Biomass-Derived Carbon Aerogels for ORR/OER Bifunctional Oxygen Electrodes
Abstract
:1. Introduction
2. Preparation and Modification of Biomass-Based CAs
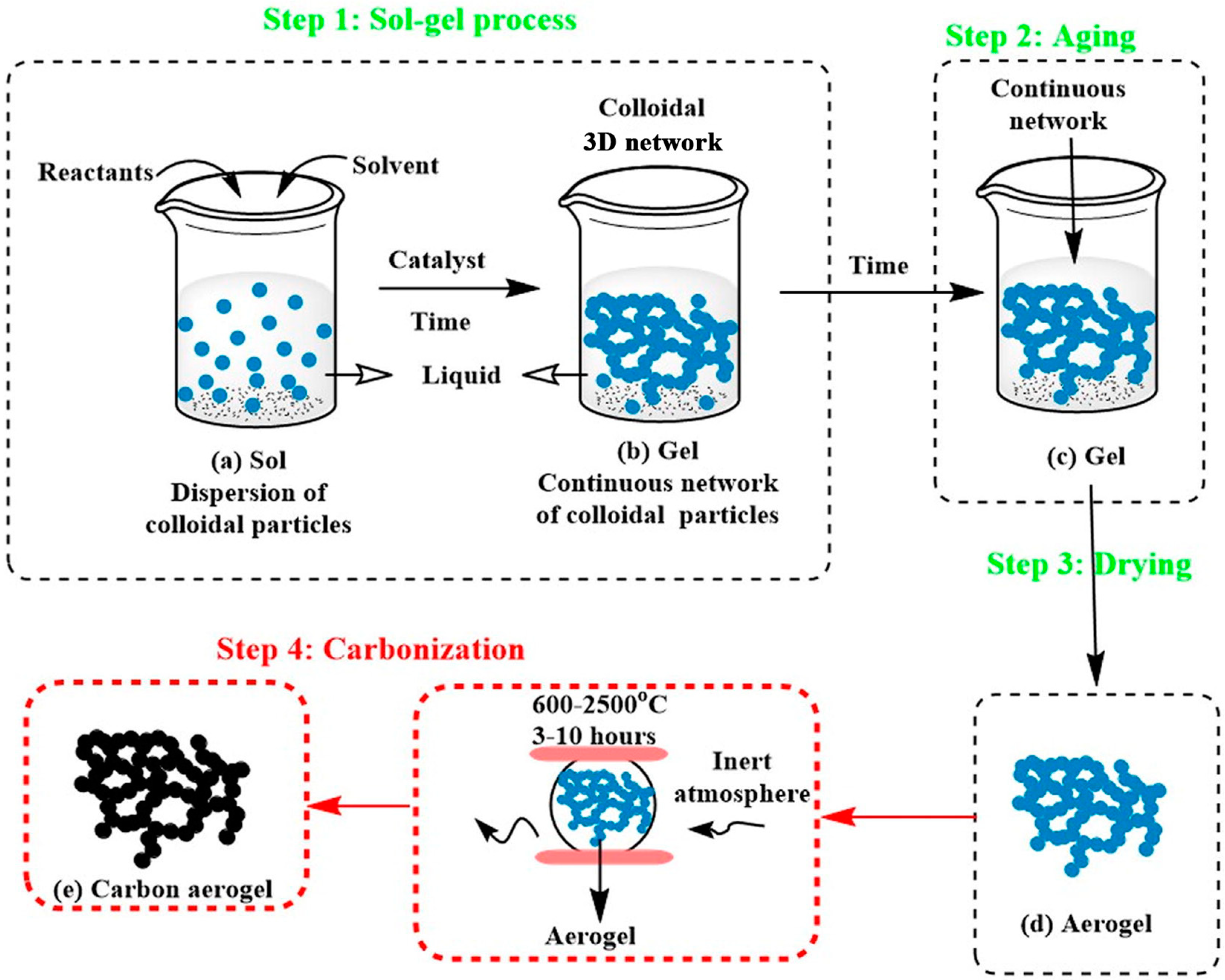
2.1. Sol-Gelation of Biomass-Based CAs
2.2. Drying of Biomass-Based CAs
- (1)
- Ambient pressure drying
- (2)
- Supercritical drying
- (3)
- Freeze drying
2.3. Carbonization of Biomass-Based CAs
2.4. Functional Modification
3. Research Progress of Biomass-Based CA for ORR/OER
3.1. Mechanism
3.1.1. ORR Reaction Mechanism
- (1)
- Under alkaline electrolytes:
- (2)
- Under acidic electrolytes:
3.1.2. OER Reaction Mechanism
- (1)
- Under alkaline electrolytes:
- (2)
- Under acidic electrolytes:
3.2. Non-Metal-Doped Biomass-Based CA Modified Catalyst
- (1)
- (2)
- Also, the multiscale porosity of CA is a crucial factor in determining their ORR catalytic activity. Micropores can provide a larger surface area and more active sites, which are beneficial for catalytic reactions. Mesopores can offer better transportation channels, facilitating the diffusion and transfer of reactants and products. Macropores can provide better fluidity, enabling the uniform distribution of reactants and regeneration of the catalyst [70,71].
- (3)
- The large specific surface area and 3D hierarchical porous structure are conducive to the full exposure of the ORR active sites.
- (4)
3.2.1. Mono-Doped CA
3.2.2. Dual-Doped CAs
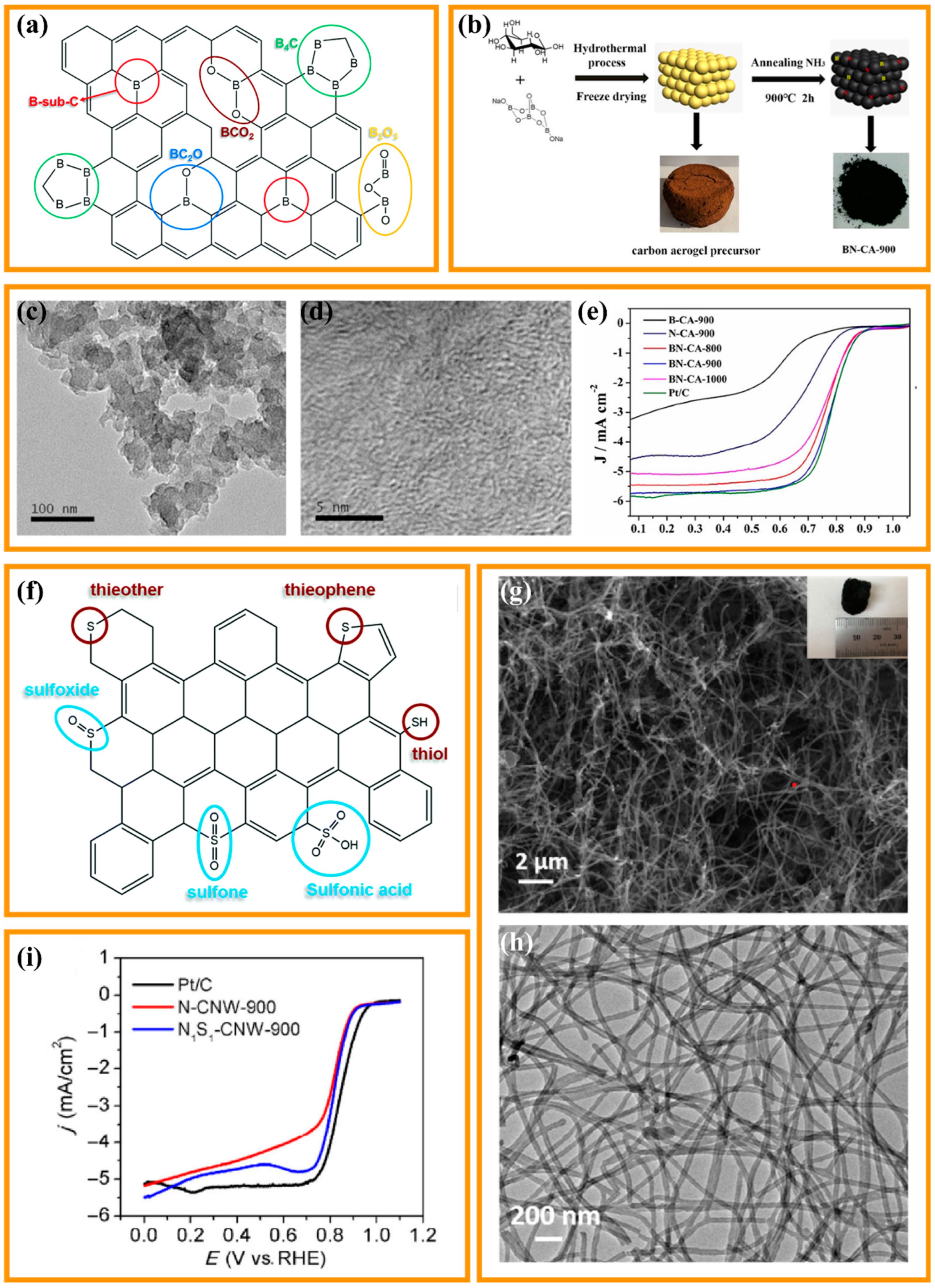
3.3. Biomass-Based CA Supported Non-Noble (Transition) Metal Modified Catalysts
3.3.1. Single Atom
3.3.2. Transition Metal Compounds
3.3.3. Metal Alloy
| Catalyst | Electrolyte | ORR and OER Performance | Metal–Air Batteries Performance | Ref | |||
|---|---|---|---|---|---|---|---|
| ORR Performance | OER Performance | Open Circuit Potential (V) | Peak Power Density (mW cm−2) | ||||
| Eonset (V vs. RHE) | E1/2 (V vs. RHE) | E10 (mV vs. RHE) | |||||
| N-CNFs | 0.1 M KOH | −0.23 (V vs. Ag/AgCl) | −0.18 (V vs. Ag/AgCl) | [79] | |||
| Pt/C | 0.1 M KOH | −0.18 (V vs. Ag/AgCl) | −0.11 (V vs. Ag/AgCl) | ||||
| N-CNF | 0.1 M KOH | 0.83 | 0.80 | 1.5 | [80] | ||
| Pt/C | 0.1 M KOH | 0.85 | 0.75 | 1.5 | |||
| BN-CA-900 | 0.1 MKOH | 0.91 | 0.77 | [81] | |||
| Pt/C | 0.1 M KOH | 0.93 | 0.78 | ||||
| N1S1-CNW-900 | 0.1 M KOH | 0.905 | 0.838 | [82] | |||
| Pt/C | 0.1 M KOH | 0.931 | 0.851 | ||||
| Fe-N-CA | 0.1 M KOH | 0.918 | 0.798 | [87] | |||
| 0.5 M H2SO4 | 0.801 | 0.592 | |||||
| Pt/C | 0.1 M KOH | 0.958 | 0.833 | ||||
| 0.5 M H2SO4 | 0.921 | 0.701 | |||||
| NCALR/Fe | 0.1 M KOH | 1.05 | 0.88 | 1.81 | 181.1 | [88] | |
| Pt/C | 0.1 M KOH | 0.99 | 0.84 | 1.80 | 175.1 | ||
| FeNC-900-8 | 0.1 M KOH | 0.88 | 1.67 | 124.9 | [89] | ||
| Pt/C | 0.1 M KOH | 0.84 | 1.50 | 87.6 | |||
| Fe-N-CNTA | 0.1 M KOH | 0.97 | 0.88 | [90] | |||
| Pt/C | 0.1 M KOH | 0.96 | 0.86 | ||||
| Co-SAs/NCNA | 0.1 M KOH | 0.98 | 0.86 | 1.49 | 206 | [91] | |
| Pt/C | 0.1 M KOH | 0.95 | 0.84 | 167 | |||
| FeP/Fe2O3@NPCA | 0.1 M KOH | 0.95 | 0.838 | 464 | 1.428 | 130 | [92] |
| Pt/C | 0.1 M KOH | 0.926 | 0.842 | 402 | 1.3 | 108 | |
| CoFe2O4/CNF | 0.1 M KOH | −0.09 (V vs. Ag/AgCl) | −0.21 (V vs. Ag/AgCl) | 800 (mV vs. Ag/AgCl) | [93] | ||
| Pt/C | 0.1 M KOH | −0.07 (V vs. Ag/AgCl) | −0.138(V vs. Ag/AgCl) | 760 (mV vs. Ag/AgCl) | |||
| HDCA-800 | 0.1 M KOH | 0.94 | 0.79 | [94] | |||
| Pt/C | 0.1 M KOH | 0.98 | 0.80 | ||||
| Co9S8/N,P-APC | 0.1 M KOH | 0.89 | 0.78 | 363 | [16] | ||
| Pt/C | 0.1 M KOH | 0.98 | 0.83 | 368 | |||
| FeCo@NS-CA | 0.1 M KOH | 0.97 | 0.85 | 450 | 1.40 | 140 | [98] |
| Pt/C | 0.1 M KOH | 1.00 | 0.85 | 420 | 1.40 | 122 | |
| FeCo/N-DNC | 0.1 M KOH | 0.98 | 0.81 | 390 | 1.50 | 115 | [14] |
| Pt/C | 0.1 M KOH | 0.98 | 0.84 | 380 | 1.50 | 109 | |
| NCAG/Fe-Co | 0.1 M KOH | 1.04 | 0.89 | 293 | 1.47 | 117 | [101] |
| Pt/C | 0.1 M KOH | 0.96 | 0.83 | 297 | 1.36 | 92 | |
| NiCo/PFC | 0.1 M KOH | 0.92 | 0.79 | 400 | [102] | ||
| Pt/C | 0.1 M KOH | 0.94 | 0.84 | 390 | |||
| NSCA/FeCo | 0.1 M KOH | 0.97 | 0.82 | 335 | 1.496 | 132 | [103] |
| Pt/C | 0.1 M KOH | 0.92 | 0.821 | 332 | 1.496 | 101.8 | |
| Fe-SA@PNC | 0.1 M KOH | 0.98 | 0.87 | 1.45 | 149 | [104] | |
| Pt/C | 0.1 M KOH | 0.95 | 0.83 | 1.40 | 119 | ||
4. Summary and Outlook
- (1)
- Complex process for preparing CAs. Commonly used synthesis processes are supercritical drying and freeze drying, which consume a large amount of energy for the drying process. In addition, the existing technology cannot freely adjust the structure of the holes in a wide range. Also, the poor mechanical properties of CA are limited in industrial production.
- (2)
- At present, research on the modulation of the electronic structure of catalysts by CAs, the regulation of heteroatom doping configuration, the interaction mechanism between CAs and catalysts, and the catalytic mechanism of CA-based catalysts is inadequate. Therefore, it is imperative to conduct a deep and systematic study of the active sites and reaction mechanisms of carbon-based catalysts through theoretical calculations, in situ characterization, and electrochemical testing techniques, and to prepare and optimize the proper catalyst structure under the guidance of basic theories.
- (3)
- The wide variety of biomass leads to certain differences in the structures and compositions among them. Therefore, it is particularly significant to focus on improving the universality of the preparation method of biomass carbon-based catalysts by adjusting the uniformity of catalyst structure and catalytic active sites.
- (4)
- Due to the diversity and compositional complexity of biomass, the specific chemical composition of biomass carbon obtained after the activated carbonization of biomass, including the percentage of heteroatoms, cannot be precisely controlled, making it difficult to ensure the consistency of its performance as an electrode for metal–air batteries.
- (5)
- Multiple steps are required to prepare most biomass-based CA materials such as biomass decomposition and carbonization. For the preparation of air electrodes, powder carbon materials need to be additionally sprayed onto carbon cloth, reducing the energy efficiency. Therefore, the rational maintaining of the original structure of biomass to prepare 3D integrated catalytic electrode materials has significant application prospects.
- (6)
- CA-supported non-noble metal catalysts and non-metal doped CA catalysts are the main directions for the future development of fuel cell catalysts. However, both still face application problems such as low catalytic activity, poor stability, doping technology load, etc. Therefore, further improvements in the performance of these two catalysts are necessary. It is believed that CA-based catalyst technology could be applicable in electrochemical catalysis in the near future.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.X.; Yang, X.; Liu, H.; Han, T.; Hu, J.; Li, H.; Wu, G. Air Electrodes for Flexible and Rechargeable Zn−Air Batteries. Small Struct. 2021, 3, 2100103. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.P.; Wang, J.; Jiang, Y.; Cui, G.; Shui, L.; Yu, A.; Wang, X.; Chen, Z. Recent Progress on Flexible Zn-Air Batteries. Energy Stor. Mater. 2021, 35, 538–549. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced Architectures and Relatives of Air Electrodes in Zn-Air Batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Gomez, I.; Lizundia, E. Biomimetic Wood-Inspired Batteries: Fabrication, Electrochemical Performance, and Sustainability within a Circular Perspective. Adv. Sustain. Syst. 2021, 5, 2100236. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Jarvis, K.A.; Therese, S.; Ferreira, P.J.; Manthiram, A. Spinel-type lithium cobalt oxide as a bifunctional electrocatalyst for the oxygen evolution and oxygen reduction reactions. Nat. Commun. 2014, 5, 3949. [Google Scholar] [CrossRef]
- Huang, Z.F.; Wang, J.; Peng, Y.; Jung, C.Y.; Fisher, A.; Wang, X. Design of Efficient Bifunctional Oxygen Reduction/Evolution Electrocatalyst: Recent Advances and Perspectives. Adv. Energy Mater. 2017, 7, 1700544. [Google Scholar] [CrossRef]
- Paul, R.; Dai, Q.; Hu, C.; Dai, L. Ten years of carbon-based metal-free electrocatalysts. Carbon Energy 2019, 1, 19–31. [Google Scholar] [CrossRef]
- Aasen, D.; Clark, M.P.; Ivey, D.G. (Co,Fe)3O4 Decorated Nitrogen-Doped Carbon Nanotubes in Nano-Composite Gas Diffusion Layers as Highly Stable Bifunctional Catalysts for Rechargeable Zinc-Air Batteries. Batter. Supercaps 2019, 3, 174–184. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, N.; Wu, C.; Xie, Y. Surface/interface nanoengineering for rechargeable Zn–air batteries. Energy Environ. Sci. 2020, 13, 1132–1153. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, L.; Luo, H.; Mustain, W.; Xu, H. Recent progress and perspectives of bifunctional oxygen reduction/evolution catalyst development for regenerative anion exchange membrane fuel cells. Nano Energy 2018, 47, 172–198. [Google Scholar] [CrossRef]
- Liu, X.; Park, M.; Kim, M.G.; Gupta, S.; Wu, G.; Cho, J. Integrating NiCo Alloys with Their Oxides as Efficient Bifunctional Cathode Catalysts for Rechargeable Zinc-Air Batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 9654–9658. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Yan, X.; Chen, Y.; Xu, L.; Sun, D.; Lee, J.M.; Tang, Y. Boosting Bifunctional Oxygen Electrocatalysis with 3D Graphene Aerogel-Supported Ni/MnO Particles. Adv. Mater. 2018, 30, 1704609. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Liu, Y.; Chen, Y.; Tang, Y.; Goodenough, J.B.; Lee, J.M. Robust N-doped carbon aerogels strongly coupled with iron-cobalt particles as efficient bifunctional catalysts for rechargeable Zn-air batteries. Nanoscale 2018, 10, 19937–19944. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, R.F.; Chen, X.M.; Tang, Y.H.; Qiao, S.Z. Fe-N decorated hybrids of CNTs grown on hierarchically porous carbon for high-performance oxygen reduction. Adv. Mater. 2014, 26, 6074–6079. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Zhang, M.; Fu, G.; Sun, D.; Lee, J.M.; Tang, Y. Alveolate porous carbon aerogels supported Co9S8 derived from a novel hybrid hydrogel for bifunctional oxygen electrocatalysis. Carbon 2019, 144, 557–566. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Wu, G.; Santandreu, A.; Kellogg, W.; Gupta, S.; Ogoke, O.; Zhang, H.; Wang, H.L.; Dai, L. Carbon nanocomposite catalysts for oxygen reduction and evolution reactions: From nitrogen doping to transition-metal addition. Nano Energy 2016, 29, 83–110. [Google Scholar] [CrossRef]
- Kobina Sam, D.; Kobina Sam, E.; Lv, X. Application of Biomass-Derived Nitrogen-Doped Carbon Aerogels in Electrocatalysis and Supercapacitors. ChemElectroChem 2020, 7, 3695–3712. [Google Scholar] [CrossRef]
- Peles-Strahl, L.; Persky, Y.; Elbaz, L. Design of advanced aerogel structures for oxygen reduction reaction electrocatalysis. SusMat 2023, 3, 44–57. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Tang, C.; Wang, J.; Zhang, Q.; Wang, Y.; Zhang, J. Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal–Air Batteries. Adv. Mater. 2019, 31, 1803800. [Google Scholar] [CrossRef]
- Shao, W.; Yan, R.; Zhou, M.; Ma, L.; Roth, C.; Ma, T.; Cao, S.; Cheng, C.; Yin, B.; Li, S. Carbon-Based Electrodes for Advanced Zinc-Air Batteries: Oxygen-Catalytic Site Regulation and Nanostructure Design. Electrochem. Energy R. 2023, 6, 11. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.J. Recent advances in preparations and applications of carbon aerogels: A review. Carbon 2020, 163, 1–18. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, H.; Zhu, Q.; Bediako, B.B.A.; Han, B. Boosting CO2 Electroreduction on N,P-Co-doped Carbon Aerogels. Angew. Chem. Int. Ed. Engl. 2020, 59, 11123–11129. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Cheng, J.; Yu, J.H.; Tian, X.L.; Zhu, M.-Q. Graphene aerogel with excellent property prepared by doping activated carbon and CNF for free-binder supercapacitor. Carbohydr. Polym. 2022, 286, 119287. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Cai, C.; Liu, K.; Liu, W.; Zhang, M.; Du, H.; Si, C.; Zhang, K. Multifunctional Superelastic, Superhydrophilic, and Ultralight Nanocellulose-Based Composite Carbon Aerogels for Compressive Supercapacitor and Strain Sensor. Adv. Funct. Mater. 2022, 32, 2113082. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Du, T.; Tian, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. Green and sustainable carbon aerogels from starch for supercapacitors and oil-water separation. Ceram. Int. 2021, 47, 22080–22087. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, L.J.; Yang, S.L.; Li, D.H.; Xie, Z.X.; Wang, B.B.; Xia, Y.Z.; Quan, F.Y. Facile strategy to produce N-doped carbon aerogels derived from seaweed for lithium-ion battery anode. J. Alloys Compd. 2017, 701, 256–261. [Google Scholar] [CrossRef]
- Tian, Y.; Estevez, D.; Wei, H.J.; Peng, M.Y.; Zhou, L.P.; Xu, P.; Wu, C.; Yan, M.; Wang, H.A.; Peng, H.X.; et al. Chitosan-derived carbon aerogels with multiscale features for efficient microwave absorption. Chem. Eng. J. 2021, 421, 129781. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Long, S.S.; Feng, Y.C.; He, F.-L.; Zhao, J.Z.; Bai, T.; Lin, H.B.; Cai, W.L.; Mao, C.W.; Chen, Y.H.; Gan, L.H.; et al. Biomass-derived, multifunctional and wave-layered carbon aerogels toward wearable pressure sensors, supercapacitors and triboelectric nanogenerators. Nano Energy 2021, 85, 105973. [Google Scholar] [CrossRef]
- Lai, F.; Miao, Y.E.; Zuo, L.; Zhang, Y.; Liu, T. Carbon Aerogels Derived from Bacterial Cellulose/Polyimide Composites as Versatile Adsorbents and Supercapacitor Electrodes. ChemNanoMat 2016, 2, 212–219. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Schneider, M.; Baiker, A. Aerogels in Catalysis. Catal. Rev. 1995, 37, 515–556. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Ye, Y.; Qiu, Z.; Zhang, Y.; Yu, Z.; Sun, X.; Bressler, D.C.; Jiang, F. Superelastic and Ultralight Aerogel Assembled from Hemp Microfibers. Adv. Funct. Mater. 2023, 33, 2300893. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Zhu, T.; Chang, S.; Wang, W. CoFe2O4/N-doped reduced graphene oxide aerogels for high-performance microwave absorption. Chem. Eng. J. 2020, 388, 124317. [Google Scholar] [CrossRef]
- Wan, C.; Lu, Y.; Jiao, Y.; Jin, C.; Sun, Q.; Li, J. Fabrication of hydrophobic, electrically conductive and flame-resistant carbon aerogels by pyrolysis of regenerated cellulose aerogels. Carbohydr. Polym. 2015, 118, 115–118. [Google Scholar] [CrossRef]
- Meng, F.; Li, L.; Wu, Z.; Zhong, H.; Li, J.; Yan, J. Facile preparation of N-doped carbon nanofiber aerogels from bacterial cellulose as an efficient oxygen reduction reaction electrocatalyst. Chin. J. Catal. 2014, 35, 877–883. [Google Scholar] [CrossRef]
- Li, Y.Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon Aerogel from Winter Melon for Highly Efficient and Recyclable Oils and Organic Solvents Absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Luo, W.; Wang, B.; Heron, C.G.; Allen, M.J.; Morre, J.; Maier, C.S.; Stickle, W.F.; Ji, X. Pyrolysis of Cellulose under Ammonia Leads to Nitrogen-Doped Nanoporous Carbon Generated through Methane Formation. Nano Lett. 2014, 14, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, M.; Li, B.; Song, X.; Yu, G.; Zhang, J.; Zhang, H.; Xu, D. Synergy mechanism of biochar and petcoke co-combustion based on potassium migration and transformation. Fuel Process. Technol. 2023, 250, 107927. [Google Scholar] [CrossRef]
- Ramirez, N.; Zámbó, D.; Sardella, F.; Kißling, P.A.; Schlosser, A.; Graf, R.T.; Pluta, D.; Deiana, C.; Bigall, N.C. Pd-Doped Cellulose Carbon Aerogels for Energy Storage Applications. Adv. Mater. Interfaces 2021, 8, 2100310. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Zhang, L.; Huang, Y.; Lu, H.; Fan, W.; Liu, T. Cotton Wool Derived Carbon Fiber Aerogel Supported Few-Layered MoSe2 Nanosheets As Efficient Electrocatalysts for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2016, 8, 7077–7085. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kou, S.; Li, Z.; Chang, Z.; Wang, M.; Liu, Z.; Lu, G. 3D interconnected nitrogen-self-doped carbon aerogels as efficient oxygen reduction electrocatalysts derived from biomass gelatin. RSC Adv. 2019, 9, 40301–40308. [Google Scholar] [CrossRef]
- Hu, Y.; Tong, X.; Zhuo, H.; Zhong, L.; Peng, X.; Wang, S.; Sun, R. 3D hierarchical porous N-doped carbon aerogel from renewable cellulose: An attractive carbon for high-performance supercapacitor electrodes and CO2 adsorption. RSC Adv. 2016, 6, 15788–15795. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Chu, M.; Wang, X.; Wang, Y.; Lin, X.; Cao, X. Mono-Doped Carbon Nanofiber Aerogel as a High-Performance Electrode Material for Rechargeable Zinc-Air Batteries. ChemElectroChem 2021, 8, 829–838. [Google Scholar] [CrossRef]
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, 1804881. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.-K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef]
- Cai, B.; Sayevich, V.; Gaponik, N.; Eychmüller, A. Emerging Hierarchical Aerogels: Self-Assembly of Metal and Semiconductor Nanocrystals. Adv. Mater. 2018, 30, 1707518. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal–air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Bhoyate, S.D.; Kim, J.; de Souza, F.M.; Lin, J.; Lee, E.; Kumar, A.; Gupta, R.K. Science and engineering for non-noble-metal-based electrocatalysts to boost their ORR performance: A critical review. Coord. Chem. Rev. 2023, 474, 214854. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, X.; Li, Q.; Luo, L.; Ding, M.; Zhang, B.; Li, Y.; Zou, J.; Jiang, B. Recent progress of electrocatalysts for oxygen reduction in fuel cells. J. Colloid Interface Sci. 2022, 607, 791–815. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Gao, R.; Wang, J.; Huang, Z.F.; Zhang, R.; Wang, W.; Pan, L.; Zhang, J.; Zhu, W.; Zhang, X.; Shi, C.; et al. Pt/Fe2O3 with Pt–Fe pair sites as a catalyst for oxygen reduction with ultralow Pt loading. Nat. Energy 2021, 6, 614–623. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Siahrostami, S.; Patel, A.; Nørskov, J.K. Understanding Catalytic Activity Trends in the Oxygen Reduction Reaction. Chem. Rev. 2018, 118, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Qu, Z.W.; Zhu, H.; Kroes, G.J.; Nørskov, J.K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 2007, 607, 83–89. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent Progress on Multimetal Oxide Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Ren, S.; Duan, X.; Liang, S.; Zhang, M.; Zheng, H. Bifunctional electrocatalysts for Zn–air batteries: Recent developments and future perspectives. J. Mater. Chem. A 2020, 8, 6144–6182. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Chen, X.; Pan, M.; Mu, S. Egg derived nitrogen-self-doped carbon/carbon nanotube hybrids as noble-metal-free catalysts for oxygen reduction. J. Power Sources 2014, 271, 522–529. [Google Scholar] [CrossRef]
- Monteverde Videla, A.H.A.; Zhang, L.; Kim, J.; Zeng, J.; Francia, C.; Zhang, J.; Specchia, S. Mesoporous carbons supported non-noble metal Fe–NXelectrocatalysts for PEM fuel cell oxygen reduction reaction. J. Appl. Electrochem. 2013, 43, 159–169. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.P.; Wu, G.; Chung, H.T.; Johnston, C.-M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, K.; Su, B.; Jiang, L. Superhydrophobicity-Mediated Electrochemical Reaction Along the Solid–Liquid–Gas Triphase Interface: Edge-Growth of Gold Architectures. Adv. Mater. 2014, 26, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Y. Microfluidic Synthesis of Nanohybrids. Small 2017, 13, 1604084. [Google Scholar] [CrossRef] [PubMed]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Yang, W.; Jia, J. Recent advances in carbon-based electrocatalysts for oxygen reduction reaction. Chin. Chem. Lett. 2020, 31, 626–634. [Google Scholar] [CrossRef]
- Sharifi, T.; Gracia-Espino, E.; Chen, A.; Hu, G.; Wågberg, T. Oxygen Reduction Reactions on Single- or Few-Atom Discrete Active Sites for Heterogeneous Catalysis. Adv. Energy Mater. 2020, 10, 1902084. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, M.; Tan, C.; Li, M.; Liang, L.; Li, K.; Su, P. KOH activated N-doped novel carbon aerogel as efficient metal-free oxygen reduction catalyst for microbial fuel cells. Chem. Eng. J. 2018, 348, 775–785. [Google Scholar] [CrossRef]
- Song, L.T.; Wu, Z.Y.; Liang, H.W.; Zhou, F.; Yu, Z.Y.; Xu, L.; Pan, Z.; Yu, S.H. Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Liang, H.W.; Wu, Z.Y.; Chen, L.F.; Li, C.; Yu, S.H. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: An efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 2015, 11, 366–376. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Yuan, W.; Shen, Y.; Xie, A. B, N Co-Doped Three-Dimensional Carbon Aerogels with Excellent Electrochemical Performance for the Oxygen Reduction Reaction. Chemistry 2019, 25, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhu, C.; Song, J.; Engelhard, M.H.; Li, X.; Zhang, P.; Xia, H.; Du, D.; Lin, Y. Template-directed synthesis of nitrogen- and sulfur-codoped carbon nanowire aerogels with enhanced electrocatalytic performance for oxygen reduction. Nano Res. 2016, 10, 1888–1895. [Google Scholar] [CrossRef]
- Jiang, W.J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.J.; Wang, J.Q.; Hu, J.S.; Wei, Z.; Wan, L.J. Understanding the High Activity of Fe–N–C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe–Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Gao, D.; Miao, S.; Wang, G.; Bao, X. High-density iron nanoparticles encapsulated within nitrogen-doped carbon nanoshell as efficient oxygen electrocatalyst for zinc–air battery. Nano Energy 2015, 13, 387–396. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single-Atom Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Wu, N.; Wang, B.; He, W.; Lei, Y.; Wang, Y. N-Doped 3D Carbon Aerogel with Trace Fe as an Efficient Catalyst for the Oxygen Reduction Reaction. ChemElectroChem 2017, 4, 514–520. [Google Scholar] [CrossRef]
- He, T.; Zhang, Y.; Chen, Y.; Zhang, Z.; Wang, H.; Hu, Y.; Liu, M.; Pao, C.W.; Chen, J.L.; Chang, L.Y.; et al. Single iron atoms stabilized by microporous defects of biomass-derived carbon aerogels as high-performance cathode electrocatalysts for aluminum–air batteries. J. Mater. Chem. A 2019, 7, 20840–20846. [Google Scholar] [CrossRef]
- Hao, R.; Gu, S.; Chen, J.; Wang, Z.; Gan, Q.; Wang, Z.; Huang, Y.; Liu, P.; Zhang, K.; Liu, K.; et al. Microporous Fe–N4 cataysts derived from biomass aerogel for a high-performance Zn–air battery. Mater. Today Energy 2021, 21, 100826. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Song, J.; Shi, Q.; Su, D.; Engelhard, M.-H.; Li, X.; Xiao, D.; Li, D.; Estevez, L.; et al. Self-Assembled Fe-N-Doped Carbon Nanotube Aerogels with Single-Atom Catalyst Feature as High-Efficiency Oxygen Reduction Electrocatalysts. Small 2017, 13, 1603407. [Google Scholar] [CrossRef]
- Shen, M.; Hu, W.; Duan, C.; Li, J.; Ding, S.; Zhang, L.; Zhu, J.; Ni, Y. Cellulose nanofibers carbon aerogel based single-cobalt-atom catalyst for high-efficiency oxygen reduction and zinc-air battery. J. Colloid Interface Sci. 2023, 629, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, L.; Yuan, Y.; Zhong, L.; Chen, Z.; Chi, X.; Lu, H.; Chen, Z.; Zou, R.; Li, T.; et al. An Iron-Decorated Carbon Aerogel for Rechargeable Flow and Flexible Zn-Air Batteries. Adv. Mater. 2020, 32, 2002292. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yan, W.; Cao, X.; Zhou, Z.; Yang, R. Bacterial-cellulose-derived carbon nanofiber-supported CoFe2O4 as efficient electrocatalyst for oxygen reduction and evolution reactions. Int. J. Hydrog. Energy 2016, 41, 5351–5360. [Google Scholar] [CrossRef]
- Li, C.; Sun, F.; Lin, Y. Refining cocoon to prepare (N, S, and Fe) ternary-doped porous carbon aerogel as efficient catalyst for the oxygen reduction reaction in alkaline medium. J. Power Sources 2018, 384, 48–57. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.X.; Liu, J.N.; Song, Y.W.; Huang, J.-Q.; Li, B.-Q. Dual-atom catalysts for oxygen electrocatalysis. Nano Energy 2022, 104, 107927. [Google Scholar] [CrossRef]
- Yu, D.; Ma, Y.; Hu, F.; Lin, C.C.; Li, L.; Chen, H.Y.; Han, X.; Peng, S. Dual-Sites Coordination Engineering of Single Atom Catalysts for Flexible Metal–Air Batteries. Adv. Energy Mater. 2021, 11, 2101242. [Google Scholar] [CrossRef]
- Wang, Y.; Kumar, A.; Ma, M.; Jia, Y.; Wang, Y.; Zhang, Y.; Zhang, G.; Sun, X.; Yan, Z. Hierarchical peony-like FeCo-NC with conductive network and highly active sites as efficient electrocatalyst for rechargeable Zn-air battery. Nano Res. 2020, 13, 1090–1099. [Google Scholar] [CrossRef]
- Pang, H.; Sun, P.; Gong, H.; Zhang, N.; Cao, J.; Zhang, R.; Luo, M.; Li, Y.; Sun, G.; Li, Y.; et al. Wood-Derived Bimetallic and Heteroatomic Hierarchically Porous Carbon Aerogel for Rechargeable Flow Zn-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 39458–39469. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, J.; Hu, Y.; Jin, Z.; Hu, K.; Reddy, K.M.; Yuan, Q.; Lin, X.; Qiu, H.-J. Theoretically Revealed and Experimentally Demonstrated Synergistic Electronic Interaction of CoFe Dual-Metal Sites on N-doped Carbon for Boosting Both Oxygen Reduction and Evolution Reactions. Nano Lett. 2022, 22, 3392–3399. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R.; Sun, Y.; Liu, Q.; Xia, M.; Li, Y.; Gao, F.; Zhao, Y.; Tse, J.-S. Identifying the Zn–Co binary as a robust bifunctional electrocatalyst in oxygen reduction and evolution reactions via shifting the apexes of the volcano plot. J. Energy Chem. 2021, 55, 162–168. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, S.; Nichols, F.; Bridges, F.; Kan, S.; He, T.; Zhang, Y.; Chen, S. Carbon aerogels with atomic dispersion of binary iron-cobalt sites as effective oxygen catalysts for flexible zinc-air batteries. J. Energy Chem. A 2020, 8, 11649–11655. [Google Scholar] [CrossRef]
- Fu, G.; Chen, Y.; Cui, Z.; Li, Y.; Zhou, W.; Xing, S.; Tang, Y.; Goodenough, J.B. Novel Hydrogel-Derived Bifunctional Oxygen Electrocatalyst for Rechargeable Air Cathodes. Nano Lett. 2016, 16, 6516–6522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Li, Y.; Wang, J.; Kawi, S.; Zhong, Q. FeCo alloy/N, S co-doped carbon aerogel derived from directional-casting cellulose nanofibers for rechargeable liquid flow and flexible Zn-air batteries. Nano Res. 2023, 16, 6870–6880. [Google Scholar] [CrossRef]
- Shen, M.; Qi, J.; Gao, K.; Duan, C.; Liu, J.; Liu, Q.; Yang, H.; Ni, Y. Chemical vapor deposition strategy for inserting atomic FeN4 sites into 3D porous honeycomb carbon aerogels as oxygen reduction reaction catalysts in high-performance Zn-air batteries. Chem. Eng. J. 2023, 464, 142719. [Google Scholar] [CrossRef]






| Aerogel Categories | High Specific Surface Area | Hierarchical Porous Structure | Conducting Skeleton | Self-Supportability | Renewable Resource |
|---|---|---|---|---|---|
| Biomass-derived carbon aerogels | Yes | Yes | Yes | Yes | Yes |
| Oxide aerogels (e.g., Silica aerogel) | Yes | Yes | No | Yes | No |
| Pure metallic aerogels | Yes | Yes | Yes | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Xu, K.; Xiao, H.; Mei, C.; Li, J. Biomass-Derived Carbon Aerogels for ORR/OER Bifunctional Oxygen Electrodes. Nanomaterials 2023, 13, 2397. https://doi.org/10.3390/nano13172397
Jiao Y, Xu K, Xiao H, Mei C, Li J. Biomass-Derived Carbon Aerogels for ORR/OER Bifunctional Oxygen Electrodes. Nanomaterials. 2023; 13(17):2397. https://doi.org/10.3390/nano13172397
Chicago/Turabian StyleJiao, Yue, Ke Xu, Huining Xiao, Changtong Mei, and Jian Li. 2023. "Biomass-Derived Carbon Aerogels for ORR/OER Bifunctional Oxygen Electrodes" Nanomaterials 13, no. 17: 2397. https://doi.org/10.3390/nano13172397







