In Situ Fabrication of CdS/Cd(OH)2 for Effective Visible Light-Driven Photocatalysis
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of CdS
2.2. Preparation of CdS/Cd(OH)2
2.3. Characterization
2.4. Photocatalytic Performance
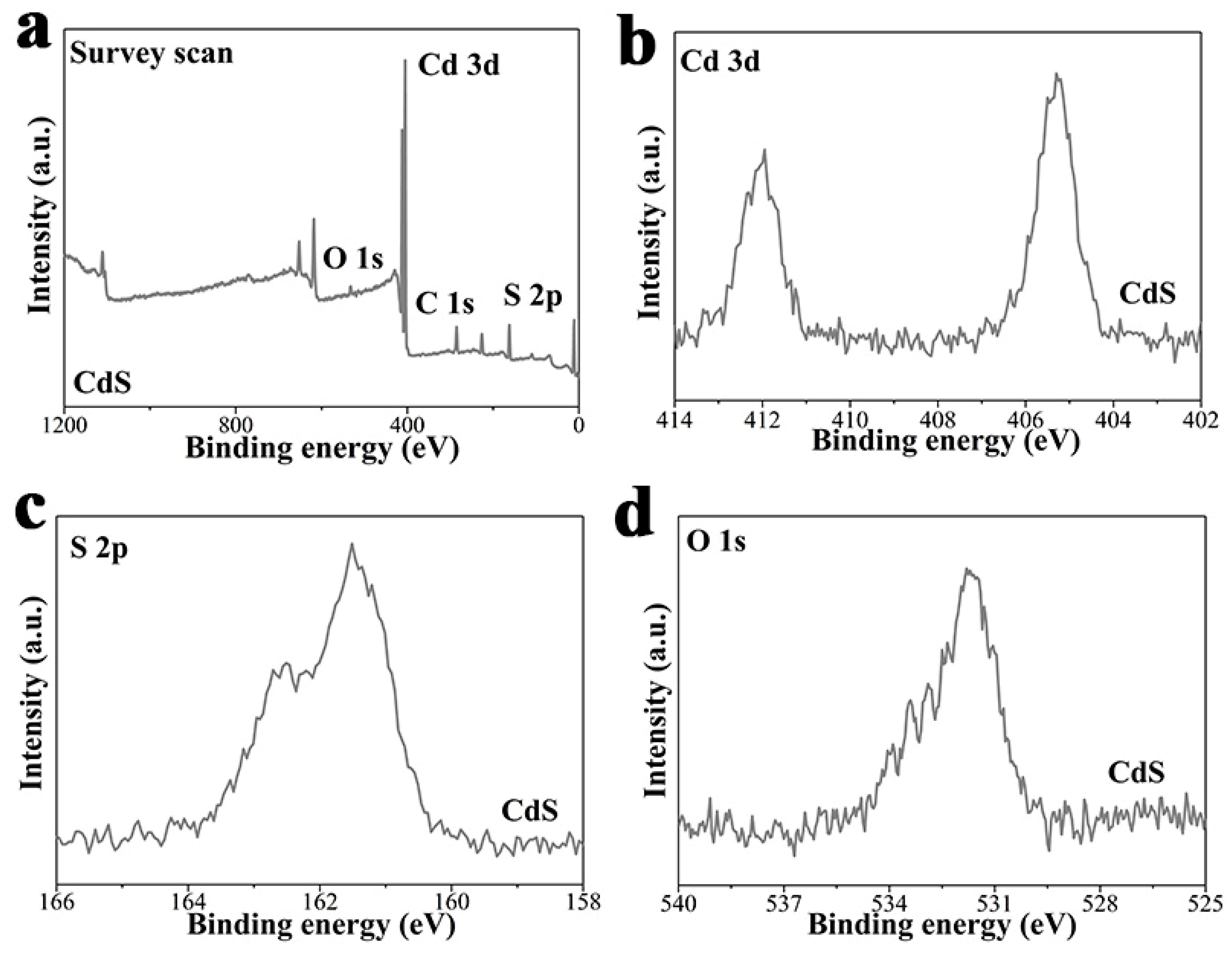
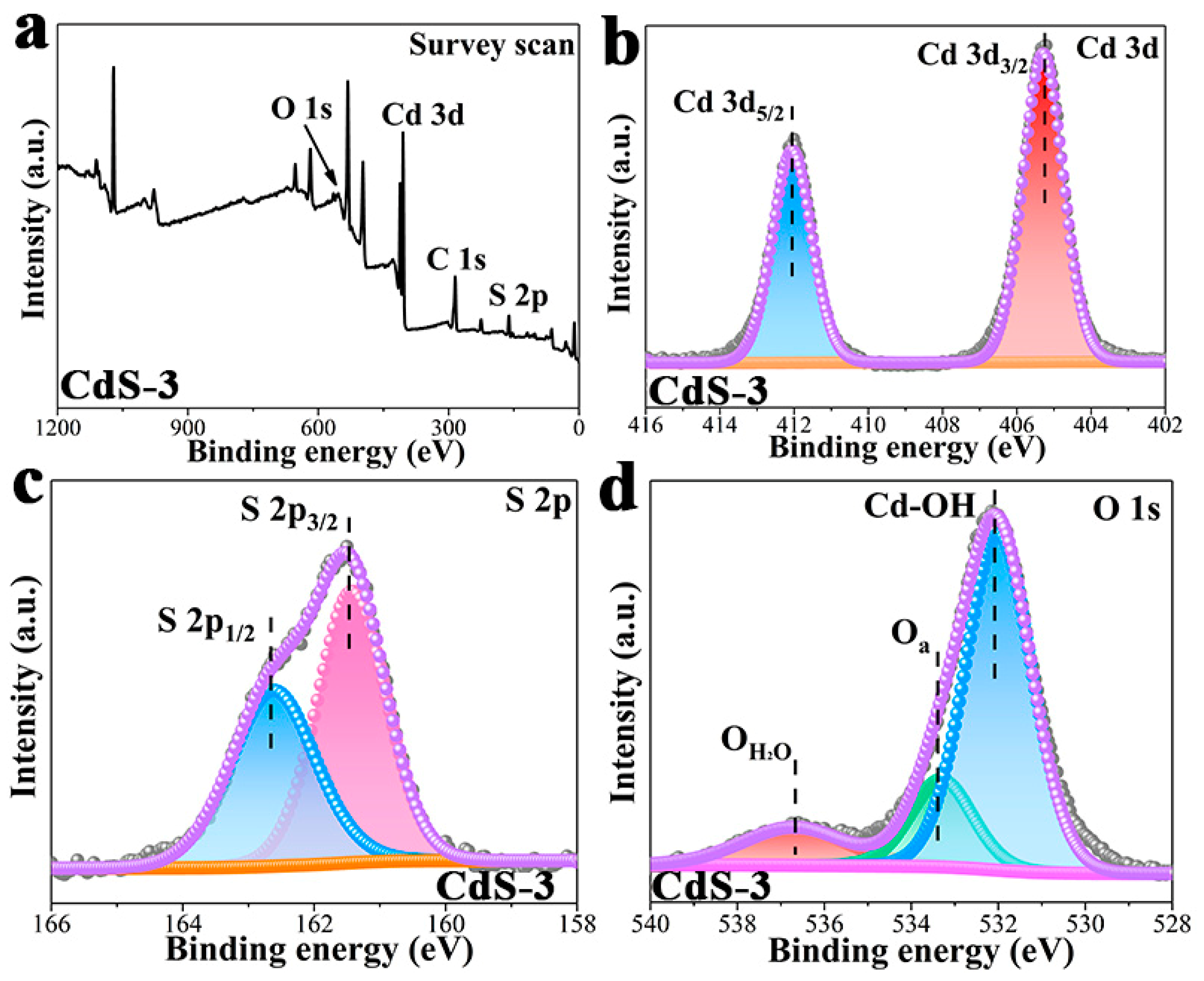
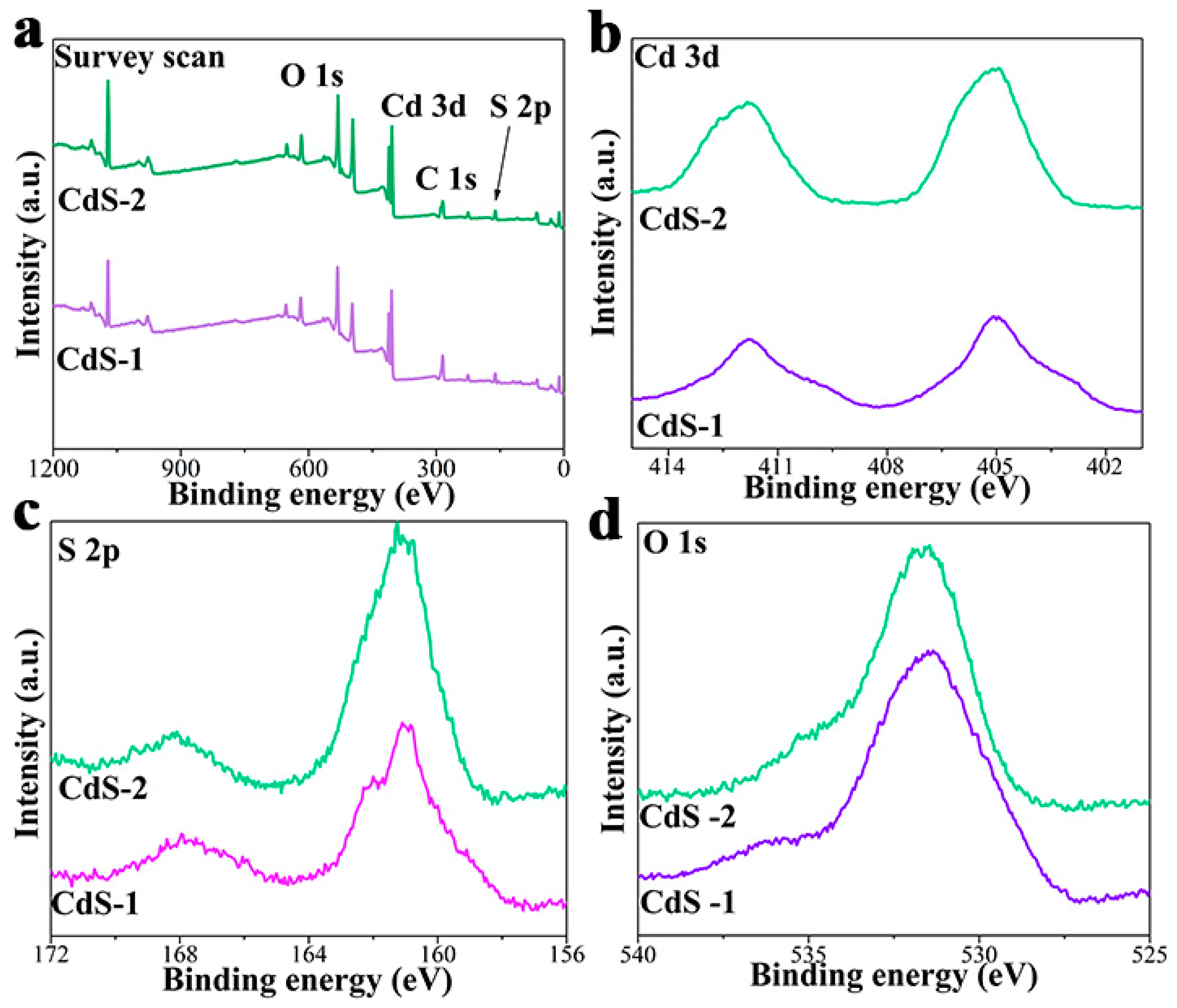
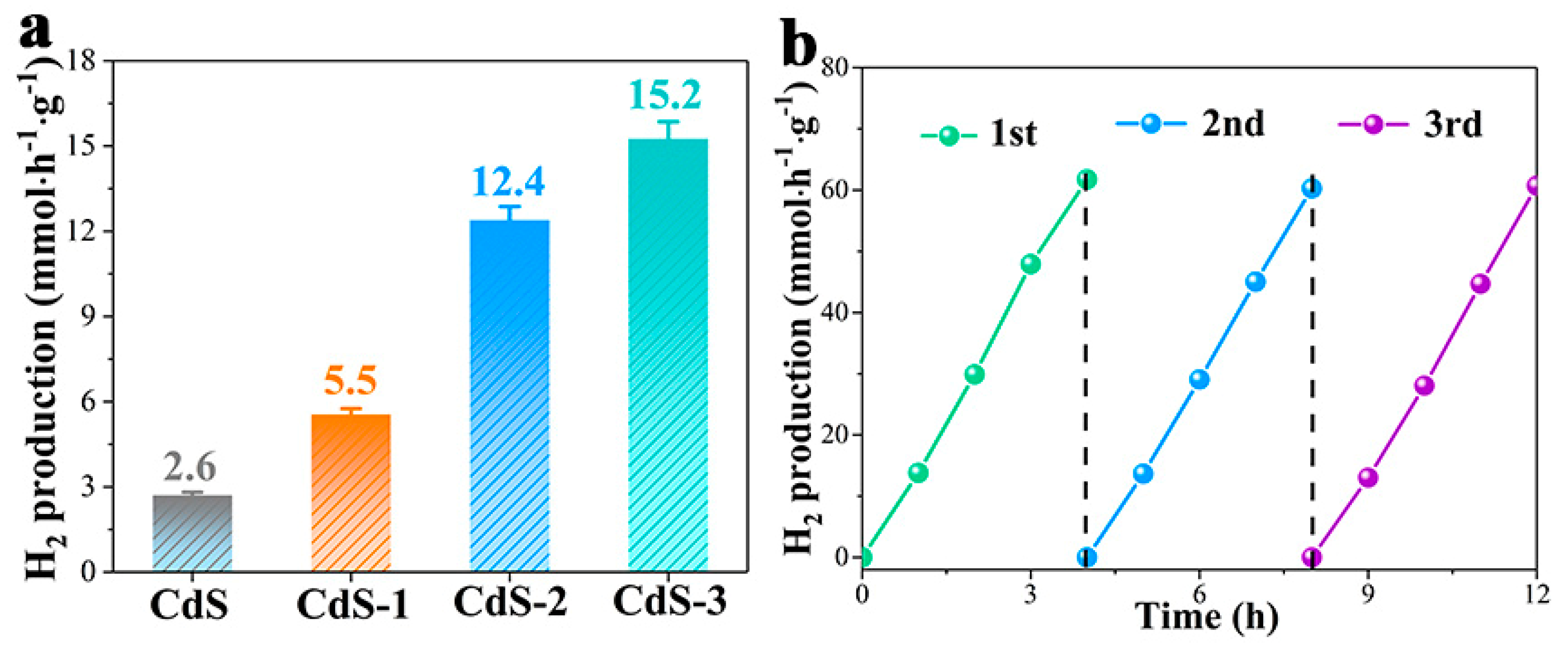
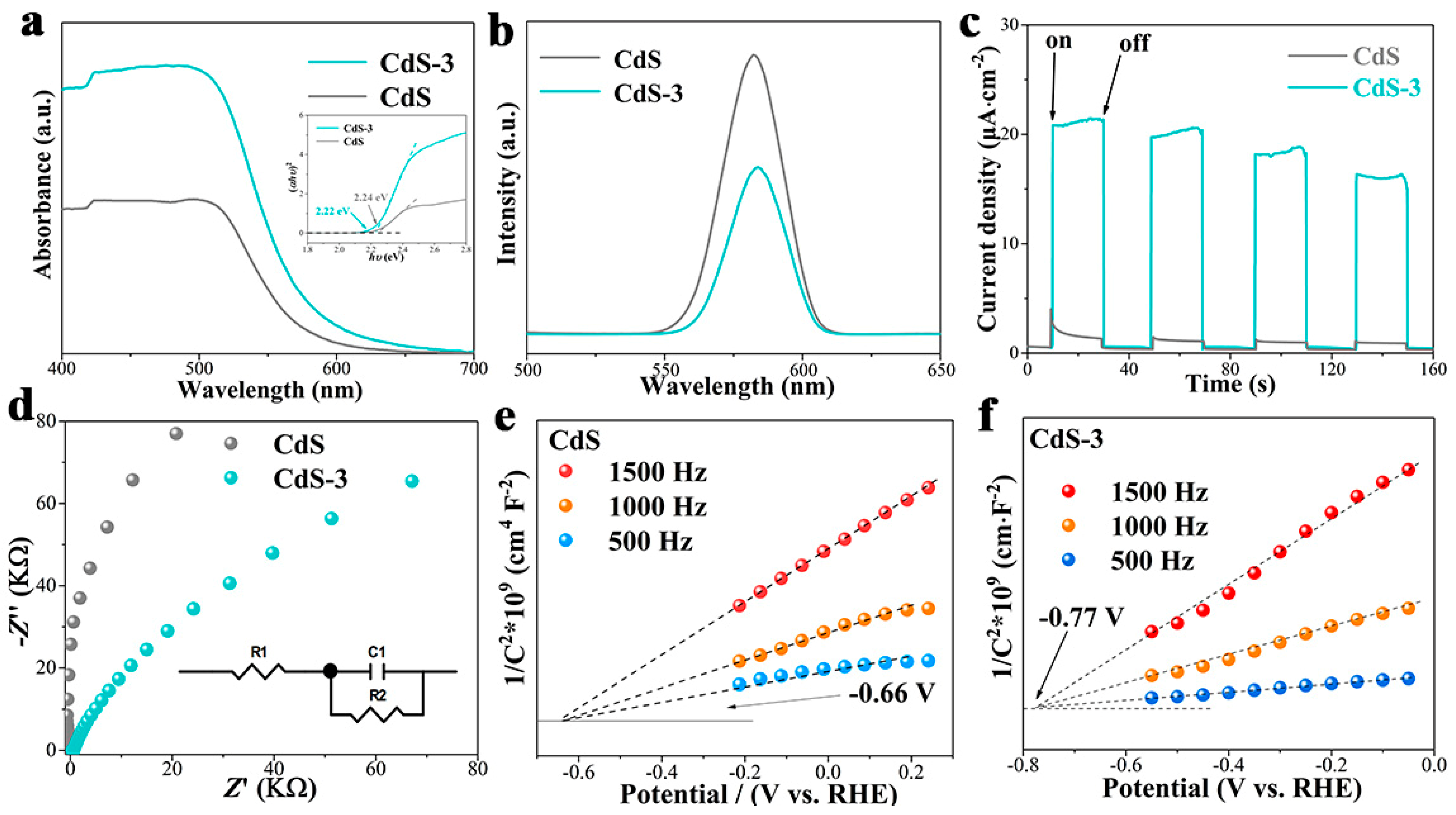
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Chen, J.; Gao, X.; Ao, Y.H.; Wang, P.F. Probing the role of surface acid sites on the photocatalytic degradation of tetracycline hydrochloride over cerium doped CdS via experiments and theoretical calculations. Dalton Trans. 2021, 50, 16620–16630. [Google Scholar] [CrossRef]
- Chen, R.; Wang, P.F.; Chen, J.; Wang, C.; Ao, Y.H. Synergetic effect of MoS2 and MXene on the enhanced H2 evolution performance of CdS under visible light irradiation. Appl. Surf. Sci. 2019, 473, 11–19. [Google Scholar] [CrossRef]
- Chen, R.; Chen, J.; Che, H.N.; Zhou, G.; Ao, Y.H.; Liu, B. Atomically dispersed main group magnesium on cadmium sulfide as the active site for promoting photocatalytic hydrogen evolution catalysis. Chin. J. Struc. Chem. 2022, 41, 2201014–2201018. [Google Scholar]
- Liu, Q.Q.; Shen, J.Y.; Yang, X.F.; Zhang, T.R.; Tang, H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B Environ. 2018, 232, 562–573. [Google Scholar] [CrossRef]
- Che, H.N.; Che, G.B.; Zhou, P.J.; Liu, C.B.; Dong, H.J.; Li, C.X.; Song, N.; Li, C.M. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]
- Wu, X.H.; Ma, H.Q.; Zhong, W.; Fan, J.J.; Yu, H.G. Porous crystalline g-C3N4: Bifunctional NaHCO3 template-mediated synthesis and improved photocatalytic H2-evolution rate. Appl. Catal. B Environ. 2020, 271, 118899. [Google Scholar] [CrossRef]
- Jiang, X.H.; Zhang, L.S.; Liu, H.Y.; Wu, D.S.; Wu, F.Y.; Tian, L.; Liu, L.L.; Zou, J.P.; Luo, S.L.; Chen, B.B. Silver single atom in carbon nitride catalyst for highly efficient photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2020, 59, 23112–23116. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Ye, H.F.; Liang, F.; Wang, Z.; Li, K.; Weng, Y.X.; Lin, Z.S.; Fu, W.F.; Che, C.M.; Chen, Y. Interstitial P-doped CdS with long-lived photogenerated electrons for photocatalytic water splitting without sacrificial agents. Adv. Mater. 2018, 30, 1705941. [Google Scholar] [CrossRef]
- Chen, R.; Luo, Y.; Qian, P.; Ma, M.H.I.; She, X.S. Rational designing of dual-functional photocatalysts for simultaneous hydrogen generation and organic pollutants degradation over Cd0.5Mn0.5S/CoP. Int. J. Hydrogen Energy 2022, 47, 32921–32927. [Google Scholar] [CrossRef]
- Chen, R.; Ma, M.H.; Luo, Y.; Qian, L.P.; Xu, S.Y. Enhanced interfacial effect between CdS and ReS2 on boosted hydrogen evolution performance via phase structure engineering. J. Solid State Chem. 2022, 312, 123238. [Google Scholar] [CrossRef]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Amirav, L.; Alivisatos, A.P. Photocatalytic hydrogen production with tunable nanorod heterostructures. J. Phys. Chem. Lett. 2010, 1, 1051–1054. [Google Scholar] [CrossRef]
- Shiga, Y.; Umezawa, N.; Srinivasan, N.; Koyasu, S.; Sakai, E.; Miyauchi, M. A metal sulfide photocatalyst composed of ubiquitous elements for solar hydrogen production. Chem. Commun. 2016, 52, 7470–7473. [Google Scholar] [CrossRef] [PubMed]
- Han, H.T.; Meng, X.C. Hydrothermal preparation of C3N4 on carbonized wood for photothermal-photocatalytic water splitting to efficiently evolve hydrogen. J. Colloid Interface Sci. 2023, 650, 846–856. [Google Scholar] [CrossRef]
- Jia, Y.C.; Tong, X.; Zhang, J.Z.; Zhang, R.; Yang, Y.; Zhang, L.; Ji, X. A facile synthesis of coral tubular g-C3N4 for photocatalytic degradation RhB and CO2 reduction. J. Alloys Compd. 2023, 965, 171432. [Google Scholar] [CrossRef]
- Bai, P.H.; Wang, C.J.; Xie, J.; Wang, H.; Kang, X.L.; Chen, M.; Wang, X. Ni-anchored g-C3N4 for improved hydrogen evolution in photocatalysis. Nanotechnology 2023, 34, 365402. [Google Scholar] [CrossRef]
- Liu, S.Q.; Qi, W.L.; Liu, J.; Meng, X.J.; Adimi, S.; Attfield, P.; Yang, M.H. Modulating electronic structure to improve the solar to hydrogen efficiency of cobalt nitride with lattice doping. ACS Catal. 2023, 13, 2214–2222. [Google Scholar] [CrossRef]
- Fateh, M.; Tessa, G.; Pelagia-Iren, G. Photochemical water splitting via transition metal oxides. Catalysts 2022, 12, 1303. [Google Scholar]
- Zhao, H.; Jian, L.; Gong, M.; Jing, M.Y.; Li, H.X.; Mao, Q.Y.; Lu, T.B.; Guo, Y.X.; Ji, R.; Chi, W.W.; et al. Transition-metal-based cocatalysts for photocatalytic water splitting. Small Struct. 2022, 3, 2100229. [Google Scholar] [CrossRef]
- Lv, K.L.; Fang, S.; Si, L.; Xia, Y.; Ho, W.; Li, M. Fabrication of TiO2 nanorod assembly grafted rGO (rGO@TiO2-NR) hybridized flake-like photocatalyst. Appl. Surf. Sci. 2017, 391, 218–227. [Google Scholar] [CrossRef]
- Yu, F.; Wang, L.C.; Xing, Q.J.; Wang, D.K.; Jiang, X.H.; Li, G.C.; Zheng, A.M.; Li, F.R.; Zou, J.P. Functional groups to modify g-C3N4 for improved photocatalytic activity of hydrogen evolution from water splitting. Chin. Chem. Lett. 2020, 31, 1648–1653. [Google Scholar] [CrossRef]
- Zou, J.P.; Wang, L.C.; Luo, J. Synthesis and efficient visible light photocatalytic H2 evolution of a metal-free g-C3N4/graphene quantum dots hybrid photocatalyst. Appl. Catal. B Environ. 2016, 193, 103–109. [Google Scholar] [CrossRef]
- Yu, J.; Li, Q.; Liu, S.; Jaroniec, M. Ionic-liquid-assisted synthesis of uniform fluorinated B/C-codoped TiO2 nanocrystals and their enhanced visible-light photocatalytic activity. Chemistry 2013, 19, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, T.; Lv, K.L.; Liu, F.L.; Li, H.Y.; Lei, M. Remarkable positive effect of Cd(OH)2 on CdS semiconductor for visible-light photocatalytic H2 production. Appl. Catal. B Environ. 2018, 229, 8–14. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.Y.; Hong, S.; Lee, S.; Lee, S.; Park, B.S.; Park, H.; Lee, C.; Lee, J. Effects of inorganic oxidants on kinetics and mechanisms of WO3-mediated photocatalytic degradation. Appl. Catal. B Environ. 2015, 162, 515–523. [Google Scholar] [CrossRef]
- Park, Y.; Kim, W.; Park, H.; Tachikawa, T.; Majima, T.; Choi, W. Carbon-doped TiO2 photocatalyst synthesized without using an external carbon precursor and the visible light activity. Appl. Catal. B Environ. 2009, 91, 355–361. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, Y.; Liu, B.; Wang, L.; Xiao, H.; Wang, S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018, 236, 396–403. [Google Scholar] [CrossRef]
- Frame, F.A.; Osterloh, F.E. CdSe-MoS2: A quantum size-confined photocatalyst for hydrogen evolution from water under visible light. J. Phys. Chem. C 2010, 114, 10628–10633. [Google Scholar] [CrossRef]
- Keimer, B.; Moore, J. The physics of quantum materials. Nat. Phys. 2017, 13, 1045–1055. [Google Scholar] [CrossRef]
- Zamin, M.; Narmina, O.B. Metal sulfide photocatalysts for hydrogen generation: A review of recent advances. Catalysts 2022, 12, 1316. [Google Scholar]
- Wang, Y.B.; Wang, Y.S.; Xu, R. Photochemical deposition of Pt on CdS for H2 evolution from water: Markedly enhanced activity by controlling Pt reduction environment. J. Phys. Chem. C 2013, 117, 783–790. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.X.; Zhang, W.D. MoS2/CdS heterojunction with high photoelectrochemical activity for H2 evolution under visible light: The role of MoS2. J. Phys. Chem. C 2013, 117, 12949–12957. [Google Scholar] [CrossRef]
- Jiang, D.C.; Sun, Z.J.; Jia, H.X.; Lu, D.P.; Du, P.W. A cocatalyst-free CdS nanorod/ZnS nanoparticle composite for high-performance visible-light-driven hydrogen production from water. J. Mater. Chem. A 2016, 4, 675–683. [Google Scholar] [CrossRef]
- Li, W.; Lee, J.R.; Jackel, F. Simultaneous optimization of colloidal stability and interfacial charge transfer efficiency in photocatalytic Pt/CdS nanocrystals. ACS Appl. Mater. Interfaces 2016, 8, 9434–29441. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Al-Oufi, M.; Nadeem, M.A.; Waterhouse, G.I.N.; Badshah, A.; Metson, J.B.; Idriss, H. On the role of metal particle size and surface coverage for photo-catalytic hydrogen production: A case study of the Au/CdS system. Appl. Catal. B Environ. 2016, 182, 266–276. [Google Scholar] [CrossRef]
- Chang, K.; Li, M.; Wang, T.; Ouyang, S.X.; Li, P.; Liu, L.Q.; Ye, J.H. Drastic layer-number-dependent activity enhancement in photocatalytic H2 evolution over nMoS2/CdS (n≥1) under visible light. Adv. Energy Mater. 2015, 5, 9491–9501. [Google Scholar] [CrossRef]
- Du, P.; Zhu, Y.; Zhang, J.; Xu, D.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Metallic 1T phase MoS2 nanosheets as a highly efficient co-catalyst for the photocatalytic hydrogen evolution of CdS nanorods. RSC Adv. 2016, 6, 74394–74399. [Google Scholar] [CrossRef]
- Stavroula, K.; Kyriakos, S.C. Dual-functional photocatalysis for simultaneous hydrogen production and oxidation of organic substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar]
- Zhang, J.F.; Wageh, S.; Al-Ghamdi, A.; Yu, J.G. New understanding on the different photocatalytic activity of wurtzite and zinc-blende CdS. Appl. Catal. B Environ. 2016, 192, 101–107. [Google Scholar] [CrossRef]
- Ran, J.R.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Iqbal, S.; Pan, Z.W.; Zhou, K.B. Enhanced photocatalytic hydrogen evolution from in situ formation of few-layered MoS2/CdS nanosheet-based van der Waals heterostructures. Nanoscale 2017, 9, 6638–6642. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Tong, B.; Yu, H.J.; Waterhouse, G.I.N.; Zhou, C.; Zhao, Y.F.; Tahir, M.; Wu, L.Z.; Tung, C.H.; Zhang, T.R. CdS nanoparticle-decorated Cd nanosheets for efficient visible light-driven photocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1501241–1501247. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Li, J.J.; Bai, Y.; Lian, J.H.; Huang, H.H.; Li, Z.M.; Lei, Z.Q.; Shangguan, W.F. Photochemical preparation of Cd/CdS photocatalysts and their efficient photocatalytic hydrogen production under visible light irradiation. Green Chem. 2014, 16, 2728–2735. [Google Scholar] [CrossRef]
- Ran, J.R.; Yu, J.G.; Jaroniec, M. Ni(OH)2 modified CdS nanorods for highly efficient visible-light-driven photocatalytic H2 generation. Green Chem. 2011, 13, 2708–2713. [Google Scholar] [CrossRef]
- Ran, J.R.; Yu, J.G. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364–1371. [Google Scholar]
- Marta, I.L. Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–104. [Google Scholar]
- Leung, D.Y.C.; Fu, X.L.; Wang, C.F.; Ni, M.; Leung, M.K.H.; Wang, X.X.; Fu, X.Z. Hydrogen production over titania-based photocatalysts. Chemsuschem 2010, 3, 681–694. [Google Scholar] [CrossRef]
- Kudo, A.; Sekizawa, M. Photocatalytic H2 evolution under visible light irradiation on Ni-doped ZnS photocatalyst. Chem. Commun. 2000, 15, 1371–1372. [Google Scholar] [CrossRef]
- Zou, Z.G.; Ye, J.H.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef]
- Xing, C.J.; Zhang, Y.J.; Yan, W.; Guo, L.J. Band structure-controlled solid solution of Cd1-xCd1-x ZnxSZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrogen Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Lee, Y.; Teramura, K.; Hara, M.; Domen, K. Modification of (Zn1+xGe)(N2Ox) solid solution as a visible light driven photocatalyst for overall water splitting. Chem. Mater. 2007, 19, 2120–2127. [Google Scholar] [CrossRef]
- Yao, W.F.; Huang, C.P.; Ye, J.H. Hydrogen production and characterization of MLaSrNb2NiO9 (M = Na, Cs, H) based photocatalysts. Chem. Mater. 2010, 22, 1107–1113. [Google Scholar] [CrossRef]
- Wang, T.; Guo, C.Y.; Zhang, L.G.; Cao, X.L.; Niu, Y.N.; Li, J.; Akram, N.; Wang, J.D. Effect of different CdS morphologies on photocatalytic water splitting performance of CdS@ZIF-67 composites. Int. J. Hydrogen Energy 2023, 58, 22021–22023. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Wang, Y.; Yin, L.X.; Li, J.Y.; Huang, J.F.; Kong, X.G.; Feng, Q. Building {0001} and {101} facet heterojunctions on hexagonal pyramid CdS single crystals with high photoactivity and photostability for hydrogen evolution. Chem. Eng. J. 2021, 426, 130777. [Google Scholar] [CrossRef]
- Ye, L.Q.; Ma, Z.Y.; Deng, Y.; Ye, Y.H.; Wang, L.; Kou, M.P.; Xie, H.Q.; Xu, Z.K.; Zhou, Y.; Xia, D.H.; et al. Robust and efficient photocatalytic hydrogen generation of ReS2/CdS and mechanistic study by on-line mass spectrometry and in situ infrared spectroscopy. Appl. Catal. B Environ. 2019, 257, 117897–117905. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Zheng, P.X.; Liu, Z.Q.; Lei, J.L.; Wu, H.; Li, N.; Ma, T.Y. Embedding au quantum dots in rimous cadmium sulfide nanospheres for enhanced photocatalytic hydrogen evolution. Small 2016, 28, 6735–6744. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhu, Y.J.; Cheng, G.F.; Huang, Y.H. SiO2/Cd(OH)2 composite nanotubes prepared by microwave-solvothermal transformation using water-dissolvable KCdCl3 nanowires as precursor and template. Mater. Lett. 2011, 65, 343–345. [Google Scholar] [CrossRef]
- Yang, Z.X.; Zhong, W.; Yin, Y.X.; Du, X.; Deng, Y.; An, C.; Du, Y.W. Controllable synthesis of single-crystalline CdO and Cd(OH)2 nanowires by a simple hydrothermal approach. Nanoscale Res. Lett. 2010, 5, 961–965. [Google Scholar] [CrossRef]
- Xu, J.X.; Yan, X.M.; Qi, Y.H.; Fu, Y.L.; Wang, C.; Wang, L. Novel phosphidated MoS2 nanosheets modified CdS semiconductor for an efficient photocatalytic H2 evolution. Chem. Eng. J. 2019, 375, 122053. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.Y.; Ji, Y.J.; Xu, J.; Yang, D.R. Synthesis of cadmium hydroxide nanoflake and nanowisker by hydrothermal method. Mater. Lett. 2005, 59, 56–58. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; Kong, L.; Guo, Z.; Fu, X.; Li, M.; Liu, J. Walnut-like CdS micro-particles/single-walled carbon nanotube hybrids: One-step hydrothermal route to synthesis and their properties. J. Mater. Chem. 2010, 20, 352–359. [Google Scholar] [CrossRef]
- Russat, J. Characterization of polyamic acid/polyimide films in the nanometric thickness range from spin-deposited polyamic acid. Surf. Interface Anal. 1988, 11, 414–420. [Google Scholar] [CrossRef]
- Singh, N.; Charan, S.; Patil, K.R.; Viswanath, A.K.; Khanna, P.K. Unusual formation of nano-particles of CdO and Cd(OH)2 from the reaction of dimethyl cadmium with DMF. Mater. Lett. 2006, 60, 3492–3498. [Google Scholar] [CrossRef]
- Wu, Y.X.; Zhao, X.S.; Li, Y.H.; Ling, Y.; Zhang, Y.Q.; Zhang, X.Q.; Huang, S.B. New insights into the efficient charge transfer by construction of adjustable dominant facet of BiOI/CdS heterojunction for antibiotics degradation and chromium Cr(VI) reduction under visible-light irradiation. Chemosphere 2022, 302, 134862. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ao, Y.; Wang, P. Enhancing the hydrogen evolution performance of CdS by synergistic effect of Ni doping and SnO2 coupling: Improved efficiency of charge transfer and H2O disassociation. Int. J. Hydrogen Energy 2021, 46, 6299–6309. [Google Scholar] [CrossRef]
- Hong, S.; Kumar, D.P.; Kim, E.H.; Park, H.; Gopannagari, M.; Reddy, D.A.; Kim, T.K. Earth abundant transition metal-doped few-layered MoS2 nanosheets on CdS nanorods for ultra-efficient photocatalytic hydrogen production. J. Mater. Chem. A 2017, 5, 20851–20859. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Qian, L.; Xu, S.; Wan, S.; Ma, M.; Zhang, L.; Jiang, R. In Situ Fabrication of CdS/Cd(OH)2 for Effective Visible Light-Driven Photocatalysis. Nanomaterials 2023, 13, 2453. https://doi.org/10.3390/nano13172453
Chen R, Qian L, Xu S, Wan S, Ma M, Zhang L, Jiang R. In Situ Fabrication of CdS/Cd(OH)2 for Effective Visible Light-Driven Photocatalysis. Nanomaterials. 2023; 13(17):2453. https://doi.org/10.3390/nano13172453
Chicago/Turabian StyleChen, Ran, Liping Qian, Shengyou Xu, Shunli Wan, Minghai Ma, Lei Zhang, and Runren Jiang. 2023. "In Situ Fabrication of CdS/Cd(OH)2 for Effective Visible Light-Driven Photocatalysis" Nanomaterials 13, no. 17: 2453. https://doi.org/10.3390/nano13172453
APA StyleChen, R., Qian, L., Xu, S., Wan, S., Ma, M., Zhang, L., & Jiang, R. (2023). In Situ Fabrication of CdS/Cd(OH)2 for Effective Visible Light-Driven Photocatalysis. Nanomaterials, 13(17), 2453. https://doi.org/10.3390/nano13172453





