.; Wu, C. Three-dimensional porous Ni, N-codoped C networks for highly sensitive and selective non-enzymatic glucose sensing. Sens. Actuators B Chem. 2019, 299, 126945. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Chu, X.; Liu, Y.; Zhang, Y.; Xiao, Q. Nitrogen, cobalt co-doped fluorescent magnetic carbon dots as ratiometric fluorescent probes for cholesterol and uric acid in human blood serum. ACS Omega 2019, 4, 9333–9342. [Google Scholar] [CrossRef] [Green Version]
- Meng, A.; Xu, Q.; Zhao, K.; Li, Z.; Liang, J.; Li, Q. A highly selective and sensitive “on-off-on” fluorescent probe for detecting Hg (II) based on Au/N-doped carbon quantum dots. Sens. Actuators B Chem. 2018, 255, 657–665. [Google Scholar] [CrossRef]
- Delnavaz, E.; Amjadi, M. A chemiluminescence probe enhanced by cobalt and nitrogen-doped carbon dots for the determination of a nitrosative stress biomarker. Microchim. Acta 2021, 188, 278. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.C.; Ang, W.L.; Sambudi, N.S. Nitrogen and bismuth-doped rice husk-derived carbon quantum dots for dye degradation and heavy metal removal. J. Photochem. Photobiol. A Chem. 2021, 418, 113411. [Google Scholar]
- Li, F.; Liu, C.; Yang, J.; Wang, Z.; Liu, W.; Tian, F. Mg/N double doping strategy to fabricate extremely high luminescent carbon dots for bioimaging. RSC Adv. 2014, 4, 3201–3205. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Dong, J.X.; Luo, H.Q.; Li, N.B. Fluorescence detection of mercury ions and cysteine based on magnesium and nitrogen co-doped carbon quantum dots and IMPLICATION logic gate operation. Sens. Actuators B Chem. 2016, 231, 147–153. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, Y.; Dong, J.; Liu, C.; Qi, Y.; Fang, G.; Wang, S. A strong blue fluorescent nanoprobe based on Mg/N co-doped carbon dots coupled with molecularly imprinted polymer for ultrasensitive and highly selective detection of tetracycline in animal-derived foods. Sens. Actuators B Chem. 2021, 338, 129809. [Google Scholar] [CrossRef]
- Dewangan, L.; Chawre, Y.; Korram, J.; Karbhal, I.; Nagwanshi, R.; Jain, V.; Satnami, M.L. N-doped, silver, and cerium co-doped carbon quantum dots based sensor for detection of Hg2+ and captopril. Microchem. J. 2022, 182, 107867. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Deng, Y.; Chen, S.; Liang, W.; Zhang, L.; Wei, X.; Gao, S.; Wan, Y. Carbon quantum dots doped with silver as lubricating oil additive for enhancing tribological performance at various temperatures. Appl. Surf. Sci. 2022, 599, 154029. [Google Scholar] [CrossRef]
- Wang, Y.; Han, C.; Yu, L.; Wu, J.; Min, Y.; Tan, J.; Zhao, Y.; Zhang, P. Etching-controlled suppression of fluorescence resonance energy transfer between nitrogen-doped carbon dots and Ag nanoprisms for glucose assay and diabetes diagnosis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118713. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, S.; Yin, K.; Hu, X.; Ng, W.J. Fluorescent N/Al Co-doped carbon dots from cellulose biomass for sensitive detection of manganese (VII). J. Fluoresc. 2019, 29, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fan, Z. Morphological analysis of chromium in carbon quantum dots pairs Co-doped with zirconium and nitrogen and their applications in imaging of living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119248. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.; Huang, C.; Su, W.; Xiao, Q. Label-free and ratiometric fluorescent nanosensor based on amino-functionalized graphene quantum dots coupling catalytic G-quadruplex/hemin DNAzyme for ultrasensitive recognition of human telomere DNA. Sens. Actuators B Chem. 2017, 245, 648–655. [Google Scholar] [CrossRef]
- Naik, V.M.; Gunjal, D.B.; Gore, A.H.; Pawar, S.P.; Mahanwar, S.T.; Anbhule, P.V.; Kolekar, G.B. Quick and low cost synthesis of sulphur doped carbon dots by simple acidic carbonization of sucrose for the detection of Fe3+ ions in highly acidic environment. Diam. Relat. Mater. 2018, 88, 262–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, S.; Huo, D.; Hou, C.; Yang, M.; Li, J.; Bian, M. Simple and sensitive fluorescence sensor for methotrexate detection based on the inner filter effect of N, S co-doped carbon quantum dots. Anal. Chim. Acta 2019, 1047, 179–187. [Google Scholar] [CrossRef]
- Wu, F.; Yang, M.; Zhang, H.; Zhu, S.; Zhu, X.; Wang, K. Facile synthesis of sulfur-doped carbon quantum dots from vitamin B1 for highly selective detection of Fe3+ ion. Opt. Mater. 2018, 77, 258–263. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Mohandoss, S.; Ganesan, S.; Palanisamy, S.; You, S.; Velsankar, K.; Sudhahar, S.; Lo, H.-M.; Lee, Y.R. Nitrogen, sulfur, and phosphorus Co-doped carbon dots-based ratiometric chemosensor for highly selective sequential detection of Al3+ and Fe3+ ions in logic gate, cell imaging, and real sample analysis. Chemosphere 2023, 313, 137444. [Google Scholar] [CrossRef]
- Jayaweera, S.; Yin, K.; Hu, X.; Ng, W.J. Facile preparation of fluorescent carbon dots for label-free detection of Fe3+. J. Photochem. Photobiol. A Chem. 2019, 370, 156–163. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Biosens. Bioelectron. 2014, 55, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem. Commun. 2012, 48, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.-S.; Zhu, C.-Q. Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 2008, 75, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, P.; Hou, X.; Lv, Y. An ascorbic acid sensor based on protein-modified Au nanoclusters. Analyst 2013, 138, 229–233. [Google Scholar] [CrossRef]
- Rong, M.-C.; Zhang, K.-X.; Wang, Y.-R.; Chen, X. The synthesis of B, N-carbon dots by a combustion method and the application of fluorescence detection for Cu2+. Chin. Chem. Lett. 2017, 28, 1119–1124. [Google Scholar] [CrossRef]
- Shahamirifard, S.; Ghaedi, M.; Montazerozohori, M.; Masoudiasl, A. Carbon dots as absorbance promoter probes for detection of Cu (II) ions in aqueous solution: Central composite design approach. Photochem. Photobiol. Sci. 2018, 17, 245–255. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, L.; Li, W.; Wang, X.; Shang, Y.; Li, B. Carbon dots doped with nitrogen and sulfur and loaded with copper (II) as a “turn-on” fluorescent probe for cystein, glutathione and homocysteine. Microchim. Acta 2016, 183, 1409–1416. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Bose, M.; Mondal, S.; Das, A.K.; Banerjee, S.; Das, N.C. A simplistic approach to green future with eco-friendly luminescent carbon dots and their application to fluorescent nano-sensor ‘turn-off’probe for selective sensing of copper ions. Mater. Sci. Eng. C 2017, 75, 1456–1464. [Google Scholar] [CrossRef]
- Sun, H.; Gao, N.; Wu, L.; Ren, J.; Wei, W.; Qu, X. Highly photoluminescent amino-functionalized graphene quantum dots used for sensing copper ions. Chem. Eur. J. 2013, 19, 13362–13368. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Wang, C.; Cao, Y.; Li, Y.; Yan, L.; Liu, M.; Lv, M.; Yang, Y.; Zhao, X.; et al. Controllable and eco-friendly synthesis of P-riched carbon quantum dots and its application for copper (II) ion sensing. Appl. Surf. Sci. 2018, 448, 589–598. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Li, X.; Zhao, B.; Wen, X.; Zhang, G.; Dong, C.; Shuang, S. Controllable synthesis of green and blue fluorescent carbon nanodots for pH and Cu2+ sensing in living cells. Biosens. Bioelectron. 2016, 77, 598–602. [Google Scholar] [CrossRef]
- Hudari, F.F.; de Almeida, L.C.; da Silva, B.F.; Zanoni, M.V.B. Voltammetric sensor for simultaneous determination of p-phenylenediamine and resorcinol in permanent hair dyeing and tap water by composite carbon nanotubes/chitosan modified electrode. Microchem. J. 2014, 116, 261–268. [Google Scholar] [CrossRef]
- Fornera, S.; Yazawa, K.; Walde, P. Spectrophotometric quantification of lactose in solution with a peroxidase-based enzymatic cascade reaction system. Anal. Bioanal. Chem. 2011, 401, 2307–2310. [Google Scholar] [CrossRef] [Green Version]
- Khattar, R.; Yadav, A.; Mathur, P. Copper (II) complexes as catalyst for the aerobic oxidation of o-phenylenediamine to 2, 3-diaminophenazine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 375–381. [Google Scholar] [CrossRef]
- Green, N.J.; Pimblott, S.M.; Tachiya, M. Generalizations of the Stern-Volmer relation. J. Phys. Chem. 1993, 97, 196–202. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N. Colorimetric determination of Amoxicillin using 4-Aminoantipyrine and the effects of different parameters. J. Phys. Conf. Ser. 2019, 1294, 052067. [Google Scholar] [CrossRef]
- Li, G.; Fu, H.; Chen, X.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. Facile and sensitive fluorescence sensing of alkaline phosphatase activity with photoluminescent carbon dots based on inner filter effect. Anal. Chem. 2016, 88, 2720–2726. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.; Huang, C.; Xie, J.; Su, W.; Sheng, J.; Xiao, Q. A carbon dots based fluorescent probe for selective and sensitive detection of hemoglobin. Sens. Actuators B Chem. 2015, 221, 1215–1222. [Google Scholar] [CrossRef]
- Svobodova, D.; Gasparič, J. Investigation of the colour reaction of phenols with 4-aminoantipyrine. Microchim. Acta 1971, 59, 384–390. [Google Scholar] [CrossRef]
- Zhai, W.; Wang, C.; Yu, P.; Wang, Y.; Mao, L. Single-layer MnO2 nanosheets suppressed fluorescence of 7-hydroxycoumarin: Mechanistic study and application for sensitive sensing of ascorbic acid in vivo. Anal. Chem. 2014, 86, 12206–12213. [Google Scholar] [CrossRef] [PubMed]
- Al Abdulmonem, W.; Aljohani, A.S.; Alhumaydhi, F.A.; Mousa, A.H.; Rasheed, Z. Protective potential of uric acid, folic acid, glutathione and ascorbic acid against the formation of toxic met-myoglobin. Protein Pept. Lett. 2021, 28, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, Q.; Tang, X.; Zhong, H.; Qiu, P.; Wang, X. A highly selective and sensitive colorimetric detection of uric acid in human serum based on MoS 2-catalyzed oxidation TMB. Anal. Bioanal. Chem. 2019, 411, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Q.; Hou, Y.; Liu, Y.; Zhang, Y. High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 2016, 155, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Deng, J.; Hou, Y.; Wang, H.; Li, H.; Zhang, Y. One-step electrochemical synthesis of ultrathin graphitic carbon nitride nanosheets and their application to the detection of uric acid. Chem. Commun. 2015, 51, 12251–12253. [Google Scholar] [CrossRef]
- Lu, J.; Xiong, Y.; Liao, C.; Ye, F. Colorimetric detection of uric acid in human urine and serum based on peroxidase mimetic activity of MIL-53 (Fe). Anal. Methods 2015, 7, 9894–9899. [Google Scholar] [CrossRef]
- Jin, D.; Seo, M.-H.; Huy, B.T.; Pham, Q.-T.; Conte, M.L.; Thangadurai, D.; Lee, Y.-I. Quantitative determination of uric acid using CdTe nanoparticles as fluorescence probes. Biosens. Bioelectron. 2016, 77, 359–365. [Google Scholar] [CrossRef]
- Amjadi, M.; Hallaj, T.; Kouhi, Z. An enzyme-free fluorescent probe based on carbon dots–MnO2 nanosheets for determination of uric acid. J. Photochem. Photobiol. A Chem. 2018, 356, 603–609. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, T.; Xin, X.; Wu, D.; Huang, Y.; Liu, Y.; Cao, Y.; Guo, M.; Yu, Y. Europium (III) modified silicone nanoparticles for ultrasensitive visual determination of tetracyclines by employing a fluorescence color switch. Microchim. Acta 2019, 186, 442. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, S.; Dou, Y.; Zhuo, Y.; Luo, Y.; Feng, Y. Novel and remarkable enhanced-fluorescence system based on gold nanoclusters for detection of tetracycline. Talanta 2014, 122, 36–42. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S. Design of fluorescent assays for cyanide and hydrogen peroxide based on the inner filter effect of metal nanoparticles. Anal. Chem. 2009, 81, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, X.; Liu, H.; Ge, S.; Yan, M.; Yu, J.; Song, X. An inner filter effect fluorescent sensor based on g-C3N4 nanosheets/chromogenic probe for simple detection of glutathione. Sens. Actuators B Chem. 2017, 248, 639–645. [Google Scholar] [CrossRef]
- Fu, Z.; Yao, M.; Niu, X.; Cui, F. Facile synthesis of highly luminescent co-doped carbon nanodots for rapid, sensitive, and label-free detection of Hg2+. Sens. Actuators B Chem. 2016, 226, 486–494. [Google Scholar] [CrossRef]
- Li, J.; Zeng, W.; Lai, X.; Wang, X.; Xu, X.; Cai, H.; Wei, L.; Cheng, X.L. Selective and sensitive determination of tetracyclines by HPLC with chemiluminescence detection based on a cerium (IV)-methoxylated cypridina luciferin analogue system. J. Sep. Sci. 2018, 41, 4115–4121. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Li, Z. Excitation-depended fluorescence emission of boron-doped graphene quantum dot as an optical probe for detection of oxytetracycline in food and information encryption patterns. Microchim. Acta 2023, 190, 278. [Google Scholar]
- Algar, W.R.; Tavares, A.J.; Krull, U.J. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal. Chim. Acta 2010, 673, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Jojoa-Sierra, S.D.; Berrio-Perlaza, K.E.; Ferraro, F.; Torres-Palma, R.A. Structure-reactivity relationship in the degradation of three representative fluoroquinolone antibiotics in water by electrogenerated active chlorine. Chem. Eng. J. 2017, 315, 552–561. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Chen, H.; Li, Z.; Song, Z.; Guo, L.; Fu, F. A sensitive colorimetric assay for cholesterol based on the peroxidase-like activity of MoS2 nanosheets. Microchim. Acta 2017, 184, 1233–1237. [Google Scholar] [CrossRef]
- Sun, X.-T.; Zhang, Y.; Zheng, D.-H.; Yue, S.; Yang, C.-G.; Xu, Z.-R. Multitarget sensing of glucose and cholesterol based on Janus hydrogel microparticles. Biosens. Bioelectron. 2017, 92, 81–86. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z. Determination of free cholesterol based on a novel flow-injection chemiluminescence method by immobilizing enzyme. Luminescence 2008, 23, 338–343. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, W.; Han, Y.; Chen, X.; Zhu, L.; Tang, W.; Wang, J.; Yue, T.; Li, Z. N, S co-doped carbon dots based fluorescent “on-off-on” sensor for determination of ascorbic acid in common fruits. Food Chem. 2018, 258, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, C.; Liu, K.; Wang, Y.; Liu, K.; Lin, Y. Hexagonal cobalt oxyhydroxide–carbon dots hybridized surface: High sensitive fluorescence turn-on probe for monitoring of ascorbic acid in rat brain following brain ischemia. Anal. Chem. 2015, 87, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, X.; Zhao, H.; Li, Z. A highly sensitive and selective detection of Cr (VI) and ascorbic acid based on nitrogen-doped carbon dots. Talanta 2018, 181, 318–325. [Google Scholar] [CrossRef]
- Liu, J.-J.; Chen, Z.-T.; Tang, D.-S.; Wang, Y.-B.; Kang, L.-T.; Yao, J.-N. Graphene quantum dots-based fluorescent probe for turn-on sensing of ascorbic acid. Sens. Actuators B Chem. 2015, 212, 214–219. [Google Scholar] [CrossRef]
- Fong, J.F.Y.; Chin, S.F.; Ng, S.M. A unique “turn-on” fluorescence signalling strategy for highly specific detection of ascorbic acid using carbon dots as sensing probe. Biosens. Bioelectron. 2016, 85, 844–852. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.J.; Li, N.; Liu, S.G.; Liang, J.Y.; Gao, X.; Fan, Y.Z.; Luo, H.Q.; Li, N.B. Proton-controlled synthesis of red-emitting carbon dots and application for hematin detection in human erythrocytes. Anal. Bioanal. Chem. 2019, 411, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-Y.; Cheng, Y.-S.; Wang, D.-M.; Li, M.-L.; Lu, W.-S.; Xu, X.-Y.; Zhou, X.-H.; Wei, X.-W. Nitrogen-doped MoS2 quantum dots: Facile synthesis and application for the assay of hematin in human blood. Mater. Sci. Eng. C 2020, 112, 110898. [Google Scholar] [CrossRef]
- Bojanowska, I. Recovery of chromium from sludge formed after neutralisation of chromic wastewater. Pol. J. Environ. Stud. 2002, 11, 117–122. [Google Scholar]
- Zhu, J.; Zhao, Z.-J.; Li, J.-J.; Zhao, J.-W. Fluorescent detection of ascorbic acid based on the emission wavelength shift of CdTe quantum dots. J. Lumin. 2017, 192, 47–55. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Su, X. Detection of ascorbic acid and folic acid based on water-soluble CuInS2 quantum dots. Analyst 2012, 137, 4598–4604. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, Y.; Ge, Y.; Song, G. “Turn-off-on” fluorescence switching of ascorbic acid-reductive silver Nanoclusters: A sensor for ascorbic acid and arginine in biological fluids. J. Fluoresc. 2017, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, N.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen based on a ferrocene derivative functional Au NPs/carbon dots nanocomposite and graphene. Anal. Chim. Acta 2016, 903, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Bose, M.; Mondal, S.; Choudhary, S.; Gangopadhyay, S.; Das, A.K.; Banerjee, S.; Das, N.C. Zinc and nitrogen ornamented bluish white luminescent carbon dots for engrossing bacteriostatic activity and Fenton based bio-sensor. Mater. Sci. Eng. C 2018, 88, 115–129. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, Z.; Raza, A.; Mehdi, M.; Arshad, A.; Deng, X.; Sun, S. Recent Advancements in Metal and Non-Metal Mixed-Doped Carbon Quantum Dots: Synthesis and Emerging Potential Applications. Nanomaterials 2023, 13, 2336. https://doi.org/10.3390/nano13162336
Akram Z, Raza A, Mehdi M, Arshad A, Deng X, Sun S. Recent Advancements in Metal and Non-Metal Mixed-Doped Carbon Quantum Dots: Synthesis and Emerging Potential Applications. Nanomaterials. 2023; 13(16):2336. https://doi.org/10.3390/nano13162336
Chicago/Turabian StyleAkram, Zubair, Ali Raza, Muhammad Mehdi, Anam Arshad, Xiling Deng, and Shiguo Sun. 2023. "Recent Advancements in Metal and Non-Metal Mixed-Doped Carbon Quantum Dots: Synthesis and Emerging Potential Applications" Nanomaterials 13, no. 16: 2336. https://doi.org/10.3390/nano13162336




