Construction of Metal Organic Framework-Derived Fe-N-C Oxidase Nanozyme for Rapid and Sensitive Detection of Alkaline Phosphatase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Apparatus
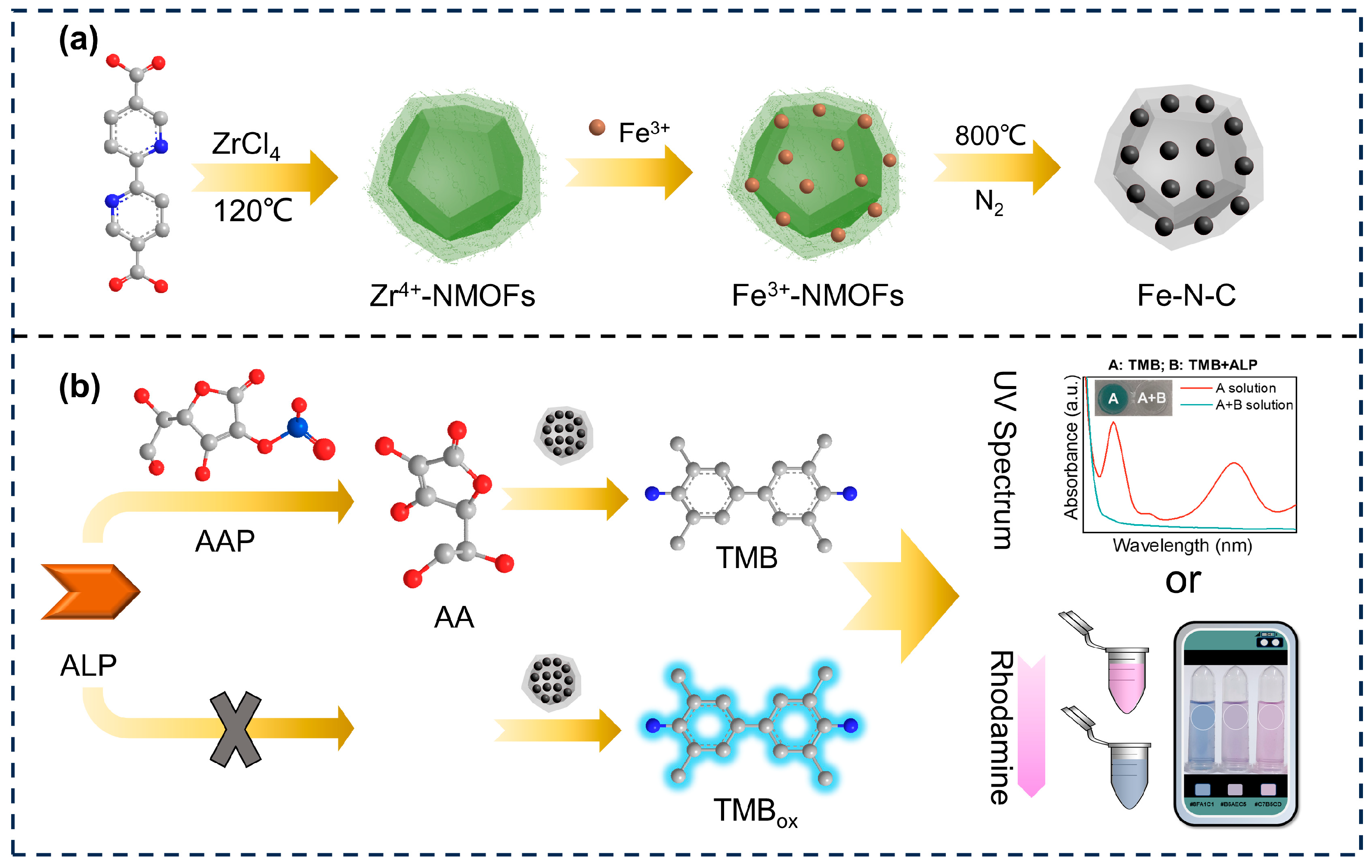
2.3. Synthesis of Fe-N-C Nanozyme
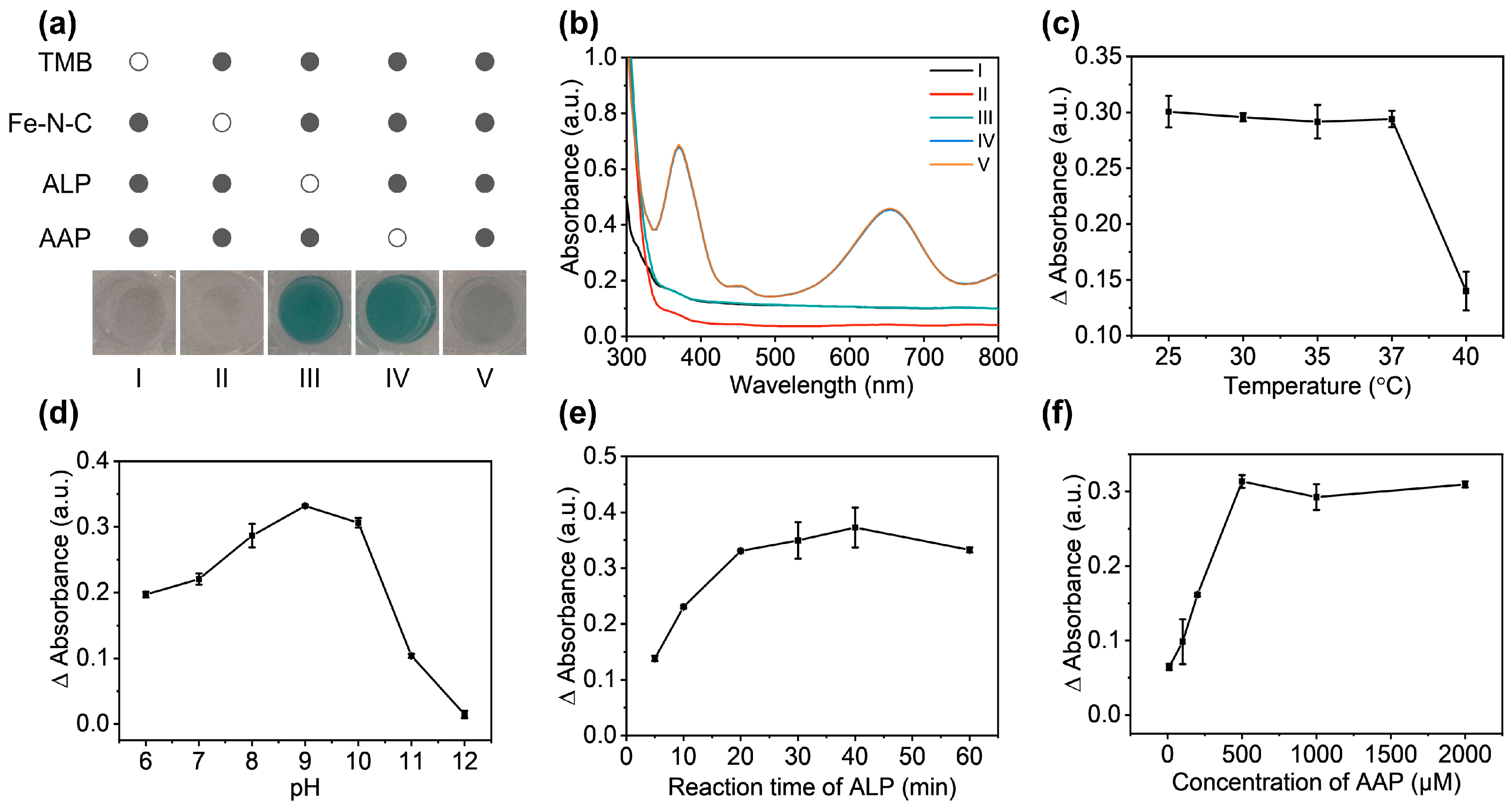
2.4. Catalytic Kinetic Experiment of TMB
2.5. Identification of ROS in the Fe-N-C Nanozyme Catalyzed System
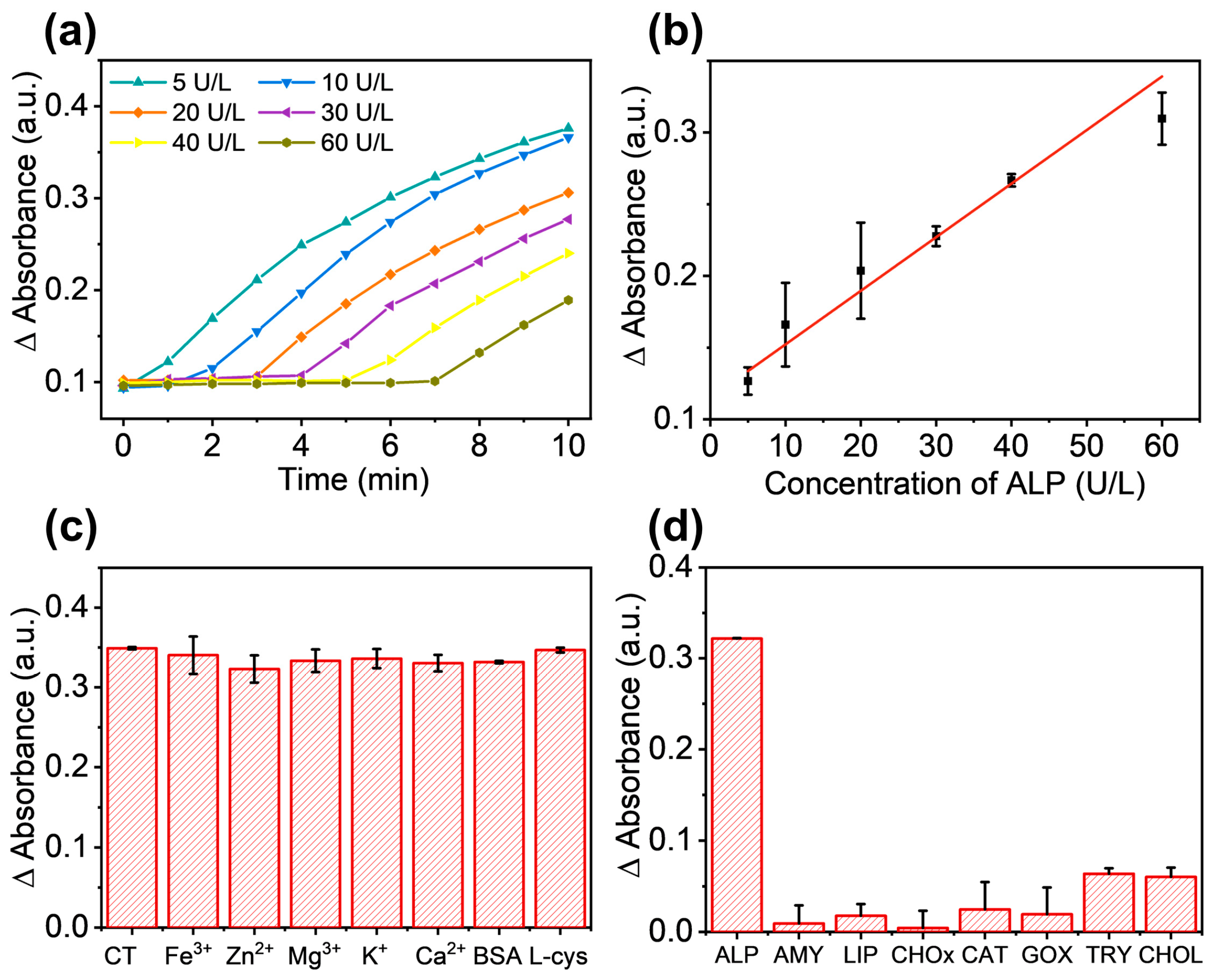
2.6. Detection of ALP with the Fe-N-C Nanozyme-Based Method
2.7. Evaluation of the Fe-N-C Nanozyme-Based Method for the Detection of ALP
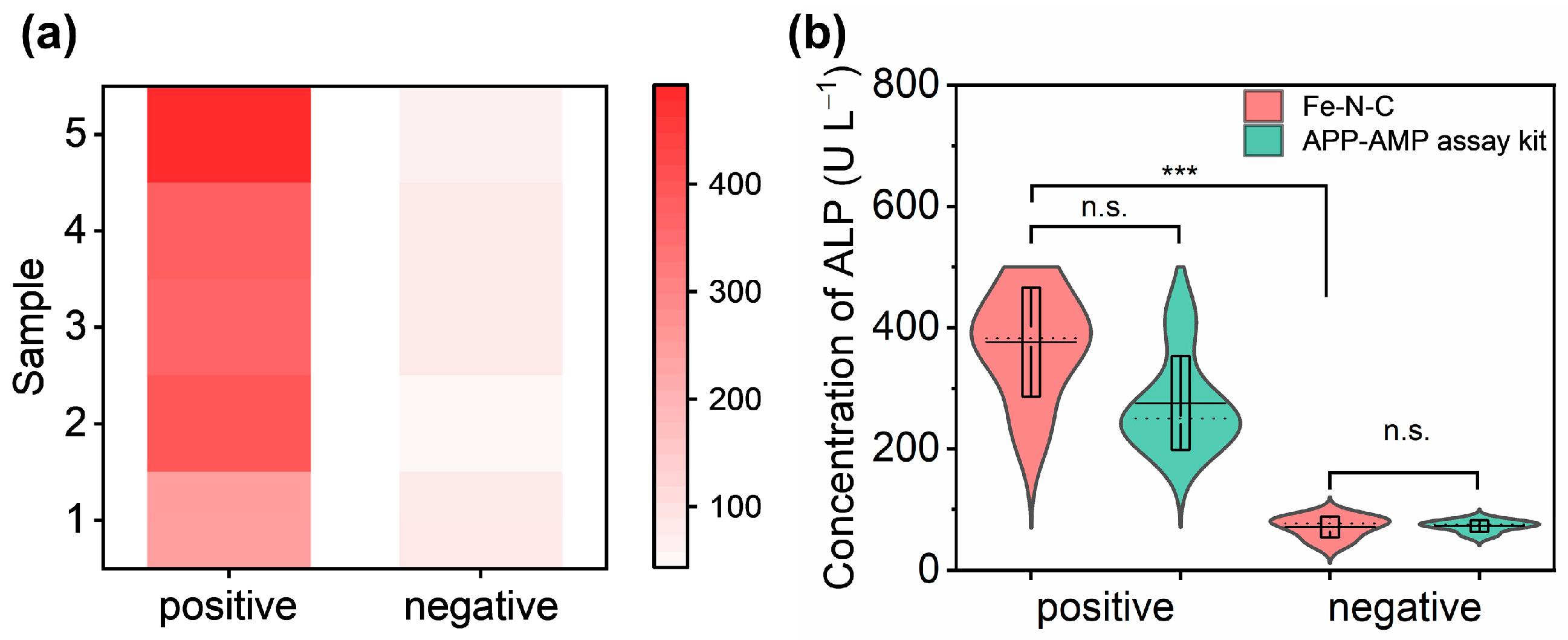
2.8. Detection of ALP in Clinical Samples
3. Results and Discussion
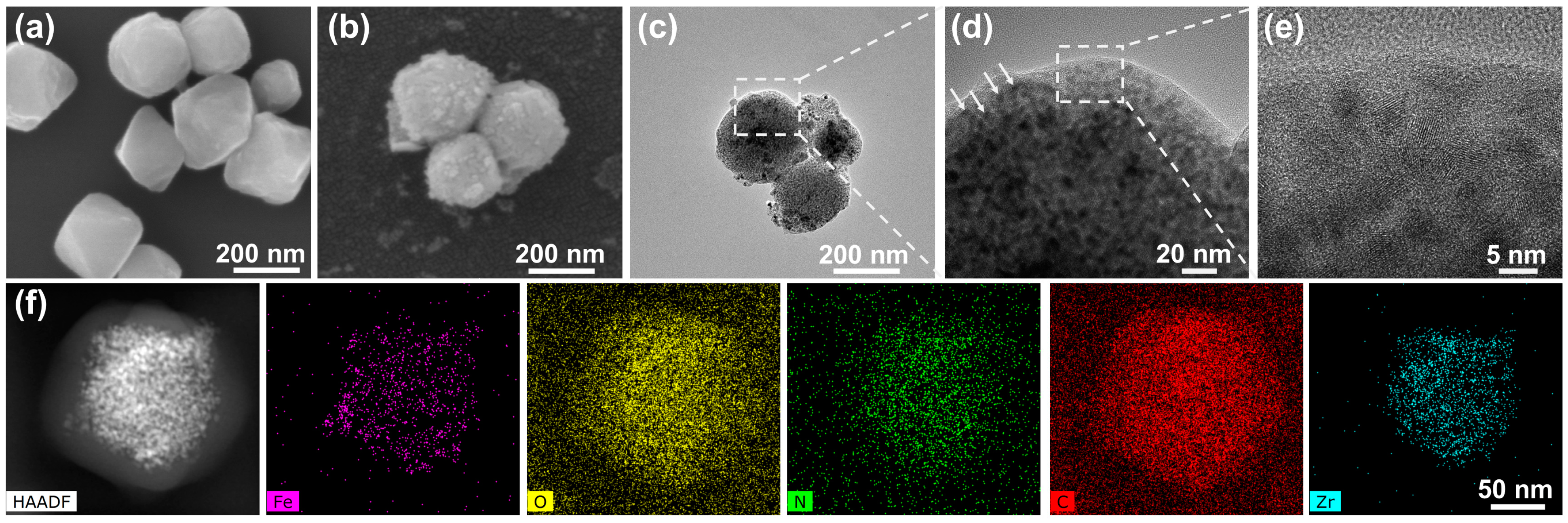
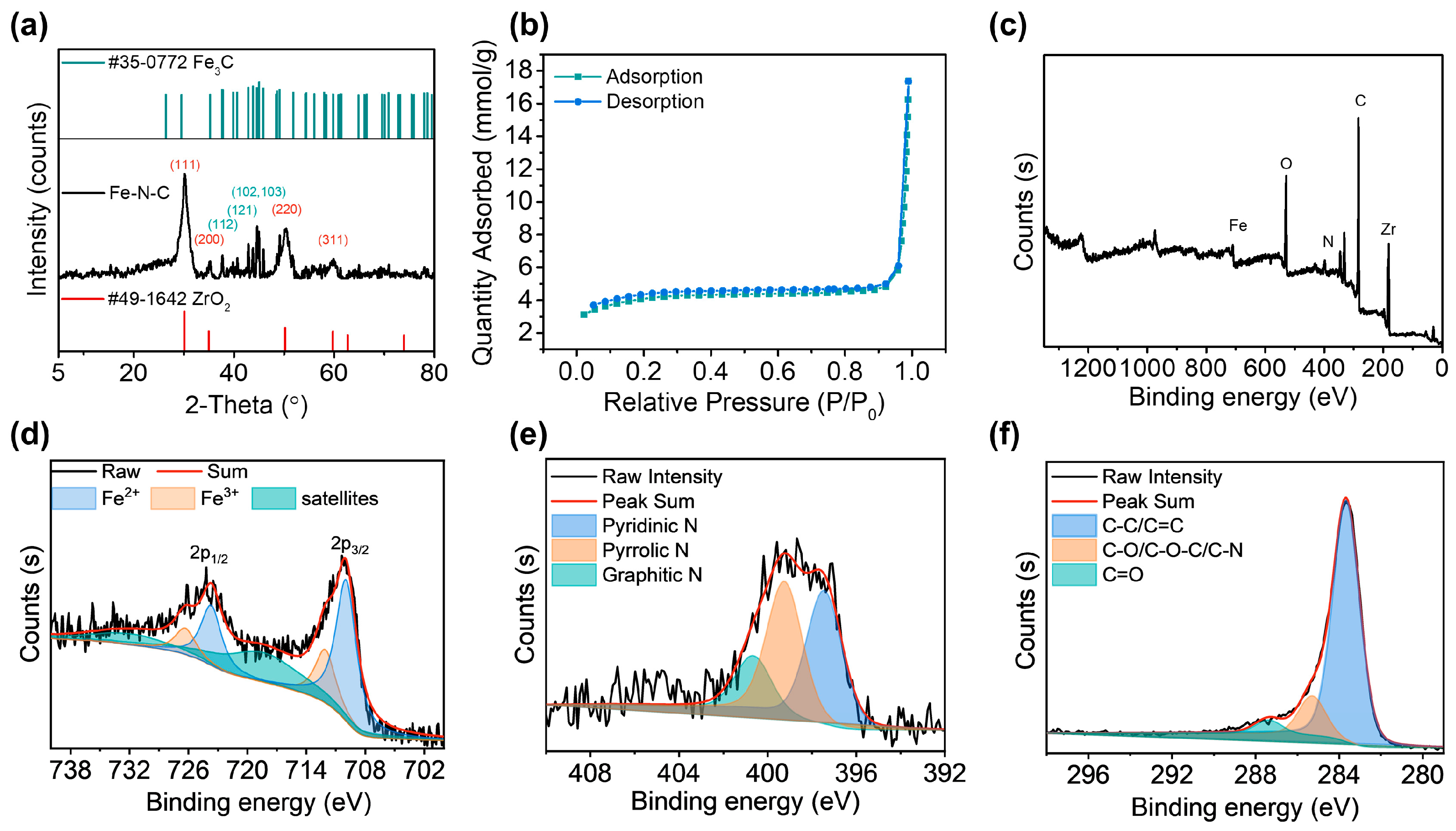
3.1. Characterization of Fe-N-C Nanozyme
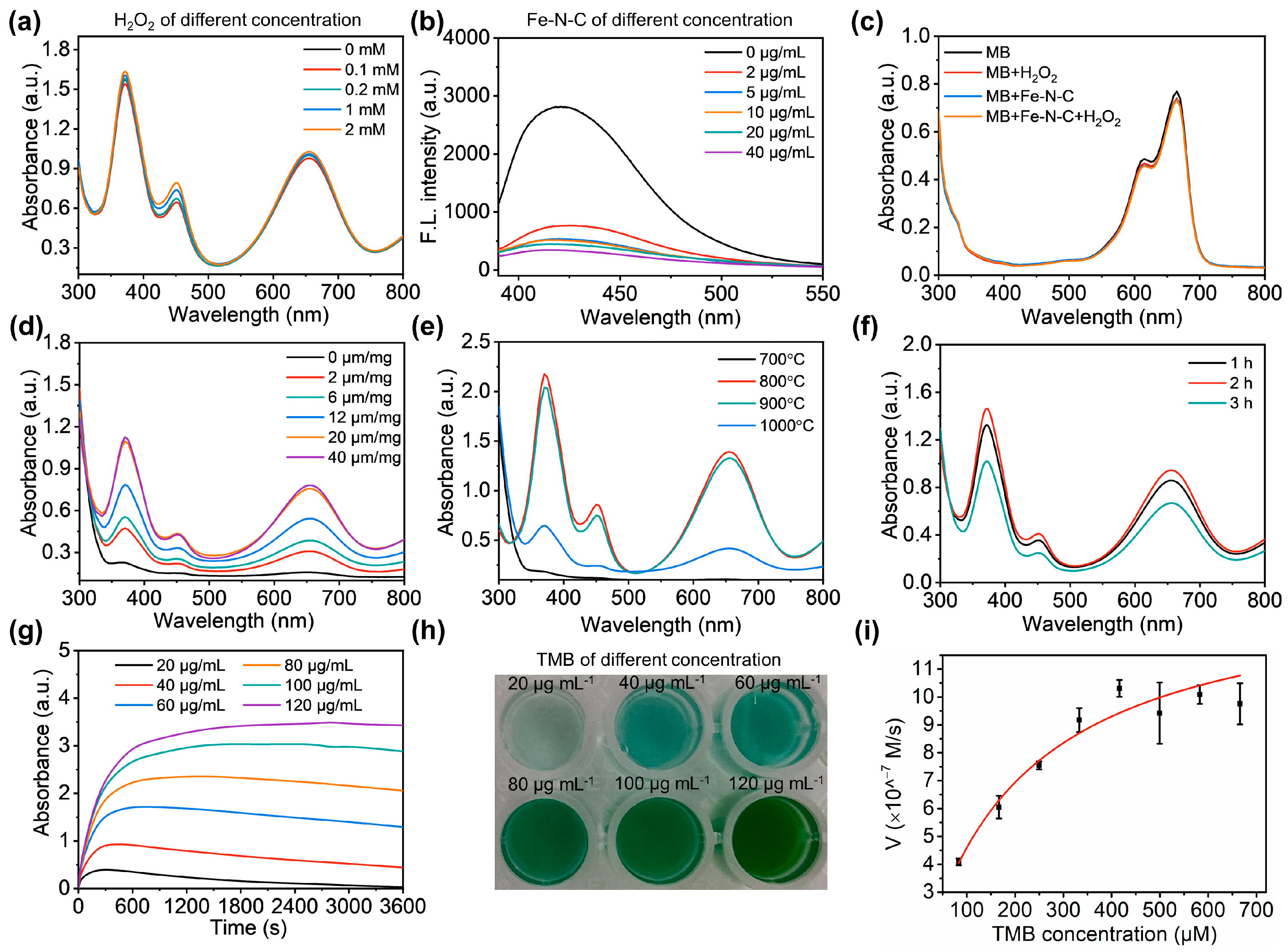
3.2. Verification and Optimization of the Enzymatic Activity of Fe-N-C Nanozyme
3.3. Detection of ALP by the Fe-N-C Nanozyme Based System
3.4. Detection of ALP in Clinical Samples
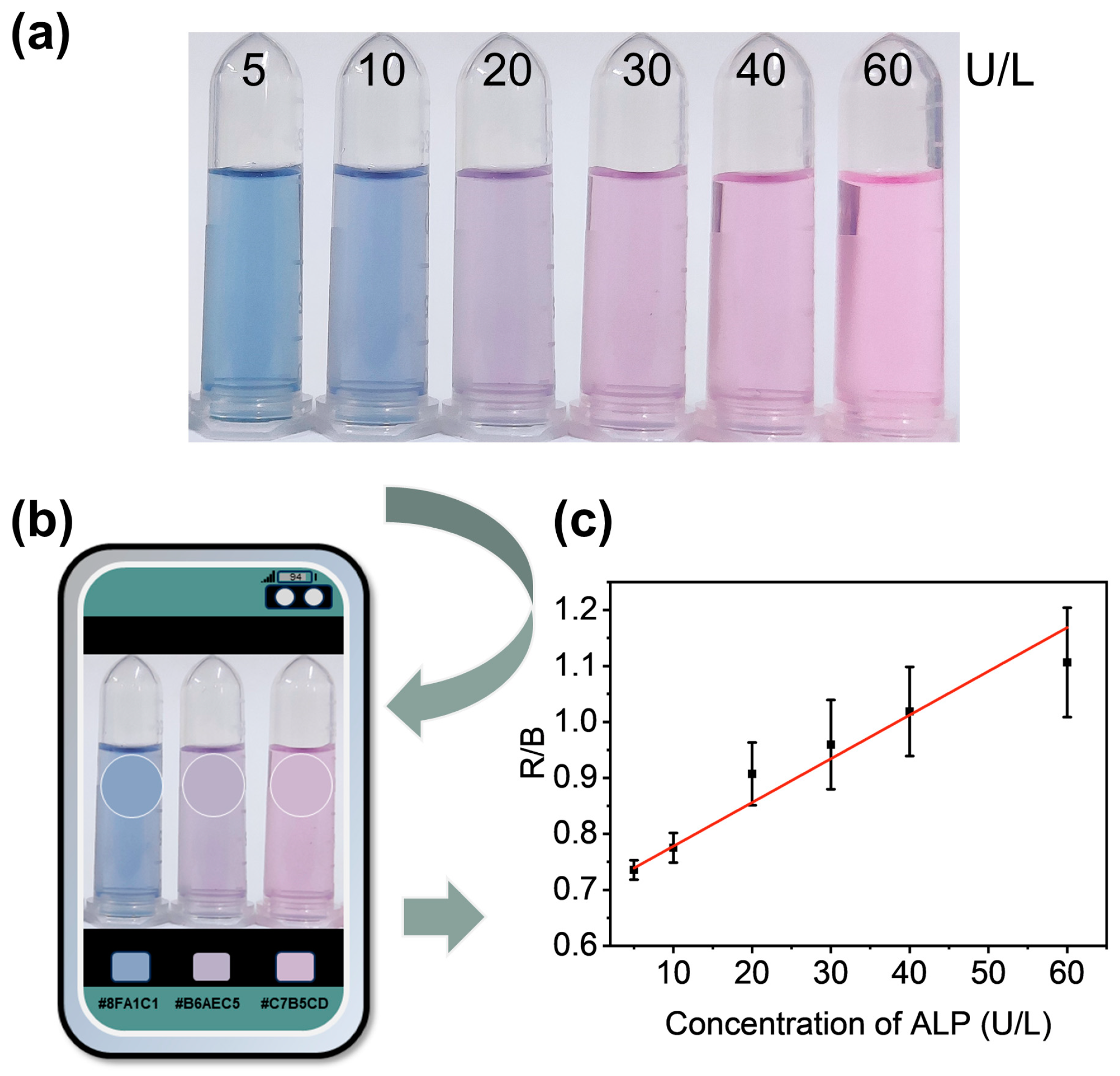
3.5. Visual Detection of ALP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaban, S.M.; Byeok Jo, S.; Hafez, E.; Ho Cho, J.; Kim, D.-H. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coord. Chem. Rev. 2022, 465, 214567. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; He, H.; Ma, C. Assays for alkaline phosphatase activity: Progress and prospects. TrAC Trends Anal. Chem. 2019, 113, 32–43. [Google Scholar] [CrossRef]
- Mahato, K.; Chandra, P. Paper-based miniaturized immunosensor for naked eye ALP detection based on digital image colorimetry integrated with smartphone. Biosens. Bioelectron. 2019, 128, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Kang, J.; Liu, J.; Wang, G. A novel electrochemical sensing strategy based on poly (3, 4-ethylenedioxythiophene): Polystyrene sulfonate, AuNPs, and Ag+ for highly sensitive detection of alkaline phosphatase. Nanomaterials 2022, 12, 3392. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Yang, Y.-C.; Shih, Y.-C.; Hung, S.-Y.; Lu, C.-Y.; Tseng, W.-L. Photoinduced electron transfer between Fe(III) and adenosine triphosphate-bodipy conjugates: Application to alkaline-phosphatase-linked immunoassay. Biosens. Bioelectron. 2016, 77, 242–248. [Google Scholar] [CrossRef]
- Madhu, M.; Chao, C.-M.; Ke, C.-Y.; Hsieh, M.-M.; Tseng, W.-L. Directed self-assembly of Ag+-deposited MoS2 quantum dots for colorimetric, fluorescent and fluorescence-lifetime sensing of alkaline phosphatase. Anal. Bioanal. Chem. 2022, 414, 1909–1919. [Google Scholar] [CrossRef]
- You, J.-G.; Lu, C.-Y.; Krishna Kumar, A.S.; Tseng, W.-L. Cerium(III)-directed assembly of glutathione-capped gold nanoclusters for sensing and imaging of alkaline phosphatase-mediated hydrolysis of adenosine triphosphate. Nanoscale 2018, 10, 17691–17698. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, Y.; Liu, F.; Wu, J.; Tian, L.; Zhao, S.; Ye, F. Smartphone flashlight-triggered covalent organic framework nanozyme activity: A universal scheme for visual point-of-care testing. Sens. Actuator-B Chem. 2023, 381, 133422. [Google Scholar] [CrossRef]
- Rao, H.; Xue, X.; Luo, M.; Liu, H.; Xue, Z. Recent advances in the development of colorimetric analysis and testing based on aggregation-induced nanozymes. Chin. Chem. Lett. 2021, 32, 25–32. [Google Scholar] [CrossRef]
- Chen, W.-H.; Yu, X.; Liao, W.-C.; Sohn, Y.S.; Cecconello, A.; Kozell, A.; Nechushtai, R.; Willner, I. ATP-responsive aptamer-based metal-organic framework nanoparticles (NMOFs) for the controlled release of loads and drugs. Adv. Funct. Mater. 2017, 27, 1702102. [Google Scholar] [CrossRef]
- Pan, M.-M.; Wang, Y.-F.; Wang, L.; Yu, X.; Xu, L. Recent advances in visual detection for cancer biomarkers and infectious pathogens. J. Mater. Chem. B 2021, 9, 35–52. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, S.; Muhammad, F.; Lin, Y.-W.; Wei, H. Rationally modulate the oxidase-like activity of nanoceria for self-regulated bioassays. ACS Sens. 2016, 1, 1336–1343. [Google Scholar] [CrossRef]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Almeida Paz, F.A. Metal-organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Liu, J.; Ren, J.; Tang, F.; Meng, X. One-pot synthesis of active copper-containing carbon dots with laccase-like activities. Nanoscale 2015, 7, 19641–19646. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Z.; Shu, J.; Jiang, X.; Wang, W.; Shi, Z.-H.; Lin, Y.-W. Mimicking a natural enzyme system: Cytochrome c oxidase-like activity of Cu2O nanoparticles by receiving electrons from cytochrome c. Inorg. Chem. 2017, 56, 9400–9403. [Google Scholar] [CrossRef] [PubMed]
- Navyatha, B.; Singh, S.; Nara, S. Auperoxidase nanozymes: Promises and applications in biosensing. Biosens. Bioelectron. 2021, 175, 112882. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, J.; Qian, X.; An, L.; Fan, L. Using a visible light-triggered pH switch to activate nanozymes for antibacterial treatment. RSC Adv. 2020, 10, 909–913. [Google Scholar] [CrossRef]
- Li, B.; Ju, Z.; Zhou, M.; Su, K.; Yuan, D. A reusable MOF-supported single-site zinc(II) catalyst for efficient intramolecular hydroamination of o-alkynylanilines. Angew. Chem. Int. Ed. 2019, 58, 7687–7691. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Mian, M.R.; Son, F.A.; Zhang, K.; Cao, R.; Chen, Z.; Lee, S.-J.; Idrees, K.B.; Goetjen, T.A.; et al. Structural diversity of zirconium metal-organic frameworks and effect on adsorption of toxic chemicals. J. Am. Chem. Soc. 2020, 142, 21428–21438. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Liu, C.; Wang, Z.; Kang, L.; Huang, Y.; Dong, K.; Ren, J.; Qu, X. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Tseng, W.-B.; Arputharaj, E.; Huang, P.-J.; Tseng, W.-L.; Bajda, T. Covalent organic framework nanosheets as an enhancer for light-responsive oxidase-like nanozymes: Multifunctional applications in colorimetric sensing, antibiotic degradation, and antibacterial agents. ACS Sustain. Chem. Eng. 2023, 11, 6956–6969. [Google Scholar] [CrossRef]
- Sha, M.; Xu, W.; Wu, Y.; Jiao, L.; Chen, Y.; Huang, J.; Tang, Y.; Gu, W.; Zhu, C. Histidine-engineered metal-organic frameworks with enhanced peroxidase-like activity for sensitive detection of metallothioneins. Sens.Actuator-B Chem. 2022, 366, 131927. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, L.; Yan, H.; Xu, W.; Wu, Y.; Zheng, L.; Gu, W.; Zhu, C. Fe-N-C single-atom catalyst coupling with Pt clusters boosts peroxidase-like activity for cascade-amplified colorimetric immunoassay. Anal. Chem. 2021, 93, 12353–12359. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-atom nanozymes. Sci. Adv. 2019, 5, eaav5490. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Qin, J.; Zhou, Y.; Yue, Q.; Wei, J. Spherical mesoporous Fe-N-C single-atom nanozyme for photothermal and catalytic synergistic antibacterial therapy. J. Colloid Interface Sci. 2022, 606, 826–836. [Google Scholar] [CrossRef]
- Pan, M.M.; Li, P.; Yu, Y.P.; Jiang, M.; Yang, X.; Zhang, P.; Nie, J.; Hu, J.; Yu, X.; Xu, L. Bimetallic ions functionalized metal-organic-framework nanozyme for tumor microenvironment regulating and enhanced photodynamic therapy for hypoxic tumor. Adv. Healthc. Mater. 2023, e2300821. [Google Scholar] [CrossRef]
- Pan, M.M.; Ouyang, Y.; Song, Y.L.; Si, L.Q.; Jiang, M.; Yu, X.; Xu, L.; Willner, I. Au3+-functionalized UiO-67 metal-organic framework nanoparticles: O2•− and •OH generating nanozymes and their antibacterial functions. Small 2022, 18, e2200548. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Z.-L.; Zheng, S.-Y. Highly sensitive DNA detection using cascade amplification strategy based on hybridization chain reaction and enzyme-induced metallization. Biosens. Bioelectron. 2015, 66, 520–526. [Google Scholar] [CrossRef]
- Sun, J.; Hu, T.; Xu, X.; Wang, L.; Yang, X. A fluorescent ELISA based on the enzyme-triggered synthesis of poly(thymine)-templated copper nanoparticles. Nanoscale 2016, 8, 16846–16850. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Y.; Sun, K.; Li, H.; Lin, X.; Zhang, P.; Zhou, L.; Wang, A.; Mehdi, S.; Wu, X.; et al. Coupling Fe3C nanoparticles and N-doping on wood-derived carbon to construct reversible cathode for Zn-air batteries. Small 2022, 18, e2202014. [Google Scholar] [CrossRef]
- Liu, B.; Yao, H.; Daniels, R.A.; Song, W.; Zheng, H.; Jin, L.; Suib, S.L.; He, J. A facile synthesis of Fe3C@mesoporous carbon nitride nanospheres with superior electrocatalytic activity. Nanoscale 2016, 8, 5441–5445. [Google Scholar] [CrossRef]
- Liang, S.; Niu, H.-Y.; Guo, H.; Niu, C.-G.; Liang, C.; Li, J.-S.; Tang, N.; Lin, L.-S.; Zheng, C.-W. Incorporating Fe3C into B, N co-doped CNTs: Non-radical-dominated peroxymonosulfate catalytic activation mechanism. Chem. Eng. J. 2021, 405, 126686. [Google Scholar] [CrossRef]
- Ghanbarlou, H.; Nasernejad, B.; Nikbakht Fini, M.; Simonsen, M.E.; Muff, J. Synthesis of an iron-graphene based particle electrode for pesticide removal in three-dimensional heterogeneous electro-fenton water treatment system. Chem. Eng. J. 2020, 395, 125025. [Google Scholar] [CrossRef]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Huang, J.; Zeng, F.; Wu, S. A fluorescent probe with aggregation-induced emission for detecting alkaline phosphatase and cell imaging. Chem.-Asian J. 2019, 14, 802–808. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Deng, H.; Li, H.; Tang, W.; Guan, L.; Qiu, Y.; Donovan, M.J.; Chen, Z.; Tan, W. In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of Helicobacter pylori. Nat. Commun. 2021, 12, 2002. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sun, Y.; Lin, L.; Shi, C.; Wang, G.; Zhang, X. Naked-eye sensitive detection of alkaline phosphatase (ALP) and pyrophosphate (PPi) based on a horseradish peroxidase catalytic colorimetric system with Cu(II). Analyst 2016, 141, 5549–5554. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Ma, F.; Yang, M.; Li, X.; Chen, X. A halide perovskite/lead sulfide heterostructure with enhanced photoelectrochemical performance for the sensing of alkaline phosphatase (ALP). Chem. Commun. 2023, 59, 1361–1364. [Google Scholar] [CrossRef]
- Wang, A.-L.; Teng, J.-X.; Yang, C.-G.; Xu, Z.-R. Rapid and facile electrospray preparation of CsPbBr3@PMMA fluorescent microspheres for fluorescent detection of alp in biological samples. Colloid Surf. A-Physicochem. Eng. Asp. 2022, 634, 127909. [Google Scholar] [CrossRef]
- Chen, C.; Geng, F.; Wang, Y.; Yu, H.; Li, L.; Yang, S.; Liu, J.; Huang, W. Design of a nanoswitch for sequentially multi-species assay based on competitive interaction between DNA-templated fluorescent copper nanoparticles, Cr3+ and pyrophosphate and ALP. Talanta 2019, 205, 120132. [Google Scholar] [CrossRef] [PubMed]
| Material | Method | Liner Range (U L−1) | LOD (U L−1) | Detection Time | Ref. |
|---|---|---|---|---|---|
| TPE-CN-pho a | Fluorescence | 25~175 | 14.2 | 60 min | [36] |
| P/DS/EDC-NHS/Anti-ALP biosensor b | Visual detection | 104~106 | 870 | 13 min | [6] |
| HRP-TMB-H2O2 with the Cu2+ system | UV spectrum | 0–120 | 5.4 | 60 min | [38] |
| CsPbBr3 NC | Photocurrent responses | 50~1000 | 42.1 | 30 min | [39] |
| CsPbBr3@PMMA | Fluorescence | 10~100 | 4.8 | 70 min | [40] |
| CuNPs-Cr3+-PPi | Fluorescence | 0~62.5 | 3.3 | 30 min | [41] |
| Fe-N-C | UV spectrum/Visual detection | 5~60 | 3.38/2.12 | 50 min | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Wang, M.; Yang, L.; Song, Y.; Jiang, M.; Yu, X.; Xu, L. Construction of Metal Organic Framework-Derived Fe-N-C Oxidase Nanozyme for Rapid and Sensitive Detection of Alkaline Phosphatase. Nanomaterials 2023, 13, 2496. https://doi.org/10.3390/nano13182496
Pan M, Wang M, Yang L, Song Y, Jiang M, Yu X, Xu L. Construction of Metal Organic Framework-Derived Fe-N-C Oxidase Nanozyme for Rapid and Sensitive Detection of Alkaline Phosphatase. Nanomaterials. 2023; 13(18):2496. https://doi.org/10.3390/nano13182496
Chicago/Turabian StylePan, Mengmeng, Ming Wang, Linjiao Yang, Yongli Song, Ming Jiang, Xu Yu, and Li Xu. 2023. "Construction of Metal Organic Framework-Derived Fe-N-C Oxidase Nanozyme for Rapid and Sensitive Detection of Alkaline Phosphatase" Nanomaterials 13, no. 18: 2496. https://doi.org/10.3390/nano13182496
APA StylePan, M., Wang, M., Yang, L., Song, Y., Jiang, M., Yu, X., & Xu, L. (2023). Construction of Metal Organic Framework-Derived Fe-N-C Oxidase Nanozyme for Rapid and Sensitive Detection of Alkaline Phosphatase. Nanomaterials, 13(18), 2496. https://doi.org/10.3390/nano13182496







