3.1. Materials Synthesis and Characterization
The synthesis of mesoporous ZnS/CdS nanocomposites was accomplished via an evaporation-induced self-assembly (EISA) sol-gel method, in which ZnS and CdS colloidal NCs self-organized into 3D mesoporous gel-like networks with the aid of block copolymer (POE
20-
b-C
16; Brij 58) micelles, as illustrated in
Figure 1a. To synthesize the CdS and ZnS colloidal NCs, a thiol-terminated ligand (3-mercaptopropionic acid) was employed, which can exert control over the nanoparticle growth by binding to the NC surface via the thiol group and can facilitate enthalpic interactions with the polar POE fragments of the polymer template via the propionic acid end-group [
28]. The pre-formed NCs were subsequently utilized as building-block units that co-assemble with the polymer micelles under solvent evaporation conditions to create hybrid NC/polymer mesostructures. Key to this step is the addition of a small amount of dilute H
2O
2 solution (3%
v/
v) to the NC/polymer dispersion (as outlined in the experimental section), which instigates the gradual oxidation and stripping of the thiolate ligands from the NCs’ surface and prompts the oxidative polymerization of metal-sulfide NCs into a 3D network (on the surface of the polymer template) via interparticle disulfide or polysulfide linking bonds [
38,
39,
40]. It is noteworthy that the oxidant concentration and the related oxidation rates of the stabilizing thiolate capping groups constitute critical parameters that play a pivotal role in the controlled aggregative assembly of colloidal NCs and the preparation of robust gel-like porous frameworks. This observation builds upon earlier research into the formation of metal chalcogenide gels and aerogels, where it was demonstrated that the addition of a small quantity of oxidant (e.g., H
2O
2) below a specific threshold value (minimum ratio of oxidant/thiolate, X
min) did not lead to sufficient removal of thiolate capping groups to initiate the gelation–polymerization of NCs [
41]. Above X
min, efficient oxidation of surface thiolate ligands was achieved, resulting in interparticle linking and the assembly of stable gel networks, which can only be disassembled under (electro)chemical reductive environments [
38,
40]. Conversely, the utilization of a high amount of oxidant (~5X
min) triggered a rapid loss of surface thiolate groups, resulting in uncontrolled NC agglomeration and the precipitation of densely packed aggregates [
41,
42,
43]. Therefore, by employing the appropriate amount of H
2O
2, the creation of an interconnected NC-network provided sufficient structural stability to enable the effective removal of the organic template from the hybrid mesostructures via a gentle post-preparative treatment with ethanol and water (as detailed in the experimental section), thus yielding mesoporous ZnS/CdS NCAs. Consequently, via this procedure and the concentration of the colloidal CdS and ZnS nano-building blocks were appropriately adjusted, a series of mesoporous ZnS/CdS nanocomposites with varying mass loadings (wt.%) of ZnS were prepared, designated as x-ZnS/CdS (x = 10, 30, 50, 70 and 90 wt.% ZnS). Additionally, single-component mesoporous CdS and ZnS NCAs were prepared using the same synthetic process and extensively studied for comparison purposes. The chemical composition of the resultant materials was determined via energy-dispersive X-ray spectroscopy (EDS), and the obtained results are summarized in
Table S1 (in Supplementary Materials). For the CdS NCAs, EDS analysis revealed the presence of Cd and S elements with a Cd:S atomic ratio very close to the 1:1 stoichiometry of CdS. In contrast, the corresponding Zn:S atomic ratio in the ZnS NCAs sample was found to be ~1:0.86, implying a small sulfur deficiency (approximately 14%) in the lattice of constituent ZnS NCs. The EDS results of the ZnS/CdS nanocomposites indicated the presence of Cd and Zn atoms at varying Cd/Zn ratios, based on which the ZnS loading amounts were estimated and found to be very close to the nominal compositions of 10–90 wt.% (see
Table S1).
The crystallinity of the as-prepared materials was characterized via powder X-ray diffraction (XRD). As seen in
Figure 1b, the mesoporous CdS and ZnS NCAs exhibit three broad diffraction peaks at the scattering angle (2θ) range of 20−60°, which can be assigned to the (111), (220), and (311) reflections of the cubic (hawleyite) structure of CdS (JCPDS no. 42–1411) and the sphalerite (cubic) crystal phase of ZnS (JCPDS no. 05–0566). The broadening of the XRD peaks reflects the presence of very small nanocrystallites with an average domain size of ~2.8 nm for CdS and ~3.1 nm for ZnS, as determined via Scherrer analysis of the (111) diffraction peak [
44]. The ZnS/CdS NCAs showed XRD patterns that resemble a combination of those of CdS and ZnS, where the corresponding peaks of ZnS gradually increase in intensity with increasing the ZnS content. This suggests the formation of dual-component ZnS/CdS composites rather than solid solution structures via incorporation of ZnS nanoclusters into the CdS matrix.
The morphology of the ZnS/CdS nanocomposite with 50 wt.% ZnS content (50-ZnS/CdS), which is the most active catalyst in this study, was assessed via field emission scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM). The representative FE-SEM image in
Figure 2a reveals an agglomerated morphology comprising small-sized nanoparticles within the range of 5 to 10 nm. In
Figure 2b, the SEM-EDS elemental mapping shows a uniform dispersion of Cd, Zn and S elements, indicating that the ZnS and CdS nanoparticles are evenly distributed throughout the assembled structure. The TEM image in
Figure 2c further reveals an open-pore structure configuration, while the high magnification image (
Figure 2c inset) also demonstrates that the pore walls consist of approximately 5–6 nm-sized CdS and ZnS nanoparticles that are in intimate contact, which is advantageous for promoting efficient interfacial charge transfer and separation. Furthermore, the high-resolution TEM (HRTEM) image in
Figure 2d displays distinct lattice fringes with interplanar spacings of 3.4 Å and 3.1 Å, corresponding to the (111) crystal planes of the cubic CdS and ZnS, respectively, in agreement with the XRD results. Taken together, the above findings attest to the successful synthesis of porous ZnS/CdS nanocomposites, comprising small-sized ZnS and CdS nanoparticles that are linked together to form a robust 3D network.
The chemical state of elements in the 50-ZnS/CdS material was analyzed via X-ray photoelectron spectroscopy (XPS). The XPS spectra of pure CdS and ZnS NCAs were also obtained as reference. Typical survey spectra are shown in
Figure S1 in Supplementary Materials, while high-resolution spectra of the Cd 3d, Zn 2p and S 2p regions are shown in
Figure 3. The Cd 3d spectra show strong signals at binding energies of 412.2 and 405.5 eV for CdS NCAs and 411.9 and 405.2 eV for the 50-ZnS/CdS nanocomposite (
Figure 3a), which are respectively assigned to the Cd 3d
3/2 and Cd 3d
5/2 core-levels of CdS [
45,
46]. The Zn 2p XPS spectrum of ZnS NCAs in
Figure 3b presents two peaks at 1045.0 and 1022.0 eV that correspond to the Zn 2p
1/2 and Zn 2p
3/2 core-levels of ZnS, respectively [
47,
48]. The respective Zn 2p binding energies of 50-ZnS/CdS are located at 1045.3 and 1022.3 eV. Furthermore, the high-resolution S 2p spectra (
Figure 3c) of all the examined samples display a prominent peak at 161.8–162.2 eV that is characteristic of the S
2− valence state in metal sulfides [
45,
46,
47,
48]. The additional small S 2p XPS peaks observed at higher binding energies (168.5–169 eV) can be attributed to some oxidized sulfur species (SO
x2−) formed on the particle surface during the synthesis process and/or air handling of the samples [
45,
49]. Noticeably, the binding energies of Cd 3d in the 50-ZnS/CdS nanocomposite are shifted by 0.3 eV towards lower energy in comparison with those of pure CdS NCAs, while the respective Zn 2p binding energies exhibit an upshift of 0.3 eV relative to the pure ZnS NCAs. These particular shift patterns in binding energies can be attributed to the altered chemical environment at the ZnS/CdS interface resulting from an electron transfer from ZnS to CdS upon hybridization, indicating the strong electronic interaction between the CdS and ZnS components.
The ideal outcome of the employed polymer-templated oxidative polymerization process would be the creation of materials with a porous interconnected network of NCs devoid of any organic molecules. In practice, however, it is common to observe a certain quantity of residual organics (<8 wt.%) on the surface of the NC-assembled structure, even after thorough washing procedures to remove the polymer template [
28]. The presence of residual organics can influence factors such as wetting properties (hydrophobicity) and accessible surface area, and hence, the photocatalytic activity of the resulting porous structures [
39]. In light of this, we assessed the efficiency of organic matter removal from the fabricated mesoporous NCA structures via thermogravimetric analysis (TGA). An illustrative example of TGA characterization performed on the mesoporous 50-ZnS/CdS nanocomposite (which is the best-performing catalyst of this work) is presented in
Figure S2. In the TGA profile, the initial weight loss (~2.4%) observed in the temperature range of 50–200 °C can be attributed to the removal of adsorbed water, indicating the hydrophilic nature of the surface. Furthermore, the analysis revealed that approximately 7.4 wt.% of organic residue remains within the assembled structure, as indicated by the weight loss in the temperature range of 200–400 °C. This observation aligns with our earlier findings regarding a CdS NCA structure [
28]. Nevertheless, as we show below (in N
2 physisorption and photocatalytic H
2 production experiments), this small percentage of residual organic content does not exert any significant constraints on pore accessibility or adversely impact the catalytic surface properties of the synthesized mesoporous nanocomposites. The porosity of the prepared materials was examined via N
2 physisorption measurements conducted at −196 °C.
Figure 4a presents a comparison of the N
2 adsorption–desorption isotherms and the corresponding pore size distribution plots for the mesoporous CdS and ZnS NCAs, along with those of the 50-ZnS/CdS nanocomposite. The corresponding plots for the other ZnS/CdS NCAs (with 10, 30, 70 and 90 wt.% ZnS content) are provided in
Figure S3. All the isotherms feature typical type-IV curves with H2-type hysteresis loop, which are characteristic of porous solids with interconnected pores [
50]. Additionally, all the N
2 adsorption isotherms exhibit a small but distinguishable capillary condensation step in the relative pressure (P/P
o) range of ~0.4–0.6, suggesting N
2 condensation in narrow-sized mesopores [
51]. The Brunauer−Emmett−Teller (BET) surface area (S
BET) and total pore volume (V
p) of the mesoporous ZnS/CdS NCAs were measured to be in the range of 243–285 m
2 g
−1 and 0.29–0.32 cm
3 g
−1, respectively, which fall between those of pure CdS (S
BET = 234 m
2 g
−1, V
p = 0.28 cm
3 g
−1) and ZnS (S
BET = 334 m
2 g
−1, V
p = 0.33 cm
3 g
−1) NCAs. As shown in
Table 1, the S
BET and V
p of the ZnS/CdS nanocomposites progressively increased as the ZnS content increased from 10 to 90 wt.%. This trend is likely attributed to the relatively lower density of ZnS (4.09 g cm
−3) compared to that of CdS (4.29 g cm
−3). Moreover, the pore width of the as-prepared mesoporous materials was estimated by applying the non-local density functional theory (NLDFT) fitting model to the adsorption data [
52]. The NLDFT analysis indicated that all the samples have quite narrow pore-size distributions (insets of
Figure 4a and
Figure S3a–d) with an average pore size (D
p) of ~6.8 and ~5.8 nm for the CdS and ZnS NCAs, respectively, and in the range of ~5.8–6.3 nm for the ZnS/CdS nanocomposites (see
Table 1).
The effect of ZnS loading on the electronic structure and optical absorption properties of the synthesized ZnS/CdS nanocomposites was examined via UV-visible/near-IR diffuse reflectance spectroscopy (UV-vis/NIR DRS). The optical absorption spectra (
Figure 4b) of the reference CdS and ZnS NCAs, obtained by transforming the diffuse reflection data using the Kubelka–Munk function, indicate sharp absorption onsets at 495 and 322 nm that correspond to the interband (i.e., from valence band to conduction band) electron transitions in CdS and ZnS, respectively. Interestingly, the spectra of the heterostructured ZnS/CdS samples display two well-defined absorption onsets, one in the visible light range (400–500 nm) and the other in the UV range (260–360 nm). These distinct absorption bands correspond to the respective light absorption from the constituent CdS and ZnS NCs, thereby signifying the dual-semiconductor nature of the as-synthesized ZnS/CdS NCAs. Additionally, a noticeable trend is observed in the absorption edges of the CdS and ZnS components of the ZnS/CdS nanocomposites. Specifically, the absorption edge of CdS shows a consistent blue-shift from 488 nm to 475 nm as the ZnS content increases from 10 to 90 wt.%. Conversely, the absorption edge of ZnS exhibits a red-shift from 327 nm to around 355 nm with the increase in ZnS content. This trend is related to the strong electron interactions between the electronic states in CdS and ZnS NCs, demonstrating their good interfacial connectivity in the assembled frameworks, in line with the results from TEM and XPS analyses. The energy band gaps (E
g) of the CdS, ZnS and ZnS/CdS mesoporous structures were determined from the corresponding Tauc plots for direct band gap semiconductors (i.e., (αhν)
2 vs. hν plots, derived from the UV−vis/NIR DRS spectra), as shown in
Figure 4c. The E
g values of pure CdS and ZnS NCAs were estimated to be 2.51 ± 0.01 eV and 3.85 ± 0.02 eV, respectively (see
Table 1). Compared to the band gap energies of bulk CdS (2.3–2.4 eV) and ZnS (3.6–3.7 eV), both mesoporous samples exhibit a small but resolved blue-shift in E
g, which is indicative of a quantum confinement effect in the constituent CdS and ZnS nanoparticles due to their very small size (circa 5–6 nm according to the TEM analysis). Meanwhile, the strong interfacial interactions between CdS and ZnS NCs are also reflected in the band gaps of the ZnS/CdS nanocomposites, where their E
g values systematically vary from 2.54 eV to 2.61 eV with increasing the ZnS content from 10 to 90 wt.% (
Table 1). Note that the referred E
g values for the ZnS/CdS nanocomposites were estimated from the respective absorption bands in the visible light region of the Tauc plots, as shown in the inset of
Figure 4c. Despite the widening of the band gap, however, all the prepared mesoporous ZnS/CdS NCAs exhibit visible light semiconducting properties, allowing them to effectively absorb a significant portion of the solar spectrum.
3.2. Photocatalytic Hydrogen Evolution Study
Owing to their intriguing optoelectronic properties and unique mesoporous nano-architecture, the present mesoporous NC-based materials are expected to promote photocatalytic reactions. For this purpose, the photocatalytic performance of the synthesized materials was evaluated for the H
2 evolution reaction from water. The effect of ZnS content on the photocatalytic H
2 evolution activity of the nanocomposites was investigated under visible light irradiation (λ ≥ 420 nm), using 20 mg of catalyst dispersed in an aqueous solution (20 mL) containing 0.35 M Na
2S and 0.25 M Na
2SO
3 as sacrificial reagents.
Figure 5a compares the rates of H
2 evolution (averaged over 3-h reaction period) obtained for the pure CdS and ZnS NCAs, along with the different ZnS/CdS catalysts. It is evident that the pure ZnS NCAs demonstrate nearly negligible H
2 production activity (~4 μmol h
−1), which is attributed to the insufficient visible light absorption due to the wide band gap of ZnS NCs (E
g = 3.85 eV). Additionally, despite the visible light response of the CdS NCAs (E
g = 2.51 eV), their H
2 production performance was relatively poor (~30 μmol h
−1), primarily due to the rapid recombination of photo-excited electron-hole pairs. Conversely, ZnS and CdS hybridization has a significant impact on the photocatalytic performance, markedly improving the H
2 evolution rate. In particular, the photo-activity of ZnS/CdS NCAs was remarkably enhanced with increasing the loading amount of ZnS NCs, reaching a maximum H
2-evolution rate of ~0.32 mmol h
−1 at 50 wt.% ZnS content (50-ZnS/CdS catalyst). This activity is about 10.7 and 80 times higher than that of single CdS and ZnS NCAs, respectively (
Figure 5a). Further increase in the ZnS content to 70 wt.% leads to a small decrease in the H
2 evolution activity (0.27 mmol h
−1 for the 70-ZnS/CdS catalyst), while the H
2 evolution rate of the 90 wt.% ZnS-loaded sample (90-ZnS/CdS) was significantly reduced to 0.1 mmol h
−1. As we will discuss in more detail later, the noticeably lower catalytic activities observed in the ZnS/CdS samples with low (10 wt.%) and high (90 wt.%) ZnS content can be attributed to the poorer charge transfer and separation ability, likely due to inadequate interfacial connectivity between unevenly distributed ZnS and CdS NCs in these assembled structures. The above findings thus indicate that the even (~1:1) mass distribution of ZnS and CdS NCs in the assembled structures plays a prominent role in the H
2-evolution performance of the resulting materials. Consequently, we focused our further catalytic studies on reactions with the 50-ZnS/CdS catalyst. Note that blank experiments conducted in the absence of light or catalyst did not exhibit any evolution of H
2, indicating that the observed production of H
2 is exclusively attributed to photocatalytic reactions.
In addition to chemical composition, morphological effects may also affect the catalytic performance of ZnS/CdS catalysts. To assess this, we prepared a reference ZnS/CdS bulk catalyst with 50 wt.% ZnS content by physically mixing equal amounts of pre-formed ZnS and CdS microparticles (see experimental section). XRD and EDS characterization results indicated that the bulk reference catalyst consists of a polycrystalline mixture of ZnS and CdS (
Figure S4a) with 50 wt.% ZnS composition (
Table S1). Additionally, compared to the mesoporous 50-ZnS/CdS nanocomposite, the bulk ZnS/CdS microparticles possess a significantly lower surface area and pore volume (S
BET = 75 m
2 g
−1, V
p = 0.15 cm
3 g
−1) and a smaller energy band gap (E
g = 2.32 eV), as determined via N
2 physisorption and UV–vis/NIR DRS measurements (see
Figure S4b,c and
Table 1). Interestingly, the reference ZnS/CdS bulk sample demonstrated a H
2 evolution rate of only ~30 μmol h
−1, which is comparable to that of pure CdS NCAs and considerably lower than that of the mesoporous 50-ZnS/CdS nanocomposite (see
Figure 5a). In addition, to better highlight the positive impact of the 3D mesoporous NC network structure on catalytic activity, we also prepared an untemplated reference nanocomposite, ZnS/CdS RNAs (RNAs: random NC-aggregates) containing 50 wt.% ZnS and compared its photocatalytic performance with that of the polymer-templated 50-ZnS/CdS catalyst. ZnS/CdS RNAs was synthesized following a template-free oxidative coupling of ZnS and CdS NCs, where the evaporation of solvent in the absence of polymer template resulted in the formation of close-packed NC-aggregates. EDS analysis indicated a ZnS content of 49.82 wt.% (
Table S1), which closely aligns with that of the 50-ZnS/CdS sample. However, in contrast to the polymer-templated nanocomposite, the ZnS/CdS RNAs exhibited a different isotherm profile, resembling a combination of type-I and type-IV with an H3 hysteresis loop, indicative of a dense microporous structure (S
BET ~114 m
2 g
−1, V
p = 0.07 cm
3 g
−1) with small interstitial voids (~1.5 nm) (see
Figure S5). Under the same reaction conditions (i.e., 20 mg of catalyst dispersed in a 0.35 M Na
2S and 0.25 M Na
2SO
3 solution), the ZnS/CdS RNAs catalyst yielded a visible light H
2-evolution rate of ~0.21 mmol h
−1 (see
Figure 5a), which is approximately 1.5 times lower than that of the mesoporous 50-ZnS/CdS nanocomposite. Therefore, based on the above results, it can be deduced that the 3D open-pore morphology with high surface area and the NC-network structure with nanoscale interfaces are contributing factors that enhance mass transport phenomena and the number of accessible active sites and thus play a crucial role in boosting the H
2 production performance of our mesoporous ZnS/CdS nanocomposite catalysts.
To further optimize the reaction conditions, we investigated the effects of different sacrificial electron donors and mass loadings of catalyst on photocatalytic H
2 production performance. To this end, we examined a series of customary sacrificial reagents, including tertiary amines, such as triethanolamine (TEOA) and triethylamine (TEA), lactic acid, methanol and a Na
2S/Na
2SO
3 mixture. The experiments were conducted in 20 mL of DI water, using a fixed amount (20 mg) of the mesoporous 50-ZnS/CdS catalyst. As depicted in
Figure S6, the most favorable results were achieved when using a mixture of Na
2S and Na
2SO
3 as sacrificial reagents. It is generally recognized that the utilization of S
2−/SO
32− species as electron donor serves as an effective strategy to enhance the H
2-evolution photoactivity of metal-sulfide catalysts. The S
2−/SO
32− pairs not only consume the surface-reaching holes but also mitigate the anodic photocorrosion of sulfur-containing catalysts (such as CdS and ZnS) by replenishing the loss of lattice sulfur with S
2− ions from the reaction solution [
9,
15,
53]. On this basis, the concentration of S
2−/SO
32− species during the photocatalytic reaction also plays an important role, as demonstrated by the control experiments performed using different concentrations of the Na
2S/Na
2SO
3 mixture (
Figure 5b). The results indicate that the H
2-evolution activity of 50-ZnS/CdS scales almost linearly with the Na
2S/Na
2SO
3 concentration and reaches a maximum of 0.58 mmol h
−1 in a saturated aqueous electrolyte containing 1.4 M Na
2S and 1.0 M Na
2SO
3. Furthermore, photocatalytic tests using different mass loadings of the 50-ZnS/CdS catalyst (dispersed in a 1.4 M Na
2S and 1.0 M Na
2SO
3 aqueous solution) indicated that the rate of H
2 evolution increased with the catalyst concentration, reaching a maximum at 1 g L
−1 (
Figure 5c). This result can be attributed to the saturation of light absorption by the catalyst particles. A further increase of the catalyst concentration (to 1.5 and 2 g L
−1) results in a gradual reduction in H
2 production performance (0.42 and 0.37 mmol h
−1, respectively), likely due to the light-scattering and shielding effects of the excessive number of catalyst particles in the reaction mixture.
Under the optimized reaction conditions (i.e., 1 g L
−1 catalyst concentration and 1.4 M Na
2S and 1.0 M Na
2SO
3 sacrificial reagents), the 50-ZnS/CdS catalyst also demonstrated excellent photochemical stability. The stability was evaluated by conducting four consecutive 6-h photocatalytic H
2-evolution runs under visible light irradiation (details shown in
Section 2.6). As inferred from the results presented in
Figure 5d, the 50-ZnS/CdS catalyst maintains its high H
2-evolution activity without any signs of decline (within 5% experimental error) even after 24 h of irradiation. After stability tests, we performed EDS, XRD, XPS and N
2 physisorption characterizations to investigate the chemical and structural stability of the reused catalyst. EDS analysis of the retrieved catalyst indicated a Cd/Zn atomic ratio of 15.62:23.42, which corresponds to a ZnS content of 50.29 wt.% (see
Table S1), very close to the initial ZnS loading amount of the fresh sample (50.37 wt.%). Additionally, no changes were observed in the crystal structure and chemical state of elements in the reused catalyst, as evidenced by its XRD pattern (
Figure S7a) and XPS spectra (
Figure S7c–e). The obtained results closely resembled those of the as-prepared 50-ZnS/CdS NCAs, substantiating the excellent chemical and structural stability of the catalyst during the examined conditions. However, N
2 physisorption measurements on the reused sample revealed a relatively lower BET surface area (~200 m
2 g
−1) and pore volume (~0.26 cm
3 g
−1) compared to the fresh material (S
BET = 266 m
2 g
−1, V
p = 0.31 cm
3 g
−1) (
Figure S7b). In addition, pore size analysis indicated a slightly broader pore-size distribution, with a peak maximum at ~6.2 nm. These small changes in the textural parameters are probably related to some minor photocorrosion, which leads to a rearrangement of nanoparticles within the porous structure. Regardless, the mesoporosity and surface area of 50-ZnS/CdS NCAs still remain high enough after the prolonged catalytic use. The 4-times reused catalyst also demonstrated a persistent H
2-evolution activity for two additional photocatalytic cycles (see
Figure S8), providing further confirmation of its impressive photocatalytic stability and reusability under the examined conditions. Remarkably, over the 36-h irradiation period, an average H
2-evolution rate of 0.58 mmol h
−1 (or ~29 mmol g
−1 h
−1 mass activity) was achieved, which is associated with an apparent quantum yield (AQY) of 60% at λ = 420 nm, assuming that all incident photons are absorbed by the catalyst. To the best of our knowledge, this efficiency is among the highest reported thus far for CdZnS-based photocatalytic systems, according to previous studies [
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
54,
55,
56,
57,
58]. A comparison of photocatalytic H
2-evolution activities of some high-performance CdZnS-based photocatalysts is presented in
Table S2. Furthermore, to assess the influence of S
2−/SO
32− concentration on the photochemical stability of 50-ZnS/CdS, we also conducted photocatalytic H
2-evolution recycling tests (i.e., successive 4-h photocatalytic runs) using the typical 0.35 M Na
2S and 0.25 M Na
2SO
3 concentrations of sacrificial reagents. As shown in
Figure S9, the lower S
2−/SO
32− concentration not only yielded an overall lower H
2-evolution activity but also failed to prevent catalyst photocorrosion, as inferred from the gradual decline in H
2-evolution rates observed during the second and third catalytic runs. Hence, this observation underscores the profound influence of the S
2−/SO
32− concentration on both the photoactivity and stability of the prepared mesoporous nanocomposites. Due to the large surface area and abundance of exposed active sites, the lower S
2−/SO
32− concentration is inadequate for the efficient consumption of surface-reaching holes, thus leading to lower rates of H
2-evolution and gradual photocorrosion of the nanocomposites.
3.3. Photocatalytic Mechanism
To clarify our understanding of the interfacial electronic interactions between the CdS and ZnS components and gain insight into the photocatalytic H
2 production mechanism of the CdS/ZnS nanocomposites, we performed electrochemical impedance spectroscopy (EIS) and photoluminescence (PL) measurements.
Figure 6a displays the Mott–Schottky (M–S) plots, i.e., the inverse square capacitance (1/C
2) versus applied potential diagrams, for the mesoporous CdS, ZnS and ZnS/CdS NCAs. The M–S plot of the bulk ZnS/CdS material with 50 wt.% ZnS is shown in
Figure S10a. All the samples showed positive slopes in the M–S plots, indicating n-type conductivity. Furthermore, by extrapolating the linear fits of the M–S curves to 1/C
2 = 0, we determined the flat band potential (E
FB) of all the materials, and the results are compared in
Table 2. All the measured potentials are reported relative to the reversible hydrogen electrode (RHE) at pH 7. For single-component CdS and ZnS NCAs, the estimated E
FB levels were −0.70 V and −1.12 V, respectively, while the E
FB positions of the ZnS/CdS materials range from −0.74 V to −1.06 V. Generally, E
FB represents the electrochemical Fermi level energy (E
F) of the materials under the examined conditions, which is approximately 0.1–0.3 eV below the conduction band (CB) edge in highly doped n-type semiconductors [
59]. In fact, the location of the CB edge potential (E
CB) can be calculated from E
FB using Equations (4) and (5), presuming that the donor dopants are completely ionized [
60].
where E
F is the Fermi level energy, N
D is the electron donor density and N
c is the density of the effective states in the CB, which is given by:
where h is the Planck constant, k is the Boltzmann constant, T is the temperature in Kelvin and
is the effective mass of electrons in the CB.
In Equation (5), assuming that m
e* ≈ m
o, in which m
o is the mass of a free electron, N
c, was estimated to be ~2.5 × 10
19 cm
−3. In addition, the donor density (N
D) of the studied catalysts was determined from the slope of the Mott–Schottky linear fits (see
Table 2). The resultant N
D values presented a progressive increase from 2.02 × 10
16 to 4.90 × 10
16 cm
−3 with an increase in the ZnS ratio from 0 to 50 wt.%, followed by a relative decrease (to 2.32 × 10
16 and 3.32 × 10
16 cm
−3) for the samples with higher ZnS contents (70 and 90 wt.%). Therefore, based on the obtained E
FB and N
D results, the E
CB levels of the prepared materials were calculated, and the valence band (VB) edge potentials (E
VB) were subsequently determined by adding the respective optical band gaps (E
g) obtained from UV-vis/NIR spectra.
Table 2 summarizes all the calculated band-edge potentials, from which a representative band-edge diagram for each catalyst was constructed, as illustrated in
Figure 6b. By analyzing the band diagrams in conjunction with the corresponding electrochemical results, several important conclusions can be drawn. Firstly, the CB edge of all the prepared catalysts is positioned well above the H
2O/H
2 reduction potential (−0.41 V vs. RHE at pH 7), demonstrating the thermodynamic ability of these materials to drive water reduction. Moreover, a clear cathodic shift is observed in the CB edge of the ZnS/CdS nanocomposites with increasing ZnS content (from 10 to 90 wt.%). This shift can be explained by the higher E
FB level of ZnS (−1.12 V) relative to that of CdS (−0.7 V), which creates a built-in potential difference after their contact. As a result, an electron flow from ZnS to CdS will take place at the ZnS/CdS contact interface until thermodynamic equilibrium is reached between the two materials. Consequently, upon enrichment with ZnS NCs, the accumulation of electrons in CdS will progressively shift the CB edge level of the nanocomposites in a more negative (cathodic) direction, positioning it between those of pure ZnS and CdS, as evidenced by the obtained EIS results. The electron transfer from ZnS to CdS in the nanocomposites is also supported by the XPS results as well as the increasing N
D values with the loading amount of ZnS. The relatively lower N
D values obtained for the nanocomposites with high ZnS content (70 and 90 wt.%) and the ZnS/CdS bulk reference (see
Table 2) likely suggest a weaker charge transfer effect between the ZnS and CdS components in these materials. To further elucidate this, the charge-transfer properties of the prepared catalysts were investigated via Nyquist plot analysis. EIS Nyquist measurements were conducted in 0.5 M Na
2SO
4 electrolyte over a frequency span of 1 Hz–100 kHz using an AC amplitude of 10 mV and a DC bias of −1.3 V (vs. Ag/AgCl, saturated KCl).
Figure 6c compares the Nyquist plots of pure CdS and ZnS NCAs with those of the ZnS/CdS nanocomposites with different ZnS content. The corresponding Nyquist plot of the ZnS/CdS bulk analogue is shown in
Figure S10b. The charge-transfer resistance (R
ct) of each sample was obtained by fitting the Nyquist plots to a Randles equivalent circuit model (inset of
Figure 6c and
Figure S10b), and the results are listed in
Table 2 and
Table S3. In brief, the simulated results indicate that all the ZnS/CdS nanocomposites possess lower R
ct values (~285–747 Ω) than the pure CdS (~1312 Ω) and ZnS (~1362 Ω) NCAs. This suggests more favorable charge-transfer dynamics at the ZnS/CdS heterojunctions, which contribute to their enhanced photocatalytic performance. Among them, the 50-ZnS/CdS catalyst exhibits the lowest R
ct (285 Ω), which aligns with its superior charge transfer and photoreduction activity. Comparatively, the bulk reference catalyst (ZnS/CdS bulk) features a significantly higher R
ct (1499 Ω), despite having a similar composition and band-edge structure to the 50-ZnS/CdS nanocomposite. Collectively, these findings suggest that, in addition to the chemical composition, the nanoscale dimensionality of the ZnS/CdS contact interfaces plays a prominent role in enhancing the charge transfer dynamics and achieving superior photocatalytic efficiency.
Additional evidence for the importance of ZnS/CdS nanoscale heterojunctions in governing the efficiency of charge carrier transfer and separation processes is provided by time-resolved photoluminescence (TR-PL) measurements.
Figure 6d depicts the TR-PL emission spectra of mesoporous CdS and 50-ZnS/CdS NCAs along with that of the ZnS/CdS bulk material, recorded using 375 nm pulse laser excitation. To obtain the carrier lifetimes, the PL decay curves were fitted with a biexponential function: F(t) = y
0 + Σ
iA
i e
−t/τi (i = 1, 2), where A
i is the amplitude fraction (Σ
iA
i = 1) and τ
i is the carrier lifetime of each component. The two time-components in the equation reflect the fast radiative charge-carrier recombination at the surface (τ
1) and slow recombination of excitons in the bulk (τ
2), respectively. The estimated fast and slow lifetimes of carriers are tabulated in
Table S4, from which the average lifetimes (τ
av) were calculated to be approximately 3.20 ± 0.017 ns and 3.70 ± 0.025 ns for the mesoporous CdS and 50-ZnS/CdS NCAs, respectively and 3.16 ± 0.011 ns for the ZnS/CdS bulk catalyst. These results attest to an improved interfacial charge transfer and separation process within the ZnS/CdS NC-assembled structure and thereby to a more efficient utilization of photo-excited electrons for H
2 evolution. Moreover, as shown in
Table S4, 50-ZnS/CdS has a lower percentage (65.0%) of τ
1-carriers than pure CdS (72.5%), indicating that the surface recombination of electron-hole pairs over CdS was inhibited upon hybridization with ZnS NCs. The efficient charge separation in the 50-ZnS/CdS catalyst was also confirmed via steady-state PL emission spectra obtained via excitation at 380 nm. As depicted in
Figure S11a, the PL spectrum of pure CdS NCAs exhibits a strong emission at ~494 nm (2.51 eV) corresponding to the band-edge excitonic relaxation of CdS NCs. Comparatively, the PL intensity of this peak is almost vanished in the 50-ZnS/CdS sample, indicating that the majority of photogenerated electron-hole pairs are dissociated over the ZnS/CdS structure, thus suppressing their recombination rate. However, this condition implies the spatial separation of charge carriers across the ZnS/CdS junctions, which is not supported by the apparent band-edge alignment between ZnS and CdS in the nanocomposites. In particular, as inferred from the band diagram of
Figure 6b, the junction between ZnS and CdS is expected to form a type-I (straddling gap) band-alignment, which cannot promote the spatial separation of charge-carriers. Instead, the type-I junction configuration favours the accumulation of electrons and holes in CdS, thus increasing their recombination probability. Therefore, a more complex charge-dissociation mechanism likely occurs at the ZnS/CdS junctions, potentially involving interfacial charge transfer pathways between the band-edges of CdS and defect-induced energy levels commonly found in ZnS. This assumption is supported by the PL spectrum of ZnS NCAs (
Figure S11b), which exhibits not only an emission peak at 322 nm (3.85 eV) due to the band-edge relaxation but also multiple emission bands with peaks at 398 nm (3.12 eV), 432 nm (2.87 eV), 473 nm (2.62 nm) and 562 nm (2.21 eV) that are attributed to the radiative recombination of excitons through defect states. Typically, four types of point defects can be present in ZnS particles: sulfur vacancies (V
S), zinc vacancies (V
Zn), interstitial zinc atoms (I
Zn) and interstitial sulfur atoms (I
S). These defects introduce energy states within the band gap of ZnS, with the V
S and I
Zn levels located bellow the CB (donor states), while V
Zn and I
S create energy levels above the VB (acceptor states) [
61]. It has been reported that the energy levels of vacancies are deeper than those of interstitial states, with I
Zn situated closer to the CB than V
S, and I
S closer to the VB than V
Zn [
62]. In this context, the PL emission bands centered at 3.12 eV and 2.87 eV can be attributed to electron transitions from the I
Zn and V
S states to the VB of ZnS, while the bands around 2.62 eV and 2.21 eV are likely associated with excitonic relaxations from V
S to I
S and from I
Zn to V
Zn, respectively, as illustrated in
Figure 7a [
63].
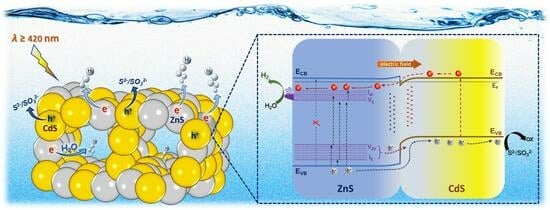
On the basis of the above results, a plausible charge transfer and photocatalytic H
2 production mechanism is proposed for the ZnS/CdS nanocomposite catalysts (
Figure 7b). As revealed by the EIS analysis, CdS has a lower E
FB level than ZnS and hence, upon hybridization, electrons tend to flow from ZnS to CdS until their electrochemical potentials reach equilibrium. During this process, the loss of electrons from ZnS will result to an upward band bending near the interface (depletion layer), while the simultaneous increase in electron density at the CdS side will lead to a downward bending of the energy bands (accumulation layer). We found that, at optimum ZnS composition (50 wt.%), a built-in potential of about 0.4 V (as determined from Mott–Schottky results) will be established at the ZnS/CdS interfaces, which consequently creates a strong internal electric field with a direction from ZnS to CdS. Upon visible light irradiation, CdS undergoes excitation and generates electron and holes in the CB and VB, respectively. On the other hand, as inferred via PL measurements (see
Figure 7a), electrons in ZnS can only be excited to the interband V
S and I
Zn states through single or multiple visible light-photon absorption, leaving behind holes in the VB, as well as the V
Zn and I
S acceptor states. Due to the built-in electric field at the ZnS/CdS interfaces, the photoexcited electrons in the CB of CdS will drift towards ZnS and be captured at the V
S and I
Zn donor states, together with the photogenerated electrons in ZnS. Such electron transfer route is supported by the direction of the internal electric field and the fact that the energy levels of V
S and I
Zn donor states (ranging from −0.3 to −0.6 V) are located just below the E
CB of CdS (−0.7 V), as illustrated in
Figure 7b. On the other hand, the holes in the VB, V
Zn and I
S states of ZnS are driven by the electric field to the VB of CdS, where they accumulate alongside the holes in CdS. This hole transport pathway from ZnS to CdS is not only influenced by the internal electric field but is also thermodynamically feasible, as the E
VB of ZnS (2.56 V) as well as the energy levels of V
Zn and I
S acceptor states (~1.65–2.31 V) are located below the E
VB of CdS (1.62 V). Additional indication for the accumulation of holes in CdS was obtained through EDS analysis on the 50-ZnS/CdS catalyst retrieved after the control photocatalytic experiment in which TEOA was used as sacrificial electron donor. Regarding this, because TEOA exhibits a slower scavenging ability for the photogenerated holes compared to the S
2−/SO
32− species (see
Figure S6), it is not expected to efficiently prevent the anodic photocorrosion of CdS by the accumulated photoholes. Indeed, as shown in
Table S1, the EDS results of the retrieved catalyst (50-ZnS/CdS-c) revealed a Zn/Cd atomic ratio of ~1.82, representing an increase of approximately 17.6% compared to the fresh sample (Zn/Cd ≈ 1.50). Additionally, the ZnS content increased from 50.34 to 55.14 wt.% after the photocatalytic reaction. Therefore, these results explicitly demonstrate a significant loss of Cd due to the photocorrosion of CdS, further proving its role as the hole acceptor in the ZnS/CdS heterojunctions. Consequently, via this defect-induced quasi-type-II charge transfer scheme, the photogenerated charge carriers in CdS and ZnS can be efficiently separated at the ZnS/CdS nanojunctions. The proposed charge transfer and separation mechanism endows electrons trapped in the donor states of ZnS with prolonged ability to participate in the catalytic reduction of H
2O to generate H
2. At the same time, the holes gathered in the VB of CdS can effectively oxidize the sacrificial S
2−/SO
32− species, thus increasing the photochemical activity and stability of the catalyst. Such photocatalytic mechanism is consistent with the results from the above electrochemical and spectroscopic studies. In addition, the mesoporous ZnS/CdS NCAs, owing to the 3D open-pore structure and high internal surface area, provide a high density of surface-active sites for redox reactions. As thus, the overall photocatalytic performance of these catalysts is significantly improved, highlighting their potential for efficient solar-fuel production.