Unraveling the Position Effect of Spiroxanthene-Based n-Type Hosts for High-Performance TADF–OLEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. OLED Fabrication
3. Results and Discussion
3.1. Synthesis and Structure Characterization
3.2. Theoretical Simulation and Electrochemistry
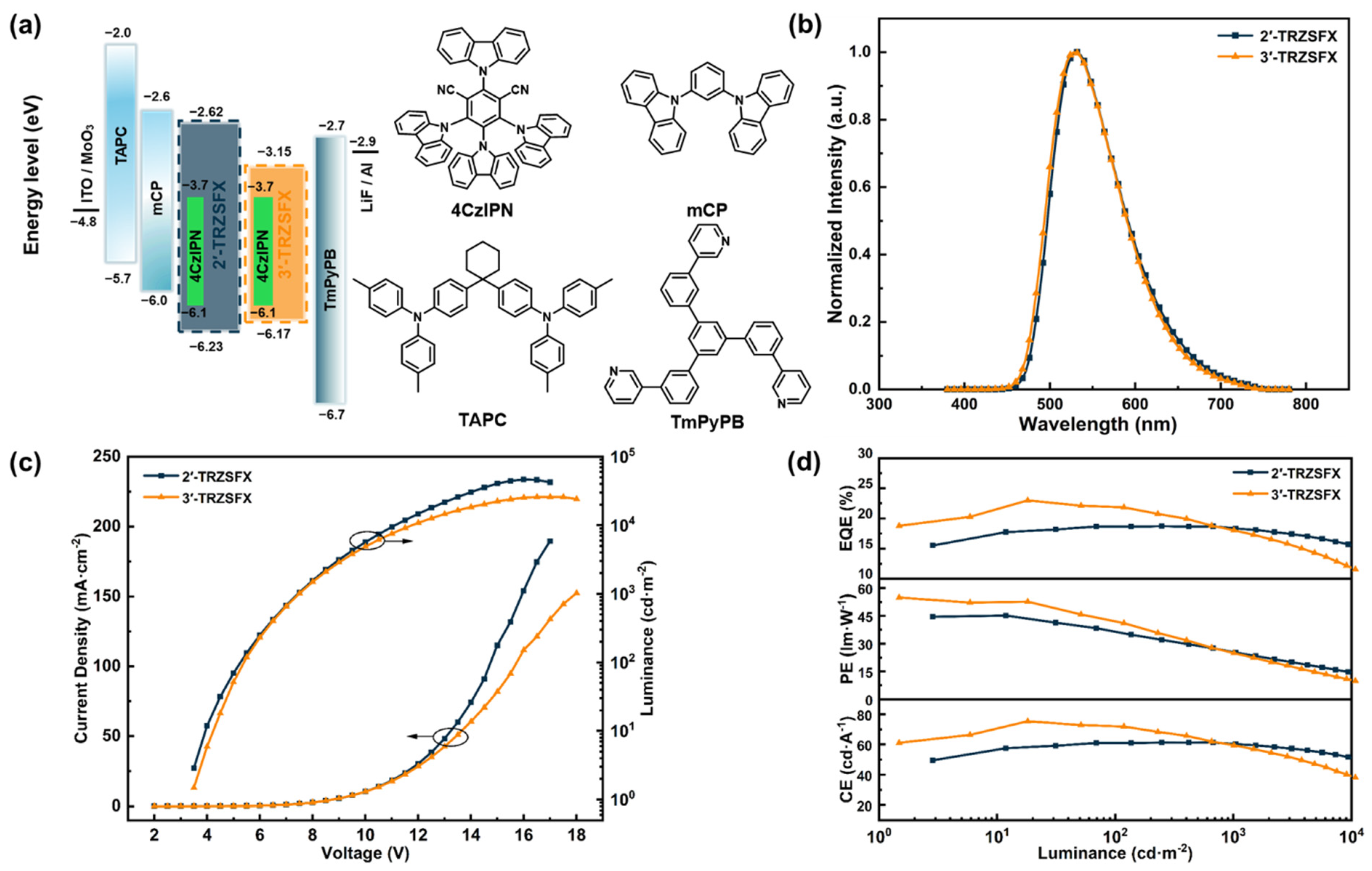
3.3. Photophysical Properties
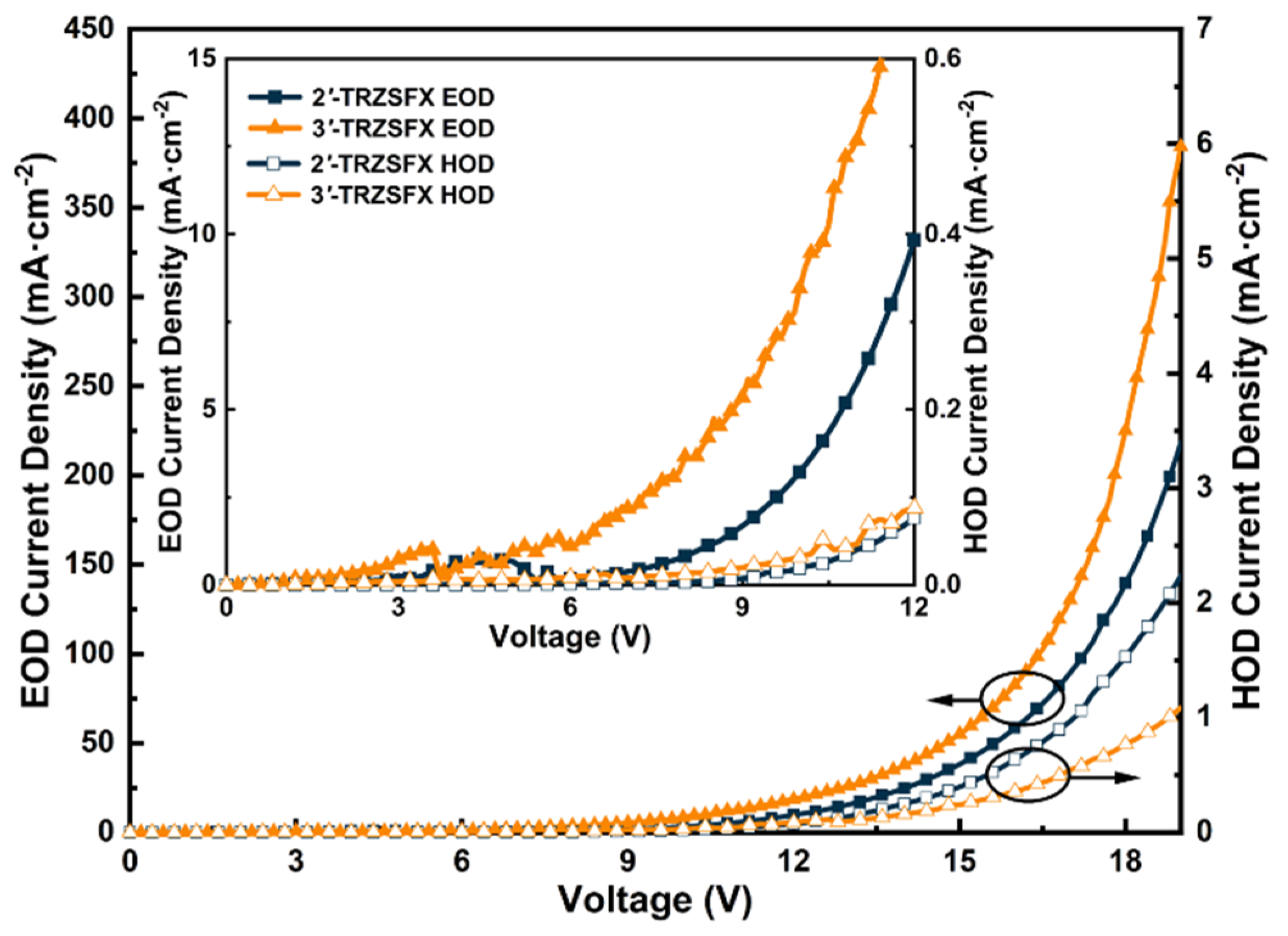
3.4. Carrier Transport Properties
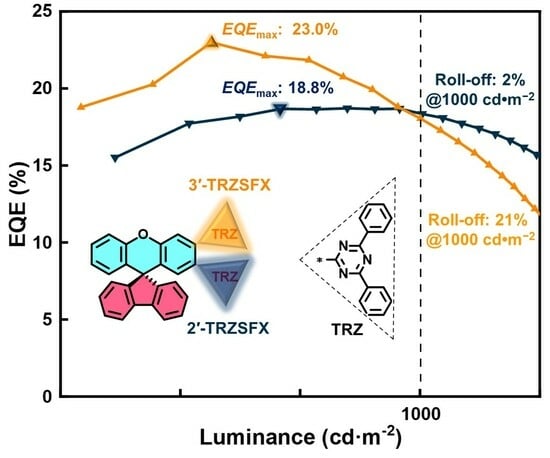
3.5. OLED Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A brief history of OLEDs—Emitter development and industry milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-Y.; Wu, J.; Wu, Z.-G.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Rational design of phosphorescent iridium(III) complexes for emission color tunability and their applications in OLEDs. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, W.; Duan, L. Recent progress in solution processable TADF materials for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 5577–5596. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 2012, 134, 14706–14709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cao, X.; Wu, Q.; Zhang, M.; Sun, N.; Zhang, X.; Tao, Y. Purely organic materials for extremely simple all-TADF white OLEDs: A new carbazole/oxadiazole hybrid material as a dual-role non-doped light blue emitter and highly efficient orange host. J. Mater. Chem. C 2018, 6, 3675–3682. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Huang, T.; Gillett, A.J.; Liu, Y.; Hu, D.; Cui, L.; Bin, Z.; Li, G.; Wei, J.; et al. Multi-Resonance Deep-Red Emitters with Shallow Potential-Energy Surfaces to Surpass Energy-Gap Law**. Angew. Chem. Inter. Ed. 2021, 60, 20498–20503. [Google Scholar] [CrossRef]
- Jiang, P.; Miao, J.; Cao, X.; Xia, H.; Pan, K.; Hua, T.; Lv, X.; Huang, Z.; Zou, Y.; Yang, C. Quenching-Resistant Multiresonance TADF Emitter Realizes 40% External Quantum Efficiency in Narrowband Electroluminescence at High Doping Level. Adv. Mater. 2022, 34, 2106954. [Google Scholar] [CrossRef]
- Han, C.; Zhang, Z.; Ding, D.; Xu, H. Dipole-Dipole Interaction Management for Efficient Blue Thermally Activated Delayed Fluorescence Diodes. Chem 2018, 4, 2154–2167. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Zhou, C.; Ni, F.; Huang, Z.; Cao, X.; Yang, C. Versatile Host Materials for Both D-A type and Multi-Resonance TADF Emitters towards Solution-Processed OLEDs with Nearly 30% EQE. Adv. Mater. 2023, 35, 2300510. [Google Scholar] [CrossRef]
- Wu, X.; Su, B.-K.; Chen, D.-G.; Liu, D.; Wu, C.-C.; Huang, Z.-X.; Lin, T.-C.; Wu, C.-H.; Zhu, M.; Li, E.Y.; et al. The role of host–guest interactions in organic emitters employing MR-TADF. Nat. Photonics 2021, 15, 780–786. [Google Scholar] [CrossRef]
- Wang, Y.; Yun, J.H.; Wang, L.; Lee, J.Y. High triplet energy hosts for blue organic light-emitting diodes. Adv. Funct. Mater. 2021, 31, 2008332. [Google Scholar] [CrossRef]
- Chatterjee, T.; Wong, K.T. Perspective on host materials for thermally activated delayed fluorescence organic light emitting diodes. Adv. Opt. Mater. 2019, 7, 1800565. [Google Scholar] [CrossRef]
- Baldo, M.A.; Adachi, C.; Forrest, S.R. Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys. Rev. B 2000, 62, 10967–10977. [Google Scholar] [CrossRef]
- Malliaras, G.G.; Scott, J.C. The roles of injection and mobility in organic light emitting diodes. J. Appl. Phys. 1998, 83, 5399–5403. [Google Scholar] [CrossRef]
- Ihn, S.-G.; Lee, N.; Jeon, S.O.; Sim, M.; Kang, H.; Jung, Y.; Huh, D.H.; Son, Y.M.; Lee, S.Y.; Numata, M.; et al. An Alternative Host Material for Long-Lifespan Blue Organic Light-Emitting Diodes Using Thermally Activated Delayed Fluorescence. Adv. Sci. 2017, 4, 1600502. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, D.; Wei, Y.; Xu, H. Extremely condensing triplet states of DPEPO-type hosts through constitutional isomerization for high-efficiency deep-blue thermally activated delayed fluorescence diodes. Chem. Sci. 2016, 7, 2870–2882. [Google Scholar] [CrossRef]
- Cui, L.-S.; Ruan, S.-B.; Bencheikh, F.; Nagata, R.; Zhang, L.; Inada, K.; Nakanotani, H.; Liao, L.-S.; Adachi, C. Long-lived efficient delayed fluorescence organic light-emitting diodes using n-type hosts. Nat. Commun. 2017, 8, 2250. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yasuda, T.; Yang, Y.S.; Zhang, Q.; Adachi, C. Luminous butterflies: Efficient exciton harvesting by benzophenone derivatives for full-color delayed fluorescence OLEDs. Angew. Chem. Inter. Ed. 2014, 53, 6402–6406. [Google Scholar] [CrossRef]
- Li, B.; Li, Z.; Hu, T.; Zhang, Y.; Wang, Y.; Yi, Y.; Guo, F.; Zhao, L. Highly efficient blue organic light-emitting diodes from pyrimidine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2018, 6, 2351–2359. [Google Scholar] [CrossRef]
- Stachelek, P.; Ward, J.S.; Dos Santos, P.L.; Danos, A.; Colella, M.; Haase, N.; Raynes, S.J.; Batsanov, A.S.; Bryce, M.R.; Monkman, A.P. Molecular Design Strategies for Color Tuning of Blue TADF Emitters. ACS Appl. Mater. Interfaces 2019, 11, 27125–27133. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ding, D.; Tao, Y.; Wei, Y.; Chen, R.; Xie, L.; Huang, W.; Xu, H. A Significantly Twisted Spirocyclic Phosphine Oxide as a Universal Host for High-Efficiency Full-Color Thermally Activated Delayed Fluorescence Diodes. Adv. Mater. 2016, 28, 3122–3130. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Noda, H.; Nakanotani, H.; Adachi, C. Effect of Carrier Balance on Device Degradation of Organic Light-Emitting Diodes Based on Thermally Activated Delayed Fluorescence Emitters. Adv. Electron. Mater. 2019, 5, 1800708. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Nakanotani, H.; Hatakeyama, T.; Adachi, C. The Role of Reverse Intersystem Crossing Using a TADF-Type Acceptor Molecule on the Device Stability of Exciplex-Based Organic Light-Emitting Diodes. Adv. Mater. 2020, 32, 1906614. [Google Scholar] [CrossRef] [PubMed]
- Mac Ciarnáin, R.; Mo, H.W.; Nagayoshi, K.; Fujimoto, H.; Harada, K.; Gehlhaar, R.; Ke, T.H.; Heremans, P.; Adachi, C. A Thermally Activated Delayed Fluorescence Green OLED with 4500 h Lifetime and 20% External Quantum Efficiency by Optimizing the Emission Zone using a Single-Emission Spectrum Technique. Adv. Mater. 2022, 34, 2201409. [Google Scholar] [CrossRef]
- Fan, X.-C.; Wang, K.; Shi, Y.-Z.; Cheng, Y.-C.; Lee, Y.-T.; Yu, J.; Chen, X.-K.; Adachi, C.; Zhang, X.-H. Ultrapure green organic light-emitting diodes based on highly distorted fused π-conjugated molecular design. Nat. Photonics 2023, 17, 280–285. [Google Scholar] [CrossRef]
- Ren, B.-Y.; Guo, R.-D.; Zhong, D.-K.; Ou, C.-J.; Xiong, G.; Zhao, X.-H.; Sun, Y.-G.; Jurow, M.; Kang, J.; Zhao, Y.; et al. A Yellow-Emitting Homoleptic Iridium(III) Complex Constructed from a Multifunctional Spiro Ligand for Highly Efficient Phosphorescent Organic Light-Emitting Diodes. Inorg. Chem. 2017, 56, 8397–8407. [Google Scholar] [CrossRef]
- Chiykowski, V.A.; Cao, Y.; Tan, H.; Tabor, D.P.; Sargent, E.H.; Aspuru-Guzik, A.; Berlinguette, C.P. Precise Control of Thermal and Redox Properties of Organic Hole-Transport Materials. Angew. Chem. Inter. Ed. 2018, 57, 15529–15533. [Google Scholar] [CrossRef]
- Han, C.; Xie, G.; Xu, H.; Zhang, Z.; Yan, P.; Zhao, Y.; Liu, S. Xanthene-based phosphine oxide host with the planar multi-insulating structure for efficient electrophosphorescence. Dyes Pigment. 2012, 94, 561–569. [Google Scholar] [CrossRef]
- Du, R.; Duan, C.; Li, Y.; Zhang, J.; Han, C.; Xu, H. Symmetrical spirobi[xanthene] based locally asymmetrical phosphine oxide host for low-voltage-driven highly efficient white thermally activated delayed fluorescence diodes. Chem. Eng. J. 2020, 392, 124870. [Google Scholar] [CrossRef]
- Ren, B.-Y.; Lan, X.; Tan, Y.-M.; Xiong, G.; Sun, Y.-G.; Guo, R.-D.; Lv, M.-H.; Xie, L.-H. Finely tuning electronic and steric structures of spiro[fluorene-9,9′-xanthene] (SFX)-based emitters by isomerization strategy towards efficient electroluminescence. Dyes Pigment. 2022, 198, 110035. [Google Scholar] [CrossRef]
- Ren, B.-Y.; Zhong, D.-K.; Sun, Y.-G.; Zhao, X.-H.; Zhang, Q.-J.; Liu, Y.; Jurow, M.; Sun, M.-L.; Zhang, Z.-S.; Zhao, Y. Quinolyl functionalized spiro[fluorene-9,9′-xanthene] host materials with bipolar characteristics for green and red phosphorescent organic light-emitting diodes. Org. Electron. 2016, 36, 140–147. [Google Scholar] [CrossRef]
- Hu, Y.-N.; Fan, X.-C.; Huang, F.; Shi, Y.-Z.; Wang, H.; Cheng, Y.-C.; Chen, M.; Wang, K.; Yu, J.; Zhang, X. Novel Multiple Resonance Type TADF Emitter as Blue Component for Highly Efficient Blue-Hazard-Free White Organic Light-Emitting Diodes. Adv. Opt. Mater. 2023, 11, 2202267. [Google Scholar] [CrossRef]
- Xie, L.-H.; Liu, F.; Tang, C.; Hou, X.-Y.; Hua, Y.-R.; Fan, Q.-L.; Huang, W. Unexpected One-Pot Method to Synthesize Spiro[fluorene-9,9‘-xanthene] Building Blocks for Blue-Light-Emitting Materials. Org. Lett. 2006, 8, 2787–2790. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-L.; Yue, S.-Z.; Lin, J.-R.; Ou, C.-J.; Qian, Y.; Zhang, Y.; Li, Y.; Wei, Q.; Zhao, Y.; Xie, L.-H. Dimeric SFX host materials for red, green and blue phosphorescent organic light-emitting devices. Synth. Met. 2014, 195, 321–327. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wang, S.-S.; Yu, Y.; Zhang, H.; Wang, W.-Y.; Yang, R.-Q.; Xie, L.H.; Liu, F.; Lin, Z.Q.; Shi, N.E.; et al. SMART Design of a Bulk-Capped Supramolecular Segment for the Assembly into Organic Interdigital Lipid Bilayer-Like (ILB) Nanosheets. Small 2018, 14, 1703151. [Google Scholar] [CrossRef]
- Shih, P.-I.; Chiang, C.-L.; Dixit, A.K.; Chen, C.-K.; Yuan, M.-C.; Lee, R.-Y.; Chen, C.-T.; Diau, E.W.-G.; Shu, C.-F. Novel Carbazole/Fluorene Hybrids: Host Materials for Blue Phosphorescent OLEDs. Org. Lett. 2006, 8, 2799–2802. [Google Scholar] [CrossRef]
- Okazawa, K.; Tsuji, Y.; Yoshizawa, K. Graph-theoretical exploration of the relation between conductivity and connectivity in heteroatom-containing single-molecule junctions. J. Chem. Phys. 2022, 156, 091102. [Google Scholar] [CrossRef]
- Yi, R.-H.; Shao, C.-M.; Lin, C.-H.; Fang, Y.-C.; Shen, H.-L.; Lu, C.-W.; Wang, K.-Y.; Chang, C.-H.; Chen, L.-Y.; Chang, Y.-H. Dicyano-Imidazole-Based Host Materials Possessing a Balanced Bipolar Nature to Realize Efficient OLEDs with Extremely High Luminance. J. Phys. Chem. C 2020, 124, 20410–20423. [Google Scholar] [CrossRef]

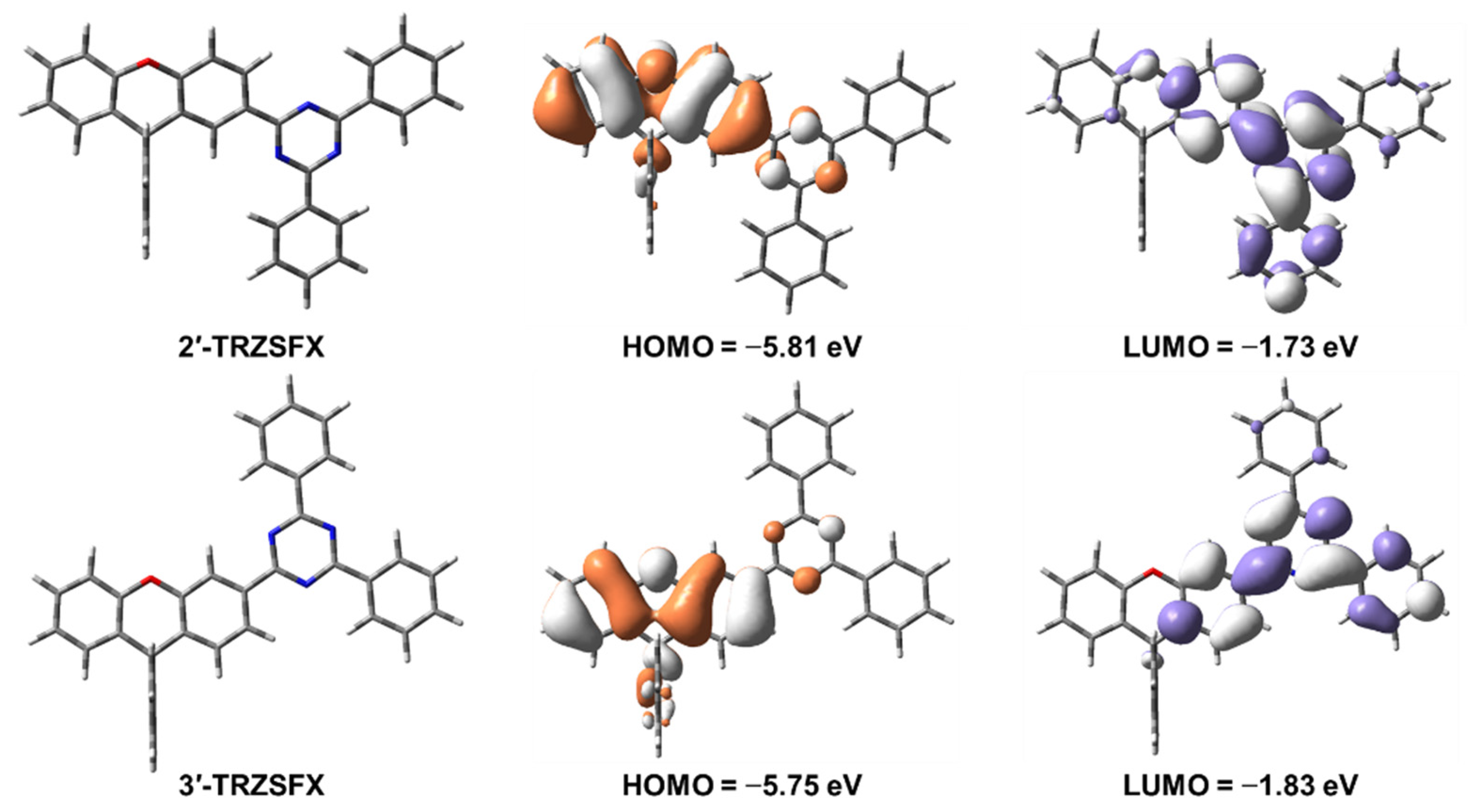

| Compound | λabsa [nm] | S1/T1b [eV] | Egc [eV] | HOMO/LUMO d [eV] | Td [°C] | Tm [°C] |
|---|---|---|---|---|---|---|
| 2′-TRZSFX | 270, 307, 324 | 3.44/2.82 | 3.68 | −6.23/−2.62 | 375 | 298.8 |
| 3′-TRZSFX | 272, 306, 335 | 3.31/2.89 | 3.61 | −6.17/−3.15 | 370 | 295.5 |
| Device | V a [V] | CE b [cd·A−1] | PE b [lm·W−1] | EQE b [%] | Lmax [cd·m−2] | CIE c |
|---|---|---|---|---|---|---|
| Device A | 3.4, 5.3, 7.5 | 61.5, 61.1, 60.3 | 45.4, 36.3, 25.2 | 18.8, 18.7, 18.4 | 47 260 | (0.35, 0.57) |
| Device B | 3.5, 5.4, 7.5 | 75.5, 72.3, 59.7 | 55.0, 42.1, 24.7 | 23.0, 21.9, 18.1 | 25 556 | (0.36, 0.58) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Deng, Y.; Ren, B.; Lan, X.; Zhang, Y.; Guo, R.; Li, C.; Xiong, G.; Sun, Y.; Zhao, Z. Unraveling the Position Effect of Spiroxanthene-Based n-Type Hosts for High-Performance TADF–OLEDs. Nanomaterials 2023, 13, 2517. https://doi.org/10.3390/nano13182517
Liu Q, Deng Y, Ren B, Lan X, Zhang Y, Guo R, Li C, Xiong G, Sun Y, Zhao Z. Unraveling the Position Effect of Spiroxanthene-Based n-Type Hosts for High-Performance TADF–OLEDs. Nanomaterials. 2023; 13(18):2517. https://doi.org/10.3390/nano13182517
Chicago/Turabian StyleLiu, Qinglin, Yun Deng, Baoyi Ren, Xia Lan, Yuehong Zhang, Runda Guo, Chensheng Li, Gang Xiong, Yaguang Sun, and Zujin Zhao. 2023. "Unraveling the Position Effect of Spiroxanthene-Based n-Type Hosts for High-Performance TADF–OLEDs" Nanomaterials 13, no. 18: 2517. https://doi.org/10.3390/nano13182517
APA StyleLiu, Q., Deng, Y., Ren, B., Lan, X., Zhang, Y., Guo, R., Li, C., Xiong, G., Sun, Y., & Zhao, Z. (2023). Unraveling the Position Effect of Spiroxanthene-Based n-Type Hosts for High-Performance TADF–OLEDs. Nanomaterials, 13(18), 2517. https://doi.org/10.3390/nano13182517