Advancements in Plasma-Enhanced Chemical Vapor Deposition for Producing Vertical Graphene Nanowalls
Abstract
:1. Introduction
2. Methods and Discussion
| Ref./Year | Gas | Temperature (°C) | Pressure | Technology Frequency | Power (W) | Coating/Substrate | Main Conclusions |
|---|---|---|---|---|---|---|---|
| [23]/2019 | CH4/H2 | 625 | 400 mTorr | RF-PECVD (13.56 MHz) | 20–80 | VGNWs/Ge<111> | VAGNAs can be used as an efficient SERS substrate |
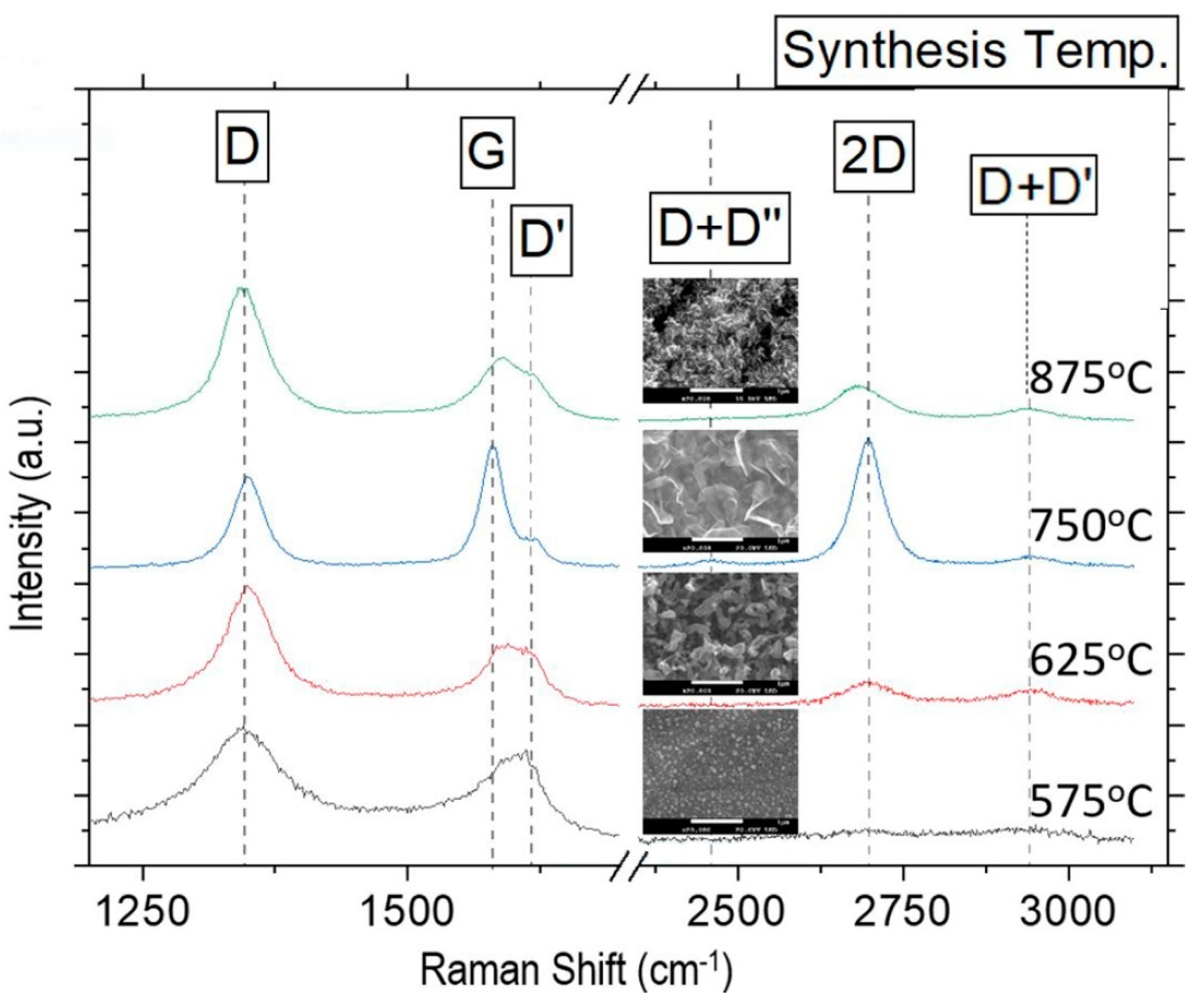
| [7]/2023 | CH4 | 575 to 900 | 400 mTorr | ICP-CVD (13.56 MHz) | 400 | VGNWs/Stainless-steel SS310 | Impact of temperature on morphology and structure of VGNWs |
| [52]/2020 | Ar/H2/C2H2 | 700 | 10–150 Pa | PECVD | 300 | VGNWs/SiO2, SiO2/Ti, SiO2/Ti/Pt | cross-section micrograph about 18 μm, width of edges less than 10 nm. |
| [19]/2022 | CH4 | 750 | 400 mTorr | ICP-CVD (13.56 MHz) | 440 | VGNWs/Stainless-steel SS310, Polycrystalline-Cu,Papiex© | growth of VGNWs in a variety of metallic and non-metallic substrates insights on morphology and crystalline quality |
| [21]/2023 | CH4 | 750 | 400 mTorr | ICP-CVD (13.56 MHz) | 400 | Mo2C/Papiex© | VGNWs as template with abundant defects favoring bonding of ns-Mo2C |
| [24]/2020 | CTAB/deionized water | 200 (24 h) | - | hydrothermal process | - | MoS2@rGO | Fabrication of MoS2@rGO nanowall structure |
| [25]/2016 | CH4/N2 + CH4 | - | 20 mTorr to 760 Torr | MW plasma torch (MPT) (2.45 GHz)/PECVD | 500–1500 | GNW/Ti NGNW/Ti | GNW/Ti and NGNW/Ti electrodes extend upper potential limit of a positive electrode of EDLCs from 0.1 V to 1.3–1.5 V |
| [9]/2021 | - | - | - | PEALD | - | GNWs/Si | GNW-Si Schottky junction-based selfpowered IR PD with high responsivity |
| [53]/2015 | C2H2/Ar/H2 | 550–750 | 200–400 Pa | PECVD | 150 | CNWs/SiC | field emission properties of the CNWs |
| [34]/2023 | - | - | - | PECVD | - | VGNWs/textured c-Si | PEDOT doped textured VGNWs/Si Schottky junction |
| [49]/2018 | Al acetylacetonate | 350–425–500 | 8 Pa | ICP-PECVD | 500 | CNWs | CNWs morphologies depending on process |
| [40]/2019 | Glucose/ureaAr | 850 | 70 kPa | Spin-coating/CVD | - | N:VGNs/304SS | growing intrinsic and nitrogen-doped VGNs on stainless steel |
| [15]/2019 | C precursor | - | - | MW-PECVD/ALD | - | VGNWs/ZnO nanorods | Hierarchical Graphene/Nanorods-Based H2O2 Electrochemical Sensor |
| [28]/2020 | H2/C2H4 | 450–620 | 29 Pa | CC-PECVD 13.56 MHz | - | VGNWs | Growth VGNWs by CC-PECVD at low temperature (450 °C), using Ni catalyst |
| [10]/2020 | CH4/H2 | 650 | - | (ns)-RI-PECVD | 400 | CNWs | isolated carbon nanowalls via high-voltage ns pulses (ns)-RI-PECVD |
| [54]/2013 | CH4/H2 | - | - | ICPCVD | - | VGNWs | Synthesis of VGNWs for field emitters |
| [50]/2022 | C precursor | 700 | - | PECVD | - | VGNWs/c-Si VGNWs/3C-SiC | VGNWs/SiC interfacial layers for heterojunction devices |
| [48]/2009 | C2H6/H2 | 930 | 160 Pa | RI-PECVD 2.45 GHz | 250–270 | CNWs/Si,SiO2,Al2O3,Ni | CNWs growth by RI-PECVD |
| [55]/2022 | C precursor | 450 | - | PECVD | - | VGNWs | VGNWs growth at low temperature plasma |
| [43]/2021 | C precursor | 600 | 500 mTorr | MW-PECVD | 1300 | CNWs/SiO2/p-Si | CNWs/SiO2/Si gas sensor |
| [13]/2018 | p-xylene | 415 | 4.7 Pa | ICPCVD | 150 | Hierarchical CNW | hCNW synthesized by a PECVD |
| [17]/2020 | - | - | - | - | - | (Li3O)n,(Na3O)n,(K3O)n @GDY | Design of Graphdiyne-based materials for optoelectronic applications |
| [35]/2023 | C precursor + Nafion | - | - | - | - | VGNWs/Si | VGNWs/Si Schottky junction solar cells with Nafion doping |
| [41]/2020 | Ar/H2/CH4 | 800 | 7 Pa | PECVD | 200 | VGNWs/VO2(B) | VGNWs/VO2(B phase) for IR detector |
| [5]/2023 | PDMS | 400 | - | HF-CVD | - | VGNWs | VGNWs for flexible pressure sensor |
| [56]/2020 | CH4 | 750 | 50 to 60 Pa | ICP-CVD | 440 | CNSs/SS304 | Photoluminescence from CNSs |
| [27]/2019 | PAN+DMF CH4/H2 | 600 | 600 Pa | Electrospinning MW-PECVD | 350 | G-CNFs | G-CNFs-MnO2 electrodes for supercapacitors |
| [33]/2020 | CH4/H2 | 750 | - | PECVD | 50 | VGNH/Si | VGNHs/c-Si Shottky junction solar cells |
| [22]/2018 | Ar/H2/CH4 | 1050 | 800 Pa | Mesoplasma, MPCVD | 10 kW | VGN/Ni@Li foam | VGN/Ni@Li foam for pseudocapacitance induced fast Li+ ion transfer |
| [26]/2017 | Ar/CH4 | 800 | - | (ECR)-PECVD | 375 | VGNWs/Ni | VGNWs/Ni for supercapacitor application |
| [51]/2022 | C2H2 | - | - | PECVD | - | VGNWs/GaN-NWVGNWs/np-SiO2 | Growth of VGNWs/GaN-NW and VGNWs/np-SiO2 by PECVD |
| [57]/2020 | Ar | 350 | 14.5 Pa | PECVD 13.56 MHz | 500 | np-Pt/CNWs | synthesis of Pt/CNW sheet electrocatalysts |
| [58]/2017 | Ar/H2/C2H2 | 700 | 10 to 150 Pa | Ar plasma jet | - | CNWs | wettability of plasma deposited CNWs |
| [37]/2022 | C2H2 | 150 | - | HF-CVD | - | VGNWs | Synthesis of VGNWs on dielectrics |
| [42]/2019 | gaseous camphor | 600 | 30 Pa | CVD | - | Graphene/ZnO/Graphene | Graphene/ZnO-NWs/Graphene Heterojunction for NO2 Gas Sensor |
| [38]/2022 | ChloroformC precursor | 650 | - | Electric field assisted PECVD | 250 | VG arrays | Rapid growth of VG arrays for TIM |
| [36]/2020 | methane, ethanol, methanol | 650 | - | AEF-PECVD | 250 | VG arrays/Cu, glass, c-Si | Vertical Graphene Arrays for TIM |
| [39]/2023 | C precursor | - | - | PECVD | - | VGNs/CF/ss | VGNWs/C fibers for TIM |
| [59]/2019 | Ar/H2/CH4 | 750–900 | - | CC-PECVD | 550–770 | VGNWs | VGNWs for Li-ion batteries |
| [60]/2023 | C precursor | - | - | RF and RI-PECVD | - | CNWs/Al2O3 nanopores | Creation of CNWs/Al2O3 nanopores |
| [61]/2021 | Ar/CH4 | 800 | - | ICP-PECVD | 140 | CNWs | Properties of CNWs |
| [31]/2022 | C precursor | - | 500 Pa | HF-CVD | - | VGNWs/substrate | VGNWs for hydrovoltaic power generation |
| [62]/2023 | C precursor | - | - | CVD | - | ns-G/W/dielectric | Multimode THz absorber based on ns-G |
| [63]/2023 | C precursor | - | - | CVD on Cu catalyst | - | SLG/SiO2/Au | SLG/SiO2/Au for absorber on SPR |
| [64]/2022 | - | - | - | - | - | PIT/ns-G/dielectric subst. | Theoretical study of PIT/ns-G/substrate |
| [65]/2023 | C precursor | - | - | CVD on Cu catalyst | - | SLG/SiO2/Au | SLG/SiO2/Au for THz absorber on SPR |
3. Growth Mechanism of VGNWs
4. Applications of VGNWs
4.1. Advancements in Photocatalysis Using Graphdiyne
4.2. Electrocatalyst for Highly Efficient Hydrogen Evolution Reaction
4.3. Rechargeable Battery Technology
4.4. Innovations in Supercapacitor Technology
4.5. Unlocking Hydrovoltaic Power Generation with VGNWs
4.6. Advancements in Solar Energy Conversion Using VGNWs
4.7. Efficient Thermal Interfaces for Enhanced Electronic Device Performance
4.8. Advancing Field Emission Technology through Vertical Graphene Nanosheets
4.9. IR Detectors with VGNWs/VO2 Composite Films
4.10. Photonic Devices Based on Graphene Nanostructures
4.11. Gas Sensing with Innovative Heterojunctions and Carbon Nanowalls
5. Future Challenges
- (a)
- Catalyst-Free Growth: Tanaka et al. [66] reported the catalyst-free growth of CNWs, which simplifies the fabrication process. However, further investigations are required to optimize this approach and to understand the factors that influence catalyst-free growth. Eliminating the need for catalysts can reduce costs and simplify the overall production process.
- (b)
- Control of Morphology: While recent studies have explored different morphological forms of CNWs, achieving precise control over their structure remains a challenge. The understanding of how the process parameters influence the growth and morphology of CNWs is essential for tailoring their properties for specific applications.
- (c)
- Uniformity and Scalability: The uniformity of CNWs over large areas is crucial for their practical applications. As the demand for CNWs in industrial and commercial settings increases, scalability becomes a vital consideration. Developing techniques that can ensure uniform and large-scale CNWs production is necessary for their widespread implementation.
- (d)
- Characterization and Standardization: As the field advances, it is essential to establish standardized characterization techniques to accurately evaluate the quality, structure, and properties of CNWs. Standardization will facilitate comparison between studies and accelerate the progress in this area.
- (e)
- Surface and Interface Engineering: CNWs’ surface and interface engineering is crucial for tailoring their properties for specific applications. By functionalizing or doping CNWs, their electrical, mechanical, and chemical characteristics can be tuned to meet the requirements of various devices and technologies.
- (f)
- Integration with Devices: For practical applications, CNWs need to be seamlessly integrated with various electronic and optoelectronic devices. Research on the compatibility and effective integration of CNWs into existing device architectures is essential for realizing their potential in real-world applications.
- (g)
- Cost-Effectiveness: As with any new technology, cost-effectiveness plays a crucial role in determining its commercial viability. Finding more cost-efficient synthesis methods, optimizing precursor gases, and improving the deposition rates will be key factors in making CNWs commercially competitive.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Synthesis of Vertically Oriented Graphene Sheets or Carbon Nanowalls—Review and Challenges. Materials 2019, 12, 2968. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, M.; Hori, M. Carbon Nanowalls; Springer: Wien, Vienna, 2010. [Google Scholar] [CrossRef]
- Wu, Y.H.; Qiao, P.W.; Chong, T.C.; Shen, Z.X. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Wei, L.; Jin, Y.; Hou, J.; Wang, X.; Guo, X. In-plane flexible solid-state microsupercapacitors for on-chip electronics. Energy 2019, 170, 338–348. [Google Scholar] [CrossRef]
- Mao, W.; Shen, H.; Wang, Z.; Liao, B.; Zhang, J.; Zhu, J.; Li, Y.; Wu, T. Flexible Pressure Sensors with a Sandwich Structure Based on Vertical Graphene Nanowalls by HFCVD. J. Electron. Mater. 2023, 52, 1526–1533. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Ohkohchi, M. Production of petal-like graphite sheets by hydrogen arc discharge. Carbon 1997, 35, 153–158. [Google Scholar] [CrossRef]
- Bertran-Serra, E.; Musheghyan-Avetisyan, A.; Chaitoglou, S.; Amade-Rovira, R.; Alshaikh, I.; Pantoja-Suárez, F.; Andújar-Bella, J.-L.; Jawhari, T.; Perez-del-Pino, A.; Gyorgy, E. Temperature-modulated synthesis of vertically oriented atomic bilayer graphene nanowalls grown on stainless steel by inductively coupled plasma chemical vapour deposition. Appl. Surf. Sci. 2023, 610, 155530. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Nihashi, Y.; Kondo, H.; Hori, M. Nucleation Control of Carbon Nanowalls Using Inductively Coupled Plasma-Enhanced Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2013, 52, 01AK05. [Google Scholar] [CrossRef]
- Cong, J.; Khan, A.; Li, J.; Wang, Y.; Xu, M.; Yang, D.; Yu, X. Direct Growth of Graphene Nanowalls on Silicon Using Plasma-Enhanced Atomic Layer Deposition for High-Performance Si-Based Infrared Photodetectors. ACS Appl. Electron. Mater. 2021, 3, 5048–5058. [Google Scholar] [CrossRef]
- Ichikawa, T.; Shimizu, N.; Ishikawa, K.; Hiramatsu, M.; Hori, M. Synthesis of isolated carbon nanowalls via high-voltage nanosecond pulses in conjunction with CH4/H2 plasma enhanced chemical vapor deposition. Carbon 2020, 161, 403–412. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Bertran, E. Effect of temperature on graphene grown by chemical vapor deposition. J. Mater. Sci. 2017, 52, 8348–8356. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.-B.; Gao, L.; Wu, H.-L.; Yang, J.; Cai, L.; Ma, T.-B.; Tung, C.-H.; Wu, L.-Z. Three-Dimensional Graphene Networks with Abundant Sharp Edge Sites for Efficient Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, K.; Yurchenko, O.; Melke, J.; Fischer, A.; Urban, G. High electrocatalytic activity of metal-free and non-doped hierarchical carbon nanowalls towards oxygen reduction reaction. Electrochim. Acta 2018, 269, 657–667. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding Three-Dimensional Graphene/MnO2 Composite Networks As Ultralight and Flexible Supercapacitor Electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.; Xiao, S.; Yang, C.; Li, X.; Guo, C.; He, G.; Li, B.; Yang, C.; Chen, H.; Liu, F.; et al. Hierarchical graphene/nanorods-based H2O2 electrochemical sensor with self-cleaning and anti-biofouling properties. Sens. Actuators B Chem. 2019, 289, 15–23. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, X.; Fu, W. Review of Vertical Graphene and its Applications. ACS Appl. Mater. Interfaces 2021, 13, 9561–9579. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Lu, J. Remarkably enhanced first hyperpolarizability and nonlinear refractive index of novel graphdiyne-based materials for promising optoelectronic applications: A first-principles study. Appl. Surf. Sci. 2020, 512, 145544. [Google Scholar] [CrossRef]
- Shandilya, P.; Mandyal, P.; Kumar, V.; Sillanpää, M. Properties, synthesis, and recent advancement in photocatalytic applications of graphdiyne: A review. Sep. Purif. Technol. 2022, 281, 119825. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Amade, R.; Bertran, E. Insights into the inherent properties of vertical graphene flakes towards hydrogen evolution reaction. Appl. Surf. Sci. 2022, 592, 153327. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Giannakopoulou, T.; Papanastasiou, G.; Tsoutsou, D.; Vavouliotis, A.; Trapalis, C.; Dimoulas, A. Cu vapor-assisted formation of nanostructured Mo2C electrocatalysts via direct chemical conversion of Mo surface for efficient hydrogen evolution reaction applications. Appl. Surf. Sci. 2020, 510, 145516. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Amade, R.; Ospina, R.; Bertran-Serra, E. Hybrid Nanostructured Compounds of Mo2C on Vertical Graphene Nanoflakes for a Highly Efficient Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 6120–6131. [Google Scholar] [CrossRef]
- Ren, F.; Lu, Z.; Zhang, H.; Huai, L.; Chen, X.; Wu, S.; Peng, Z.; Wang, D.; Ye, J. Pseudocapacitance Induced Uniform Plating/Stripping of Li Metal Anode in Vertical Graphene Nanowalls. Adv. Funct. Mater. 2018, 28, 1805638. [Google Scholar] [CrossRef]
- Al-Hagri, A.; Li, R.; Rajput, N.S.; Lu, J.-Y.; Cong, S.; Sloyan, K.; Almahri, M.A.; Tamalampudi, S.R.; Chiesa, M.; Al Ghaferi, A. Direct growth of single-layer terminated vertical graphene array on germanium by plasma enhanced chemical vapor deposition. Carbon 2019, 155, 320–325. [Google Scholar] [CrossRef]
- Chen, H.; Song, T.; Tang, L.; Pu, X.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. In-situ growth of vertically aligned MoS2 nanowalls on reduced graphene oxide enables a large capacity and highly stable anode for sodium ion storage. J. Power Sources 2020, 445, 227271. [Google Scholar] [CrossRef]
- Chi, Y.-W.; Hu, C.-C.; Shen, H.-H.; Huang, K.-P. New Approach for High-Voltage Electrical Double-Layer Capacitors Using Vertical Graphene Nanowalls with and without Nitrogen Doping. Nano Lett. 2016, 16, 5719–5727. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, G.; Ghosh, S.; Polaki, S.R.; Mathews, T.; Kamruddin, M. Scalable transfer of vertical graphene nanosheets for flexible supercapacitor applications. Nanotechnology 2017, 28, 415702. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Bo, Z.; Yang, S.; Duan, L.; Yang, H.; Yan, J.; Cen, K.; Ostrikov, K. (Ken) Hierarchical nanocarbon-MnO2 electrodes for enhanced electrochemical capacitor performance. Energy Storage Mater. 2019, 16, 607–618. [Google Scholar] [CrossRef]
- Hussain, S.; Kovacevic, E.; Berndt, J.; Santhosh, N.M.; Pattyn, C.; Dias, A.; Strunskus, T.; Ammar, M.-R.; Jagodar, A.; Gaillard, M.; et al. Low-temperature low-power PECVD synthesis of vertically aligned graphene. Nanotechnology 2020, 31, 395604. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Feng, Z.; Chen, Z.; Yang, Y.; Zhou, Q.; Zhou, Q.; Peng, C.; Yang, G. Recent progress on the preparation of three-dimensional vertically aligned graphene and its applications insupercapacitors. Chin. Sci. Bull. 2021, 66, 3617–3630. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, G.; Jeong, S.M.; Rout, C.S. A review on supercapacitors based on plasma enhanced chemical vapor deposited vertical graphene arrays. J. Energy Storage 2022, 53, 105212. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, H.; Wang, Z.; Li, Y.; Wu, T.; Mao, W.; Zhang, J. Direct fabrication of high-quality vertical graphene nanowalls on arbitrary substrates without catalysts for tidal power generation. Nanoscale 2022, 14, 15119–15128. [Google Scholar] [CrossRef]
- Ren, H.; Tang, M.; Guan, B.; Wang, K.; Yang, J.; Wang, F.; Wang, M.; Shan, J.; Chen, Z.; Wei, D.; et al. Hierarchical Graphene Foam for Efficient Omnidirectional Solar-Thermal Energy Conversion. Adv. Mater. 2017, 29, 1702590. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.A.; Roy, S.B.; Gwak, D.; Akhtar, I.; Nasir, N.; Kumar, S.; Khan, M.F.; Heo, K.; Chun, S.-H.; Seo, Y. Solar cell based on vertical graphene nano hills directly grown on silicon. Carbon 2020, 164, 235–243. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Liu, L.; Jia, L.; Zhang, Y.; Yu, W. Large-area 11.75% efficient vertical graphene nanowalls/textured silicon Schottky junction solar cell based on PEDOT:Nafion doping scheme. Jpn. J. Appl. Phys. 2023, 62, 031002. [Google Scholar] [CrossRef]
- Liu, L.; Jia, L.; Huang, Y.; Zhang, Y.; Yu, W. High-performance vertical graphene nanowall/silicon Schottky junction solar cells with Nafion doping and plasma etching. J. Alloys Compd. 2023, 939, 168765. [Google Scholar] [CrossRef]
- Xu, S.; Wang, S.; Chen, Z.; Sun, Y.; Gao, Z.; Zhang, H.; Zhang, J. Electric-Field-Assisted Growth of Vertical Graphene Arrays and the Application in Thermal Interface Materials. Adv. Funct. Mater. 2020, 30, 2003302. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Zheng, P.; Shen, H.; Gao, B.; Ge, J.; Xu, Y.; Yan, X.; Zhan, R.; Yang, Y.; et al. Near Room-Temperature Synthesis of Vertical Graphene Nanowalls on Dielectrics. ACS Appl. Mater. Interfaces 2022, 14, 21348–21355. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cheng, T.; Yan, Q.; Shen, C.; Yu, Y.; Lin, C.; Ding, F.; Zhang, J. Chloroform-Assisted Rapid Growth of Vertical Graphene Array and Its Application in Thermal Interface Materials. Adv. Sci. 2022, 9, 2200737. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Jia, W.; Yang, Y.; Zhou, Q.; Ma, L.; Wang, J. Preparation and Heat Dissipation Performance of Vertical Graphene Nanosheets/Carbon Fibers Composite Film. Coatings 2023, 13, 407. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Ding, Y.; Chen, Q.; Li, J. Direct patterned growth of intrinsic/doped vertical graphene nanosheets on stainless steel via heating solid precursor films for field emission application. Mater. Des. 2019, 162, 293–299. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Wan, D. CVD preparation of vertical graphene nanowalls/VO2 (B) composite films with superior thermal sensitivity in uncooled infrared detector. J. Materiomics 2020, 6, 280–285. [Google Scholar] [CrossRef]
- Xiong, Y.; Lingmin, Y.; Haining, M.; Yuan, L.; Chun, L.; Mingli, Y.; Hongbo, D.; Hui, F.X. Direct Synthesis of Upstanding Graphene/ZnO Nanowalls/Graphene Sandwich Heterojunction and Its Application for NO2 Gas Sensor. J. Nanosci. Nanotechnol. 2019, 19, 7947–7952. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Choi, H.; Lee, S.; Lee, G.; Kim, Y.; Choi, W.; Kang, H. Room Temperature Gas Sensor Application of Carbon Nanowalls using Electrical Resistance Change by Surface Adsorption of Toxic Gases. Mater. Res. Bull. 2021, 141, 111377. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Amade, R.; Bertran, E. Evaluation of Graphene/WO3 and Graphene/CeO x Structures as Electrodes for Supercapacitor Applications. Nanoscale Res. Lett. 2017, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180. [Google Scholar] [CrossRef]
- Yu, K.; Bo, Z.; Lu, G.; Mao, S.; Cui, S.; Zhu, Y.; Chen, X.; Ruoff, R.S.; Chen, J. Growth of carbon nanowalls at atmospheric pressure for one-step gas sensor fabrication. Nanoscale Res. Lett. 2011, 6, 202. [Google Scholar] [CrossRef]
- Baranov, O.; Levchenko, I.; Xu, S.; Lim, J.W.M.; Cvelbar, U.; Bazaka, K. Formation of vertically oriented graphenes: What are the key drivers of growth? 2D Mater. 2018, 5, 044002. [Google Scholar] [CrossRef]
- Kondo, S.; Kawai, S.; Takeuchi, W.; Yamakawa, K.; Den, S.; Kano, H.; Hiramatsu, M.; Hori, M. Initial growth process of carbon nanowalls synthesized by radical injection plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2009, 106, 094302. [Google Scholar] [CrossRef]
- Giese, A.; Schipporeit, S.; Buck, V.; Wöhrl, N. Synthesis of carbon nanowalls from a single-source metal-organic precursor. Beilstein J. Nanotechnol. 2018, 9, 1895–1905. [Google Scholar] [CrossRef]
- Khan, A.; Cong, J.; Kumar, R.R.; Ahmed, S.; Yang, D.; Yu, X. Chemical Vapor Deposition of Graphene on Self-Limited SiC Interfacial Layers Formed on Silicon Substrates for Heterojunction Devices. ACS Appl. Nano Mater. 2022, 5, 17544–17555. [Google Scholar] [CrossRef]
- Sun, J.; Rattanasawatesun, T.; Tang, P.; Bi, Z.; Pandit, S.; Lam, L.; Wasén, C.; Erlandsson, M.; Bokarewa, M.; Dong, J.; et al. Insights into the Mechanism for Vertical Graphene Growth by Plasma-Enhanced Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2022, 14, 7152–7160. [Google Scholar] [CrossRef]
- Bita, B.; Vizireanu, S.; Stoica, D.; Ion, V.; Yehia, S.; Radu, A.; Iftimie, S.; Dinescu, G. On the Structural, Morphological, and Electrical Properties of Carbon Nanowalls Obtained by Plasma-Enhanced Chemical Vapor Deposition. J. Nanomater. 2020, 2020, 8814459. [Google Scholar] [CrossRef]
- Cui, L.; Chen, J.; Yang, B.; Sun, D.; Jiao, T. RF-PECVD synthesis of carbon nanowalls and their field emission properties. Appl. Surf. Sci. 2015, 357, 1–7. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, T.; Liu, F.; Dong, J.; Yao, Z.; Shen, C.; Deng, S.; Xu, N.; Liu, Y.; Gao, H.-J. Controlled Synthesis of Large-Scale, Uniform, Vertically Standing Graphene for High-Performance Field Emitters. Adv. Mater. 2013, 25, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, E.; Jagodar, A.; Strunskus, T.; Radicic, R.; Marath Santhosh, N.M.; Zavašnik, J.; Cvelbar, U.; Krstulovic, N.; Berndt, J. (Invited) Functionalization of Vertically Aligned Graphene Nanowalls for Applications in the Field of Renewable Energies: From Coatings to Decoration. ECS Meet. Abstr. 2022, MA2022-02, 890. [Google Scholar] [CrossRef]
- Musheghyan-Avetisyan, A.; Güell, F.; Martínez-Alanis, P.R.; Amade, R.; Martí-González, J.; Bertran-Serra, E. Photoluminescence from carbon structures grown by inductively coupled plasma chemical vapor deposition. J. Vac. Sci. Technol. A 2020, 38, 023405. [Google Scholar] [CrossRef]
- Tigges, S.; Wöhrl, N.; Radev, I.; Hagemann, U.; Heidelmann, M.; Nguyen, T.B.; Gorelkov, S.; Schulz, S.; Lorke, A. One-step synthesis of carbon-supported electrocatalysts. Beilstein J. Nanotechnol. 2020, 11, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Vizireanu, S.; Ionita, M.D.; Ionita, R.E.; Stoica, S.D.; Teodorescu, C.M.; Husanu, M.A.; Apostol, N.G.; Baibarac, M.; Panaitescu, D.; Dinescu, G. Aging phenomena and wettability control of plasma deposited carbon nanowall layers. Plasma Process. Polym. 2017, 14, 1700023. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, J.; Li, S.; Zhang, L.; Fu, J.; Huang, F.; Cheng, Q. Vertically-oriented graphene nanowalls: Growth and application in Li-ion batteries. Diam. Relat. Mater. 2019, 91, 54–63. [Google Scholar] [CrossRef]
- Yerlanuly, Y.; Christy, D.; Nong, N.V.; Kondo, H.; Alpysbayeva, B.Y.; Zhumadilov, R.; Nemkayeva, R.R.; Ramazanov, T.S.; Hori, M.; Gabdullin, M.T. Creation of unique shapes by coordination of alumina nanopores and carbon nanowalls. Fuller. Nanotub. Carbon Nanostructures 2023, 31, 295–301. [Google Scholar] [CrossRef]
- Yerlanuly, Y.; Zhumadilov, R.; Nemkayeva, R.; Uzakbaiuly, B.; Beisenbayev, A.R.; Bakenov, Z.; Ramazanov, T.; Gabdullin, M.; Ng, A.; Brus, V.V.; et al. Physical properties of carbon nanowalls synthesized by the ICP-PECVD method vs. the growth time. Sci. Rep. 2021, 11, 19287. [Google Scholar] [CrossRef]
- Ye, Z.; Wu, P.; Wang, H.; Jiang, S.; Huang, M.; Lei, D.; Wu, F. Multimode tunable terahertz absorber based on a quarter graphene disk structure. Results Phys. 2023, 48, 106420. [Google Scholar] [CrossRef]
- Lai, R.; Shi, P.; Yi, Z.; Li, H.; Yi, Y. Triple-Band Surface Plasmon Resonance Metamaterial Absorber Based on Open-Ended Prohibited Sign Type Monolayer Graphene. Micromachines 2023, 14, 953. [Google Scholar] [CrossRef]
- Tang, B.; Guo, Z.; Jin, G. Polarization-controlled and symmetry-dependent multiple plasmon-induced transparency in graphene-based metasurfaces. Opt. Express 2022, 30, 35554. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, P.; Wen, Q.; Chen, H.; Tang, Y.; Yi, Z.; Wei, K.; Li, G.; Tang, B.; Yi, Y. Graphene Multi-Frequency Broadband and Ultra-Broadband Terahertz Absorber Based on Surface Plasmon Resonance. Electronics 2023, 12, 2655. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshimura, M.; Okamoto, A.; Ueda, K. Growth of Carbon Nanowalls on a SiO2 Substrate by Microwave Plasma-Enhanced Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2005, 44, 2074. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertran-Serra, E.; Rodriguez-Miguel, S.; Li, Z.; Ma, Y.; Farid, G.; Chaitoglou, S.; Amade, R.; Ospina, R.; Andújar, J.-L. Advancements in Plasma-Enhanced Chemical Vapor Deposition for Producing Vertical Graphene Nanowalls. Nanomaterials 2023, 13, 2533. https://doi.org/10.3390/nano13182533
Bertran-Serra E, Rodriguez-Miguel S, Li Z, Ma Y, Farid G, Chaitoglou S, Amade R, Ospina R, Andújar J-L. Advancements in Plasma-Enhanced Chemical Vapor Deposition for Producing Vertical Graphene Nanowalls. Nanomaterials. 2023; 13(18):2533. https://doi.org/10.3390/nano13182533
Chicago/Turabian StyleBertran-Serra, Enric, Shahadev Rodriguez-Miguel, Zhuo Li, Yang Ma, Ghulam Farid, Stefanos Chaitoglou, Roger Amade, Rogelio Ospina, and José-Luis Andújar. 2023. "Advancements in Plasma-Enhanced Chemical Vapor Deposition for Producing Vertical Graphene Nanowalls" Nanomaterials 13, no. 18: 2533. https://doi.org/10.3390/nano13182533







