Effects of Temperature on the Physicochemical Properties of Bioinspired, Synthetic, and Biogenic Hydroxyapatites Calcinated under the Same Thermal Conditions
Abstract
:1. Introduction
2. Materials and Methods
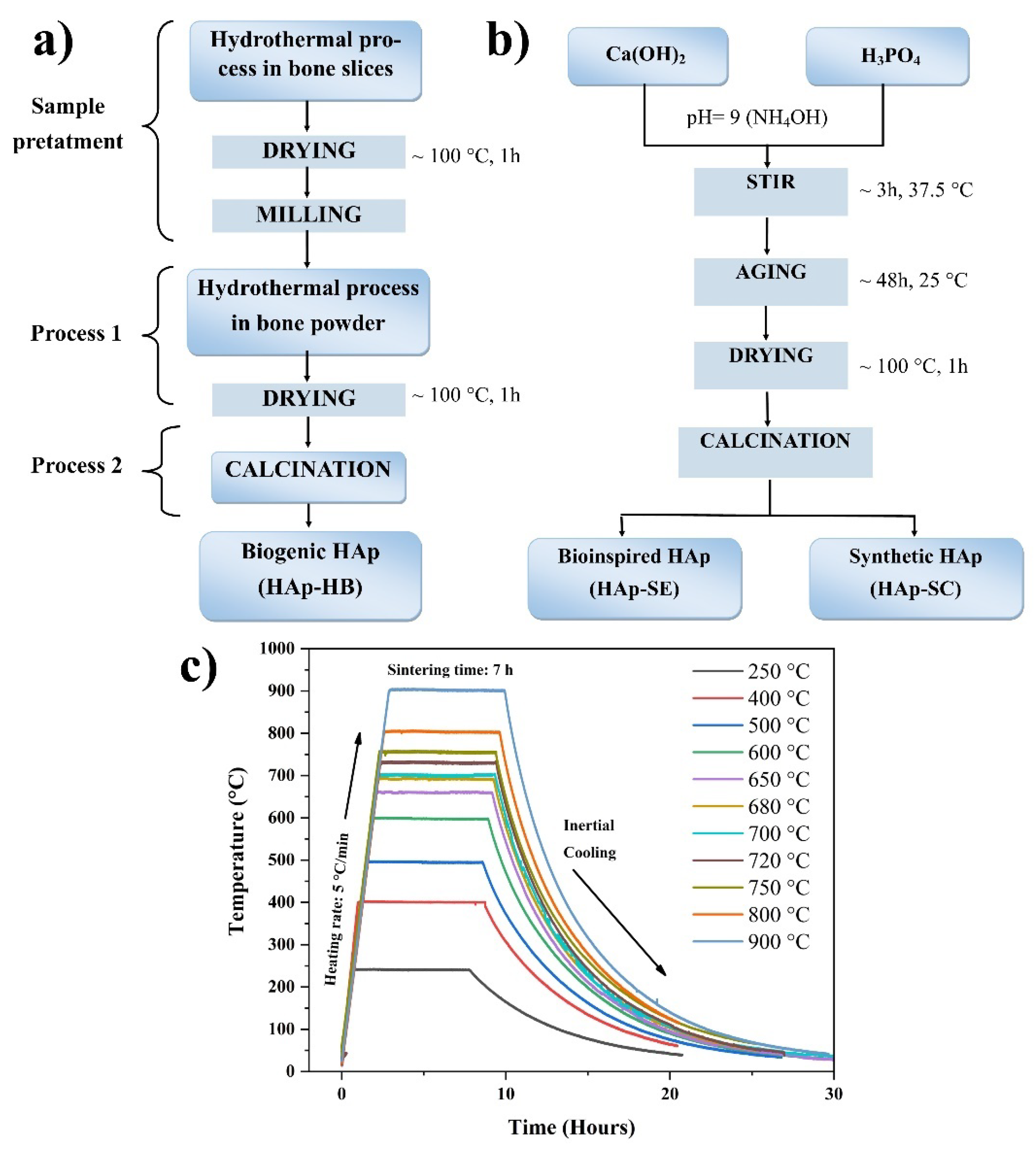
2.1. Raw Materials and Samples Obtention
2.2. Thermal Analysis—TGA
2.3. Elemental Compositions by ICP-OES
2.4. High-Resolution Scanning Electron Microscopy (HR-SEM)
2.5. Structural Properties: X-ray Diffraction (XRD)
2.6. High-Resolution Transmission Electron Microscopy (HR-TEM)
2.7. Functional Groups: FTIR Analysis
2.8. Raman Spectroscopy Analysis
3. Results and Discussion
3.1. Thermal Events by Thermogravimetric Analysis
3.2. Study of Elemental Compositions by ICP-OES
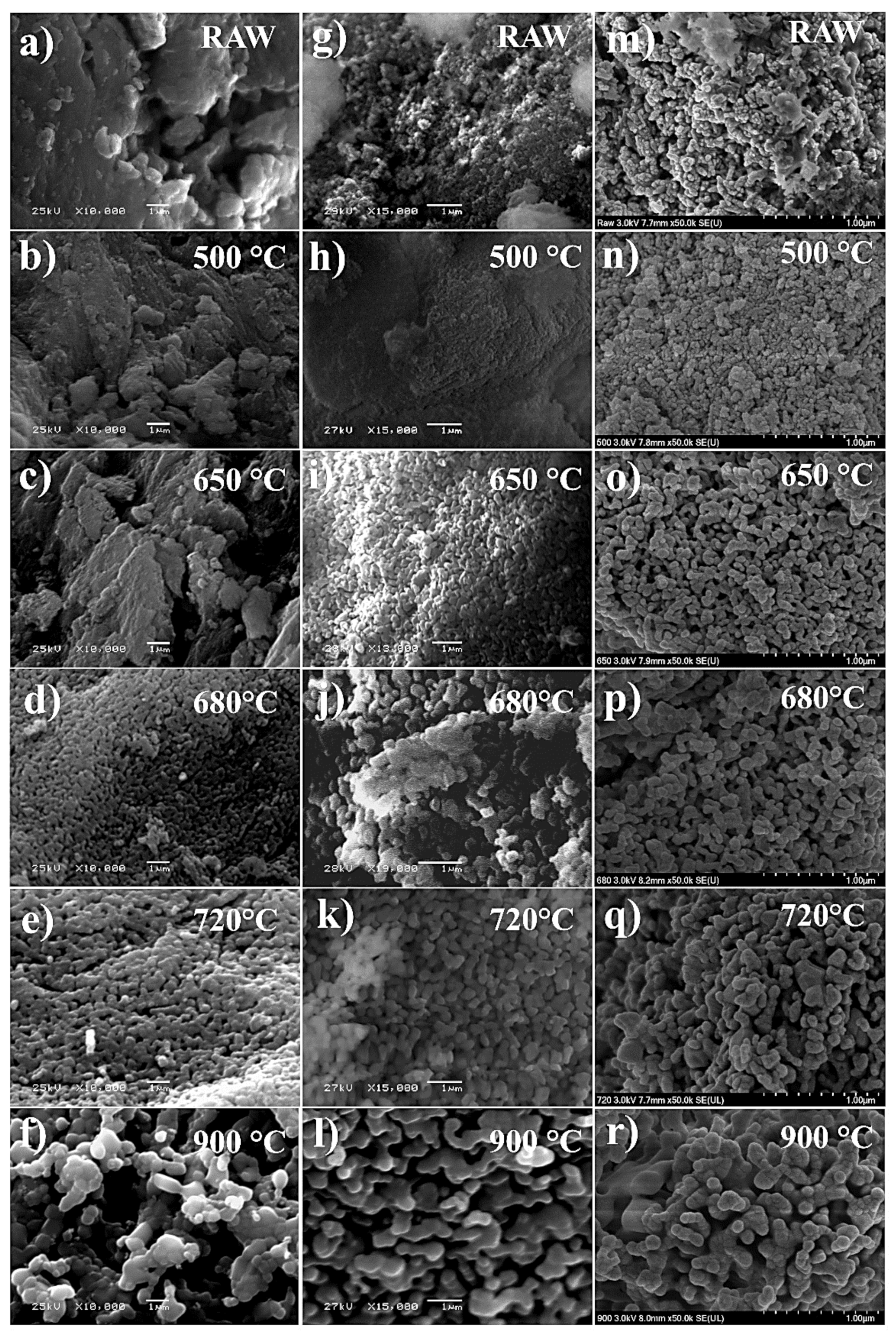
3.3. Effect of Calcination Temperature in SEM Morphology
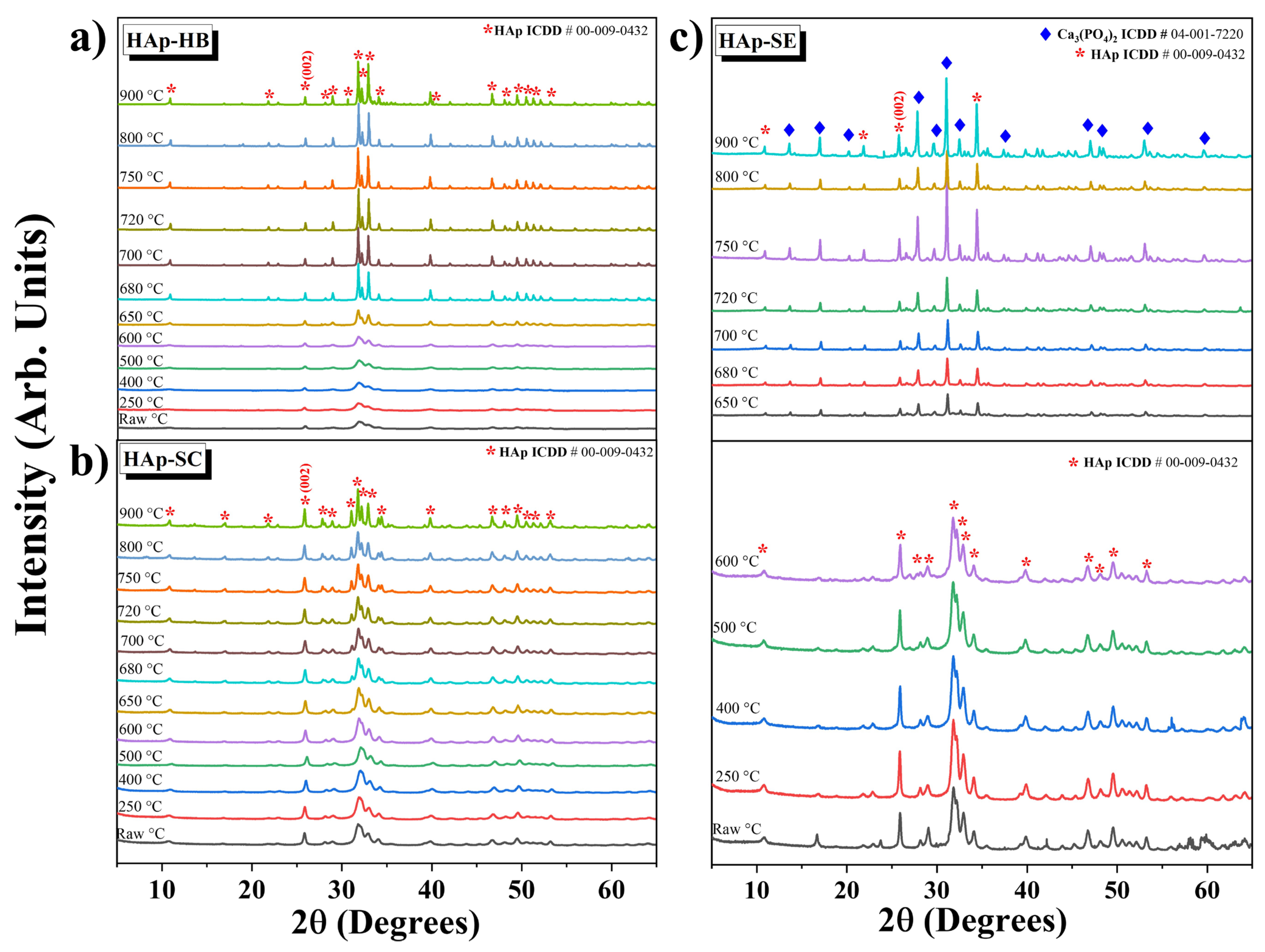
3.4. Structural Analysis by XRD
3.5. HR-TEM Analysis
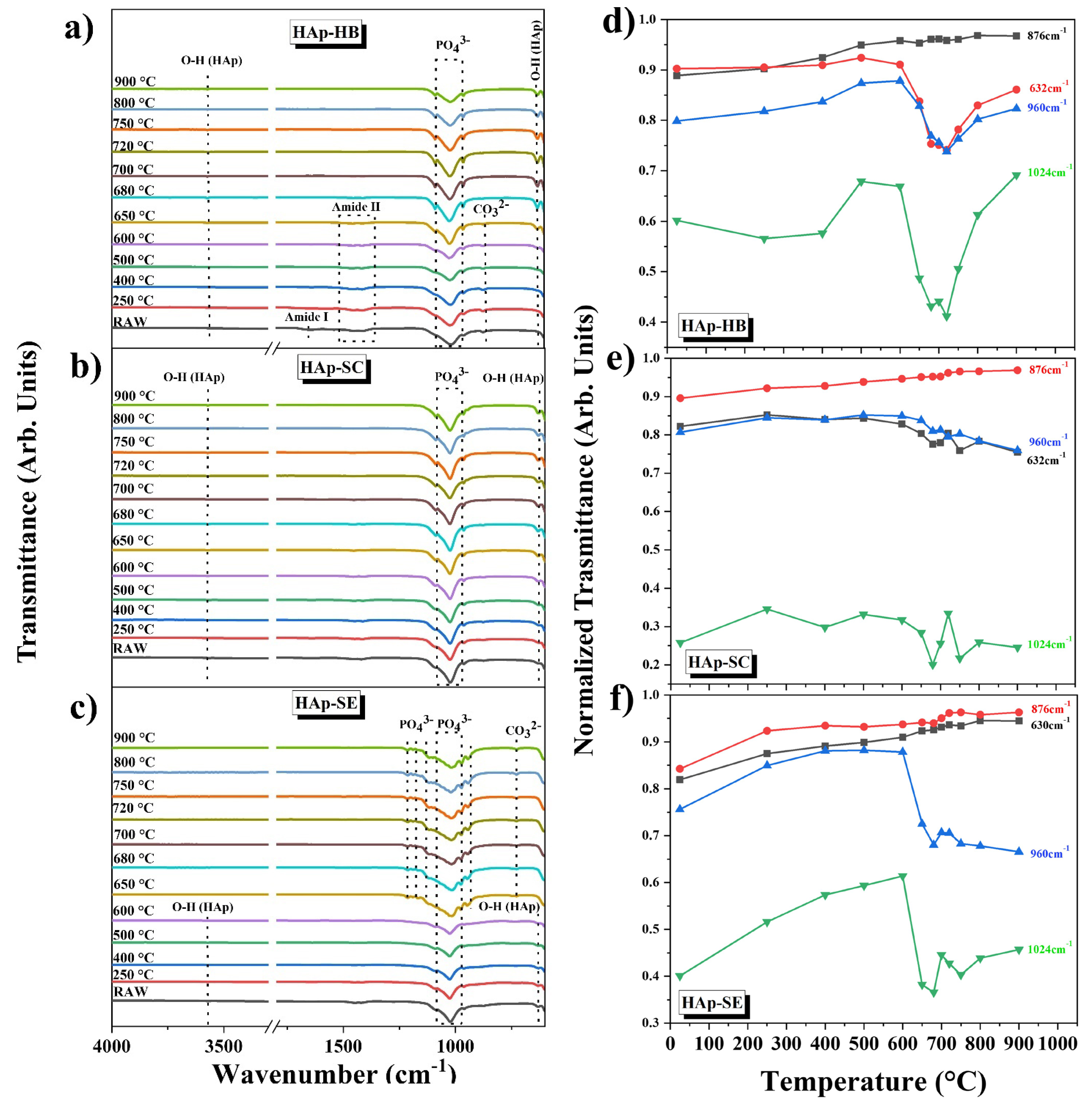
3.6. Analyses of Functional Groups
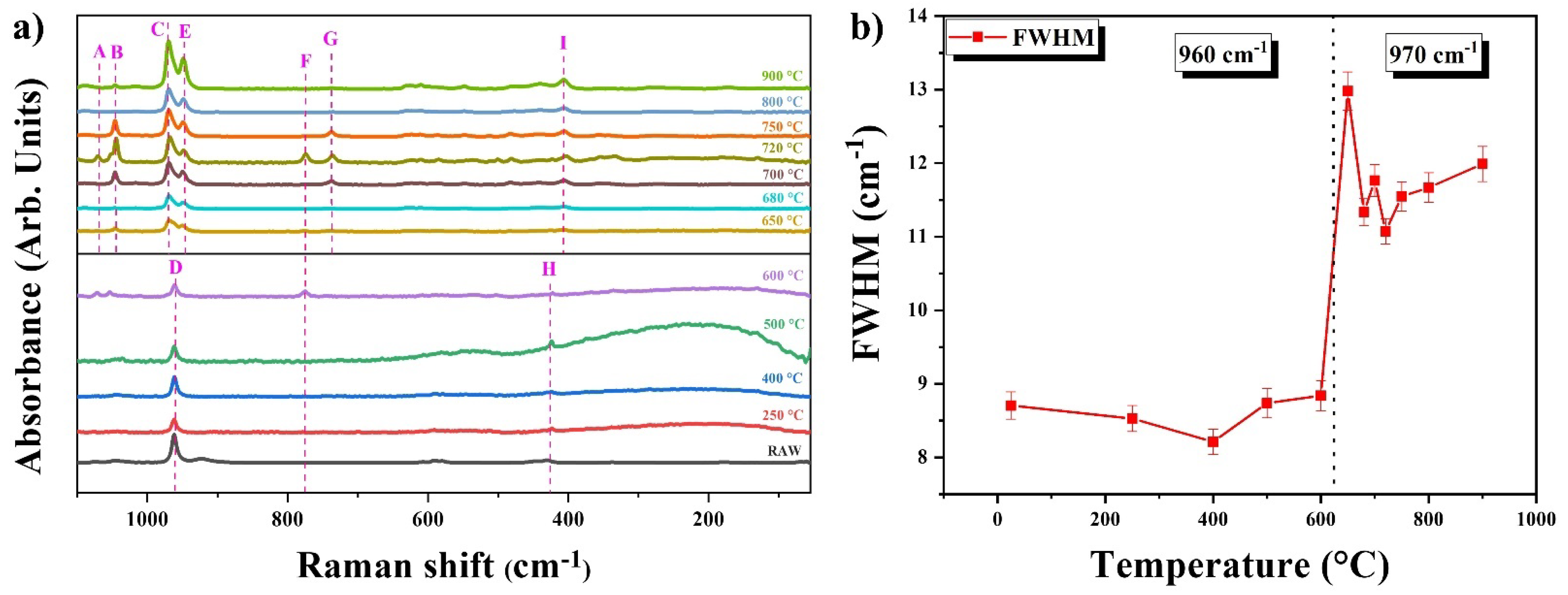
3.7. Dehydroxylation Analysis by Raman Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Millán-Malo, B.M.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. Effect of the nano crystal size on the x-ray diffraction patterns of biogenic hydroxyapatite from human, bovine, and porcine bones. Sci. Rep. 2019, 9, 5915. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Khil, M.S.; Omran, A.M.; Sheikh, F.A.; Kim, H.Y. Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J. Mater. Process. Technol. 2009, 209, 3408–3415. [Google Scholar] [CrossRef]
- Janus, A.M.; Faryna, M.; Haberko, K.; Rakowska, A.; Panz, T. Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones. Microchim. Acta. 2008, 161, 349–353. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Jeronimo-Cruz, R.; Rubio-Rosas, E.; Rodriguez-García, M.E. The effect of cyclic heat treatment on the physicochemical properties of bio hydroxyapatite from bovine bone. J. Mater. Sci. Mater. Med. 2018, 29, 52. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kumta, P.N. Sol-gel synthesis and characterization of nanostructured hydroxyapatite powder. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Earl, J.S.; Wood, D.J.; Milne, S.J. Hydrothermal synthesis of hydroxyapatite. J. Phys. Conf. Ser. 2006, 26, 268–271. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2019, 29, 233–239. [Google Scholar] [CrossRef]
- Mobasherpour, I.; Heshajin, M.S.; Kazemzadeh, A.; Zakeri, M. Synthesis of nanocrystalline hydroxyapatite by using precipitation method. J. Alloys Compd. 2007, 430, 330–333. [Google Scholar] [CrossRef]
- Correa-Piña, B.A.; Gomez-Vazquez, O.M.; Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Millan-Malo, B.M.; Rodriguez-García, M.E. Synthesis and characterization of nano-hydroxyapatite added with magnesium obtained by wet chemical precipitation. Prog. Nat. Sci. Mater. Int. 2021, 3, 575–582. [Google Scholar] [CrossRef]
- Gomez-Vazquez, O.M.; Correa-Piña, B.A.; Zubieta-Otero, L.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Synthesis and characterization of bioinspired nano-hydroxyapatite by wet chemical precipitation. Ceram. Int. 2021, 47, 32775–32785. [Google Scholar] [CrossRef]
- Cañon-Davila, D.F.; Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Pfeiffer, H.; Ramirez-Bon, R.; Rodriguez-Garcia, M.E. Study of the coalescence phenomena in biogenic nano-hydroxyapatite produced by controlled calcination processes at low temperature. Ceram. Int. 2023, 49, 17524–17533. [Google Scholar] [CrossRef]
- Šimková, L.; Šulcová, P. Characterization and thermal behavior of hydroxyapatite prepared by precipitation. J. Therm. Anal. Calorim. 2019, 138, 321–329. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Ramirez-Gutierrez, C.F.; del Real, A.; Rubio-Rosas, E.; Rodriguez-García, M.E. Study of bovine hydroxyapatite obtained by calcination at low heating rates and cooled in furnace air. J. Mater. Sci. 2016, 51, 4431–4441. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Herrera-Lara, M.; Bernal-Alvarez, L.R.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. In-situ XRD study of the crystal size transition of hydroxyapatite from swine bone. Ceram. Int. 2020, 46, 24454–24461. [Google Scholar] [CrossRef]
- Castillo-Paz, A.M.; Londoño-Restrepo, S.M.; Tirado-Mejía, L.; Mondragón, M.A.; Rodríguez-García, M.E. Nano to micro size transition of hydroxyapatite in porcine bone during heat treatment with low heating rates. Prog. Nat. Sci. Mater. Int. 2020, 30, 494–501. [Google Scholar] [CrossRef]
- Liao, C.J.; Lin, F.H.; Chen, K.S.; Sun, J.S. Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomaterials 1999, 20, 1807–1813. [Google Scholar] [CrossRef]
- Miranda, M.A.R.; Sasaki, J.M. The limit of application of the Scherrer equation. Acta Crystallogr. Sect. A Found. Adv. 2018, 74, 54–65. [Google Scholar] [CrossRef]
- Chandrasekar, A.; Sagadevan, S.; Dakshnamoorthy, A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 2013, 8, 1639–1645. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Slepko, A.; Demkov, A.A. Hydroxyapatite: Vibrational spectra and monoclinic to hexagonal phase transition. J. Appl. Phys. 2015, 117, 074701. [Google Scholar] [CrossRef]
- Londoño-Restrepo, S.M.; Zubieta-Otero, L.F.; Jeronimo-Cruz, R.; Mondragon, M.A.; Rodriguez-García, M.E. Effect of the crystal size on the infrared and Raman spectra of bio hydroxyapatite of human, bovine, and porcine bones. J. Raman Spectrosc. 2019, 50, 1120–1129. [Google Scholar] [CrossRef]
- Zubieta-Otero, L.F.; Londoño-Restrepo, S.M.; Lopez-Chavez, G.; Hernandez-Becerra, E.; Rodriguez-Garcia, M.E. Comparative study of physicochemical properties of bio-hydroxyapatite with commercial samples. Mater. Chem. Phys. 2021, 259, 124201. [Google Scholar] [CrossRef]
- Laohavisuti, N.; Boonchom, B.; Boonmee, W.; Chaiseeda, K.; Seesanong, S. Simple recycling of biowaste eggshells to various calcium phosphates for specific industries. Sci. Rep. 2021, 11, 15143. [Google Scholar] [CrossRef] [PubMed]
- Pena, J.; Vallet-Regí, M. Hydroxyapatite, tricalcium phosphate and biphasic materials prepared by a liquid mix technique. J. Eur. Ceram. Soc. 2003, 23, 1687–1696. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Le Coustumer, P.; Predoi, D. Vibrational investigations of silver-doped hydroxyapatite with antibacterial properties. J. Spectrosc. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Furko, M.; Horváth, Z.E.; Mihály, J.; Balázsi, K.; Balázsi, C. Comparison of the Morphological and Structural Characteristic of Bioresorbable and Biocompatible Hydroxyapatite-Loaded Biopolymer Composites. Nanomaterials 2021, 11, 3194. [Google Scholar] [CrossRef]
- De Aza, P.N.; Santos, C.; Pazo, A.; De Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 1. Raman Spectrum of β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 912–915. [Google Scholar] [CrossRef]
- Santos, L.F.; Almeida, R.M.; Tikhomirov, V.K.; Jha, A. Raman spectra and structure of fluoroaluminophosphate glasses. J. Non Cryst. Solids 2001, 284, 43–48. [Google Scholar] [CrossRef]



| Mineral (PPM) | * H-Raw | HAp-HB | HAp-SE | HAp-SC |
|---|---|---|---|---|
| Ca | 285,898 | 217,222 | 304,417 | 425,142 |
| Mg | 3887 | 4156 | 3621 | ND |
| P | 107,267 | 120,648 | 157,998 | 12,524 |
| Na | 7912 | 4708 | 312 | ND |
| S | 26 | 665 | 252 | ND |
| Sr | 90 | ND | 127 | ND |
| K | 56 | 127 | 316 | ND |
| Si | 120 | 21 | 125 | ND |
| Fe | 89.2 | ND | 15 | 12 |
| Al | 37 | ND | 28 | 6 |
| Zn | 179 | 83 | 124 | 4 |
| Ba | ND | ND | 16 | ND |
| Ca/P | 2.06 | 1.39 | 1.49 | 2.62 |
| Theoretical | Experimental | |||
|---|---|---|---|---|
| ICDD (00-009-0432) [19] | Sample | TEM | Sample | XRD |
| d(002) = 3.440 Å c = 6.884 Å | HAp-SC | d(300) = 2.710Å a = b = 9.387 Å d(002) = 3.344Å c = 6.688 Å | HAp-SC | 2θ(002) = 25.830° d(002) = 3.445 Å a = b = 9.413 Å 2θ(310) = 39.822° d(310) = 2.260 Å c = 6.890 Å |
| d(211) = 2.814 Å a = b = 9.418 Å c = 6.884 Å | HAp-SE | d(300) = 2.719 Å a = b = 9.418 Å d(211) = 2.829Å c = 7.007 Å | HAp-SE | 2θ(002) = 25.90° d(002) = 3.434 Å a = b = 9.398 Å 2θ(310) = 39.888° d(310) = 2.257 Å c = 6.869 Å |
| d(300) = 2.720 Å a = b = 9.418 Å | HAp-HB | d(300) = 2.712Å a = b = 9.348 Å d(301) = 2.502Å c = 6.678 Å | HAp-HB | 2θ(002) = 25.896° d(002) = 3.436 Å a = b = 9.405 Å 2q(310) = 39.858° d(310) = 2.259 Å c = 6.872 Å |
| FTIR Spectroscopy (HAp-HB) | |||
| Functional Group | Wavenumber (cm−1) * | Wavenumber (cm−1) R | Reference |
| -COOH ν | 3571 | 3570 | [21] |
| Amide I | 1639 | 1642 | [22] |
| Amide II | 1533 | 1543 | [21] |
| PO43− ν3 as | 1086 | 1088, 1014 | [21,22] |
| 1023 | 1023 | [7] | |
| PO43− ν1 s | 962 | 962 | [7] |
| 962 | 960 | [21,22] | |
| CO32− ν2 as | 877 | 873 | [7] |
| 877 | 872 | [21,22] | |
| O-H δ | 633 | 642 | [22] |
| FTIR Spectroscopy (HAp-SC) | |||
| Functional group | Wavenumber (cm−1) * | Wavenumber (cm−1) R | Reference |
| O-H ν | 3571 | 3570 | [21] |
| O-H δ | 629 | 642 | [22] |
| PO43− ν3 as | 1089 | 1088, 1014 | [21,22] |
| 1027 | 1023 | [7] | |
| CO32− ν2 as | 877 | 873 | [7] |
| 877 | 872 | [21,22] | |
| PO43− ν1 s | 963 | 962 | [7] |
| 963 | 960 | [21,22] | |
| FTIR Spectroscopy (HAp-SE) | |||
| Functional Group | Wavenumber (cm−1) * | Wavenumber (cm−1) R | Reference |
| O-H ν | 3572 | 3570 | [21] |
| O-H δ | 632 | 642 | [22] |
| PO43− νs TCP | 724 | 725 | [23] |
| PO43− ν3 as | 1096 | 1088, 1014 | [21,22] |
| 1024 | 1023 | [7] | |
| PO43− ν1 s | 968 | 962 | [7] |
| 968 | 960 | [21,22] | |
| PO43− ν1 TCP | 940 | 945 | [23] |
| CO32− ν2 as | 877 | 873 | [7] |
| 877 | 872 | [21,22] | |
| PO43− ν3 TCP | 1120 | 1120 | [23,24] |
| P-O ν TCP | 1176 | 1150 | [23] |
| P-O-H δ TCP | 1212 | 1221 | [23,24] |
| Wavenumber (cm−1) | Assignment and Normal Vibrational Modes | Wavenumber (cm−1) Reference | |
| A | 1073 | ν1 s CO3 + ν3 as P-O | [25,26] |
| B | 1047 | ν3 s P-O | [25] |
| C | 970 | ν1 s PO43− | [21] |
| D | 961 | ν1 s PO43− | [25] |
| E | 949 | ν1 s PO43− | [27] |
| F | 775 | ν -P-O-P- | [28] |
| G | 737 | ν s P-O-P | [26] |
| H | 424 | ν2 s PO43− | [27] |
| I | 407 | ν2 s PO43− | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Vazquez, O.M.; Bernal-Alvarez, L.R.; Velasquez-Miranda, J.I.; Rodriguez-Garcia, M.E. Effects of Temperature on the Physicochemical Properties of Bioinspired, Synthetic, and Biogenic Hydroxyapatites Calcinated under the Same Thermal Conditions. Nanomaterials 2023, 13, 2385. https://doi.org/10.3390/nano13172385
Gomez-Vazquez OM, Bernal-Alvarez LR, Velasquez-Miranda JI, Rodriguez-Garcia ME. Effects of Temperature on the Physicochemical Properties of Bioinspired, Synthetic, and Biogenic Hydroxyapatites Calcinated under the Same Thermal Conditions. Nanomaterials. 2023; 13(17):2385. https://doi.org/10.3390/nano13172385
Chicago/Turabian StyleGomez-Vazquez, Omar M., Leon R. Bernal-Alvarez, Jesus I. Velasquez-Miranda, and Mario E. Rodriguez-Garcia. 2023. "Effects of Temperature on the Physicochemical Properties of Bioinspired, Synthetic, and Biogenic Hydroxyapatites Calcinated under the Same Thermal Conditions" Nanomaterials 13, no. 17: 2385. https://doi.org/10.3390/nano13172385
APA StyleGomez-Vazquez, O. M., Bernal-Alvarez, L. R., Velasquez-Miranda, J. I., & Rodriguez-Garcia, M. E. (2023). Effects of Temperature on the Physicochemical Properties of Bioinspired, Synthetic, and Biogenic Hydroxyapatites Calcinated under the Same Thermal Conditions. Nanomaterials, 13(17), 2385. https://doi.org/10.3390/nano13172385