Fabrication of Non-Wetting Mg(OH)2 Composites with Photoresponsive Capabilities and Their Environmental Restoration Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Superhydrophobic Composite
2.3. Characterization
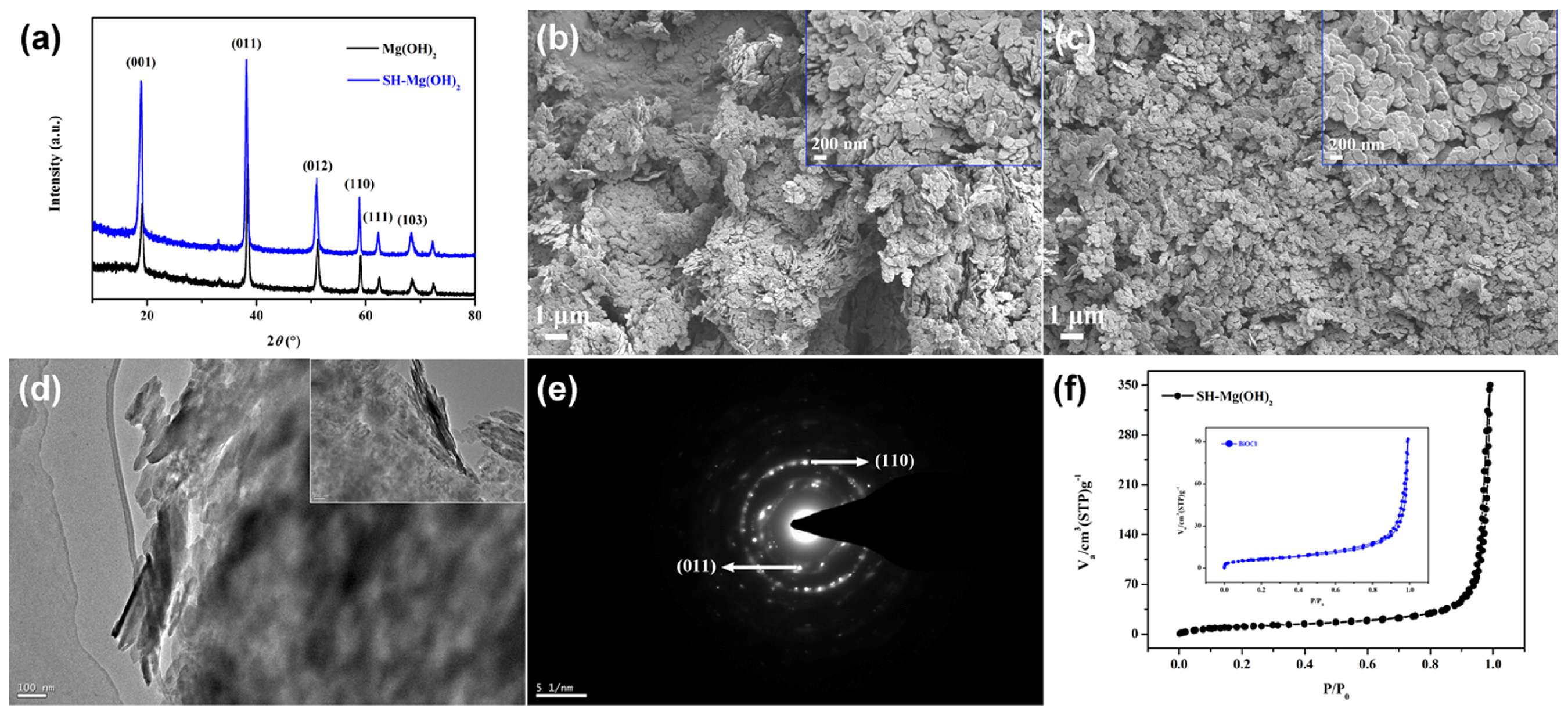
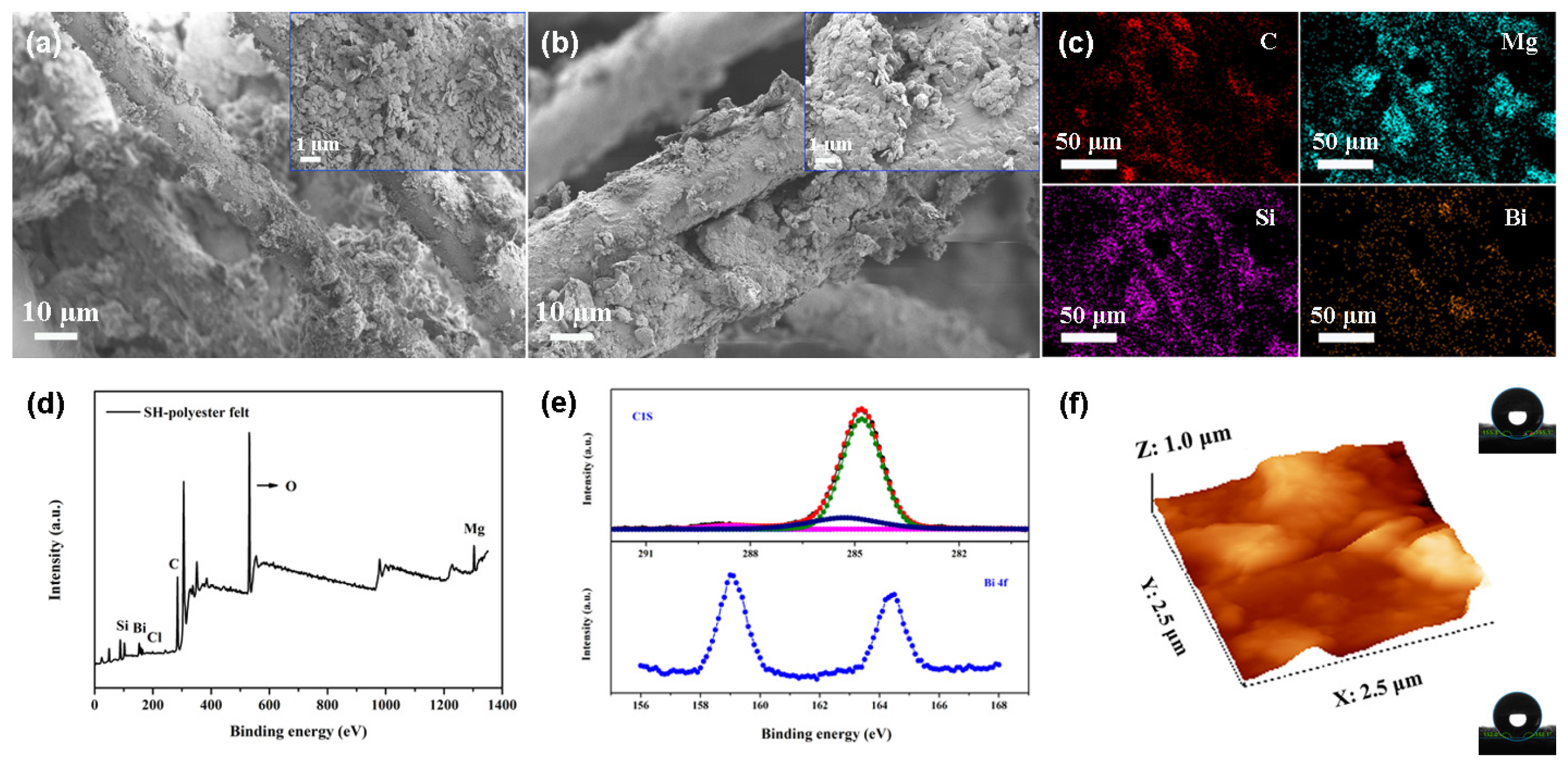
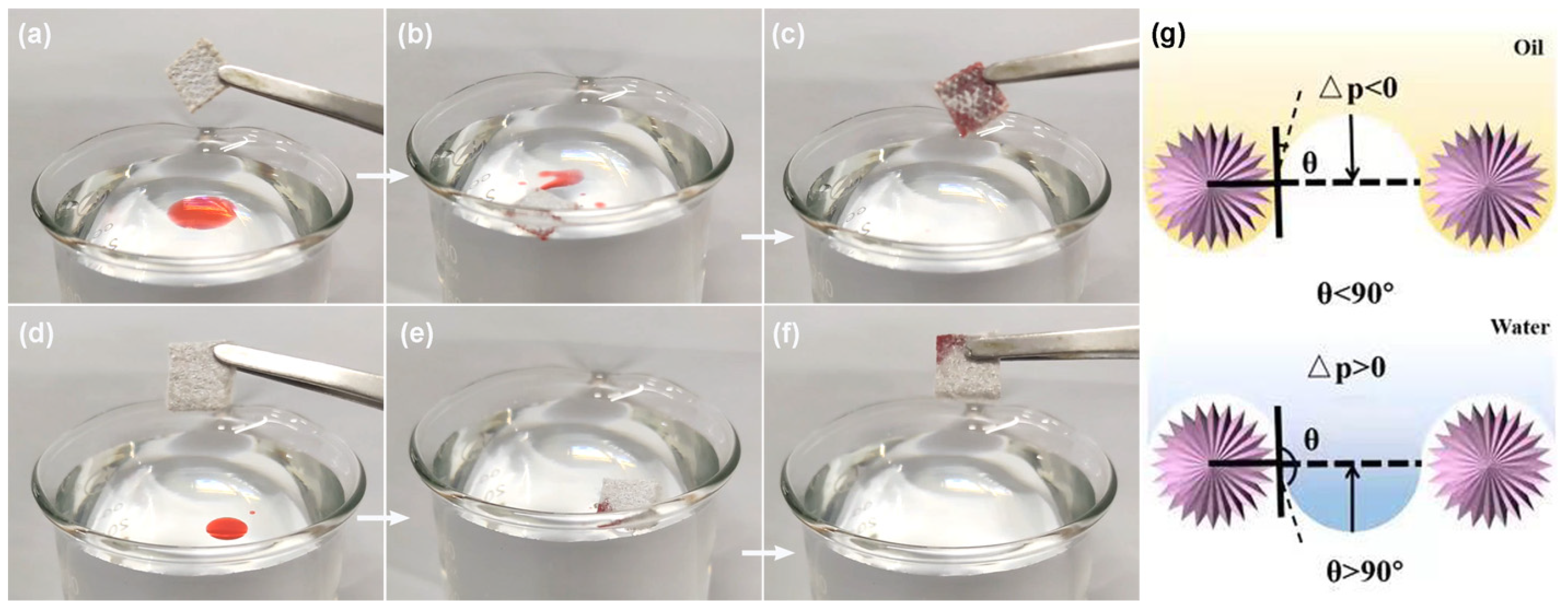
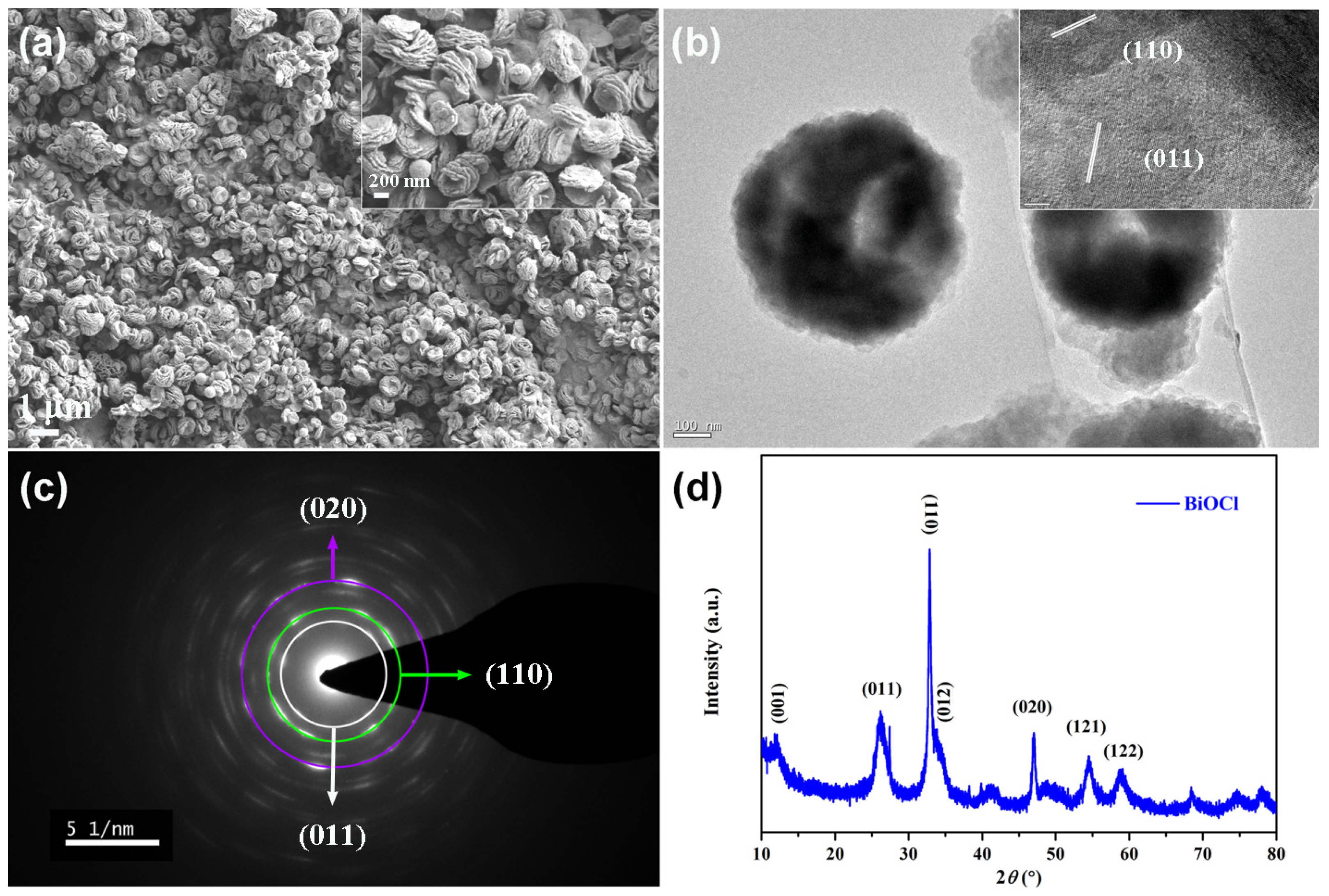
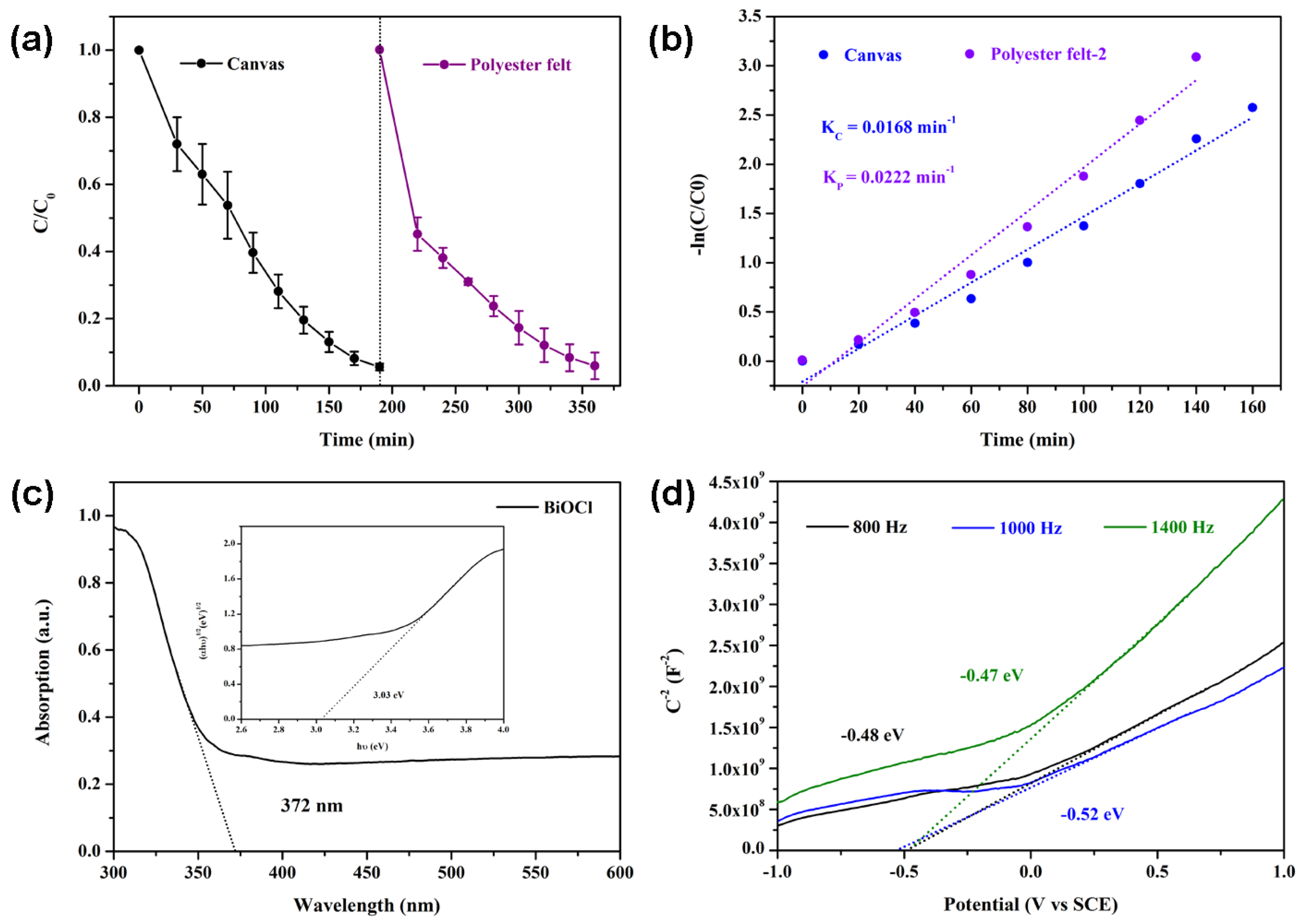
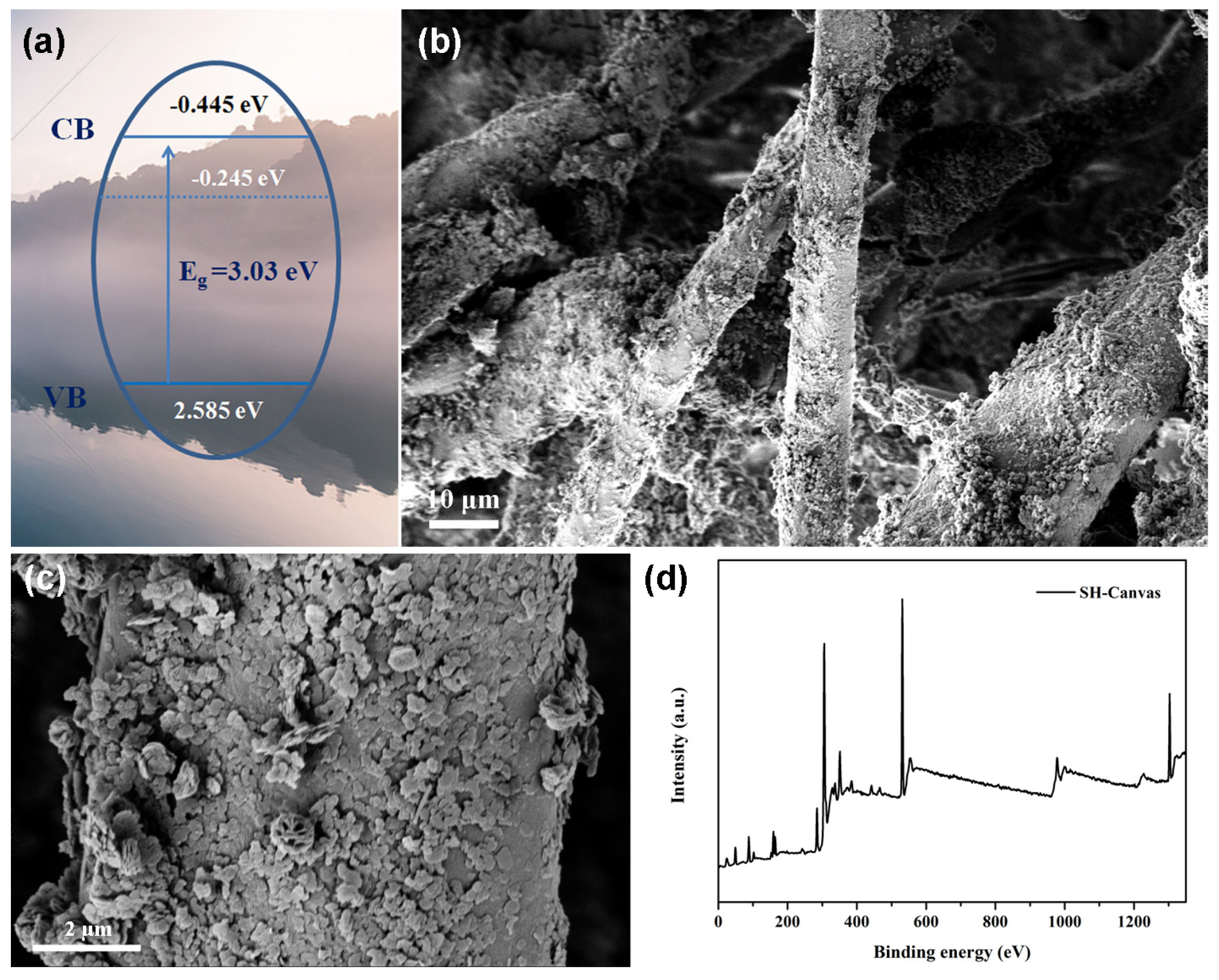
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Yang, X.Y.; Jia, X.H.; Yang, J.; Miao, X.; Shao, D.; Song, H.J.; Li, Y. Full biomass-derived multifunctional aerogel for solar-driven interfacial evaporation. Chem. Eng. J 2023, 471, 144684. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, C.L.; Tao, H.Y.; Ge, B.; Zhang, Y.L.; Zhao, L.M.; Liu, J.C.; Ren, G.N.; Zhang, Z.Z. A sponge-based iron-tannic-acid hydrogel interface evaporator designed for clean water production. Sci. China Technol. Sci. 2024, 67, 1579–1591. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Luo, Y.J.; Li, M.Y.; Ge, B.; Zhao, L.M.; Zhang, T.H.; Ren, G.N.; Zhang, Z.Z. A self-floating graphite felt evaporator: Interface wetting control and its application in environmental remediation and desalination. Chem. Eng. J. 2024, 488, 151038. [Google Scholar] [CrossRef]
- Zhou, X.; Li, D.X.; Wang, L.L.; Wang, Q.; Wang, Z.; Jing, Q.; Marisol, R.; Li, L. Recent advances in the modification of melamine sponge for oil-water separation. J. Mater. Sci. Technol. 2025, 207, 209–224. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, B.B.; Deng, J.W.; Li, H.R. One-step facile fabrication of Mg(OH)2/PVA/ZnO membrane with superior stability and oil-water separation. Sep. Purif. Technol. 2024, 351, 128105. [Google Scholar] [CrossRef]
- Ghosh, S.; Mukherjee, S.; Karthik, V.; Bera, P.; Dhakshinamorthy, A.; Biswas, S. A superhydrophobic MOF facilitating efficient solvent-free catalytic chemical fixation of CO2 and oxidation of hydrocarbons and MOF@cotton@starch composite-based selective sensing of a herbicide. J. Mater. Chem. C 2024, 12, 4460–4472. [Google Scholar] [CrossRef]
- Fu, Y.F.; Fan, Z.Z.; Liu, Q.W.; Tong, Q.L.; Qiao, S.Y.; Cai, L.; Zhang, X.S. A robust superhydrophobic and superoleophilic SA-HKUST-1 membrane for efficient oil/water mixture separation. New J. Chem. 2024, 48, 5372–5380. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, M.Q.; Tao, H.Y.; Ge, B.; Liu, S.; Liu, J.C.; Ren, G.N.; Zhang, Z.Z. Constructing of efficient interface solar evaporator: In-situ colloid foaming strategy for solar desalination and visible light response sewage purification. J. Colloids Interf. Sci. 2023, 649, 107–117. [Google Scholar] [CrossRef]
- Ghosh, S.; Rana, A.; Patel, A.; Manna, D.; Biswas, S. Superhydrophobic nanosized metal-organic framework composites for the targeted removal of hydrophobic pharmaceuticals with outstanding bacterial anti-adhesion properties. Environ. Sci. Nano 2024, 11, 1233–1244. [Google Scholar] [CrossRef]
- Zhao, H.L.; Ma, X.Z.; Xu, X.B.; Cui, M.H.; Stott, N.E.; Zhu, J.; Chen, J. Lightweight, superhydrophobic, lignin-based polyurethane foam composites for underwater pressure sensing. J. Mater. Chem. C 2024, 12, 3203–3209. [Google Scholar] [CrossRef]
- Chen, C.; Gao, C.C.; Lin, K.G.; Zhang, J.; Zhang, Z.W.; Jing, Y.F.; Xiao, Y.S.; Shan, G.H.; Xie, C. Recycling of expired cow milk for constructing multifunctional biomass nonfluorinated chromatic paint with superhydrophobicity. Chem. Eng. J. 2024, 492, 152326. [Google Scholar] [CrossRef]
- Kong, L.J.; Sun, P.Q.; Liu, J.C.; Lin, Y.X.; Xiao, C.; Bao, C.; Zheng, K.; Xue, M.; Zhang, X.; Liu, X.L.; et al. Superhydrophobic and mechanical properties enhanced the electrospinning film with a multiscale micro-nano structure for high-efficiency radiation cooling. J. Mater. Chem. A 2024, 12, 7886–7895. [Google Scholar] [CrossRef]
- Guan, Y.H.; Bi, B.Q.; Qiao, D.; Cao, S.J.; Zhang, W.J.; Wang, Z.N.; Zeng, H.B.; Li, Y.M. Bioinspired superhydrophobic polylactic acid aerogel with tree branch structure for the removal of viscous oil spills assisted by solar energy. J. Mater. Chem. A 2024, 12, 9850–9862. [Google Scholar] [CrossRef]
- Zhu, W.H.; Xing, Y.J.; Wang, H.R.; Yu, M.Y.; Cen, H.Y.; Liu, L.L.; Li, Y.Q.; Zhu, W. Fluorine-free preparation of superhydrophobic polyester fabric with directional moisture transport for efficient oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134369. [Google Scholar] [CrossRef]
- Huang, Z.H.; Wang, Z.Z.; Wang, S.Q.; Shan, X.W.; Yin, S.M.; Tao, B. Superhydrophilic–superhydrophobic integrated system based on copper mesh for continuous and efficient oil-water separation. RSC Adv. 2024, 14, 6064–6071. [Google Scholar] [CrossRef]
- He, X.T.; Lu, J.H.; Liu, J.X.; Wu, Z.X.; Li, B.Y.; Chen, Z.; Tao, W.Q.; Li, Z. Superhydrophobic Co-MOF-based sponge for efficient oil-water separation utilizing photothermal effect. J. Hazard. Mater. 2024, 469, 134090. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Sun, M.Y.Z.; Zhang, H.R.; Cao, X.Q.; Li, Y.Q.; Zhou, Y.T. All in one double pillared MXene membrane for excellent oil/water separation, pollutant removal, and anti-fouling performance. Chin. J. Struct. Chem. 2024, in press. [Google Scholar] [CrossRef]
- Zhao, D.X.; Wu, P.Y.; Zhu, H.Y.; Jiang, R.; Chen, J.W.; Qiu, C.H.; Jiang, S.T.; Lu, G.P. Construction of S-scheme heterojunctions of a Ti-doped Ce-MOF and BiOCl for efficient photocatalytic selective oxidation of amines. Inorg. Chem. Front. 2024, 11, 1583–1595. [Google Scholar] [CrossRef]
- Xu, S.F.; Zhang, J.Q.; Sun, X.F.; Yang, H.; Ma, J. Construction of ternary AuPt/Bi2WO6/ZnIn2S4 heterostructures for photocatalytic degradation of tetrabromobisphenol A. Colloids Surf. A 2024, 689, 133757. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.L.; Hu, P.; Liu, T.; Weng, B.; Ye, K.H.; Luo, Y.M.; Ji, H.B. Vacancy pair induced surface chemistry reconstruction of Cs2AgBiBr6/Bi2WO6 heterojunction to enhance photocatalytic CO2 reduction. Appl. Catal. B Environ. Energy 2024, 351, 123956. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Le, P.D.; Cao, T.M.; Pham, V.V. Establishing Z-scheme Bi2WO6/g-C3N4 interfaces toward efficient photocatalytic performance of NOx under visible light. J Alloy. Compd. 2024, 989, 174244. [Google Scholar] [CrossRef]
- Pham, H.A.L.; Nguyen, V.H.; Lee, T.; Nguyen, V.C.; Nguyen, T.D. Construction of BiOCl/bismuth-based halide perovskite heterojunctions derived from the metal-organic framework CAU-17 for effective photocatalytic degradation. Chemosphere 2024, 357, 142114. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Okab, A.A.; Graimed, B.H.; Ammar, S.H.; Taofeeq, H.; Al-Yasiri, M. Building a robust S-scheme BiOCl/CuBi2O4 system for photocatalytic oxidation of sulfamethoxazole under solar light irradiation. Sol. Energy 2024, 275, 112640. [Google Scholar] [CrossRef]
- Wang, W.T.; Liu, Z.Y.; Nie, H.W.; Kong, B. The direct Z-scheme character and roles of S vacancy in BiOCl/Bi2S3-(001) heterostructures for superior photocatalytic activity: A hybrid density functional investigation. Phys. Chem. Chem. Phys. 2024, 26, 10723–10736. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, Z.R.; Zhang, X.; Zhang, M.L.; Li, J.Y.; Zhang, D.X.; Xu, X.Y. Effective solar light-driven isothiazolinone degradation by morphology-and oxygen vacancy-modified Gd-doped BiOCl. New J. Chem. 2024, 48, 6168–6179. [Google Scholar] [CrossRef]
- Xu, M.C.; Dong, F.; Zhang, Z.P.; Shao, M.; Wan, Y.S. A Novel Z-type 0D/2D BiOCl/NiAl-LDH heterojunction for photodegradation of multiple antibiotics in industrial wastewater: Degradation pathways and toxicity analysis. J Alloy. Compd. 2024, 991, 174543. [Google Scholar] [CrossRef]
- Waehayee, A.; Phonsuksawang, P.; Falun, P.; Ngamwongwan, L.; Choklap, T.; Prachanat, J.; Chankhanittha, T.; Butburee, T.; Suthirakun, S.; Siritanon, T. Enhancing Z-scheme {001}/{110} junction in BiOCl with {110} surface oxygen vacancies for photocatalytic degradation of rhodamine B and tetracycline. J. Alloy. Compd. 2024, 997, 174915. [Google Scholar] [CrossRef]
- Chen, R.X.; Gan, W.; Guo, J.; Lu, Y.Q.; Ding, S.; Liu, R.; Zhang, M.; Sun, Z.Q. Internal electric field and oxygen vacancies synergistically boost S-scheme VO/BiOCl-TiO2 heterojunction film for photocatalytic degradation of norfloxacin. Chem. Eng. J. 2024, 489, 151260. [Google Scholar] [CrossRef]
- Farid, M.A.; Ashraf, A.R.; Sarfaraz, R.; Hassan, S.U.; Naeem, N.; Naeem, H. Simultaneous photocatalytic degradation of methylene blue and methyl orange using a green synthesized Zn0.98Mn0.02O/BiOCl nanocomposite. New J. Chem. 2024, 48, 887–897. [Google Scholar] [CrossRef]
- Fu, S.; Chu, Z.L.; Huang, Z.Q.; Dong, X.M.; Bie, J.H.; Yang, Z.; Zhu, H.J.; Pu, W.Y.; Wu, W.Z.; Liu, B. Construction of Z-scheme AgCl/BiOCl heterojunction with oxygen vacancies for improved pollutant degradation and bacterial inactivation. RSC Adv. 2024, 14, 3888–3899. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, Y.H.; Liu, X.G.; Li, X. Synthesis of double Z-scheme CdS/Bi2O2CO3/BiOCl heterojunction photocatalysts for degradation of rhodamine B under visible light. React. Chem. Eng. 2024, 9, 186–198. [Google Scholar] [CrossRef]
- Nakayama, D.; Wu, C.M.; Motora, K.G.; Koinkar, P.; Furube, A. Novel solar-light-driven Z-scheme BiOCl@WS2 nanocomposite photocatalysts for the photocatalytic removal of organic pollutants. New J. Chem. 2023, 47, 22078–22089. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.Z.; Ge, B.; Men, X.H.; Xue, Q.J. One-pot, template-free synthesis of a robust superhydrophobic polymer monolith with an adjustable hierarchical porous structure. Green Chem. 2016, 18, 5266–5272. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wu, M.; Duan, G.G.; Gong, X. One-step fabrication of eco-friendly superhydrophobic fabrics for high-efficiency oil/water separation and oil spill cleanup. Nanoscale 2022, 14, 1296–1309. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, K.L. A novel flower-like dual Z-scheme BiSI/Bi2WO6/g-C3N4 photocatalyst has excellent photocatalytic activity for the degradation of organic pollutants under visible light. Diam. Relat. Mater. 2021, 115, 108343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhao, J.; Peng, Y.; Li, Y.; Guo, W.; Liao, C. Fabrication of Non-Wetting Mg(OH)2 Composites with Photoresponsive Capabilities and Their Environmental Restoration Performance. Nanomaterials 2024, 14, 1240. https://doi.org/10.3390/nano14151240
Zhang D, Zhao J, Peng Y, Li Y, Guo W, Liao C. Fabrication of Non-Wetting Mg(OH)2 Composites with Photoresponsive Capabilities and Their Environmental Restoration Performance. Nanomaterials. 2024; 14(15):1240. https://doi.org/10.3390/nano14151240
Chicago/Turabian StyleZhang, Dongmei, Jiaqi Zhao, Yangyang Peng, Yuchao Li, Wenbin Guo, and Chengzhu Liao. 2024. "Fabrication of Non-Wetting Mg(OH)2 Composites with Photoresponsive Capabilities and Their Environmental Restoration Performance" Nanomaterials 14, no. 15: 1240. https://doi.org/10.3390/nano14151240





