Synergistic Halide- and Ligand-Exchanges of All-Inorganic Perovskite Nanocrystals for Near-Unity and Spectrally Stable Red Emission
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of CsPbBr3 NCs
2.3. Preparation of ZnI2 and ZnBr2 Precursor Solutions
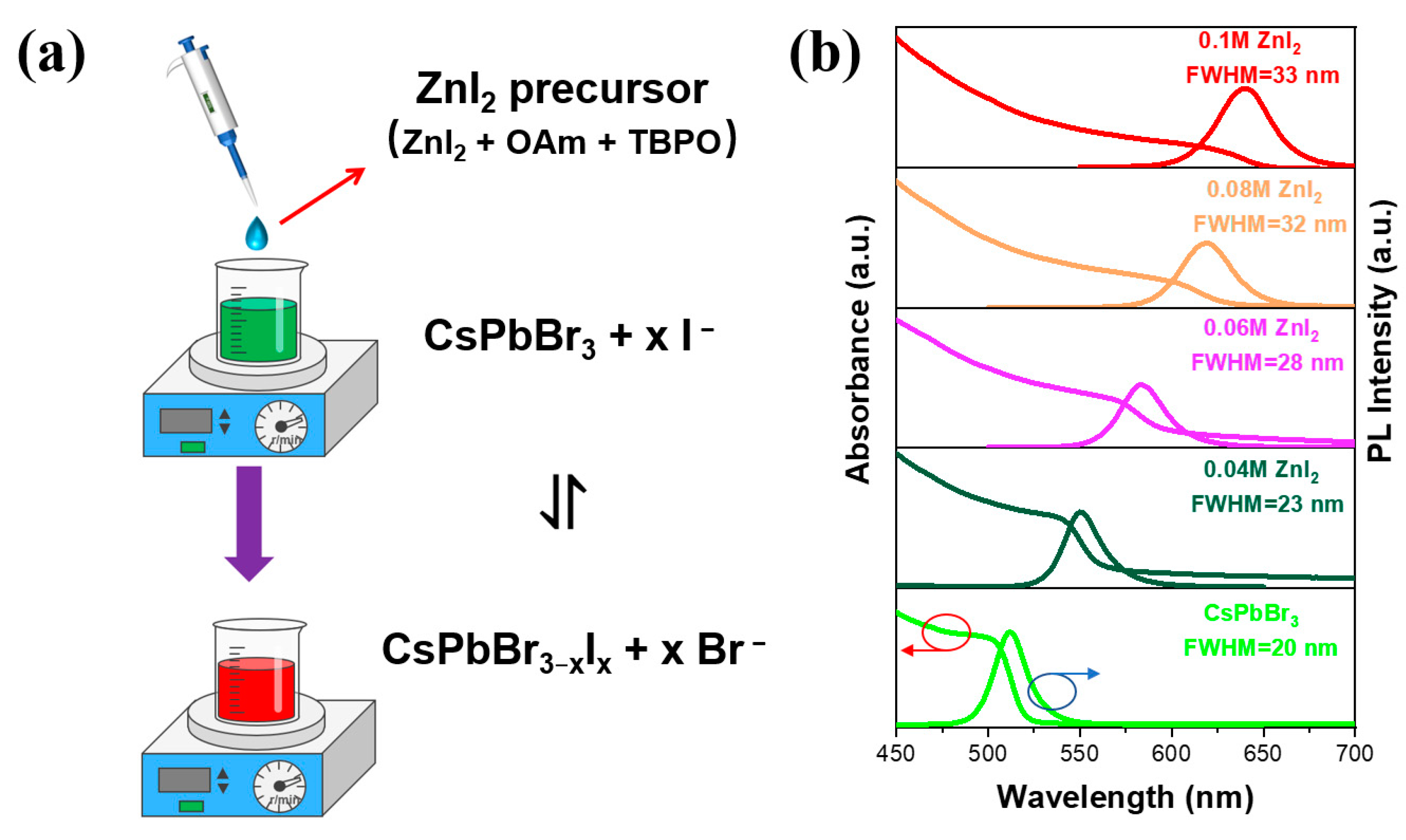
2.4. Anion Exchange Processes
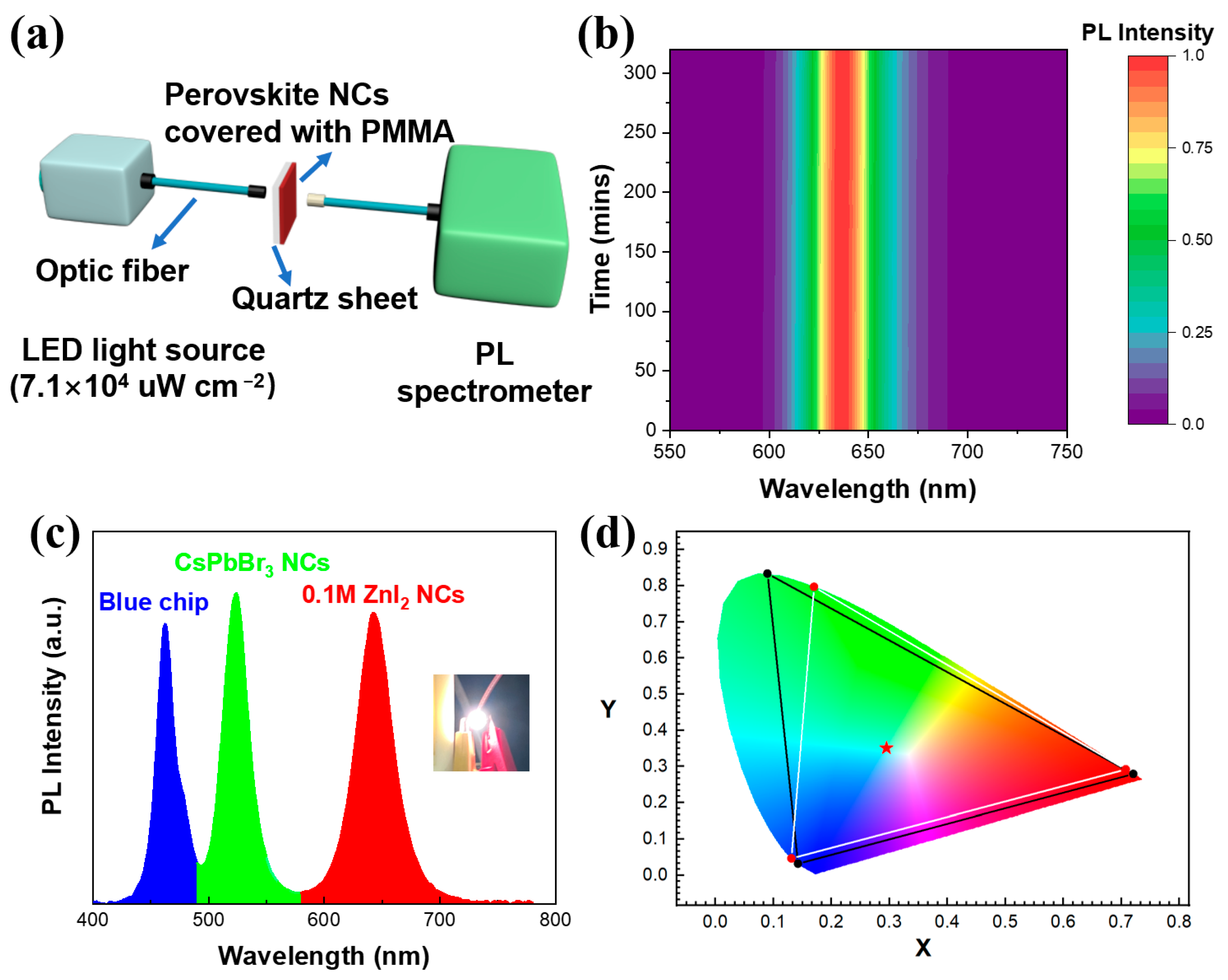
2.5. Fabrication of WLED Device
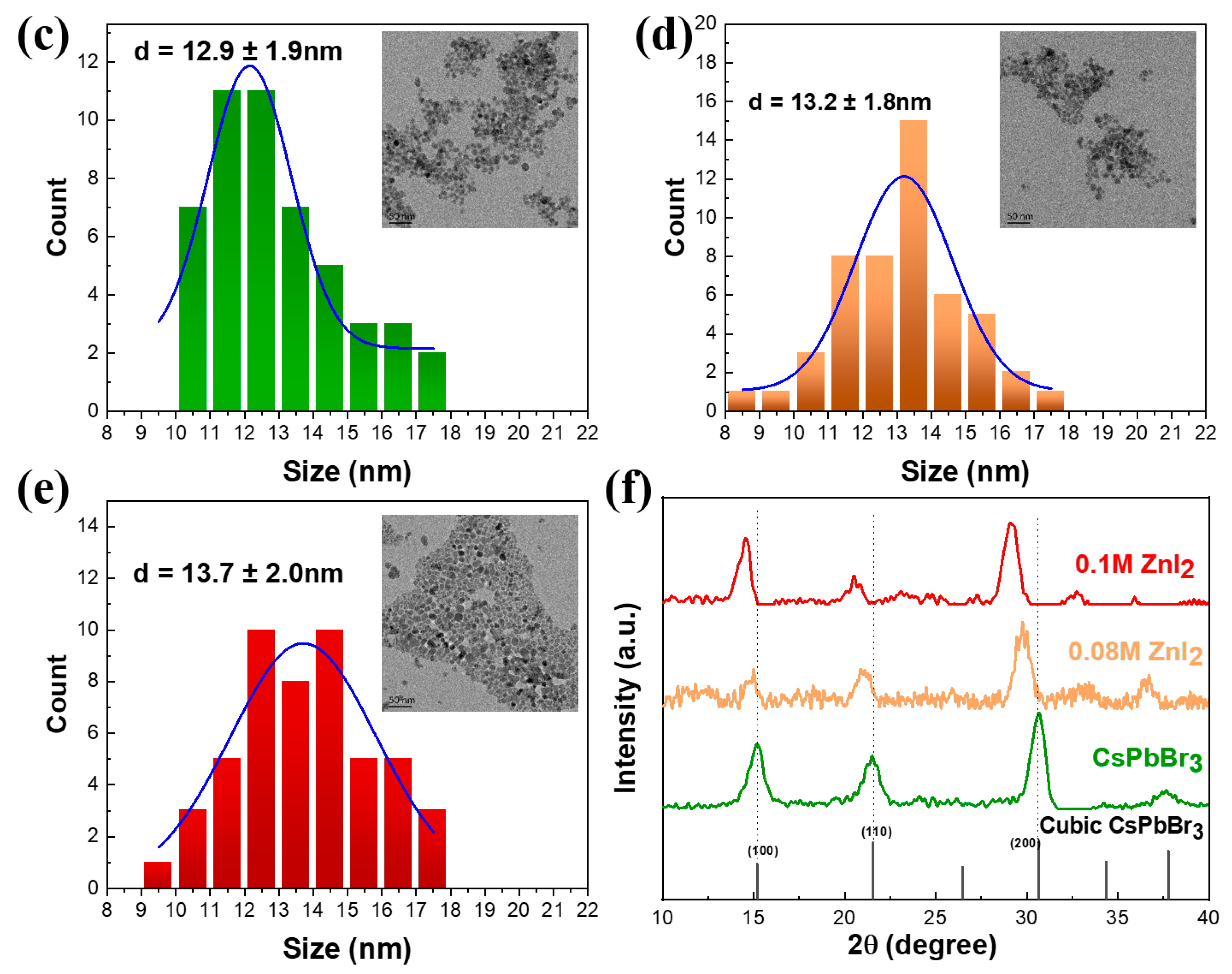
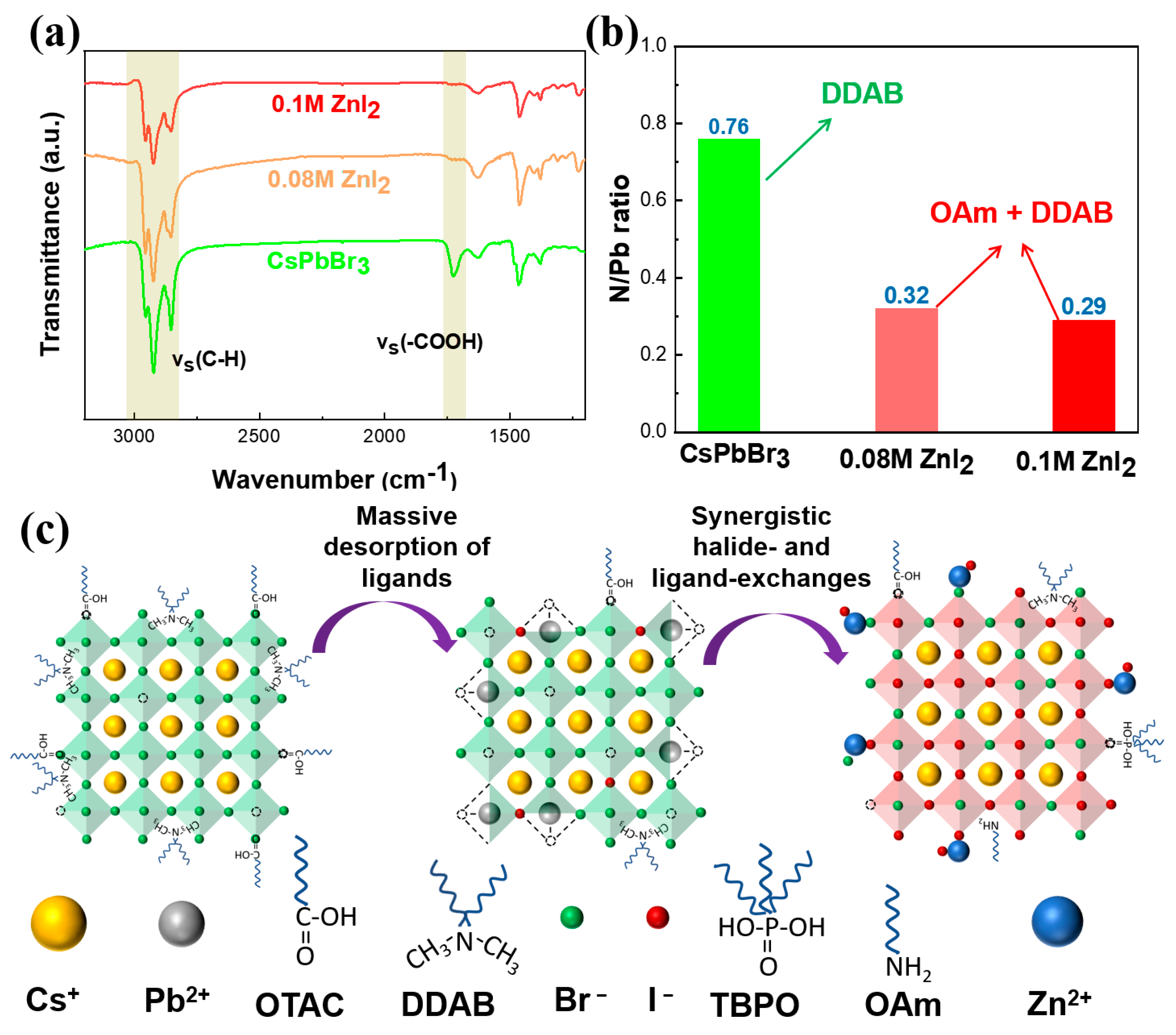
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Efros, A.L.; Brus, L.E. Nanocrystal Quantum Dots: From Discovery to Modern Development. ACS Nano 2021, 15, 6192–6210. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of Quantum Dots, Quantum Dot Films, and Quantum Dot Light-Emitting Diodes for Display Applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ali, A.; Haq, I.U.; Aziz, S.A.; Ali, Z.; Ahmad, I. The effect of potassium insertion on optoelectronic properties of cadmium chalcogenides. Mater. Sci. Semicond. Process. 2021, 122, 105466. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Ravi, P.; Sathish, M.; Vijayakumar, M.; Sakar, M.; Karthik, M.; Balakumar, S.; Reddy, K.R.; Shetti, N.P.; Aminabhavi, T.M.; et al. Metal chalcogenide-based core/shell photocatalysts for solar hydrogen production: Recent advances, properties and technology challenges. J. Hazard Mater. 2021, 415, 125588. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Su, W.; Li, J.; Xu, B.; Shan, Q.; Wu, Y.; Zhang, F.; Luo, M.; Xiang, H.; Cui, Z.; et al. A Universal Ternary-Solvent-Ink Strategy toward Efficient Inkjet-Printed Perovskite Quantum Dot Light-Emitting Diodes. Adv. Mater. 2022, 34, 2107798. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, C.; Xu, J.; Li, S.; Cui, M.; Fu, X.; Liu, Y.; Liu, Y.; Wan, H.; Wei, K.; et al. Synthesis-on-substrate of quantum dot solids. Nature 2022, 612, 679–684. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Tsai, H.; Shrestha, S.; Vilá, R.A.; Huang, W.; Liu, C.; Hou, C.-H.; Huang, H.-H.; Wen, X.; Li, M.; Wiederrecht, G.; et al. Bright and stable light-emitting diodes made with perovskite nanocrystals stabilized in metal–organic frameworks. Nat. Photonics 2021, 15, 843–849. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Zhang, Y.; Wu, H.; Ji, C.; Chuai, Y.; Wang, P.; Wen, S.; Zhang, C.; Yu, W.W. Bright Perovskite Nanocrystal Films for Efficient Light-Emitting Devices. J. Phys. Chem. Lett. 2016, 7, 4602–4610. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX(3), X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Ng, T.W.; Thachoth Chandran, H.; Chan, C.Y.; Lo, M.F.; Lee, C.S. Ionic Charge Transfer Complex Induced Visible Light Harvesting and Photocharge Generation in Perovskite. ACS Appl. Mater. Interfaces 2015, 7, 20280–20284. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Shang, Y.; Yin, J.; De Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A.M.; Hedhili, M.N.; Emwas, A.H.; et al. Bidentate Ligand-Passivated CsPbI(3) Perovskite Nanocrystals for Stable Near-Unity Photoluminescence Quantum Yield and Efficient Red Light-Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 562–565. [Google Scholar] [CrossRef]
- Yassitepe, E.; Yang, Z.; Voznyy, O.; Kim, Y.; Walters, G.; Castañeda, J.A.; Kanjanaboos, P.; Yuan, M.; Gong, X.; Fan, F.; et al. Amine-Free Synthesis of Cesium Lead Halide Perovskite Quantum Dots for Efficient Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 8757–8763. [Google Scholar] [CrossRef]
- Steele, J.A.; Jin, H.; Dovgaliuk, I.; Berger, R.F.; Braeckevelt, T.; Yuan, H.; Martin, C.; Solano, E.; Lejaeghere, K.; Rogge, S.M.J.; et al. Thermal unequilibrium of strained black CsPbI(3) thin films. Science 2019, 365, 679–684. [Google Scholar] [CrossRef]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Highly Efficient All-Inorganic Planar Heterojunction Perovskite Solar Cells Produced by Thermal Coevaporation of CsI and PbI(2). J. Phys. Chem. Lett. 2017, 8, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of alpha-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Zou, C.; Huang, C.Y.; Sanehira, E.M.; Luther, J.M.; Lin, L.Y. Highly stable cesium lead iodide perovskite quantum dot light-emitting diodes. Nanotechnology 2017, 28, 455201. [Google Scholar] [CrossRef]
- Hoffman, J.B.; Schleper, A.L.; Kamat, P.V. Transformation of Sintered CsPbBr3 Nanocrystals to Cubic CsPbI3 and Gradient CsPbBrxI3-x through Halide Exchange. J. Am. Chem. Soc. 2016, 138, 8603–8611. [Google Scholar] [CrossRef]
- Fang, F.; Chen, W.; Li, Y.; Liu, H.; Mei, M.; Zhang, R.; Hao, J.; Mikita, M.; Cao, W.; Pan, R.; et al. Employing Polar Solvent Controlled Ionization in Precursors for Synthesis of High-Quality Inorganic Perovskite Nanocrystals at Room Temperature. Adv. Funct. Mater. 2018, 28, 1706000. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.B.; Tong, X.; Sun, Y.B.; Zhang, H.Y.; Min, Y.G.; Qian, Y.N. Near-Unity Quantum Yield and Superior Stable Indium-Doped CsPbBrxI3-x Perovskite Quantum Dots for Pure Red Light-Emitting Diodes. Adv. Opt. Mater. 2022, 10, 2101517. [Google Scholar] [CrossRef]
- Chiba, T.; Hayashi, Y.; Ebe, H.; Hoshi, K.; Sato, J.; Sato, S.; Pu, Y.J.; Ohisa, S.; Kido, J. Anion-exchange red perovskite quantum dots with ammonium iodine salts for highly efficient light-emitting devices. Nat. Photonics 2018, 12, 681–687. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D′Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Vashishtha, P.; Halpert, J.E. Field-Driven Ion Migration and Color Instability in Red-Emitting Mixed Halide Perovskite Nanocrystal Light-Emitting Diodes. Chem. Mater. 2017, 29, 5965–5973. [Google Scholar] [CrossRef]
- Hoke, E.T.; Slotcavage, D.J.; Dohner, E.R.; Bowring, A.R.; Karunadasa, H.I.; McGehee, M.D. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics. Chem. Sci. 2015, 6, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Belisle, R.A.; Bush, K.A.; Bertoluzzi, L.; Gold-Parker, A.; Toney, M.F.; McGehee, M.D. Impact of Surfaces on Photoinduced Halide Segregation in Mixed-Halide Perovskites. ACS Energy Lett. 2018, 3, 2694–2700. [Google Scholar] [CrossRef]
- Yang, J.N.; Song, Y.; Yao, J.S.; Wang, K.H.; Wang, J.J.; Zhu, B.S.; Yao, M.M.; Rahman, S.U.; Lan, Y.F.; Fan, F.J.; et al. Potassium Bromide Surface Passivation on CsPbI(3-x)Br(x) Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 2956–2967. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Cao, Y.; Wang, J.P.; Fang, H.H.; Loi, M.A.; Zhao, N.; Wong, C.P. Benzylamine-Treated Wide-Bandgap Perovskite with High Thermal-Photostability and Photovoltaic Performance. Adv. Energy Mater. 2017, 7, 1701048. [Google Scholar] [CrossRef]
- Xu, F.; Chen, D.; Huang, D.; Xu, K.; Liang, S.; Hu, J.; Zhang, X.; Liu, L.; Xiong, F.; Zhu, H. Suppression of Photoinduced Phase Segregation in Mixed-Halide Perovskite Nanocrystals for Stable Light-Emitting Diodes. J. Phys. Chem. Lett. 2022, 13, 718–725. [Google Scholar] [CrossRef]
- Rehman, W.; Milot, R.L.; Eperon, G.E.; Wehrenfennig, C.; Boland, J.L.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Charge-Carrier Dynamics and Mobilities in Formamidinium Lead Mixed-Halide Perovskites. Adv. Mater. 2015, 27, 7938–7944. [Google Scholar] [CrossRef] [PubMed]
- Andaji-Garmaroudi, Z.; Abdi-Jalebi, M.; Guo, D.; Macpherson, S.; Sadhanala, A.; Tennyson, E.M.; Ruggeri, E.; Anaya, M.; Galkowski, K.; Shivanna, R.; et al. A Highly Emissive Surface Layer in Mixed-Halide Multication Perovskites. Adv. Mater. 2019, 31, 1902374. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhu, Z.; Li, J.; Jen, A.K.Y.; Wang, L. Inorganic CsPb1−xSnxIBr2 for Efficient Wide-Bandgap Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1800525. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef]
- Fang, C.; Li, Y.; Cai, Y.; Zhou, T.-L.; Tang, X.; Xie, R.-J. Facial synthesis of highly stable and bright CsPbX3 (X = Cl, Br, I) perovskite nanocrystals via an anion exchange at the water-oil interface. Sci. China Mater. 2020, 64, 158–168. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Xu, W.; Yang, L.; Liu, Y.; Yao, D.; Zhang, D.; Zhang, H.; Yang, B. Engineering the Photoluminescence of CsPbX(3) (X = Cl, Br, and I) Perovskite Nanocrystals Across the Full Visible Spectra with the Interval of 1 nm. ACS Appl. Mater. Interfaces 2019, 11, 14256–14265. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Ma, Z.; Yuan, F.; Zhou, X.; Wang, H.; Liu, Z.; Qing, J.; Chen, H.; Li, X.; et al. A Multifunctional “Halide-Equivalent” Anion Enabling Efficient CsPb(Br/I)(3) Nanocrystals Pure-Red Light-Emitting Diodes with External Quantum Efficiency Exceeding 23. Adv. Mater. 2023, 35, 2209002. [Google Scholar] [CrossRef]
- Li, H.; Lin, H.; Ouyang, D.; Yao, C.; Li, C.; Sun, J.; Song, Y.; Wang, Y.; Yan, Y.; Wang, Y.; et al. Efficient and Stable Red Perovskite Light-Emitting Diodes with Operational Stability >300 h. Adv. Mater. 2021, 33, 2008820. [Google Scholar] [CrossRef]
- Zhu, H.; Tong, G.; Li, J.; Xu, E.; Tao, X.; Sheng, Y.; Tang, J.; Jiang, Y. Enriched-Bromine Surface State for Stable Sky-Blue Spectrum Perovskite QLEDs with an EQE of 14.6. Adv. Mater. 2022, 34, 2205092. [Google Scholar] [CrossRef]
- Yang, B.; Mao, X.; Hong, F.; Meng, W.; Tang, Y.; Xia, X.; Yang, S.; Deng, W.; Han, K. Lead-Free Direct Band Gap Double-Perovskite Nanocrystals with Bright Dual-Color Emission. J. Am. Chem. Soc. 2018, 140, 17001–17006. [Google Scholar] [CrossRef]
- Song, J.; Fang, T.; Li, J.; Xu, L.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Organic-Inorganic Hybrid Passivation Enables Perovskite QLEDs with an EQE of 16.48. Adv. Mater. 2018, 30, 1805409. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.C.; Draguta, S.; Kamat, P.V.; Kuno, M. Light-Induced Anion Phase Segregation in Mixed Halide Perovskites. ACS Energy Lett. 2018, 3, 204–213. [Google Scholar] [CrossRef]
- Kuno, M.; Brennan, M.C. What Exactly Causes Light-Induced Halide Segregation in Mixed-Halide Perovskites? Matter 2020, 2, 21–23. [Google Scholar] [CrossRef]
- Gualdron-Reyes, A.F.; Yoon, S.J.; Barea, E.M.; Agouram, S.; Munoz-Sanjose, V.; Melendez, A.M.; Nino-Gomez, M.E.; Mora-Sero, I. Controlling the Phase Segregation in Mixed Halide Perovskites through Nanocrystal Size. ACS Energy Lett. 2019, 4, 54–62. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Zhang, D.; Du, Q.; Hong, W.; Liang, Y.; Duan, X.; Feng, S.; Lan, L.; Wang, L.; Chen, J.; et al. Synergistic Halide- and Ligand-Exchanges of All-Inorganic Perovskite Nanocrystals for Near-Unity and Spectrally Stable Red Emission. Nanomaterials 2023, 13, 2337. https://doi.org/10.3390/nano13162337
Chen K, Zhang D, Du Q, Hong W, Liang Y, Duan X, Feng S, Lan L, Wang L, Chen J, et al. Synergistic Halide- and Ligand-Exchanges of All-Inorganic Perovskite Nanocrystals for Near-Unity and Spectrally Stable Red Emission. Nanomaterials. 2023; 13(16):2337. https://doi.org/10.3390/nano13162337
Chicago/Turabian StyleChen, Kaiwang, Dengliang Zhang, Qing Du, Wei Hong, Yue Liang, Xingxing Duan, Shangwei Feng, Linfeng Lan, Lei Wang, Jiangshan Chen, and et al. 2023. "Synergistic Halide- and Ligand-Exchanges of All-Inorganic Perovskite Nanocrystals for Near-Unity and Spectrally Stable Red Emission" Nanomaterials 13, no. 16: 2337. https://doi.org/10.3390/nano13162337
APA StyleChen, K., Zhang, D., Du, Q., Hong, W., Liang, Y., Duan, X., Feng, S., Lan, L., Wang, L., Chen, J., & Ma, D. (2023). Synergistic Halide- and Ligand-Exchanges of All-Inorganic Perovskite Nanocrystals for Near-Unity and Spectrally Stable Red Emission. Nanomaterials, 13(16), 2337. https://doi.org/10.3390/nano13162337








