Tin/Tin Oxide Nanostructures: Formation, Application, and Atomic and Electronic Structure Peculiarities
Abstract
1. Introduction
2. Fabrication of Tin-Based Nanostructures and Thin Films
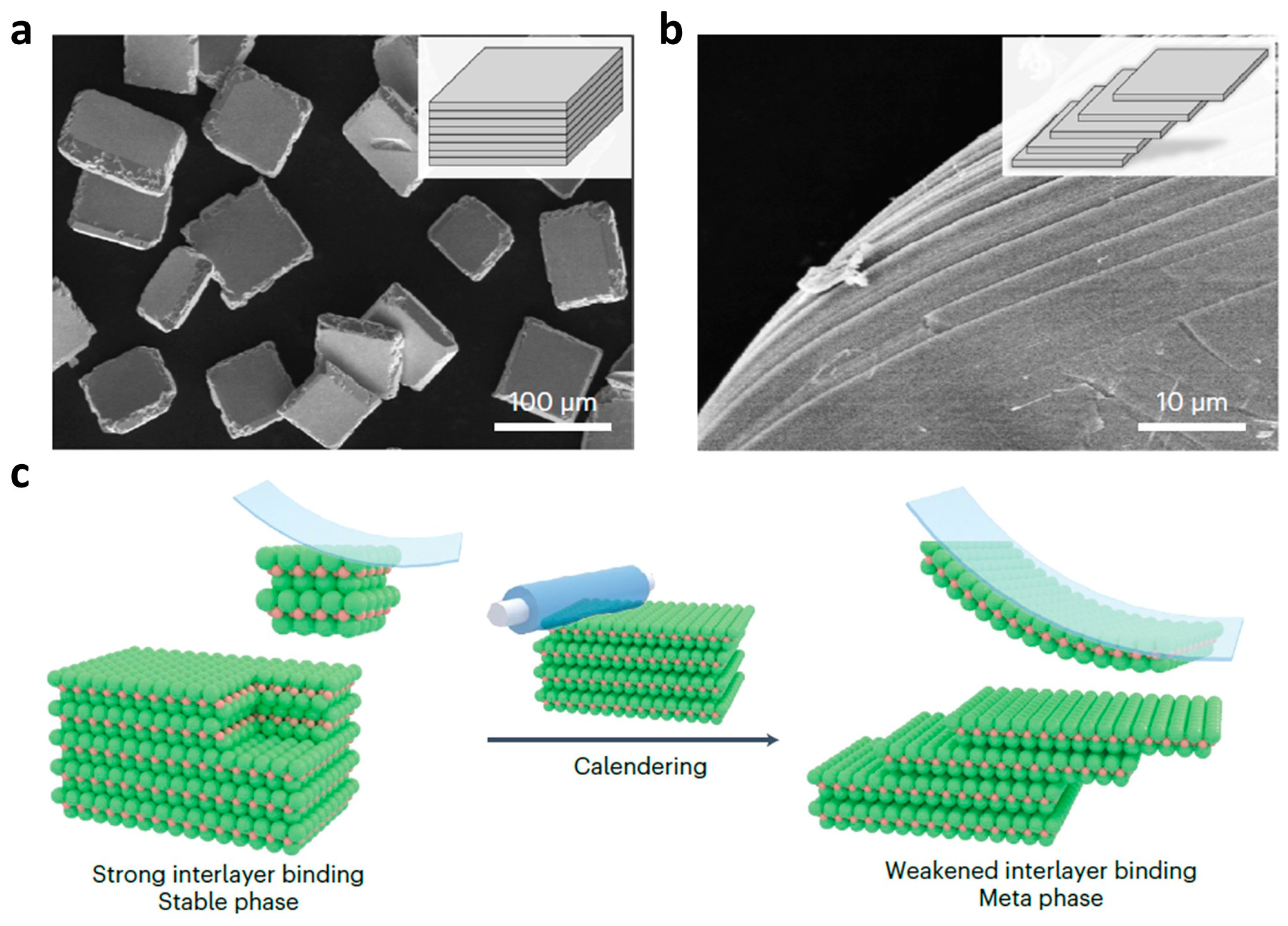
2.1. Solid-State Methods
2.2. Solution-Based Methods
2.3. Vapor-Based Methods
3. Applications
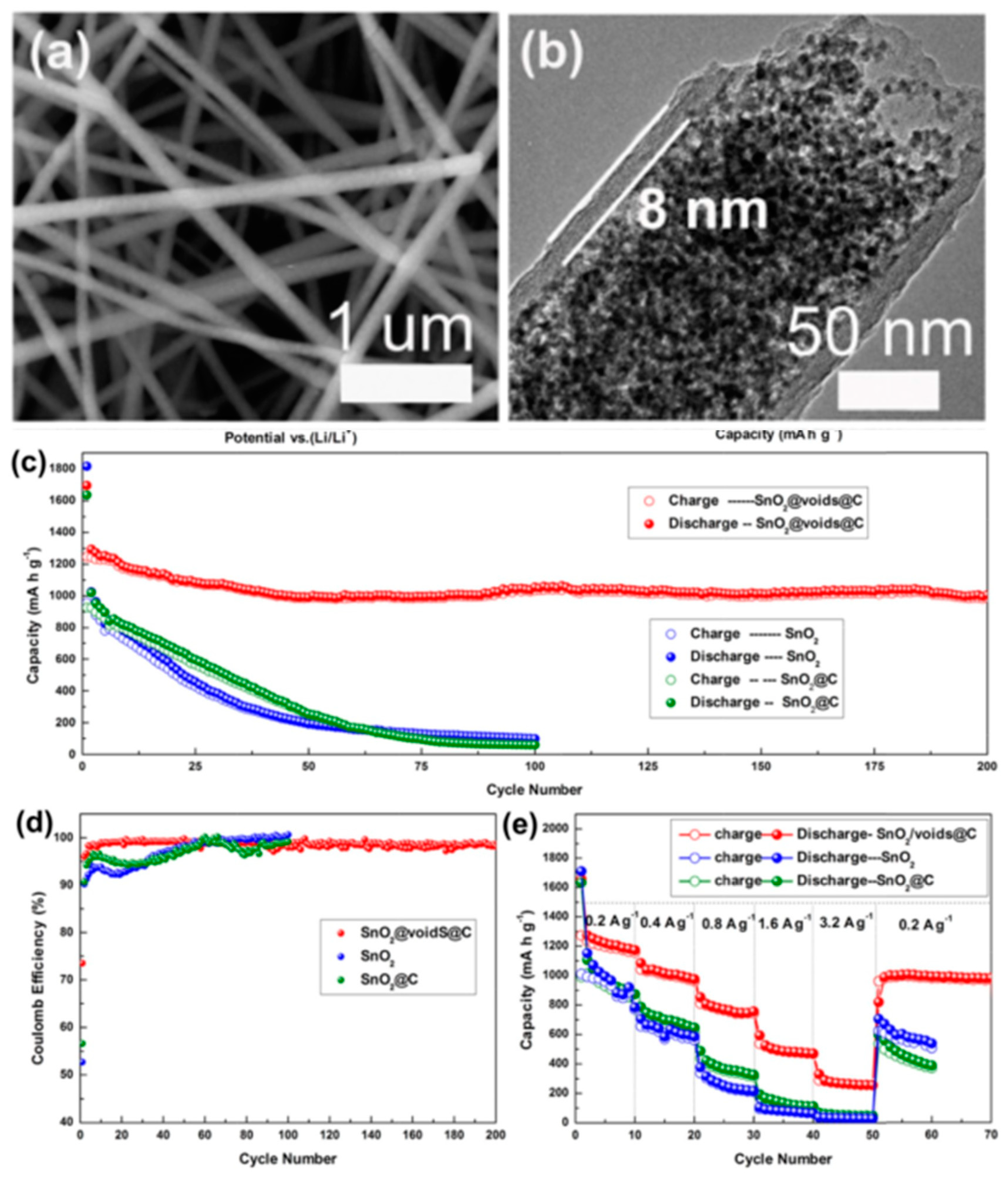
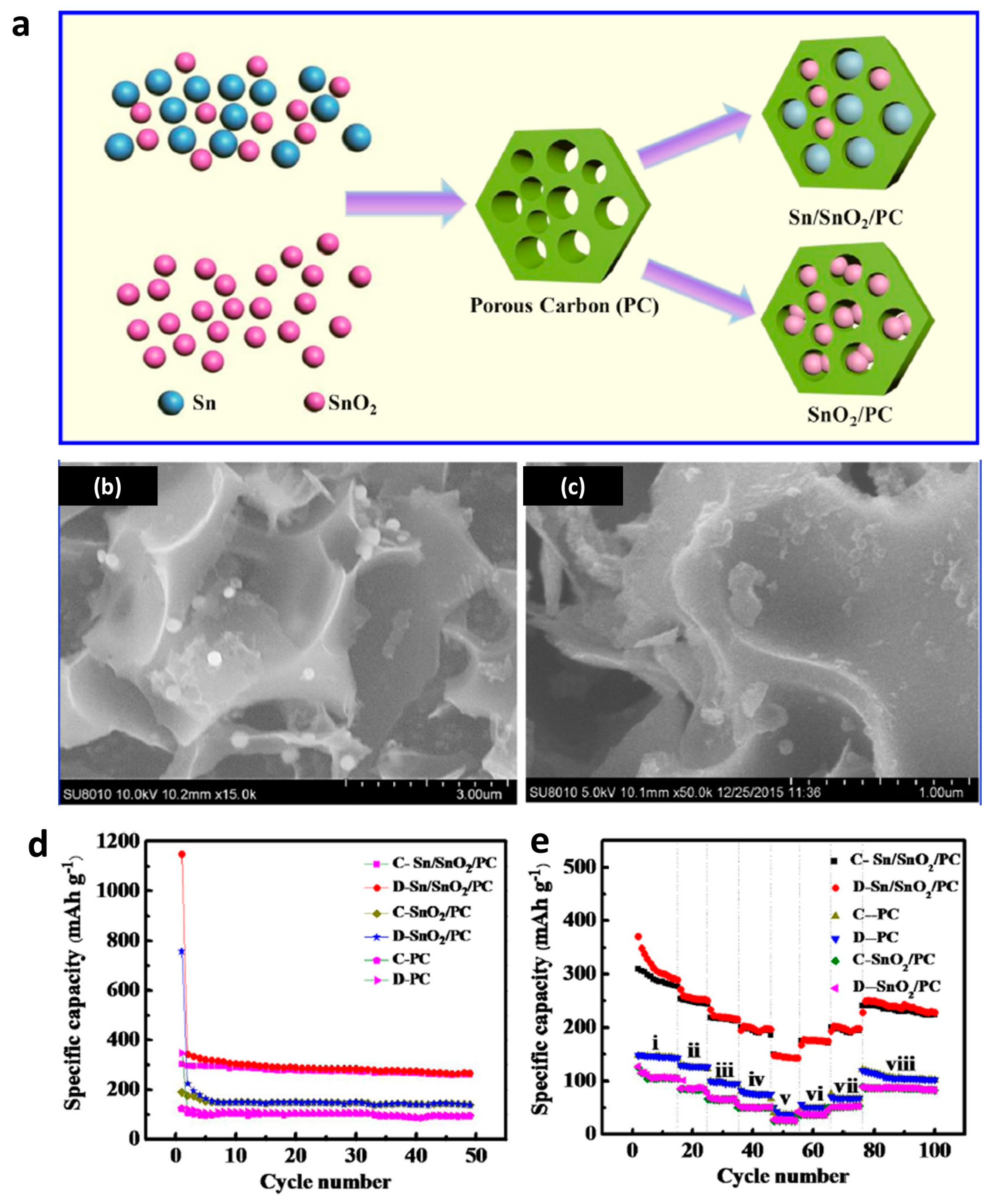
3.1. Li-Ion and Na-Ion Batteries
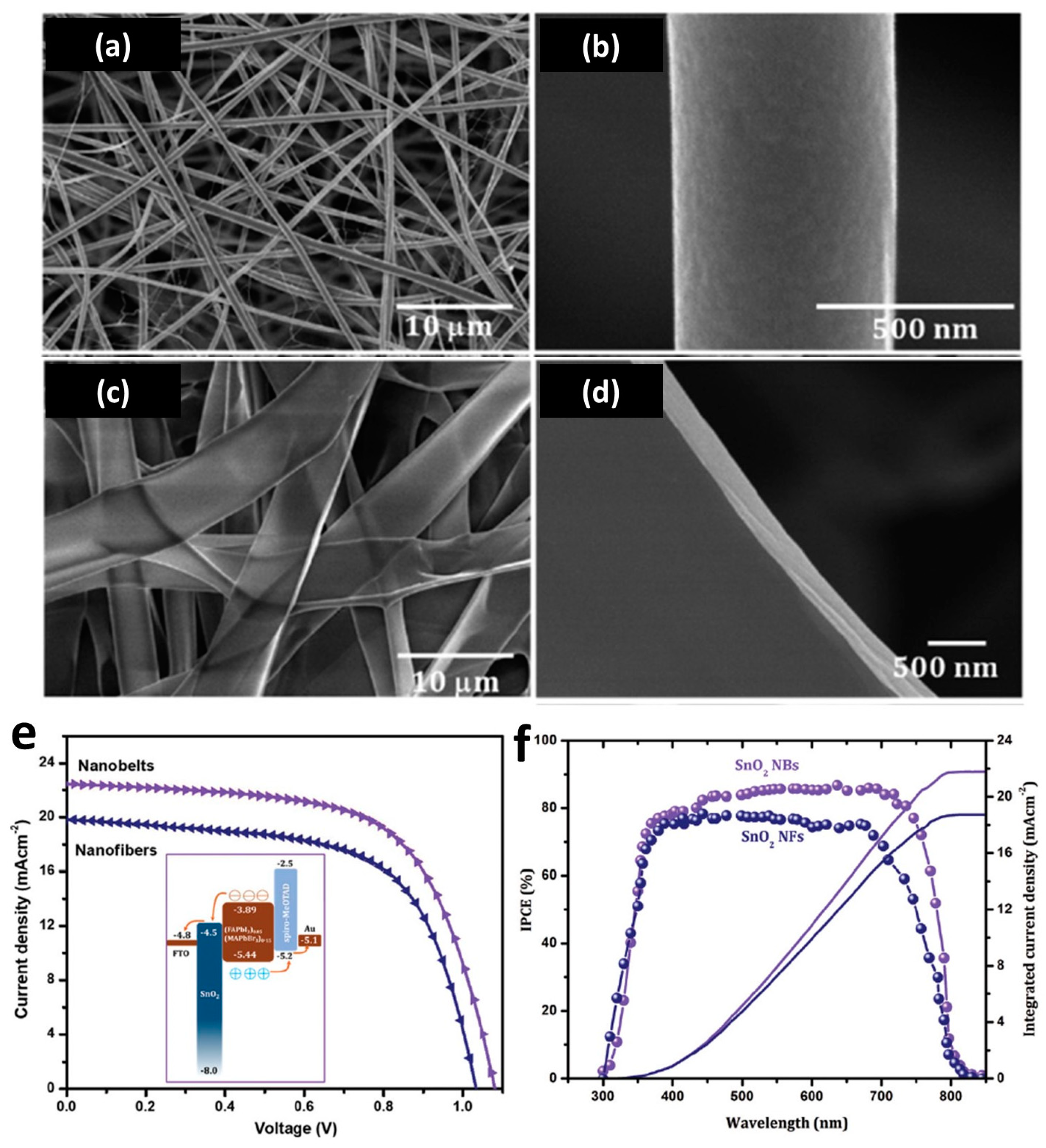
3.2. Energy Conversation and Storage
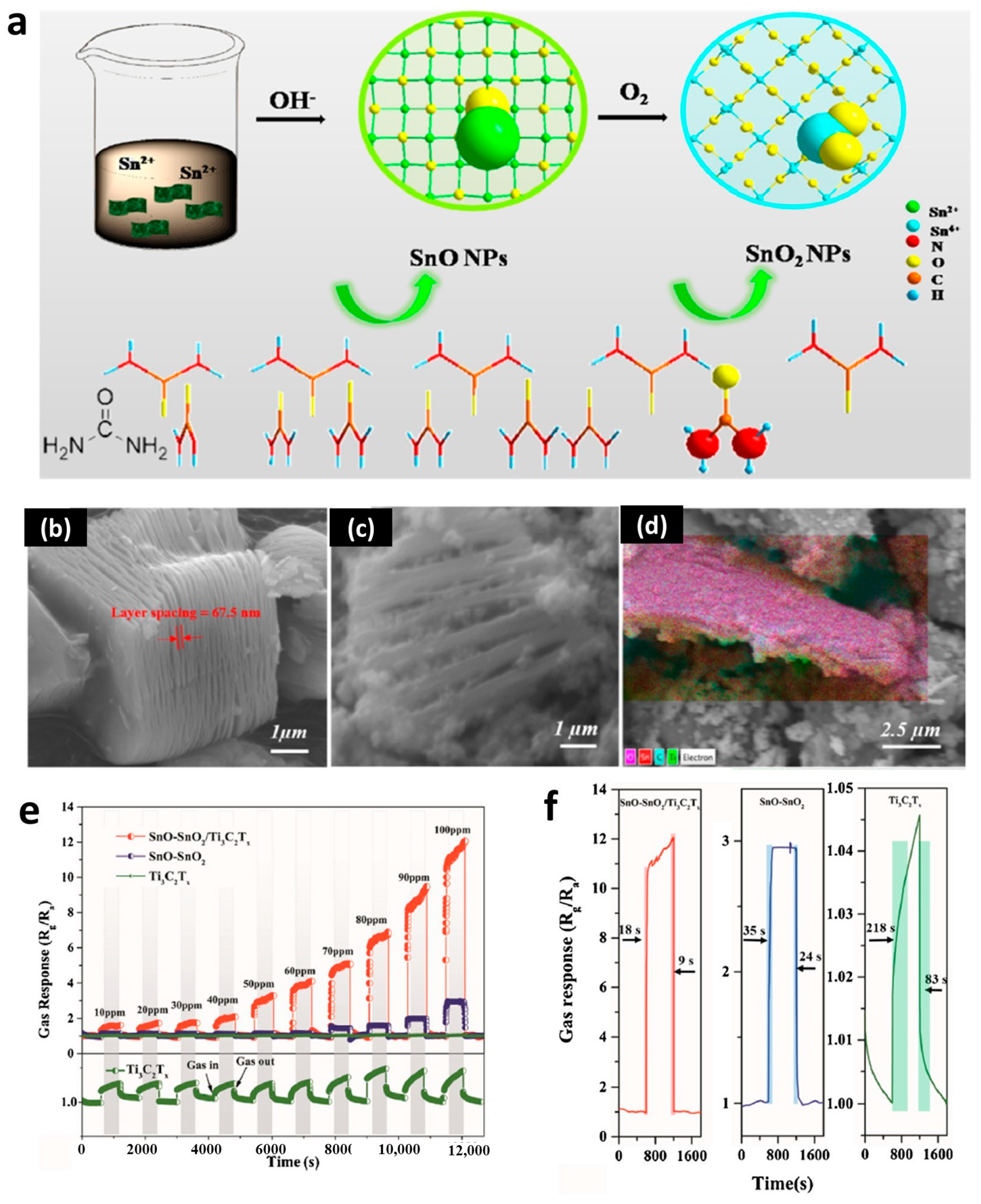
3.3. Gas Sensors
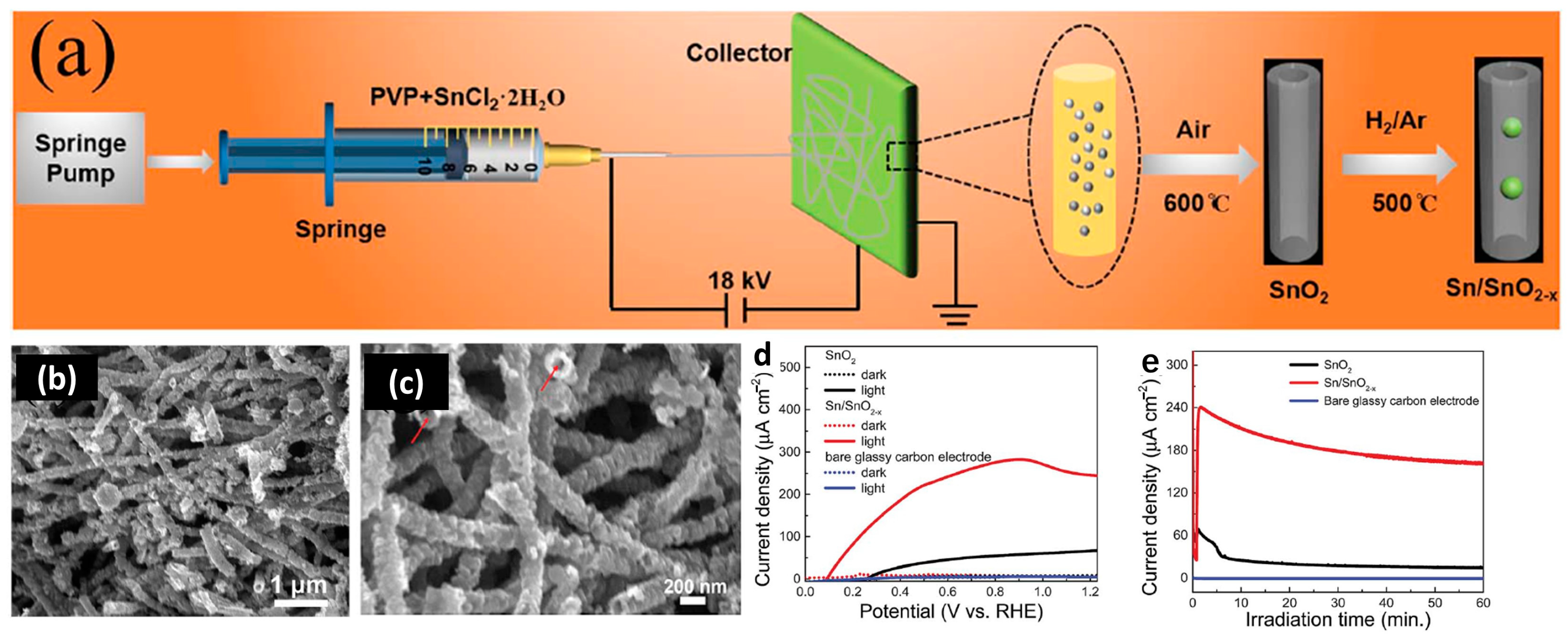
3.4. Photocatalytic and Bio-Photonic Applications
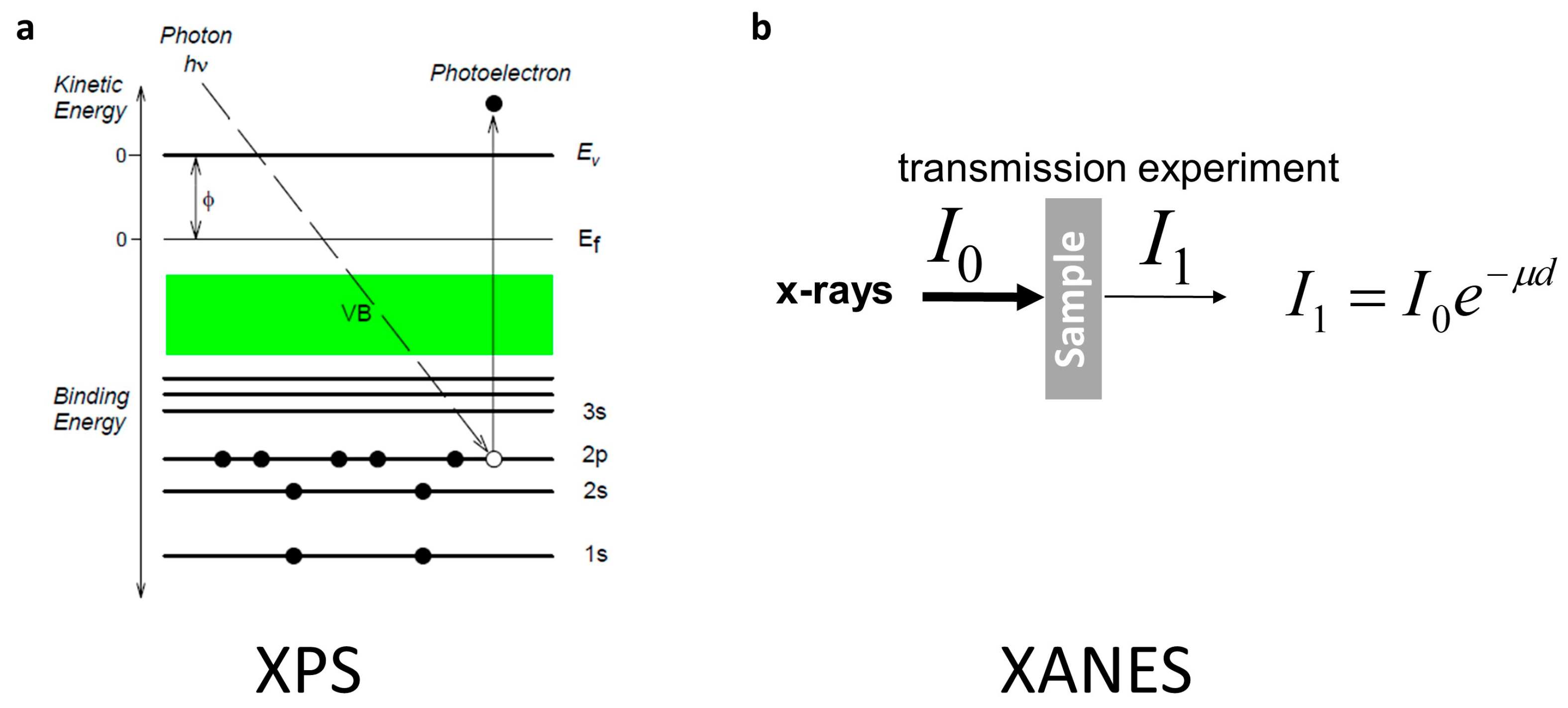
4. Atomic and Electronic Features Studies in Tin/Tin Oxide Nanostructures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanta, D.; Ahmaruzzaman, M. Tin oxide nanostructured materials: An overview of recent developments in synthesis, modifications and potential applications. RSC Adv. 2016, 6, 110996–111015. [Google Scholar] [CrossRef]
- An, H.; Yoo, M.; Ha, H.; Choi, H.; Kang, E.; Kim, H.Y. Efficient Sn Recovery from SnO2 by Alkane (CxHy=2x+2, 0 ≤ x ≤ 4) Reduction. Sci. Rep. 2019, 9, 16702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Umezawa, N. Band gap engineering of bulk and nanosheet SnO: An insight into the interlayer Sn-Sn lone pair interactions. Phys. Chem. Chem. Phys. 2015, 17, 17816–17820. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Yang, Y.; Wu, P. Band Gap Engineering of SnO2 by Epitaxial Strain: Experimental and Theoretical Investigations. J. Phys. Chem. C 2014, 118, 6448–6453. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yamaguchi, A.; Yang, Y.; Aisnada, A.N.E.; Uchida, S.; Abe, H.; Ueda, S.; Yamaguchi, K.; Tanabe, T.; Miyauchi, M. Synthesis and Characterization of the Orthorhombic Sn3O4 Polymorph. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300640. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, M.; Kar, A. Modulating the properties of SnO2 nanocrystals: Morphological effects on structural, photoluminescence, photocatalytic, electrochemical and gas sensing properties. J. Mater. Chem. C 2020, 8, 4604–4635. [Google Scholar] [CrossRef]
- Sheng, N.; Lu, J.; Hu, J.; Zhu, R.; Fadillah, L.; Wang, C.; Zhu, C.; Rao, Z.; Habazaki, H. Synthesis of Sn@SnO2 core-shell microcapsules by a self-oxidation strategy for medium temperature thermal storage. Chem. Eng. J. 2021, 420, 129906. [Google Scholar] [CrossRef]
- Kuang, Y.; Zardetto, V.; van Gils, R.; Karwal, S.; Koushik, D.; Verheijen, M.A.; Black, L.E.; Weijtens, C.; Veenstra, S.; Andriessen, R.; et al. Low-Temperature Plasma-Assisted Atomic-Layer-Deposited SnO2 as an Electron Transport Layer in Planar Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 30367–30378. [Google Scholar] [CrossRef]
- Wang, L.P.; Leconte, Y.; Feng, Z.; Wei, C.; Zhao, Y.; Ma, Q.; Xu, W.; Bourrioux, S.; Azais, P.; Srinivasan, M.; et al. Novel Preparation of N-Doped SnO2 Nanoparticles via Laser-Assisted Pyrolysis: Demonstration of Exceptional Lithium Storage Properties. Adv. Mater. 2017, 29, 1603286. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Chen, Z.; Xu, J.; Zhu, J.; Xin, X. Two-step solid-state synthesis of tin oxide and its gas-sensing property. Mater. Chem. Phys. 2002, 73, 335–338. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, Z.; Wen, G.; Zhang, X.; Xu, M.; Weng, Y.; Nie, Y.; Dou, H.; Jiang, Y.; Deng, Y.P.; et al. Dual-Scale Integration Design of Sn-ZnO Catalyst toward Efficient and Stable CO2 Electroreduction. Adv. Mater. 2022, 34, e2204637. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Tan, X.; Wu, R.; Yan, X.; Chen, C.; Zhu, Q.; Zheng, L.; Ma, J.; Zhang, J.; et al. Highly Efficient CO2 Electroreduction to Methanol through Atomically Dispersed Sn Coupled with Defective CuO Catalysts. Angew. Chem. Int. Ed. Engl. 2021, 60, 21979–21987. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Kang, Y.C. Nanofibers Comprising Yolk-Shell Sn@void@SnO/SnO2 and Hollow SnO/SnO2 and SnO2 Nanospheres via the Kirkendall Diffusion Effect and Their Electrochemical Properties. Small 2015, 11, 4673–4681. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.K.; Pradhan, M.; Sarkar, S.; Pal, T. Large-scale solid-state synthesis of Sn-SnO2 nanoparticles from layered SnO by sunlight: A material for dye degradation in water by photocatalytic reaction. Environ. Sci. Technol. 2013, 47, 2339–2345. [Google Scholar] [CrossRef]
- Li, F.; Xu, J.; Yu, X.; Chen, L.; Zhu, J.; Yang, Z.; Xin, X. One-step solid-state reaction synthesis and gas sensing property of tin oxide nanoparticles. Sens. Actuators B Chem. 2000, 81, 165–169. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Tang, A.; Jin, S.; Qiu, G. Synthesis of tin oxide nanoparticles by mechanochemical reaction. J. Alloys Compd. 2004, 363, 276–279. [Google Scholar] [CrossRef]
- Chakravarty, R.; Chakraborty, S.; Shukla, R.; Bahadur, J.; Ram, R.; Mazumder, S.; Dev Sarma, H.; Tyagi, A.K.; Dash, A. Mechanochemical synthesis of mesoporous tin oxide: A new generation nanosorbent for 68Ge/68Ga generator technology. Dalton Trans. 2016, 45, 13361–13372. [Google Scholar] [CrossRef]
- Jiang, K.; Ji, J.; Gong, W.; Ding, L.; Li, J.; Li, P.; Li, B.; Geng, F. Mechanical cleavage of non-van der Waals structures towards two-dimensional crystals. Nat. Synth. 2022, 2, 58–66. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Kumar, T.P.; Renganathan, N.G.; Gopukumar, S.; Wohlfahrt-Mehrens, M.; Garche, J. Electrochemical behavior of Sn/SnO2 mixtures for use as anode in lithium rechargeable batteries. J. Power Sources 2005, 144, 197–203. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Guo, S.; Hou, M.; Ma, Y. In Situ Synthesis of Hierarchical Flower-like Sn/SnO2 Heterogeneous Structure for Ethanol GAS Detection. Materials 2023, 16, 792. [Google Scholar] [CrossRef]
- Hassan, F.M.; Chen, Z.; Yu, A.; Chen, Z.; Xiao, X. Sn/SnO2 embedded in mesoporous carbon nanocomposites as negative electrode for lithium ion batteries. Electrochim. Acta 2013, 87, 844–852. [Google Scholar] [CrossRef]
- Wang, Z.; Song, D.; Si, J.; Jiang, Y.; Yang, Y.; Jiang, Y.; Huang, S.; Chen, Z.; Zhao, B. One-step hydrothermal reduction synthesis of tiny Sn/SnO2 nanoparticles sandwiching between spherical graphene with excellent lithium storage cycling performances. Electrochim. Acta 2018, 292, 72–80. [Google Scholar] [CrossRef]
- Lim, J.; Garcia-Esparza, A.T.; Lee, J.W.; Kang, G.; Shin, S.; Jeon, S.S.; Lee, H. Electrodeposited Sn-Cu@Sn dendrites for selective electrochemical CO2 reduction to formic acid. Nanoscale 2022, 14, 9297–9303. [Google Scholar] [CrossRef]
- Saito, G.; Azman, W.O.S.B.W.M.; Nakasugi, Y.; Akiyama, T. Optimization of electrolyte concentration and voltage for effective formation of Sn/SnO2 nanoparticles by electrolysis in liquid. Adv. Powder Technol. 2014, 25, 1038–1042. [Google Scholar] [CrossRef]
- Santiago-Giraldo, F.M.; Lugo-Ruiz, A.A.; Bailón-Ruiz, S.J. Production and characterization of tin oxide (SnO2) nanostructures. MRS Adv. 2022, 7, 249–254. [Google Scholar] [CrossRef]
- Zima, T.; Bataev, I. Morphology and phase transformations of tin oxide nanostructures synthesized by the hydrothermal method in the presence of dicarboxylic acids. J. Solid State Chem. 2016, 243, 282–289. [Google Scholar] [CrossRef]
- Akhir, M.A.M.; Mohamed, K.; Lee, H.L.; Rezan, S.A. Synthesis of Tin Oxide Nanostructures Using Hydrothermal Method and Optimization of its Crystal size by Using Statistical Design of Experiment. Procedia Chem. 2016, 19, 993–998. [Google Scholar] [CrossRef]
- Zakaria, Y.; Aissa, B.; Fix, T.; Ahzi, S.; Samara, A.; Mansour, S.; Slaoui, A. Study of wide bandgap SnOx thin films grown by a reactive magnetron sputtering via a two-step method. Sci. Rep. 2022, 12, 15294. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. P-type SnO thin films prepared by reactive sputtering at high deposition rates. J. Mater. Sci. Technol. 2019, 35, 1706–1711. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Chen, W.-C.; Tsai, Y.-T.; Kung, Y.-C.; Chang, C.-H.; Wu, C.-C.; Hsieh, H.-H. Sputtering deposition of P-type SnO films using robust Sn/SnO2 mixed target. Thin Solid Films 2014, 555, 57–61. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Tsai, S.-P.; Chang, C.-H.; Hsu, C.-J.; Chen, W.-C.; Hsieh, H.-H.; Wu, C.-C. Preparation of p-type SnO thin films and transistors by sputtering with robust Sn/SnO2 mixed target in hydrogen-containing atmosphere. Thin Solid Films 2015, 585, 50–56. [Google Scholar] [CrossRef]
- Hsu, P.C.; Hsu, C.J.; Chang, C.H.; Tsai, S.P.; Chen, W.C.; Hsieh, H.H.; Wu, C.C. Sputtering deposition of P-type SnO films with SnO2 target in hydrogen-containing atmosphere. ACS Appl. Mater. Interfaces 2014, 6, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.Y.; Lee, S.J.; Jang, Y.; Kim, J.S.; Hwang, C.S.; Cho, D.Y. X-ray spectroscopy study on the electronic structure of Sn-added p-type SnO films. J. Phys. Condens. Matter 2020, 32, 065502. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.C.; Mousa, M.B.M.; Parsons, G.N. Thermal atomic layer deposition of Sn metal using SnCl4 and a vapor phase silyl dihydropyrazine reducing agent. J. Vac. Sci. Technol. A 2018, 36, 06A106. [Google Scholar] [CrossRef]
- Knapik, A.; Syrek, K.; Kozieł, M.; Zaraska, L. Cathodic deposition of SnO2 layers on transparent conductive substrates and their photoelectrochemical activity. J. Ind. Eng. Chem. 2022, 111, 380–388. [Google Scholar] [CrossRef]
- Huang, P.H.; Zhang, Z.X.; Hsu, C.H.; Wu, W.Y.; Ou, S.L.; Huang, C.J.; Wuu, D.S.; Lien, S.Y.; Zhu, W.Z. Deposition Mechanism and Characterization of Plasma-Enhanced Atomic Layer-Deposited SnOx Films at Different Substrate Temperatures. Nanomaterials 2022, 12, 2859. [Google Scholar] [CrossRef]
- Heo, J.; Hock, A.S.; Gordon, R.G. Low Temperature Atomic Layer Deposition of Tin Oxide. Chem. Mater. 2010, 22, 4964–4973. [Google Scholar] [CrossRef]
- Vallejos, S.; Selina, S.; Annanouch, F.E.; Gracia, I.; Llobet, E.; Blackman, C. Aerosol assisted chemical vapour deposition of gas sensitive SnO2 and Au-functionalised SnO2 nanorods via a non-catalysed vapour solid (VS) mechanism. Sci. Rep. 2016, 6, 28464. [Google Scholar] [CrossRef]
- Han, S.H.; Agbenyeke, R.E.; Lee, G.Y.; Park, B.K.; Kim, C.G.; Eom, T.; Son, S.U.; Han, J.H.; Ryu, J.Y.; Chung, T.M. Novel Heteroleptic Tin(II) Complexes Capable of Forming SnO and SnO2 Thin Films Depending on Conditions Using Chemical Solution Deposition. ACS Omega 2022, 7, 1232–1243. [Google Scholar] [CrossRef]
- Mathur, S.; Barth, S.; Shen, H.; Pyun, J.C.; Werner, U. Size-dependent photoconductance in SnO2 nanowires. Small 2005, 1, 713–717. [Google Scholar] [CrossRef]
- Sivakov, V. Iron, Germanium and Tin Tert-Butoxides in the CVD Process: Thin Film Deposition and Characterization. Ph.D. Thesis, Universität des Saarlandes, Saarland, Germany, 2004. [Google Scholar]
- Liu, P.; Schleusener, A.; Zieger, G.; Bochmann, A.; van Spronsen, M.A.; Sivakov, V. Nanostructured Silicon Matrix for Materials Engineering. Small 2023, 19, e2206318. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Sivakov, V.; Shen, H.; Barth, S.; Cavelius, C.; Nilsson, A.; Kuhn, P. Nanostructured films of iron, tin and titanium oxides by chemical vapor deposition. Thin Solid Films 2006, 502, 88–93. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Akbari, M.; Sekkat, A.; Ta, H.T.T.; Resende, J.; Jimenez, C.; Musselman, K.P.; Munoz-Rojas, D. Atmospheric atomic layer deposition of SnO2 thin films with tin(II) acetylacetonate and water. Dalton Trans. 2022, 51, 9278–9290. [Google Scholar] [CrossRef]
- Kim, H.Y.; Nam, J.H.; George, S.M.; Park, J.-S.; Park, B.K.; Kim, G.H.; Jeon, D.J.; Chung, T.-M.; Han, J.H. Phase-controlled SnO2 and SnO growth by atomic layer deposition using Bis(N-ethoxy-2,2-dimethyl propanamido)tin precursor. Ceram. Int. 2019, 45, 5124–5132. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-Liquid-Solid Mechanism of Single Crystal Growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.H.; Yang, G.W. Growth mechanisms of SnO2/Sn nanocables. Nanotechnology 2006, 17, 4682–4688. [Google Scholar] [CrossRef][Green Version]
- Putnam, M.C.; Filler, M.A.; Kayes, B.M.; Kelzenberg, M.D.; Guan, Y.; Lewis, N.S.; Eiler, J.M.; Atwater, H.A. Secondary Ion Mass Spectrometry of Vapor-Liquid-Solid Grown, Au-Catalyzed, Si Wires. Nano Lett. 2008, 8, 3109–3113. [Google Scholar] [CrossRef]
- Song, J.-S.; Cho, G.-B.; Kim, K.-W.; Ahn, H.-J.; Kim, H.-S.; Ahn, J.-H.; Cho, K.-K. Fabrication of multilayer graphene-encapsulated Sn/SnO2 nanocomposite as an anode material for lithium-ion batteries and its electrochemical properties. Appl. Surf. Sci. 2019, 481, 736–740. [Google Scholar] [CrossRef]
- Thorat, G.M.; Jadhav, H.S.; Chung, W.-J.; Seo, J.G. Collective use of deep eutectic solvent for one-pot synthesis of ternary Sn/SnO2@C electrode for supercapacitor. J. Alloys Compd. 2018, 732, 694–704. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Li, L.; Xu, L.; Wang, W.; Zhang, J.; Xu, X.; Zou, J.; Tang, C. High performance UV light photodetectors based on Sn-nanodot-embedded SnO2 nanobelts. J. Mater. Chem. C 2015, 3, 5253–5258. [Google Scholar] [CrossRef]
- Li, S.; Zhong, X.; Song, Y.; Shen, X.; Sun, J.; Song, Y.; Wang, R.; Zhu, M.; Zhong, H.; Zheng, A. Controlled hybridization of Sn–SnO2 nanoparticles via simple-programmed microfluidic processes for tunable ultraviolet and blue emissions. J. Mater. Chem. C 2014, 2, 7687–7694. [Google Scholar] [CrossRef]
- Han, J.; Kong, D.; Lv, W.; Tang, D.M.; Han, D.; Zhang, C.; Liu, D.; Xiao, Z.; Zhang, X.; Xiao, J.; et al. Caging tin oxide in three-dimensional graphene networks for superior volumetric lithium storage. Nat. Commun. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Liu, J. Biomass-derived porous carbon materials for advanced lithium sulfur batteries. J. Energy Chem. 2019, 34, 171–185. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Electrochemical lithiation of tin and tin-based intermetallics and composites. Electrochim. Acta 1999, 45, 31–50. [Google Scholar] [CrossRef]
- Eom, K.; Jung, J.; Lee, J.T.; Lair, V.; Joshi, T.; Lee, S.W.; Lin, Z.; Fuller, T.F. Improved stability of nano-Sn electrode with high-quality nano-SEI formation for lithium ion battery. Nano Energy 2015, 12, 314–321. [Google Scholar] [CrossRef]
- Wang, X.-L.; Feygenson, M.; Aronson, M.C.; Han, W.-Q. Sn/SnOx Core-Shell Nanospheres: Synthesis, Anode Performance in Li Ion Batteries, and Superconductivity. J. Phys. Chem. C 2010, 114, 14697–14703. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, K.; Sun, C.; Yu, R.; Zhuang, Z.; Li, J.; Xu, W.; Wang, C.; Xu, W.; Mai, L. Compact Sn/SnO2 microspheres with gradient composition for high volumetric lithium storage. Energy Storage Mater. 2020, 25, 376–381. [Google Scholar] [CrossRef]
- He, F.; Xu, Q.; Zheng, B.; Zhang, J.; Wu, Z.; Zhong, Y.; Chen, Y.; Xiang, W.; Zhong, B.; Guo, X. Synthesis of hierarchical Sn/SnO nanosheets assembled by carbon-coated hollow nanospheres as anode materials for lithium/sodium ion batteries. RSC Adv. 2020, 10, 6035–6042. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, W.; Liu, C.; Ma, C.; Yang, Y.; Ji, H.; Yang, G. Synthesis of heterostructure Sn|SnO2 submicron particles supported by carbon fibers as binder-free anodes for highly reversible lithium storage. J. Alloys Compd. 2018, 750, 220–227. [Google Scholar] [CrossRef]
- Sun, L.; Si, H.; Zhang, Y.; Shi, Y.; Wang, K.; Liu, J.; Zhang, Y. Sn-SnO2 hybrid nanoclusters embedded in carbon nanotubes with enhanced electrochemical performance for advanced lithium ion batteries. J. Power Sources 2019, 415, 126–135. [Google Scholar] [CrossRef]
- Pol, V.G.; Wen, J.; Miller, D.J.; Thackeray, M.M. Sonochemical Deposition of Sn, SnO2 and Sb on Spherical Hard Carbon Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2014, 161, A777–A782. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Yang, J.; Wang, J.; Geng, D.; Li, R.; Cai, M.; Sham, T.K.; Sun, X. Hierarchical nanostructured core-shell Sn@C nanoparticles embedded in graphene nanosheets: Spectroscopic view and their application in lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Gu, L.; Xia, F.; Liu, B.; Hou, X.; Wang, Q.; Liu, D.; He, D. Fabrication of voids-involved SnO2@C nanofibers electrodes with highly reversible Sn/SnO2 conversion and much enhanced coulombic efficiency for lithium-ion batteries. J. Power Sources 2016, 327, 21–28. [Google Scholar] [CrossRef]
- Dong, W.; Xu, J.; Wang, C.; Lu, Y.; Liu, X.; Wang, X.; Yuan, X.; Wang, Z.; Lin, T.; Sui, M.; et al. A Robust and Conductive Black Tin Oxide Nanostructure Makes Efficient Lithium-Ion Batteries Possible. Adv. Mater. 2017, 29, 1700136. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, W.; Liu, L.; Wang, H.; Xu, Z.; Li, F.; Fu, H.; Lv, H.; Chen, L.; Kang, Y. Sandwich-like Sn/SnO2@Graphene anode composite assembled by fortissimo penetration of γ-ray and interlamellar limitation of graphene oxide. J. Alloys Compd. 2019, 779, 856–862. [Google Scholar] [CrossRef]
- Gao, S.; Wang, N.; Li, S.; Li, D.; Cui, Z.; Yue, G.; Liu, J.; Zhao, X.; Jiang, L.; Zhao, Y. A Multi-Wall Sn/SnO2@Carbon Hollow Nanofiber Anode Material for High-Rate and Long-Life Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 2465–2472. [Google Scholar] [CrossRef]
- Narsimulu, D.; Vadnala, S.; Srinadhu, E.S.; Satyanarayana, N. Electrospun Sn–SnO2/C composite nanofibers as an anode material for lithium battery applications. J. Mater. Sci. Mater. Electron. 2018, 29, 11117–11123. [Google Scholar] [CrossRef]
- Cheng, Y.; Yi, Z.; Wang, C.; Wu, Y.; Wang, L. Controllable fabrication of C/Sn and C/SnO/Sn composites as anode materials for high-performance lithium-ion batteries. Chem. Eng. J. 2017, 330, 1035–1043. [Google Scholar] [CrossRef]
- Dong, Y.; Das, S.; Zhu, L.; Ben, T.; Qiu, S. Standout electrochemical performance of SnO2 and Sn/SnO2 nanoparticles embedded in a KOH-activated carbonized porous aromatic framework (PAF-1) matrix as the anode for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 18822–18831. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Zhang, X.-H.; Wen, J.-B.; Huang, Y.-G.; Lai, F.-Y.; Li, Q.-Y. Preparation of Spherical Sn/SnO2/Porous Carbon Composite Materials as Anode Material for Lithium-Ion Batteries. J. Mater. Eng. Perform. 2015, 24, 1856–1864. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, X.; Liu, W.; Xu, B.; Hu, P.; Gao, Z.; Huang, Y. Flakes-stacked Sn/SnO2/C composite as highly stable anode material for lithium-ion batteries. J. Alloys Compd. 2020, 831, 154677. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Zheng, J.; Ye, W.; Yin, W.; Tang, B.; Rui, Y. Preparation of promising anode materials with Sn-MOF as precursors for superior lithium and sodium storage. J. Alloys Compd. 2020, 84, 155605. [Google Scholar] [CrossRef]
- Yang, J.; Chen, S.; Tang, J.; Tian, H.; Bai, T.; Zhou, X. Rod-like hierarchical Sn/SnOx@C nanostructures with enhanced lithium storage properties. Appl. Surf. Sci. 2018, 435, 203–209. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Li, J.; Xu, Z.; Cao, L.; Qi, H. Synergistic effect of the core-shell structured Sn/SnO2/C ternary anode system with the improved sodium storage performance. J. Power Sources 2016, 324, 447–454. [Google Scholar] [CrossRef]
- Tan, L.; Feng, S.; Li, X.; Wang, Z.; Peng, W.; Liu, T.; Yan, G.; Li, L.; Wu, F.; Wang, J. Oxygen-induced lithiophilicity of tin-based framework toward highly stable lithium metal anode. Chem. Eng. J. 2020, 394, 124848. [Google Scholar] [CrossRef]
- Kravchyk, K.; Protesescu, L.; Bodnarchuk, M.I.; Krumeich, F.; Yarema, M.; Walter, M.; Guntlin, C.; Kovalenko, M.V. Monodisperse and inorganically capped Sn and Sn/SnO2 nanocrystals for high-performance Li-ion battery anodes. J. Am. Chem. Soc. 2013, 135, 4199–4202. [Google Scholar] [CrossRef]
- Huang, Y.; Pan, Q.; Wang, H.; Ji, C.; Wu, X.; He, Z.; Li, Q. Preparation of a Sn@SnO2@C@MoS2 composite as a high-performance anode material for lithium-ion batteries. J. Mater. Chem. A 2016, 4, 7185–7189. [Google Scholar] [CrossRef]
- Namsar, O.; Autthawong, T.; Laokawee, V.; Boonprachai, R.; Haruta, M.; Kurata, H.; Yu, A.; Chairuangsri, T.; Sarakonsri, T. Improved electrochemical performance of anode materials for high energy density lithium-ion batteries through Sn(SnO2)–SiO2/graphene-based nanocomposites prepared by a facile and low-cost approach. Sustain. Energy Fuels 2020, 4, 4625–4636. [Google Scholar] [CrossRef]
- Zuo, D.-C.; Song, S.-C.; An, C.-S.; Tang, L.-B.; He, Z.-J.; Zheng, J.-C. Synthesis of sandwich-like structured Sn/SnOx@MXene composite through in-situ growth for highly reversible lithium storage. Nano Energy 2019, 62, 401–409. [Google Scholar] [CrossRef]
- Li, R.; Nie, S.; Miao, C.; Xin, Y.; Mou, H.; Xu, G.; Xiao, W. Heterostructural Sn/SnO2 microcube powders coated by a nitrogen-doped carbon layer as good-performance anode materials for lithium ion batteries. J. Colloid Interface Sci. 2022, 606, 1042–1054. [Google Scholar] [CrossRef]
- Jiang, B.; He, Y.; Li, B.; Zhao, S.; Wang, S.; He, Y.B.; Lin, Z. Polymer-Templated Formation of Polydopamine-Coated SnO2 Nanocrystals: Anodes for Cyclable Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Huang, Q.; Yi, R.; Dai, F.; Gordin, M.L.; Hu, S.; Chen, S.; Yu, Z.; Sohn, H.; Song, J.; et al. Room-Temperature Synthesis of Mesoporous Sn/SnO2 Composite as Anode for Sodium-Ion Batteries. Eur. J. Inorg. Chem. 2016, 2016, 1950–1954. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Fan, L.; Yu, Z.; Yan, B.; Xiong, D.; Song, X.; Li, S.; Adair, K.R.; Li, D.; et al. Rational design of Sn/SnO2 /porous carbon nanocomposites as anode materials for sodium-ion batteries. Appl. Surf. Sci. 2017, 412, 170–176. [Google Scholar] [CrossRef]
- Dahash, A.; Ochs, F.; Janetti, M.B.; Streicher, W. Advances in seasonal thermal energy storage for solar district heating applications: A critical review on large-scale hot-water tank and pit thermal energy storage systems. Appl. Energy 2019, 239, 296–315. [Google Scholar] [CrossRef]
- Tulus, V.; Boer, D.; Cabeza, L.F.; Jiménez, L.; Guillén-Gosálbez, G. Enhanced thermal energy supply via central solar heating plants with seasonal storage: A multi-objective optimization approach. Appl. Energy 2016, 181, 549–561. [Google Scholar] [CrossRef]
- Wei, H.; Qiu, C.; Wang, C.; Lin, K.; Yang, S.; Han, J.; Lu, Y.; Liu, X. Development of phase change materials using hydrolyzed Al-Bi composite powder for solar energy storage. Chem. Eng. J. 2021, 421, 127836. [Google Scholar] [CrossRef]
- Mehrali, M.; ten Elshof, J.E.; Shahi, M.; Mahmoudi, A. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material. Chem. Eng. J. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Soni, V.; Kumar, A.; Jain, V.K. Performance evaluation of nano-enhanced phase change materials during discharge stage in waste heat recovery. Renew. Energy 2018, 127, 587–601. [Google Scholar] [CrossRef]
- Omara, A.A.M. Phase change materials for waste heat recovery in internal combustion engines: A review. J. Energy Storage 2021, 44, 103421. [Google Scholar] [CrossRef]
- Farzanehnia, A.; Khatibi, M.; Sardarabadi, M.; Passandideh-Fard, M. Experimental investigation of multiwall carbon nanotube/paraffin based heat sink for electronic device thermal management. Energy Convers. Manag. 2019, 179, 314–325. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, L.; Wan, H.; Liu, H.; Wu, D.; Wang, X. Construction of polyaniline/carbon nanotubes-functionalized phase-change microcapsules for thermal management application of supercapacitors. Chem. Eng. J. 2020, 396, 125317. [Google Scholar] [CrossRef]
- Hassan, A.; Wahab, A.; Qasim, M.A.; Janjua, M.M.; Ali, M.A.; Ali, H.M.; Jadoon, T.R.; Ali, E.; Raza, A.; Javaid, N. Thermal management and uniform temperature regulation of photovoltaic modules using hybrid phase change materials-nanofluids system. Renew. Energy 2020, 145, 282–293. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ostry, M.; Paksoy, H.O.; Charvat, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Keshteli, A.N.; Sheikholeslami, M. Nanoparticle enhanced PCM applications for intensification of thermal performance in building: A review. J. Mol. Liq. 2019, 274, 516–533. [Google Scholar] [CrossRef]
- Liu, C.; Zong, J.; Zhang, J.; He, D.; Guo, C.; Xu, B.; Rao, Z. Knitting aryl network polymers (KAPs)-embedded copper foam enables highly efficient thermal energy storage. J. Mater. Chem. A 2020, 8, 15177–15186. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Bao, R.-Y.; Yi, K.; Li, M.; Peng, L.; Cai, Z.; Yang, M.-B.; Wei, D.; Yang, W. Hybridizing graphene aerogel into three-dimensional graphene foam for high-performance composite phase change materials. Energy Storage Mater. 2018, 13, 88–95. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Chang, S.J.; Kim, K.-H.; Dong, W.; Kim, S. A novel enhancement of shape/thermal stability and energy-storage capacity of phase change materials through the formation of composites with 3D porous (3,6)-connected metal–organic framework. Chem. Eng. J. 2020, 389, 124430. [Google Scholar] [CrossRef]
- Yuan, K.; Liu, J.; Fang, X.; Zhang, Z. Novel facile self-assembly approach to construct graphene oxide-decorated phase-change microcapsules with enhanced photo-to-thermal conversion performance. J. Mater. Chem. A 2018, 6, 4535–4543. [Google Scholar] [CrossRef]
- Lu, J.; Sheng, N.; Zhu, C. Fabrication of Sn@SiO2 core-shell microcapsules with high durability for medium-temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 239, 111652. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Macro-encapsulation of metallic phase change material using cylindrical-type ceramic containers for high-temperature thermal energy storage. Appl. Energy 2016, 170, 324–328. [Google Scholar] [CrossRef]
- Navarrete, N.; La Zara, D.; Goulas, A.; Valdesueiro, D.; Hernández, L.; van Ommen, J.R.; Mondragón, R. Improved thermal energy storage of nanoencapsulated phase change materials by atomic layer deposition. Sol. Energy Mater. Sol. Cells 2020, 206, 110322. [Google Scholar] [CrossRef]
- Gaponenko, S.V. Optical Properties of Semiconductor Nanocrystals; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Bukowski, T.J.; Simmons, J.H. Quantum Dot Research: Current State and Future Prospects. Crit. Rev. Solid State Mater. Sci. 2002, 27, 119–142. [Google Scholar] [CrossRef]
- Tagliente, M.A.; Bello, V.; Pellegrini, G.; Mattei, G.; Mazzoldi, P.; Massaro, M. SnO2 nanoparticles embedded in silica by ion implantation followed by thermal oxidation. J. Appl. Phys. 2009, 106, 104304. [Google Scholar] [CrossRef]
- Mu, H.; Yu, W.; Yuan, J.; Lin, S.; Zhang, G. Interface and surface engineering of black phosphorus: A review for optoelectronic and photonic applications. Mater. Futures 2022, 1, 104304. [Google Scholar] [CrossRef]
- Xu, R.; Guo, J.; Mi, S.; Wen, H.; Pang, F.; Ji, W.; Cheng, Z. Advanced atomic force microscopies and their applications in two-dimensional materials: A review. Mater. Futures 2022, 1, 032302. [Google Scholar] [CrossRef]
- Zhuang, R.; Cai, S.; Mei, Z.; Liang, H.; Zhao, N.; Mu, H.; Yu, W.; Jiang, Y.; Yuan, J.; Lau, S.; et al. Solution-grown BiI/BiI3 van der Waals heterostructures for sensitive X-ray detection. Nat. Commun. 2023, 14, 1621. [Google Scholar] [CrossRef]
- Huang, H.; Cui, P.; Chen, Y.; Yan, L.; Yue, X.; Qu, S.; Wang, X.; Du, S.; Liu, B.; Zhang, Q.; et al. 24.8%-efficient planar perovskite solar cells via ligand-engineered TiO2 deposition. Joule 2022, 6, 2186–2202. [Google Scholar] [CrossRef]
- Song, J.; Zheng, E.; Bian, J.; Wang, X.-F.; Tian, W.; Sanehira, Y.; Miyasaka, T. Low-temperature SnO2-based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A 2015, 3, 10837–10844. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Huang, Y.; Liu, F.; Lv, M.; Chen, S.; Hu, L.; Tang, J.; Yao, J.; Dai, S. Mesoporous SnO2 nanoparticle films as electron-transporting material in perovskite solar cells. RSC Adv. 2015, 5, 28424–28429. [Google Scholar] [CrossRef]
- Jung, K.-H.; Seo, J.-Y.; Lee, S.; Shin, H.; Park, N.-G. Solution-processed SnO2 thin film for a hysteresis-free planar perovskite solar cell with a power conversion efficiency of 19.2%. J. Mater. Chem. A 2017, 5, 24790–24803. [Google Scholar] [CrossRef]
- Jeong, S.; Seo, S.; Park, H.; Shin, H. Atomic layer deposition of a SnO2 electron-transporting layer for planar perovskite solar cells with a power conversion efficiency of 18.3%. Chem. Commun. 2019, 55, 2433–2436. [Google Scholar] [CrossRef]
- Dagar, J.; Castro-Hermosa, S.; Gasbarri, M.; Palma, A.L.; Cina, L.; Matteocci, F.; Calabrò, E.; Di Carlo, A.; Brown, T.M. Efficient fully laser-patterned flexible perovskite modules and solar cells based on low-temperature solution-processed SnO2/mesoporous-TiO2 electron transport layers. Nano Res. 2018, 11, 2669–2681. [Google Scholar] [CrossRef]
- Ahmad, W.; Liu, D.; Ahmad, W.; Wang, Y.; Zhang, P.; Zhang, T.; Zheng, H.; Chen, Z.D.; Li, S. Physisorption of Oxygen in SnO2 Nanoparticles for Perovskite Solar Cells. IEEE J. Photovoltaics 2019, 9, 200–206. [Google Scholar] [CrossRef]
- Tao, H.; Wang, H.; Bai, Y.; Long, H.; Zhao, H.; Fu, Q.; Ma, Z. Effects of sputtering power of SnO2 electron selective layer on perovskite solar cells. J. Mater. Sci. Mater. Electron. 2019, 30, 12036–12043. [Google Scholar] [CrossRef]
- Mohamad Noh, M.F.; Arzaee, N.A.; Safaei, J.; Mohamed, N.A.; Kim, H.P.; Mohd Yusoff, A.R.; Jang, J.; Mat Teridi, M.A. Eliminating oxygen vacancies in SnO2 films via aerosol-assisted chemical vapour deposition for perovskite solar cells and photoelectrochemical cells. J. Alloys Compd. 2019, 773, 997–1008. [Google Scholar] [CrossRef]
- Trost, S.; Behrendt, A.; Becker, T.; Polywka, A.; Görrn, P.; Riedl, T. Tin Oxide (SnOx) as Universal “Light-Soaking” Free Electron Extraction Material for Organic Solar Cells. Adv. Energy Mater. 2015, 5, 1500277. [Google Scholar] [CrossRef]
- Xie, C.; Xiao, C.; Fang, J.; Zhao, C.; Li, W. Core/shell AgNWs@SnOx electrodes for high performance flexible indoor organic solar cells with >25% efficiency. Nano Energy 2023, 107, 108153. [Google Scholar] [CrossRef]
- Yuan, R.; Cai, B.; Lv, Y.; Gao, X.; Gu, J.; Fan, Z.; Liu, X.; Yang, C.; Liu, M.; Zhang, W.-H. Boosted charge extraction of NbOx-enveloped SnO2 nanocrystals enables 24% efficient planar perovskite solar cells. Energy Environ. Sci. 2021, 14, 5074–5083. [Google Scholar] [CrossRef]
- Wu, P.; Wang, S.; Li, X.; Zhang, F. Advances in SnO2-based perovskite solar cells: From preparation to photovoltaic applications. J. Mater. Chem. A 2021, 9, 19554–19588. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S.F. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 2018, 9, 3239. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.S.; Patil, J.V.; Kim, H.; Hong, C.K. Synthesis of SnO2 nanofibers and nanobelts electron transporting layer for efficient perovskite solar cells. Nanoscale 2018, 10, 8275–8284. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ng, A.; Ren, Z.; Hu, H.; Lin, H.-C.; Chu, C.-W.; Li, G. Facile synthesis of composite tin oxide nanostructures for high-performance planar perovskite solar cells. Nano Energy 2019, 60, 275–284. [Google Scholar] [CrossRef]
- Ali, F.; Pham, N.D.; Bradford, H.J.; Khoshsirat, N.; Ostrikov, K.; Bell, J.M.; Wang, H.; Tesfamichael, T. Tuning the Amount of Oxygen Vacancies in Sputter-Deposited SnOx films for Enhancing the Performance of Perovskite Solar Cells. ChemSusChem 2018, 11, 3096–3103. [Google Scholar] [CrossRef]
- Jiang, E.; Yan, J.; Ai, Y.; Li, N.; Yan, B.; Zeng, Y.; Sheng, J.; Ye, J. Defect engineering of oxygen vacancies in SnOx electron transporting layer for perovskite solar cells. Mater. Today Energy 2019, 12, 389–397. [Google Scholar] [CrossRef]
- Xiong, Q.; Yang, L.; Zhou, Q.; Wu, T.; Mai, C.L.; Wang, Z.; Wu, S.; Li, X.; Gao, P. NdCl3 Dose as a Universal Approach for High-Efficiency Perovskite Solar Cells Based on Low-Temperature-Processed SnOx. ACS Appl. Mater. Interfaces 2020, 12, 46306–46316. [Google Scholar] [CrossRef]
- Xiong, L.; Qin, M.; Yang, G.; Guo, Y.; Lei, H.; Liu, Q.; Ke, W.; Tao, H.; Qin, P.; Li, S.; et al. Performance enhancement of high temperature SnO2-based planar perovskite solar cells: Electrical characterization and understanding of the mechanism. J. Mater. Chem. A 2016, 4, 8374–8383. [Google Scholar] [CrossRef]
- Liu, K.; Chen, S.; Wu, J.; Zhang, H.; Qin, M.; Lu, X.; Tu, Y.; Meng, Q.; Zhan, X. Fullerene derivative anchored SnO2 for high-performance perovskite solar cells. Energy Environ. Sci. 2018, 11, 3463–3471. [Google Scholar] [CrossRef]
- Bi, H.; Zuo, X.; Liu, B.; He, D.; Bai, L.; Wang, W.; Li, X.; Xiao, Z.; Sun, K.; Song, Q.; et al. Multifunctional organic ammonium salt-modified SnO2 nanoparticles toward efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2021, 9, 3940–3951. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, X.; Xue, R.; Xu, P.; Wang, S.; Xu, C.; Zeng, W.; Xiong, Y.; Sang, H.; Liang, D. Low-Temperature Growing Anatase TiO2/SnO2 Multi-dimensional Heterojunctions at MXene Conductive Network for High-Efficient Perovskite Solar Cells. Nano-Micro Lett. 2020, 12, 44. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, H.; Feng, S.; Yang, L.; Dong, H.; Wang, J.; Tian, C.; Li, L.; Lu, H.; Jeong, J.; et al. Modulation of perovskite crystallization processes towards highly efficient and stable perovskite solar cells with MXene quantum dot-modified SnO2. Energy Environ. Sci. 2021, 14, 3447–3454. [Google Scholar] [CrossRef]
- Yang, L.; Dall’Agnese, Y.; Hantanasirisakul, K.; Shuck, C.E.; Maleski, K.; Alhabeb, M.; Chen, G.; Gao, Y.; Sanehira, Y.; Jena, A.K.; et al. SnO2–Ti3C2 MXene electron transport layers for perovskite solar cells. J. Mater. Chem. A 2019, 7, 5635–5642. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Naushad, M.; Al-Muhtaseb, A.a.H.; Ruksana; Alshehri, S.M.; Alothman, Z.A.; Ahamad, T. Synthesis and characterization of highly selective and sensitive Sn/SnO2/N-doped carbon nanocomposite (Sn/SnO2@NGC) for sensing toxic NH3 gas. Chem. Eng. J. 2018, 345, 58–66. [Google Scholar] [CrossRef]
- Verma, M.; Bahuguna, G.; Saharan, A.; Gaur, S.; Haick, H.; Gupta, R. Room Temperature Humidity Tolerant Xylene Sensor Using a Sn-SnO2 Nanocomposite. ACS Appl. Mater. Interfaces 2023, 15, 5512–5520. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Y.; Wang, M.; Liu, J.; Pei, C.; Liu, B.; Zhao, H.; Liu, S.; Yang, H. Effect of Unsaturated Sn Atoms on Gas-Sensing Property in Hydrogenated SnO2 Nanocrystals and Sensing Mechanism. Sci. Rep. 2017, 7, 1231. [Google Scholar] [CrossRef]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B Chem. 2015, 208, 122–127. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Park, M.-J.; Kwon, S.-H.; Joo, H.-J.; Kwon, H.-I. Highly sensitive and selective room-temperature NO2 gas-sensing characteristics of SnOx-based p-type thin-film transistor. Sens. Actuators B Chem. 2019, 288, 625–633. [Google Scholar] [CrossRef]
- Shao, M.; Liu, J.; Ding, W.; Wang, J.; Dong, F.; Zhang, J. Oxygen vacancy engineering of self-doped SnO2-x nanocrystals for ultrasensitive NO2 detection. J. Mater. Chem. C 2020, 8, 487–494. [Google Scholar] [CrossRef]
- Rawat, R.K.; Tripathi, D.; Singh, A.; Yadav, J.; Dwivedi, P.; Chauhan, P. Concurrent Synthesis of Functional SnO2/SnO Composite for Proton Conductive Sensor and Photo-Catalytic Treatment. J. Electrochem. Soc. 2022, 169, 077506. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Chen, W. Fabrication of SnO2-SnO nanocomposites with p-n heterojunctions for the low-temperature sensing of NO2 gas. Nanoscale 2015, 7, 12133–12142. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, F.; Hermawan, A.; Asakura, Y.; Hasegawa, T.; Kumagai, H.; Kato, H.; Kakihana, M.; Zhu, J.; Yin, S. SnO-SnO2 modified two-dimensional MXene Ti3C2Tx for acetone gas sensor working at room temperature. J. Mater. Sci. Technol. 2021, 73, 128–138. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Lei, Y.; Xie, S.; Wu, N.; Gou, Y.; Han, C.; Shi, Q.; Fang, D. Vertical SnO2 nanosheet@SiC nanofibers with hierarchical architecture for high-performance gas sensors. J. Mater. Chem. C 2016, 4, 295–304. [Google Scholar] [CrossRef]
- Yan, S.; Wu, Q. Micropored Sn-SnO2/carbon heterostructure nanofibers and their highly sensitive and selective C2H5OH gas sensing performance. Sens. Actuators B Chem. 2014, 205, 329–337. [Google Scholar] [CrossRef]
- Van Toan, N.; Chien, N.V.; Van Duy, N.; Vuong, D.D.; Lam, N.H.; Hoa, N.D.; Van Hieu, N.; Chien, N.D. Scalable fabrication of SnO2 thin films sensitized with CuO islands for enhanced H2S gas sensing performance. Appl. Surf. Sci. 2015, 324, 280–285. [Google Scholar] [CrossRef]
- Fu, D.; Zhu, C.; Zhang, X.; Li, C.; Chen, Y. Two-dimensional net-like SnO2/ZnO heteronanostructures for high-performance H2S gas sensor. J. Mater. Chem. A 2016, 4, 1390–1398. [Google Scholar] [CrossRef]
- Mauraya, A.K.; Mahana, D.; Pal, P.; Muthiah, S.; Singh, P.; Muthusamy, S.K. Effect of bulk and surface modification of SnO2 thin films with PdO catalyst on CO gas sensing characteristics prepared by vacuum evaporation process. J. Alloys Compd. 2020, 843, 155979. [Google Scholar] [CrossRef]
- Manikandan, K.; Dhanuskodi, S.; Thomas, A.R.; Maheswari, N.; Muralidharan, G.; Sastikumar, D. Size–strain distribution analysis of SnO2 nanoparticles and their multifunctional applications as fiber optic gas sensors, supercapacitors and optical limiters. RSC Adv. 2016, 6, 90559–90570. [Google Scholar] [CrossRef]
- Choi, P.G.; Izu, N.; Shirahata, N.; Masuda, Y. Improvement of sensing properties for SnO2 gas sensor by tuning of exposed crystal face. Sens. Actuators B Chem. 2019, 296, 126655. [Google Scholar] [CrossRef]
- Yu, H.; Wang, S.; Xiao, C.; Xiao, B.; Wang, P.; Li, Z.; Zhang, M. Enhanced acetone gas sensing properties by aurelia-like SnO2 micro-nanostructures. CrystEngComm 2015, 17, 4316–4324. [Google Scholar] [CrossRef]
- Borah, S.; Bhattacharyya, B.; Deka, J.; Borah, A.; Devi, A.; Deka, D.; Mishra, S.; Raidongia, K.; Gogoi, N. Enhanced catalytic activity and near room temperature gas sensing properties of SnO2 nanoclusters@mesoporous Sn(iv) organophosphonate composite. Dalton Trans. 2017, 46, 8664–8672. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Equivalence between thermal and room temperature UV light-modulated responses of gas sensors based on individual SnO2 nanowires. Sens. Actuators B Chem. 2009, 140, 337–341. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, R.; Manzanares, M.; Hernandez-Ramirez, F.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. A model for the response towards oxidizing gases of photoactivated sensors based on individual SnO2 nanowires. Phys. Chem. Chem. Phys. 2009, 11, 10881–10889. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ramirez, F.; Barth, S.; Tarancon, A.; Casals, O.; Pellicer, E.; Rodriguez, J.; Romano-Rodriguez, A.; Morante, J.R.; Mathur, S. Water vapor detection with individual tin oxide nanowires. Nanotechnology 2007, 18, 424016. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Chen, S.; Sun, J.; Liu, J.; Che, Y.; Liu, X.; Zhang, J.; Yang, D. Highly Efficient Gas Sensor Using a Hollow SnO2 Microfiber for Triethylamine Detection. ACS Sens. 2017, 2, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Lee, C.-M.; Lo, Y.-J. Reducing gas-sensing performance of Ce-doped SnO2 thin films through a cosputtering method. RSC Adv. 2017, 7, 4724–4734. [Google Scholar] [CrossRef]
- Qin, G.; Gao, F.; Jiang, Q.; Li, Y.; Liu, Y.; Luo, L.; Zhao, K.; Zhao, H. Well-aligned Nd-doped SnO2 nanorod layered arrays: Preparation, characterization and enhanced alcohol-gas sensing performance. Phys. Chem. Chem. Phys. 2016, 18, 5537–5549. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.-G.; Hua, W.; Liu, H.; Wei, B. Heterostructured Sn/SnO2-x nanotube peapods with a strong plasmonic effect for photoelectrochemical water oxidation. J. Mater. Chem. A 2019, 7, 16883–16891. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Liu, Y.; Xia, M.; Finfrock, Y.Z.; Zakeeruddin, S.M.; Ren, D.; Gratzel, M. Solar reduction of carbon dioxide on copper-tin electrocatalysts with energy conversion efficiency near 20%. Nat. Commun. 2022, 13, 5898. [Google Scholar] [CrossRef]
- Esmaeili-Bafghi-Karimabad, A.; Ghanbari, D.; Salavati-Niasari, M.; Nejati-Moghadam, L.; Gholamrezaei, S. Photo-catalyst tin dioxide: Synthesis and characterization different morphologies of SnO2 nanostructures and nanocomposites. J. Mater. Sci. Mater. Electron. 2015, 26, 6970–6978. [Google Scholar] [CrossRef]
- Ma, H.; Teng, K.; Fu, Y.; Song, Y.; Wang, Y.; Dong, X. Synthesis of visible-light responsive Sn-SnO2/C photocatalyst by simple carbothermal reduction. Energy Environ. Sci. 2011, 4, 3067–3074. [Google Scholar] [CrossRef]
- Hung-Low, F.; Ramirez, D.A.; Peterson, G.R.; Hikal, W.M.; Hope-Weeks, L.J. Development of a carbon-supported Sn–SnO2 photocatalyst by a new hybridized sol–gel/dextran approach. RSC Adv. 2016, 6, 21019–21025. [Google Scholar] [CrossRef]
- Das, O.R.; Uddin, M.T.; Rahman, M.M.; Bhoumick, M.C. Highly active carbon supported Sn/SnO2 photocatalysts for degrading organic dyes. J. Phys. Conf. Ser. 2018, 1086, 012011. [Google Scholar] [CrossRef]
- Uma, H.B.; Ananda, S.; Kumar, M.S.V. Electrochemical synthesis and characterization of CuO/ZnO/SnO nano photocatalyst: Evaluation of its application towards photocatalysis, photo-voltaic and antibacterial properties. Chem. Data Collect. 2021, 32, 100658. [Google Scholar] [CrossRef]
- Hussain, S.; Kongi, N.; Erikson, H.; Rähn, M.; Merisalu, M.; Matisen, L.; Paiste, P.; Aruväli, J.; Sammelselg, V.; Estudillo-Wong, L.A.; et al. Platinum nanoparticles photo-deposited on SnO2-C composites: An active and durable electrocatalyst for the oxygen reduction reaction. Electrochim. Acta 2019, 316, 162–172. [Google Scholar] [CrossRef]
- Yin, K.; Shao, M.; Zhang, Z.; Lin, Z. A single-source precursor route to Ag/SnO2 heterogeneous nanomaterials and its photo-catalysis in degradation of Conco Red. Mater. Res. Bull. 2012, 47, 3704–3708. [Google Scholar] [CrossRef]
- Li, J.; Hu, W.; Zhong, J.; Zeng, J.; Huang, S.; Xiao, Z.; Li, M. Photo-induced charge separation and photocatalytic activity of Ga-doped SnO2. Appl. Phys. A 2014, 116, 2149–2156. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Li, Y.; Zhang, J.; Du, Y.; Yang, Y.; Jiang, Y.; Lin, K. CdS@Polydopamine@SnO2-x sandwich structure with electrostatic repulsion effect and oxygen deficiency: Enhanced photocatalytic hydrogen evolution activity and inhibited photo-corrosion. Chem. Eng. J. 2022, 434, 134602. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-Enhanced Raman Spectroscopy for Bioanalysis: Reliability and Challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Zrimsek, A.B.; Chiang, N.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.I.; Schatz, G.C.; Van Duyne, R.P. Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hayden, E.Y.; Xia, M.; Liang, O.; Cheah, L.; Teplow, D.B.; Xie, Y.H. Surface enhanced Raman spectroscopy distinguishes amyloid Beta-protein isoforms and conformational states. Protein Sci. 2018, 27, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Kurouski, D.; Sorci, M.; Postiglione, T.; Belfort, G.; Lednev, I.K. Detection and structural characterization of insulin prefibrilar oligomers using surface enhanced Raman spectroscopy. Biotechnol. Prog. 2014, 30, 488–495. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Lu, H.P. Revealing the secondary structural changes of amyloid β peptide by probing the spectral fingerprint characters. J. Raman Spectrosc. 2013, 44, 670–674. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Taguchi, A.; Kawata, S. Deep-Ultraviolet Biomolecular Imaging and Analysis. Adv. Opt. Mater. 2018, 7, 1801099. [Google Scholar] [CrossRef]
- Dörfer, T.; Schmitt, M.; Popp, J. Deep-UV surface-enhanced Raman scattering. J. Raman Spectrosc. 2007, 38, 1379–1382. [Google Scholar] [CrossRef]
- Jha, S.K.; Ahmed, Z.; Agio, M.; Ekinci, Y.; Loffler, J.F. Deep-UV surface-enhanced resonance Raman scattering of adenine on aluminum nanoparticle arrays. J. Am. Chem. Soc. 2012, 134, 1966–1969. [Google Scholar] [CrossRef]
- Taguchi, A.; Hayazawa, N.; Furusawa, K.; Ishitobi, H.; Kawata, S. Deep-UV tip-enhanced Raman scattering. J. Raman Spectrosc. 2009, 40, 1324–1330. [Google Scholar] [CrossRef]
- Ren, B.; Lin, X.F.; Yang, Z.L.; Liu, G.K.; Aroca, R.F.; Mao, B.W.; Tian, Z.Q. Surface-Enhanced Raman Scattering in the Ultraviolet Spectral Region: UV-SERS on Rhodium and Ruthenium Electrodes. J. Am. Chem. Soc. 2003, 125, 9598–9599. [Google Scholar] [CrossRef]
- Das, R.; Soni, R.K. Rhodium nanocubes and nanotripods for highly sensitive ultraviolet surface-enhanced Raman spectroscopy. Analyst 2018, 143, 2310–2322. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, Y.; Taguchi, A.; Honda, M.; Watanabe, K.; Saito, Y.; Kawata, S. Indium for Deep-Ultraviolet Surface-Enhanced Resonance Raman Scattering. Acs Photonics 2014, 1, 598–603. [Google Scholar] [CrossRef]
- Das, R.; Soni, R.K. Highly stable In@SiO2 core-shell nanostructures for ultraviolet surface-enhanced Raman spectroscopy. Appl. Surf. Sci. 2019, 489, 755–765. [Google Scholar] [CrossRef]
- Bezerra, A.G.; Machado, T.N.; Woiski, T.D.; Turchetti, D.A.; Lenz, J.A.; Akcelrud, L.; Schreiner, W.H. Plasmonics and SERS activity of post-transition metal nanoparticles. J. Nanoparticle Res. 2018, 20, 142. [Google Scholar] [CrossRef]
- Li, B.; Wu, H.-H.; Luo, P.-F.; Lin, K.; Cheng, J.-G.; Zhong, H.-H.; Jiang, Y.; Lu, Y.-W. Size-Dependent Plasmonic Mode Evolution and SERS Performance of β-Sn Nanoparticles. J. Phys. Chem. C 2018, 123, 735–738. [Google Scholar] [CrossRef]
- Gaspar, D.; Pimentel, A.C.; Mendes, M.J.; Mateus, T.; Falcão, B.P.; Leitão, J.P.; Soares, J.; Araújo, A.; Vicente, A.; Filonovich, S.A.; et al. Ag and Sn Nanoparticles to Enhance the Near-Infrared Absorbance of a-Si:H Thin Films. Plasmonics 2014, 9, 1015–1023. [Google Scholar] [CrossRef]
- Jung, J.; Pedersen, T.G.; Søndergaard, T.; Pedersen, K.; Larsen, A.N.; Nielsen, B.B. On localized surface plasmons of metallic tin nanoparticles in silicon. Phys. Status Solidi (RRL) Rapid Res. Lett. 2010, 4, 292–294. [Google Scholar] [CrossRef]
- Turishchev, S.; Schleusener, A.; Chuvenkova, O.; Parinova, E.; Liu, P.; Manyakin, M.; Kurganskii, S.; Sivakov, V. Spectromicroscopy Studies of Silicon Nanowires Array Covered by Tin Oxide Layers. Small 2023, 19, e2206322. [Google Scholar] [CrossRef]
- Ming, T.; Turishchev, S.; Schleusener, A.; Parinova, E.; Koyuda, D.; Chuvenkova, O.; Schulz, M.; Dietzek, B.; Sivakov, V. Silicon Suboxides as Driving Force for Efficient Light-Enhanced Hydrogen Generation on Silicon Nanowires. Small 2021, 17, e2007650. [Google Scholar] [CrossRef]
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kim, J. Low temperature synthesis of porous tin oxide anode for high-performance lithium-ion battery. Electrochim. Acta 2013, 109, 461–467. [Google Scholar] [CrossRef]
- Su, X.; Lin, C.; Wang, X.; Maroni, V.A.; Ren, Y.; Johnson, C.S.; Lu, W. A new strategy to mitigate the initial capacity loss of lithium ion batteries. J. Power Sources 2016, 324, 150–157. [Google Scholar] [CrossRef]
- Dutta, A.; Kuzume, A.; Kaliginedi, V.; Rahaman, M.; Sinev, I.; Ahmadi, M.; Cuenya, B.R.; Vesztergom, S.; Broekmann, P. Probing the chemical state of tin oxide NP catalysts during CO2 electroreduction: A complementary operando approach. Nano Energy 2018, 53, 828–840. [Google Scholar] [CrossRef]
- Lei, F.; Liu, W.; Sun, Y.; Xu, J.; Liu, K.; Liang, L.; Yao, T.; Pan, B.; Wei, S.; Xie, Y. Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat. Commun. 2016, 7, 12697. [Google Scholar] [CrossRef]
- Turishchev, S.Y.; Parinova, E.V.; Pisliaruk, A.K.; Koyuda, D.A.; Yermukhamed, D.; Ming, T.; Ovsyannikov, R.; Smirnov, D.; Makarova, A.; Sivakov, V. Surface deep profile synchrotron studies of mechanically modified top-down silicon nanowires array using ultrasoft X-ray absorption near edge structure spectroscopy. Sci. Rep. 2019, 9, 8066. [Google Scholar] [CrossRef] [PubMed]
- Turishchev, S.Y.; Parinova, E.V.; Nesterov, D.N.; Koyuda, D.A.; Sivakov, V.; Schleusener, A.; Terekhov, V.A. Synchrotron studies of top-down grown silicon nanowires. Results Phys. 2018, 9, 1494–1496. [Google Scholar] [CrossRef]
- Sham, T.K.; Naftel, S.J.; Kim, P.S.G.; Sammynaiken, R.; Tang, Y.H.; Coulthard, I.; Moewes, A.; Freeland, J.W.; Hu, Y.F.; Lee, S.T. Electronic structure and optical properties of silicon nanowires: A study using x-ray excited optical luminescence and x-ray emission spectroscopy. Phys. Rev. B 2004, 70, 045313. [Google Scholar] [CrossRef]
- Moscu, A.; Theodoridi, C.; Cardenas, L.; Thieuleux, C.; Motta-Meira, D.; Agostini, G.; Schuurman, Y.; Meunier, F. CO dissociation on Pt-Sn nanoparticles triggers Sn oxidation and alloy segregation. J. Catal. 2018, 359, 76–81. [Google Scholar] [CrossRef]
- Manyakin, M.D.; Kurganskii, S.I.; Dubrovskii, O.I.; Chuvenkova, O.A.; Domashevskaya, E.P.; Ryabtsev, S.V.; Ovsyannikov, R.; Parinova, E.V.; Sivakov, V.; Turishchev, S.Y. Electronic and atomic structure studies of tin oxide layers using X-ray absorption near edge structure spectroscopy data modelling. Mater. Sci. Semicond. Process. 2019, 99, 28–33. [Google Scholar] [CrossRef]
- Pelliccione, C.J.; Timofeeva, E.V.; Segre, C.U. Potential-Resolved In Situ X-ray Absorption Spectroscopy Study of Sn and SnO2 Nanomaterial Anodes for Lithium-Ion Batteries. J. Phys. Chem. C 2016, 120, 5331–5339. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Shin, H.J.; Chae, K.H.; Won, S.O. X-ray absorption spectroscopy investigations on electronic structure and luminescence properties of Eu:SnO2-SnO nanocomposites. Current Appl. Phys. 2016, 16, 1342–1348. [Google Scholar] [CrossRef]
- Korusenko, P.M.; Nesov, S.N.; Bolotov, V.V.; Povoroznyuk, S.N.; Pushkarev, A.I.; Ivlev, K.E.; Smirnov, D.A. Formation of tin-tin oxide core shell nanoparticles in the composite SnO2-x/nitrogen doped carbon nanotubes by pulsed ion beam irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B 2017, 394, 37–43. [Google Scholar] [CrossRef]
- Böhme, S.; Philippe, B.; Edström, K.; Nyholm, L. Photoelectron Spectroscopic Evidence for Overlapping Redox Reactions for SnO2 Electrodes in Lithium-Ion Batteries. J. Phys. Chem. C 2017, 121, 4924–4936. [Google Scholar] [CrossRef]
- Manikandan, D.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Boukhvalov, D.W.; Murugan, R. XANES, EXAFS, EPR, and First-Principles Modeling on Electronic Structure and Ferromagnetism in Mn Doped SnO2 Quantum Dots. J. Phys. Chem. C 2019, 123, 3067–3075. [Google Scholar] [CrossRef]
- Kronast, F.; Schlichting, J.; Radu, F.; Mishra, S.K.; Noll, T.; Dürr, H.A. Spin-resolved photoemission microscopy and magnetic imaging in applied magnetic fields. Surf. Interface Anal. 2010, 42, 1532–1536. [Google Scholar] [CrossRef]
- Maillard-Schaller, E.; Boyanov, B.I.; English, S.; Nemanich, R.J. Role of the substrate strain in the sheet resistance stability of NiSi deposited on Si(100). J. Appl. Phys. 1999, 85, 3614–3618. [Google Scholar] [CrossRef]
- Renault, O.; Barrett, N.; Bailly, A.; Zagonel, L.F.; Mariolle, D.; Cezar, J.C.; Brookes, N.B.; Winkler, K.; Krömker, B.; Funnemann, D. Energy-filtered XPEEM with NanoESCA using synchrotron and laboratory X-ray sources: Principles and first demonstrated results. Surf. Sci. 2007, 601, 4727–4732. [Google Scholar] [CrossRef]
- Turishchev, S.Y.; Parinova, E.V.; Kronast, F.; Ovsyannikov, R.; Malashchenok, N.V.; Streltsov, E.A.; Ivanov, D.K.; Fedotov, A.K. Photoemission electron microscopy of arrays of submicron nickel rods in a silicon dioxide matrix. Phys. Solid State 2014, 56, 1916–1921. [Google Scholar] [CrossRef]
- Kasrai, M.; Lennard, W.N.; Brunner, R.W.; Bancroft, G.M.; Bardwell, J.A.; Tan, K.H. Sampling depth of total electron and fluorescence measurements in Si L- and K-edge absorption spectroscopy. Appl. Surf. Sci. 1996, 99, 303–312. [Google Scholar] [CrossRef]
- Barranco, A.; Yubero, F.; Espinos, J.P.; Gröning, P.; González-Elipe, A.R. Electronic state characterization of SiOx thin films prepared by evaporation. J. Appl. Phys. 2005, 97, 113714. [Google Scholar] [CrossRef]
- Terekhov, V.A.; Turishchev, S.Y.; Kashkarov, V.M.; Domashevskaya, E.P.; Mikhailov, A.N.; Tetel’baum, D.I. Silicon nanocrystals in SiO2 matrix obtained by ion implantation under cyclic dose accumulation. Phys. E Low-Dimens. Syst. Nanostruct. 2007, 38, 16–20. [Google Scholar] [CrossRef]
- Turishchev, S.Y.; Terekhov, V.A.; Parinova, E.V.; Korolik, O.V.; Mazanik, A.V.; Fedotov, A.K. Surface modification and oxidation of Si wafers after low energy plasma treatment in hydrogen, helium and argon. Mater. Sci. Semicond. Process. 2013, 16, 1377–1381. [Google Scholar] [CrossRef]
- Kucheyev, S.; Baumann, T.; Sterne, P.; Wang, Y.; van Buuren, T.; Hamza, A.; Terminello, L.; Willey, T. Surface electronic states in three-dimensional SnO2 nanostructures. Phys. Rev. B 2005, 72, 035404. [Google Scholar] [CrossRef]
- Domashevskaya, E.P.; Yurakov, Y.A.; Ryabtsev, S.V.; Chuvenkova, O.A.; Kashkarov, V.M.; Turishchev, S.Y. Synchrotron investigations of the initial stage of tin nanolayers oxidation. J. Electron Spectrosc. Relat. Phenom. 2007, 156, 340–343. [Google Scholar] [CrossRef]
- Domashevskaya, E.P.; Chuvenkova, O.A.; Ryabtsev, S.V.; Yurakov, Y.A.; Kashkarov, V.M.; Shchukarev, A.V.; Turishchev, S.Y. Electronic structure of undoped and doped SnOx nanolayers. Thin Solid Films 2013, 537, 137–144. [Google Scholar] [CrossRef]
- Zhou, J.G.; Fang, H.T.; Maley, J.M.; Ko, J.Y.P.; Murphy, M.; Chu, Y.; Sammynaiken, R.; Sham, T.K. An X-ray Absorption, Photoemission, and Raman Study of the Interaction between SnO2 Nanoparticle and Carbon Nanotube. J. Phys. Chem. C 2009, 113, 6114–6117. [Google Scholar] [CrossRef]
- Du, W.; Wang, Q.; Saxner, D.; Deskins, N.A.; Su, D.; Krzanowski, J.E.; Frenkel, A.I.; Teng, X. Highly active iridium/iridium-tin/tin oxide heterogeneous nanoparticles as alternative electrocatalysts for the ethanol oxidation reaction. J. Am. Chem. Soc. 2011, 133, 15172–15183. [Google Scholar] [CrossRef]
- Stöhr, J. NEXAFS Spectroscopy; Springer Berlin: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Hoffmann, P.; Mikalo, R.P.; Yfantis, A.; Batchelor, D.R.; Appel, G.; Yfantis, D.; Schmeißer, D. A Spectro-Microscopic Approach for Thin Film Analysis: Grain Boundaries in mc-Si and Sn/SnO2 Nano Particles. Mikrochim. Acta 2001, 136, 109–113. [Google Scholar] [CrossRef]
- Kolmakov, A.; Potluri, S.; Barinov, A.; Mentes, T.O.; Gregoratti, L.; Nino, M.A.; Locatelli, A.; Kiskinova, M. Spectromicroscopy for Addressing the Surface and Electron Transport Properties of Individual 1-D Nanostructures and Their Networks. ACS Nano 2008, 10, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Mogk, D. X-Ray Photoelectron Spectroscopy. Available online: https://serc.carleton.edu/msu_nanotech/methods/xps.html (accessed on 17 March 2023).
- Chuvenkova, O.A.; Domashevskaya, E.P.; Ryabtsev, S.V.; Yurakov, Y.A.; Popov, A.E.; Koyuda, D.A.; Nesterov, D.N.; Spirin, D.E.; Ovsyannikov, R.Y.; Turishchev, S.Y. XANES and XPS investigations of surface defects in wire-like SnO2 crystals. Phys. Solid State 2015, 57, 153–161. [Google Scholar] [CrossRef]
- Gago, R.; Prucnal, S.; Azpeitia, J.; Esteban-Mendoza, D.; Jiménez, I. Soft X-ray absorption study of sputtered tin oxide films. J. Alloys Compd. 2022, 902, 163768. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Spanopoulos, I.; Abed, J.; Ke, W.; Wicks, J.; Kanatzidis, M.G.; Sargent, E.H. Conventional Solvent Oxidizes Sn(II) in Perovskite Inks. ACS Energy Lett. 2020, 5, 1153–1155. [Google Scholar] [CrossRef]
- Zhou, X.T.; Heigl, F.; Murphy, M.W.; Shan, T.K.; Regier, T.; Coulthard, I.; Blyth, R.I.R. Time-resolved x-ray excited optical luminescence from SnO2 nanoribbons: Direct evidence for the origin of the blue luminescence and the role of surface states. Appl. Phys. Lett. 2006, 89, 213109. [Google Scholar] [CrossRef]
- Birrozzi, A.; Mullaliu, A.; Eisenmann, T.; Asenbauer, J.; Diemant, T.; Geiger, D.; Kaiser, U.; Oliveira de Souza, D.; Ashton, T.E.; Groves, A.R.; et al. Synergistic Effect of Co and Mn Co-Doping on SnO2 Lithium-Ion Anodes. Inorganics 2022, 10, 46. [Google Scholar] [CrossRef]
- Jeon, Y.-S.; Kang, D.-H.; Kim, J.-H.; Park, N.-G. Stability and efficiency improvement of perovskite solar cells by surface hydroxyl defect passivation of SnO2 layer with 4-fluorothiophenol. J. Mater. Chem. A 2023, 11, 3673–3681. [Google Scholar] [CrossRef]
- Smith, M.A.; Chen, M.; Dai, Z.; Antolini, C.; Jayasekara, G.K.; Yadavalli, S.K.; Reinhart, B.J.; Padture, N.P.; Hayes, D. Real-Time Investigation of Sn(II) Oxidation in Pb-Free Halide Perovskites by X-ray Absorption and Mössbauer Spectroscopy. ACS Appl. Energy Mater. 2021, 4, 4327–4332. [Google Scholar] [CrossRef]
- Liu, J.; Yao, H.; Wang, S.; Wu, C.; Ding, L.; Hao, F. Origins and Suppression of Sn(II)/Sn(IV) Oxidation in Tin Halide Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2300696. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Sivakov, V. Tin/Tin Oxide Nanostructures: Formation, Application, and Atomic and Electronic Structure Peculiarities. Nanomaterials 2023, 13, 2391. https://doi.org/10.3390/nano13172391
Liu P, Sivakov V. Tin/Tin Oxide Nanostructures: Formation, Application, and Atomic and Electronic Structure Peculiarities. Nanomaterials. 2023; 13(17):2391. https://doi.org/10.3390/nano13172391
Chicago/Turabian StyleLiu, Poting, and Vladimir Sivakov. 2023. "Tin/Tin Oxide Nanostructures: Formation, Application, and Atomic and Electronic Structure Peculiarities" Nanomaterials 13, no. 17: 2391. https://doi.org/10.3390/nano13172391
APA StyleLiu, P., & Sivakov, V. (2023). Tin/Tin Oxide Nanostructures: Formation, Application, and Atomic and Electronic Structure Peculiarities. Nanomaterials, 13(17), 2391. https://doi.org/10.3390/nano13172391







