Recent Advances and Challenges in Ti-Based Oxide Anodes for Superior Potassium Storage
Abstract
:1. Introduction
1.1. Advantages of Potassium-Ion Batteries
1.2. Anodes of PIBs
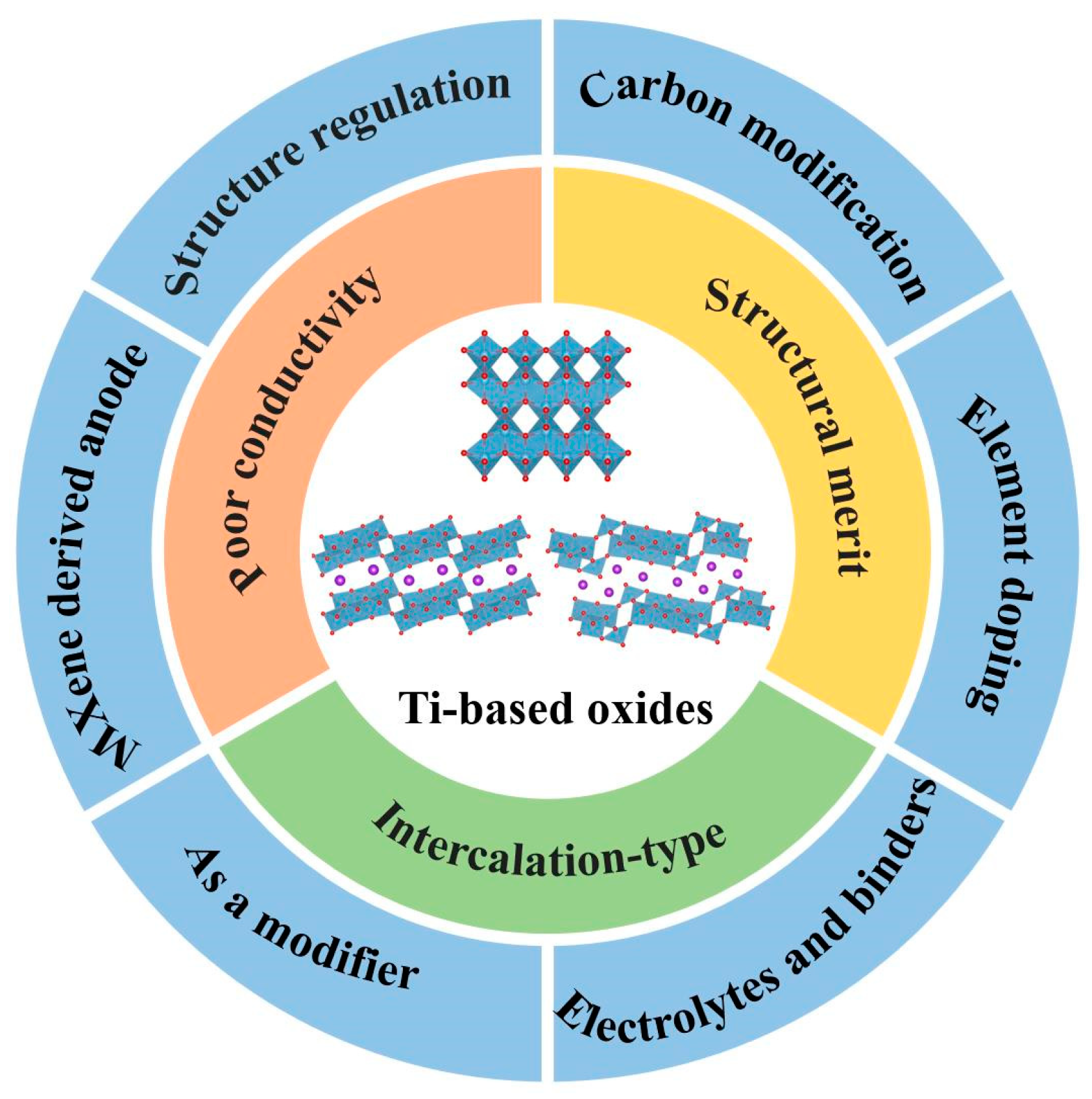
1.3. Ti-Based Oxide Anodes
2. TiO2
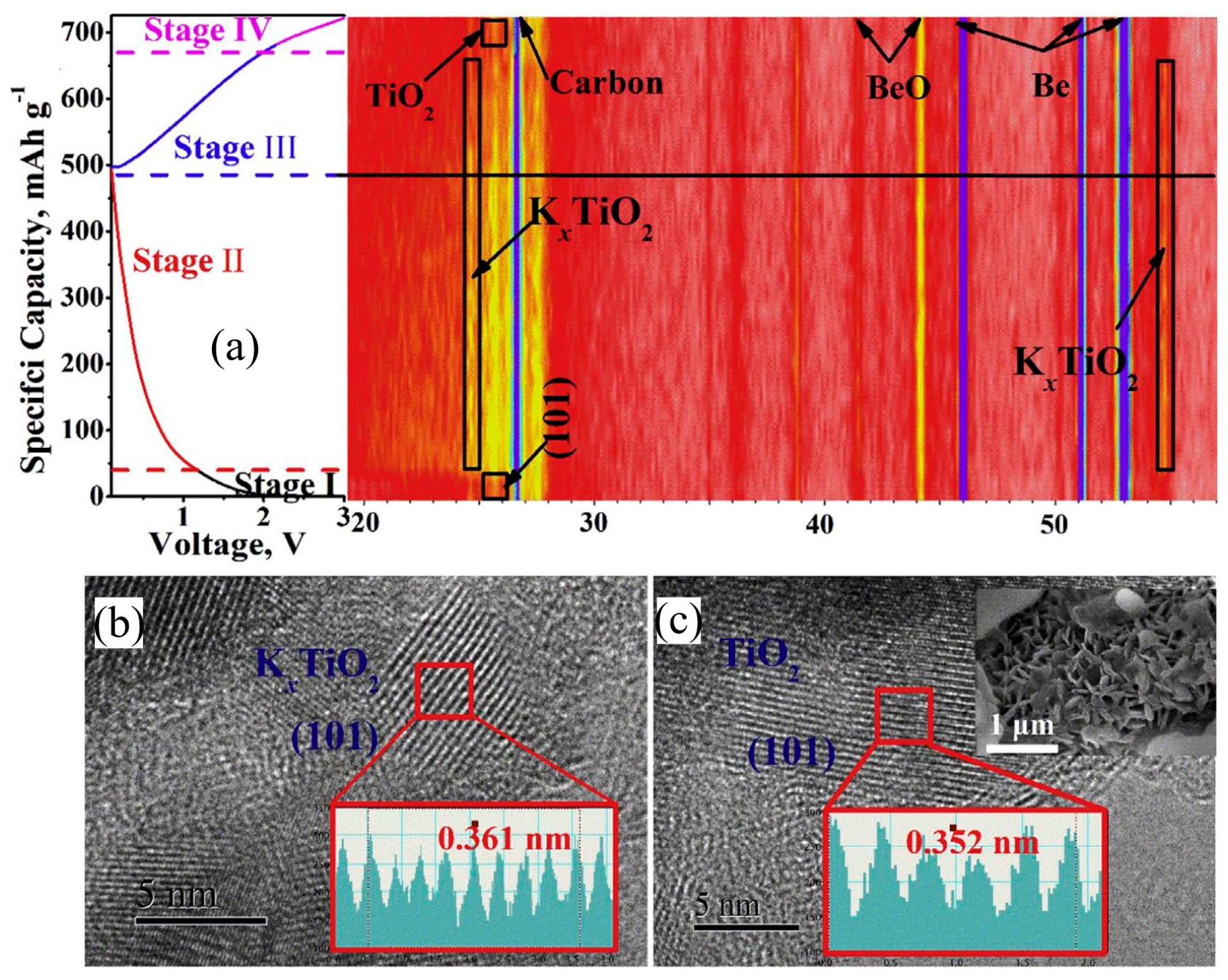
2.1. Research on Potassium Storage Mechanism
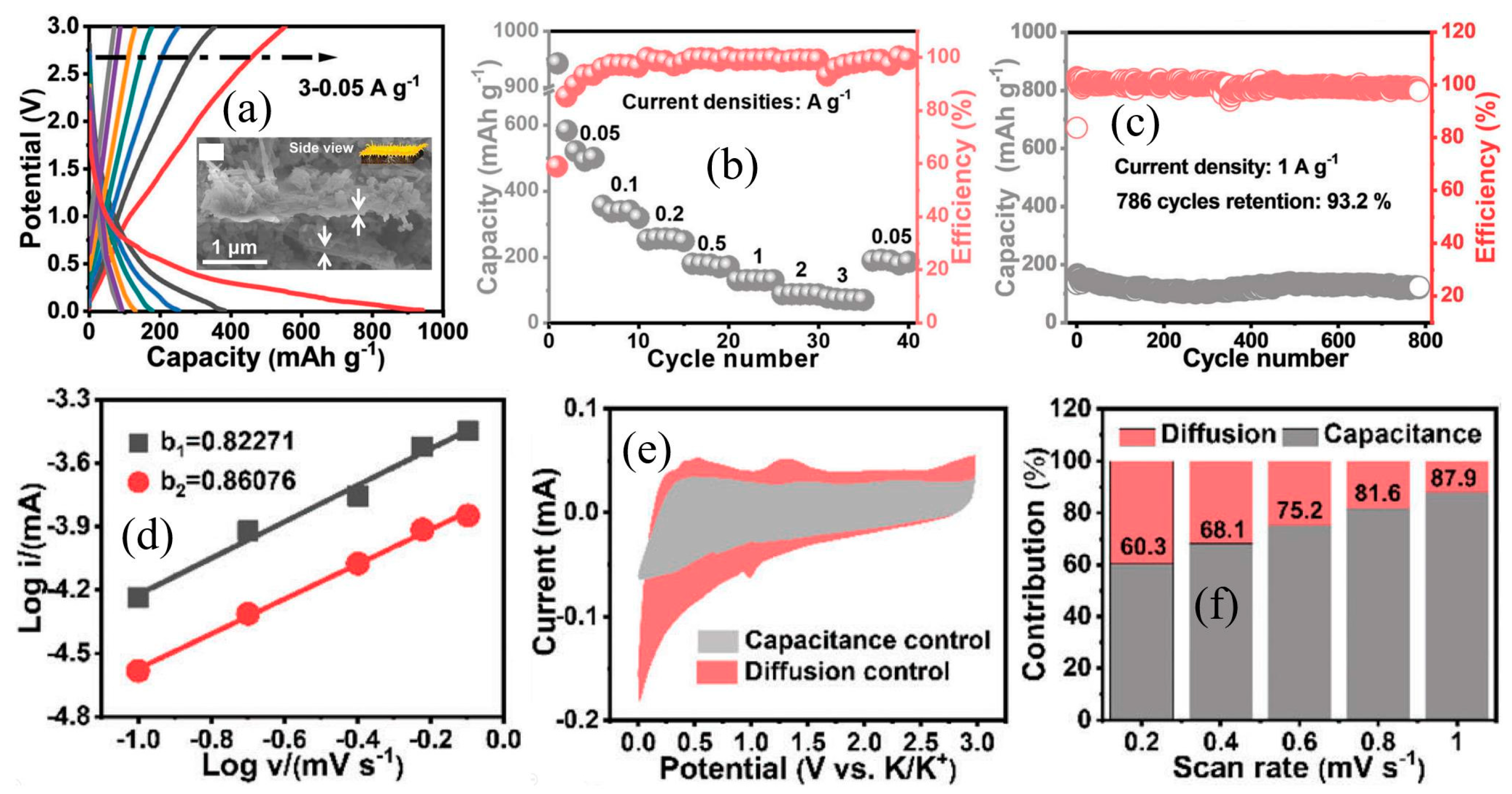
2.2. Structural Regulation, Carbon Modification and Element Doping
2.3. MXene-Derived TiO2-Based Anodes
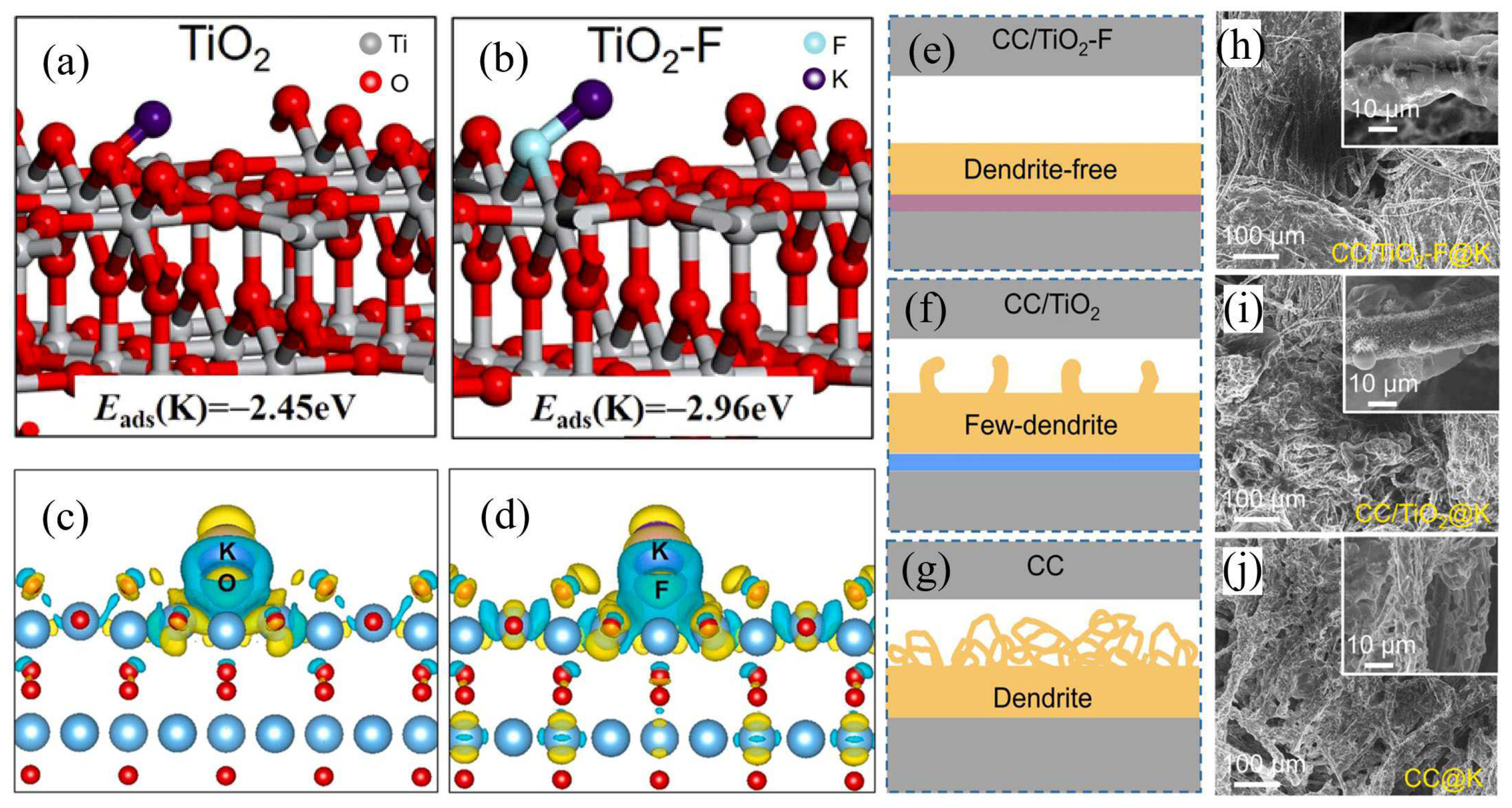
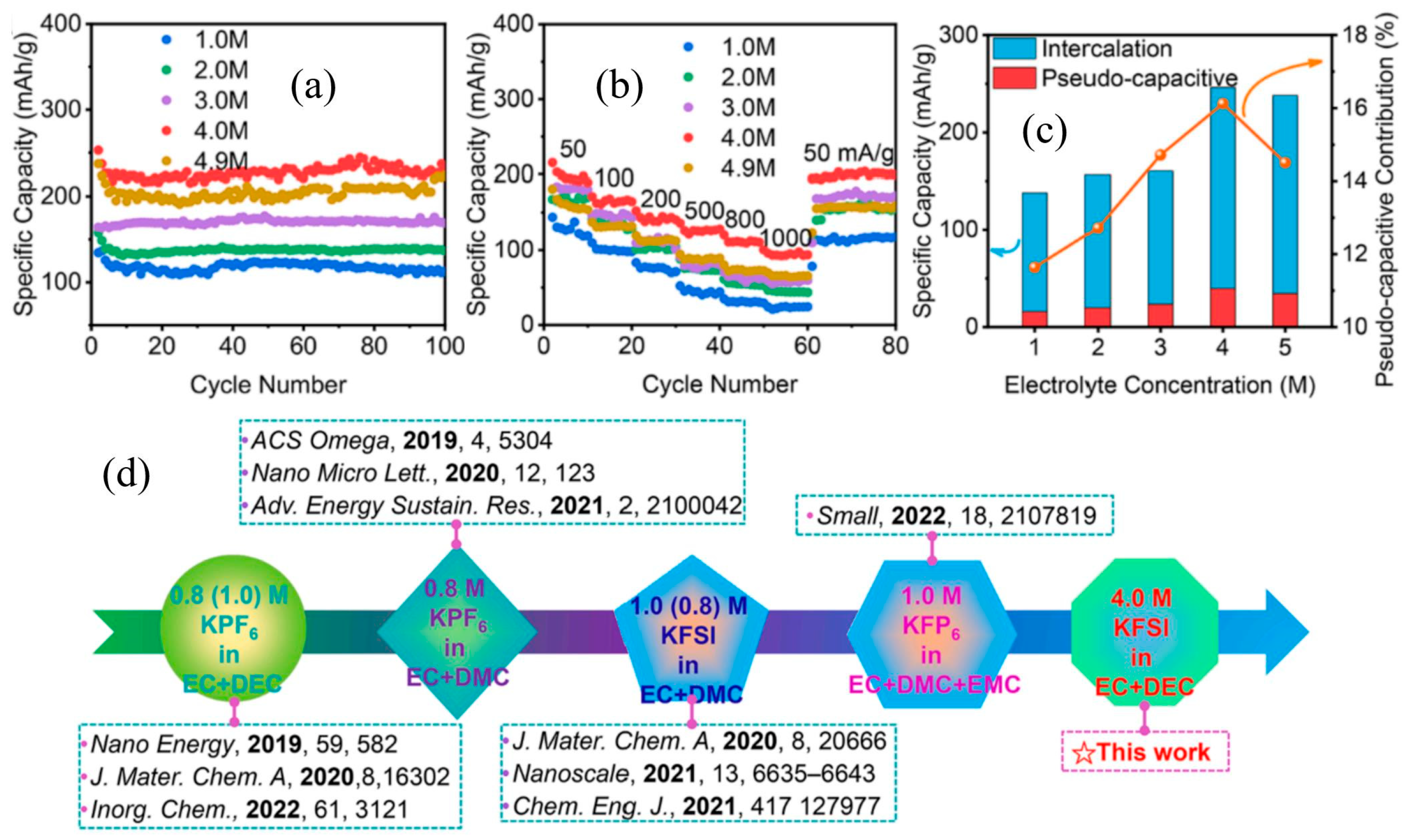
2.4. Effects of Electrolytes and Binders
2.5. The Role as a Modifier
3. Other Ti-Based Oxide Anodes
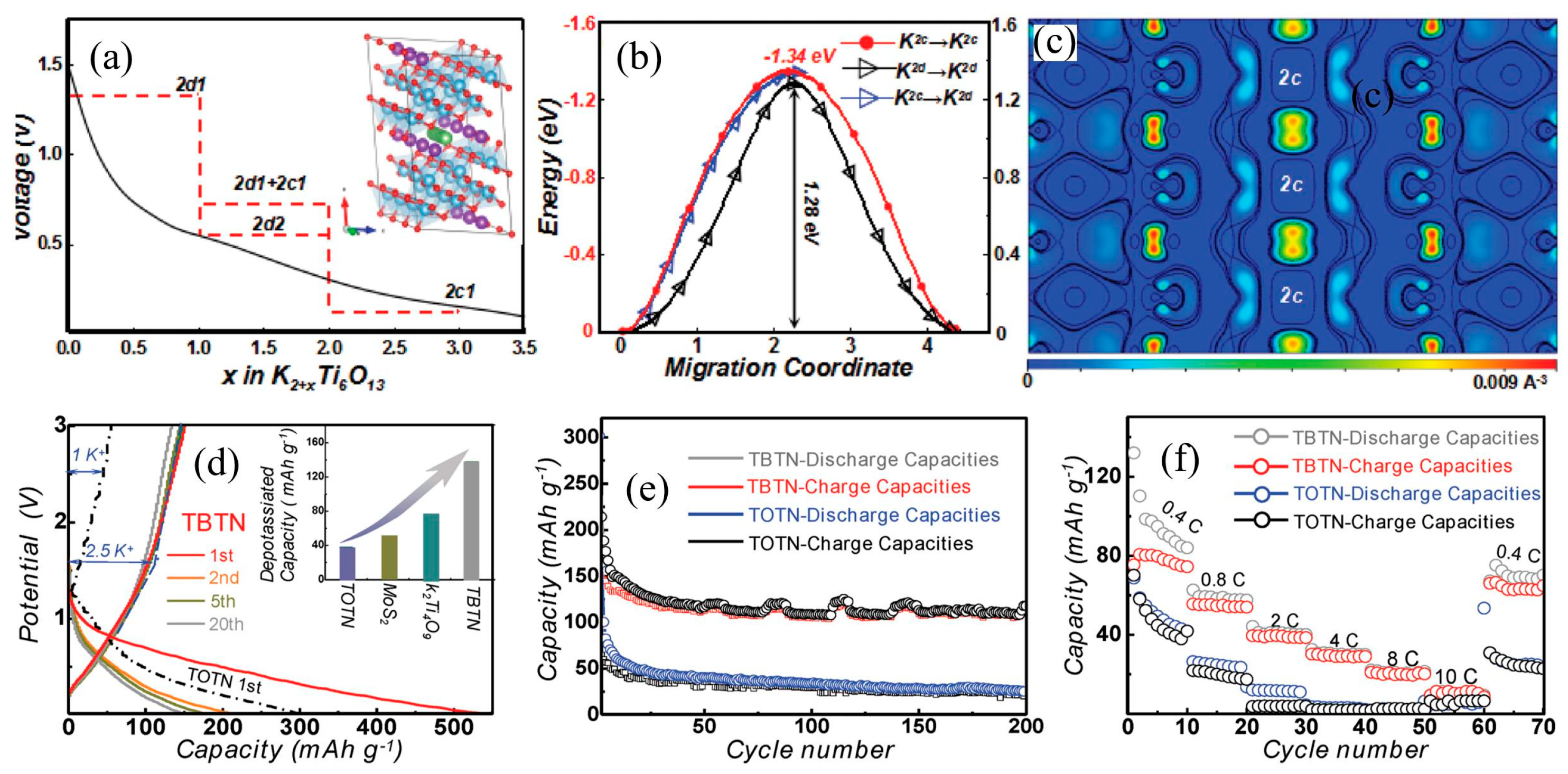
3.1. K2Ti6O13 Anode
3.2. K2Ti4O9 and K2Ti8O17 Anodes
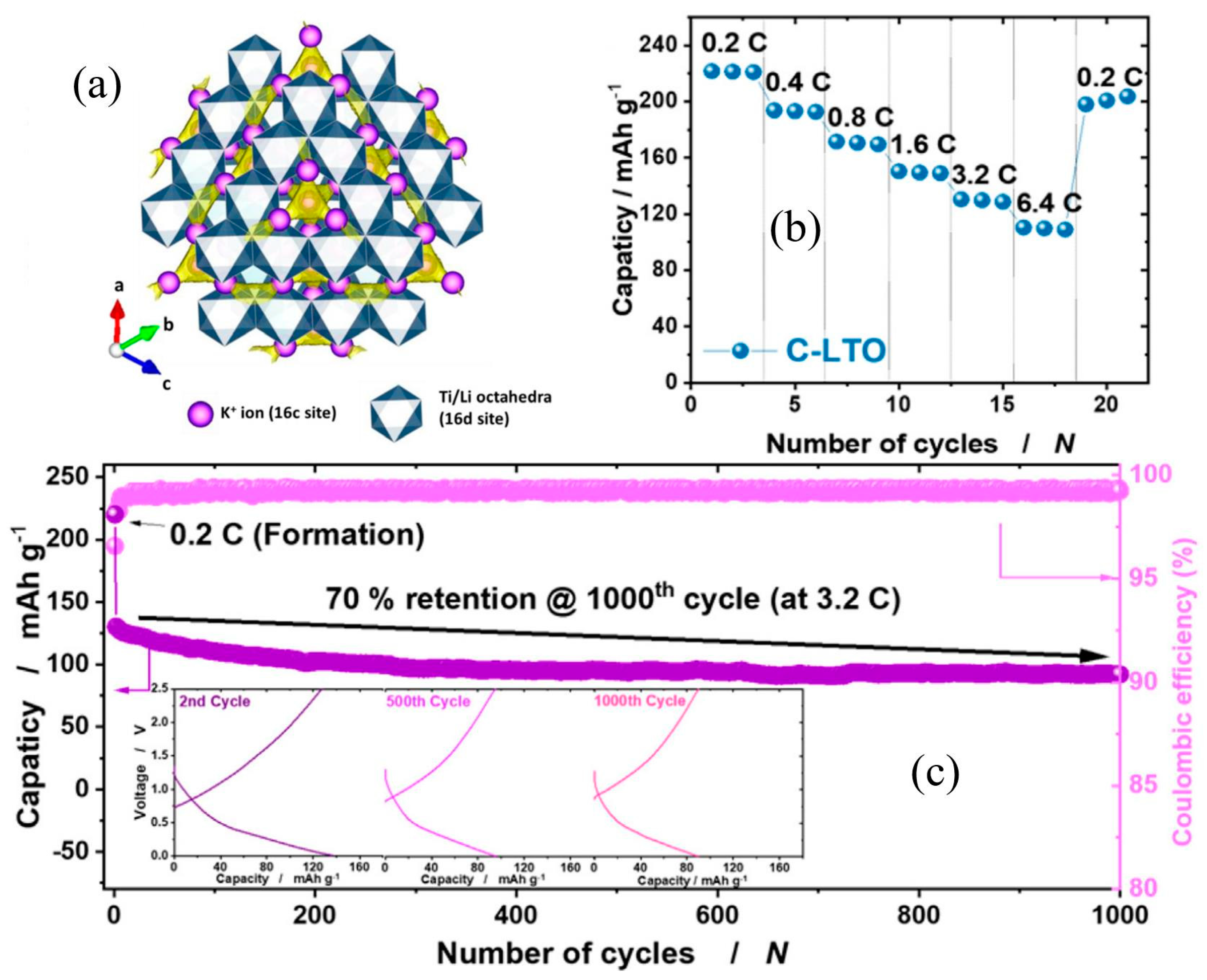
3.3. Na2Ti3O7 and Li4Ti5O12 Anodes, etc.
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries-Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.; Ji, X.; Jia, C.; Wang, H. Advancements and Challenges in Potassium Ion Batteries: A Comprehensive Review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research Development on K-Ion Batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Xing, Z.; Lam, C.W.K.; Pang, S.-S.; Zhang, W.; Ju, Z. Advanced Carbon-Based Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2019, 9, 1900343. [Google Scholar] [CrossRef]
- Min, X.; Xiao, J.; Fang, M.; Wang, W.; Zhao, Y.; Liu, Y.; Abdelkader, A.M.; Xi, K.; Kumar, R.V.; Huang, Z. Potassium-ion batteries: Outlook on present and future technologies. Energ. Environ. Sci. 2021, 14, 2186–2243. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-S.; Duan, S.-Y.; Sun, Y.-G.; Bin, D.-S.; Tao, X.-S.; Zhang, D.; Liu, Y.; Cao, A.-M.; Wan, L.-J. Recent developments in electrode materials for potassium-ion batteries. J. Mater. Chem. A 2019, 7, 4334–4352. [Google Scholar] [CrossRef]
- Wang, N.; Chu, C.; Xu, X.; Du, Y.; Yang, J.; Bai, Z.; Dou, S. Comprehensive New Insights and Perspectives into Ti-Based Anodes for Next-Generation Alkaline Metal (Na+, K+) Ion Batteries. Adv. Energy Mater. 2018, 8, 1801888. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Li, D. Recent Developments in Alloying-type Anode Materials for Potassium-Ion Batteries. Chem. Asian J. 2020, 15, 1648–1659. [Google Scholar] [CrossRef]
- Sha, M.; Liu, L.; Zhao, H.; Lei, Y. Anode materials for potassium-ion batteries: Current status and prospects. Carbon. Energy 2020, 2, 350–369. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Lei, Y. Recent Research Progress of Anode Materials for Potassium-ion Batteries. Energy Environ. Mater. 2020, 3, 105–120. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Henzie, J.; Zhang, J.; Ha, J.; Amin, M.A.; Hossain, M.S.A.; Jun, S.C.; Yamauchi, Y. Recent Advances and Perspectives of Battery-Type Anode Materials for Potassium Ion Storage. ACS Nano 2021, 15, 18931–18973. [Google Scholar] [CrossRef]
- Gu, Y.; Pei, Y.R.; Zhao, M.; Yang, C.C.; Jiang, Q. Sn-, Sb- and Bi-Based Anodes for Potassium Ion Battery. Chem. Rec. 2022, 22, e202200098. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, C.; Mi, L.; Chou, S.; Chen, W.; Shen, C. Recent Progress on the Alloy-Based Anode for Sodium-Ion Batteries and Potassium-Ion Batteries. Small 2021, 17, 1903194. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.-X.; Wang, J.; Chen, C.; Li, S.-Y.; Wang, S.-W.; Zheng, S.-J.; Li, F.-J. Recent progresses on alloy-based anodes for potassium-ion batteries. Rare Met. 2020, 39, 989–1004. [Google Scholar] [CrossRef]
- Lu, S.; Wu, H.; Xu, S.; Wang, Y.; Zhao, J.; Li, Y.; Abdelkader, A.M.; Li, J.; Wang, W.; Xi, K.; et al. Iron Selenide Microcapsules as Universal Conversion-Typed Anodes for Alkali Metal-Ion Batteries. Small 2021, 17, 2005745. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; You, Y.; Hu, R.; Cao, X.; Wei, Y.; Zhang, H.; Pan, L.; Sun, Z. Comprehensively Understanding the Role of Anion Vacancies on K-Ion Storage: A Case Study of Se-Vacancy-Engineered VSe2. Adv. Mater. 2023, 35, 2211311. [Google Scholar] [CrossRef]
- Deng, Q.; Fu, Y.; Zhu, C.; Yu, Y. Niobium-Based Oxides Toward Advanced Electrochemical Energy Storage: Recent Advances and Challenges. Small 2019, 15, 1804884. [Google Scholar] [CrossRef]
- Jian, Z.; Hwang, S.; Li, Z.; Hernandez, A.S.; Wang, X.; Xing, Z.; Su, D.; Ji, X. Hard-Soft Composite Carbon as a Long-Cycling and High-Rate Anode for Potassium-Ion Batteries. Adv. Funct. Mater. 2017, 27, 1700324. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Zhou, M.; Fu, Q.; Zhao, C.; Wu, M.; Lei, Y. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat. Commun. 2018, 9, 1720. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Zhao, S.; He, G.; Miller, T.S. Pseudohexagonal Nb2O5 Anodes for Fast-Charging Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 16664–16672. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Ma, L.; Yu, C.; Ji, Z.; Pan, L.; Mai, W. Graphite Anode for Potassium Ion batteries: Current Status and Perspective. Energy Environ. Mater. 2022, 5, 458–469. [Google Scholar] [CrossRef]
- Zhao, Z.; Cheng, J.; Li, K.; Li, C.; Zhang, S.; Pei, X.; Niu, Z.; Liu, Z.; Fu, Y.; Li, D. Heterojunction interfacial promotion of fast and prolonged alkali-ion storage of urchin-like Nb2O5@C nanospheres. J. Mater. Chem. A 2021, 9, 23467–23476. [Google Scholar] [CrossRef]
- Guo, S.; Yi, J.; Sun, Y.; Zhou, H. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries. Energ. Environ. Sci. 2016, 9, 2978–3006. [Google Scholar] [CrossRef]
- Lou, S.; Zhao, Y.; Wang, J.; Yin, G.; Du, C.; Sun, X. Ti-Based Oxide Anode Materials for Advanced Electrochemical Energy Storage: Lithium/Sodium Ion Batteries and Hybrid Pseudocapacitors. Small 2019, 15, 1904740. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Santos, M.B.; Tartaj, P.; Morales, E.; Manuel Amarilla, J. TiO2 Nanostructures as Anode Materials for Li/Na-Ion Batteries. Chem. Rec. 2018, 18, 1178–1191. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.-F.; Zheng, S. Challenges of Spinel Li4Ti5O12 for Lithium-Ion Battery Industrial Applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, L.; Su, D.; Jaroniec, M.; Qiao, S.-Z. Na2Ti3O7@N-Doped Carbon Hollow Spheres for Sodium-Ion Batteries with Excellent Rate Performance. Adv. Mater. 2017, 29, 1700989. [Google Scholar] [CrossRef]
- Li, R.; Lin, C.; Wang, N.; Luo, L.; Chen, Y.; Li, J.; Guo, Z. Advanced composites of complex Ti-based oxides as anode materials for lithium-ion batteries. Adv. Compos. Hybrid. Ma. 2018, 1, 440–459. [Google Scholar] [CrossRef]
- Paul, S.; Rahman, M.A.; Bin Sharif, S.; Kim, J.-H.; Siddiqui, S.-E.T.; Hossain, M.A.M. TiO2 as an Anode of High-Performance Lithium-Ion Batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Qi, R.; Cheng, Y.-J.; Xia, Y.; Mueller-Buschbaum, P.; Hu, X. Bronze-Phase TiO2 as Anode Materials in Lithium and Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2201675. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zheng, F.; Pan, Q.; Liu, Y.; Wang, G.; Liu, T.; Hu, J.; Liu, M. Design of TiO2eC hierarchical tubular heterostructures for high performance potassium ion batteries. Nano Energy 2019, 59, 582–590. [Google Scholar] [CrossRef]
- Cai, J.; Cai, R.; Sun, Z.; Wang, X.; Wei, N.; Xu, F.; Shao, Y.; Gao, P.; Dou, S.; Sun, J. Confining TiO2 Nanotubes in PECVD-Enabled Graphene Capsules Toward Ultrafast K-Ion Storage: In Situ TEM/XRD Study and DFT Analysis. Nano-Micro Lett. 2020, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.G.; Ma, J.; Fukunishi, M.; Salanne, M.; Komaba, S.; Dambournet, D. Insights into Li+, Na+, and K+ Intercalation in Lepidocrocite-Type Layered TiO2 Structures. ACS Appl. Energy Mater. 2018, 1, 2078–2086. [Google Scholar] [CrossRef]
- Deng, Q.; Yao, L. Self-Standing Soft Carbon-Coated MoS2 Nanofiber Film Anode for Superior Potassium Storage. Coatings 2022, 12, 1969. [Google Scholar] [CrossRef]
- Zang, Q.; Zhang, Q.; Luo, Z.; Liao, L.; Ouyang, X.; Xie, S. Construction of high conductivity carbon-coated MoS2 on porous carbon nanofibers for synergistic potassium storage. J. Power Sources 2022, 543, 231800. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Liu, Y.; Jiao, S.; Peng, B.; Li, J.; Yu, L.; Zhang, G. Nest-like TiO2-nitrogen-doped-carbon hybrid nanostructures as superior host for potassium-ion hybrid capacitors. Chem. Eng. J. 2021, 417, 127977. [Google Scholar] [CrossRef]
- Luo, R.; Ma, Y.; Qu, W.; Qian, J.; Li, L.; Wu, F.; Chen, R. High Pseudocapacitance Boosts Ultrafast, High-Capacity Sodium Storage of 3D Graphene Foam-Encapsulated TiO2 Architecture. ACS Appl. Mater. Interfaces 2020, 12, 23939–23950. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Wang, X.; Zhou, M.; Wu, K.; Lin, C.; Younus, H.A.; Zhang, M.; Zhang, S.; Cheng, F.; Zhang, Y. Carbon-Coated Flower-Like TiO2 Nanosphere as an Ultrastable Anode Material for Potassium-Ion Batteries: Structure Design and Mechanism Study. ACS Appl. Energy Mater. 2022, 5, 15586–15596. [Google Scholar] [CrossRef]
- Li, W.; Gao, N.; Cheng, S.; Wu, J.; Chen, Q. Electrochemical Performance of Sandwich-like Structured TiO2/graphene Composite as Anode for Potassium-ion Batteries. Int. J. Electrochem. Sci. 2022, 17, 221222. [Google Scholar] [CrossRef]
- Dubal, D.P.; Schneemann, A.; Ranc, V.; Kment, S.; Tomanec, O.; Petr, M.; Kmentova, H.; Otyepka, M.; Zboril, R.; Fischer, R.A.; et al. Ultrafine TiO2 Nanoparticle Supported Nitrogen-Rich Graphitic Porous Carbon as an Efficient Anode Material for Potassium-Ion Batteries. Adv. Energy Sustain. Res. 2021, 2, 2100042. [Google Scholar] [CrossRef]
- Liao, J.; Hu, Q.; Mu, J.; Chen, F.; He, X.; Chen, F.; Wen, Z.; Chen, C. Introducing a conductive pillar: A polyaniline intercalated layered titanate for high-rate and ultra-stable sodium and potassium ion storage. Chem. Commun. 2020, 56, 8392–8395. [Google Scholar] [CrossRef]
- Cao, J.; Zhong, J.; Xu, H.; Li, S.; Deng, H.; Wang, T.; Fan, L.; Wang, X.; Wang, L.; Zhu, J.; et al. N/S co-doped carbon nanosheet bundles as high-capacity anode for potassium-ion battery. Nano Res. 2022, 15, 2040–2046. [Google Scholar] [CrossRef]
- Hwang, K.; Sohn, H.; Yoon, S. Mesostructured niobium-doped titanium oxide-carbon (Nb-TiO2-C) composite as an anode for high-performance lithium-ion batteries. J. Power Sources 2018, 378, 225–234. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, N.; Huang, S.; Wu, N.-L.; Wei, M. Sulfur-Doped Anatase TiO2 as an Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3791–3797. [Google Scholar] [CrossRef]
- Fan, M.; Lin, Z.; Zhang, P.; Ma, X.; Wu, K.; Liu, M.; Xiong, X. Synergistic Effect of Nitrogen and Sulfur Dual-Doping Endows TiO2 with Exceptional Sodium Storage Performance. Adv. Energy Mater. 2021, 11, 2003037. [Google Scholar] [CrossRef]
- Su, D.; Liu, L.; Liu, Z.; Dai, J.; Wen, J.; Yang, M.; Jamil, S.; Deng, H.; Cao, G.; Wang, X. Electrospun Ta-doped TiO2/C nanofibers as a high-capacity and long-cycling anode material for Li-ion and K-ion batteries. J. Mater. Chem. A 2020, 8, 20666–20676. [Google Scholar] [CrossRef]
- Cui, J.; Yin, P.; Xu, A.; Jin, B.; Li, Z.; Shao, M. Fluorine enhanced nucleophilicity of TiO2 nanorod arrays: A general approach for dendrite-free anodes towards high-performance metal batteries. Nano Energy 2022, 93, 106837. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Lu, S. Recent Advances in Layered Ti3C2Tx MXene for Electrochemical Energy Storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef]
- Pang, J.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Bashir, T.; Zhou, S.; Yang, S.; Ismail, S.A.; Ali, T.; Wang, H.; Zhao, J.; Gao, L. Progress in 3D-MXene Electrodes for Lithium/Sodium/Potassium/Magnesium/Zinc/Aluminum-Ion Batteries. Electrochem. Energy R. 2023, 6, 5. [Google Scholar] [CrossRef]
- Wu, S.; Feng, Y.; Wu, K.; Jiang, W.; Xue, Z.; Xiong, D.; Chen, L.; Feng, Z.; Wen, K.; Li, Z.; et al. Mxene Ti3C2 generated TiO2 nanoparticles in situ and uniformly embedded in rGO sheets as high stable anodes for potassium ion batteries. J. Alloys Compd. 2023, 930, 167414. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, R.; Liu, B.; Zhang, Y.; Zhu, K.; Yan, J.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries. J. Mater. Chem. A 2019, 7, 5363–5372. [Google Scholar] [CrossRef]
- Qi, F.; Shao, L.; Lu, X.; Liu, G.; Shi, X.; Sun, Z. MXene-derived TiSe2/TiO2/C heterostructured hexagonal prisms as high rate anodes for Na-ion and K-ion batteries. Appl. Surf. Sci. 2022, 605, 154653. [Google Scholar] [CrossRef]
- Zhang, R.; Tian, Y.; Otitoju, T.; Feng, Z.; Wang, Y.; Sun, T. Sand-Fixation Model for Interface Engineering of Layered Titania and N/O-Doped Carbon Composites to Enhance Potassium/Sodium Storage. Small 2023, 2302148. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, T.; Sun, D.; Ji, X.; Zhou, X. Recent Advances in Electrolytes for Potassium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2211290. [Google Scholar] [CrossRef]
- Xing, J.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energy R. 2022, 5, 14. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Zhang, S.; Zhou, T.; Mao, J.; Guo, Z. Cathode Materials for Potassium-Ion Batteries: Current Status and Perspective. Electrochem. Energy R. 2018, 1, 625–658. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, C.; Nie, L.; Zang, S.; Chen, H.; Chen, N.; Ma, M.; Lai, Q. Electrolyte manipulation enhanced pseudo-capacitive K-storage for TiO2 anode. Appl. Surf. Sci. 2023, 611, 155617. [Google Scholar] [CrossRef]
- Wang, C.; Su, L.; Wang, N.; Lv, D.; Wang, D.; Yang, J.; Qian, Y. Unravelling binder chemistry in sodium/potassium ion batteries for superior electrochemical performances. J. Mater. Chem. A 2022, 10, 4060–4067. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Ding, T.; Tian, R.; Sun, D.; Wang, M.-S.; Zhou, X. Synthesis of yolk-shell Bi2O3@TiO2 submicrospheres with enhanced potassium storage. Sci. China Chem. 2022, 65, 1807–1816. [Google Scholar] [CrossRef]
- Wen, S.; Gu, X.; Ding, X.; Zhang, L.; Dai, P.; Li, L.; Liu, D.; Zhang, W.; Zhao, X.; Guo, Z. Constructing ultrastable electrode/electrolyte interface for rapid potassium ion storage capability via salt chemistry and interfacial engineering. Nano Res. 2022, 15, 2083–2091. [Google Scholar] [CrossRef]
- Schuppert, N.D.; Mukherjee, S.; Bates, A.M.; Son, E.-J.; Choi, M.J.; Park, S. Ex-situ X-ray diffraction analysis of electrode strain at TiO2 atomic layer deposition/alpha-MoO3 interface in a novel aqueous potassium ion battery. J. Power Sources 2016, 316, 160–169. [Google Scholar] [CrossRef]
- Huang, M.; Xi, B.; Mi, L.; Zhang, Z.; Chen, W.; Feng, J.; Xiong, S. Rationally Designed Three-Layered TiO2@amorphous MoS3@Carbon Hierarchical Microspheres for Efficient Potassium Storage. Small 2022, 18, 2107819. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Chen, F.; Liu, S.; Bayaguud, A.; Feng, Y.; Zhang, Z.; Fu, Y.; Yu, Y.; Zhu, C. Advantageous Functional Integration of Adsorption-Intercalation-Conversion Hybrid Mechanisms in 3D Flexible Nb2O5@Hard Carbon@MoS2@Soft Carbon Fiber Paper Anodes for Ultrafast and Super-Stable Sodium Storage. Adv. Funct. Mater. 2020, 30, 1908665. [Google Scholar] [CrossRef]
- Su, D.; Dai, J.; Yang, M.; Wen, J.; Yang, J.; Liu, W.; Hu, H.; Liu, L.; Feng, Y. Red phosphorus embedded in TiO2/C nanofibers to enhance the potassium-ion storage performance. Nanoscale 2021, 13, 6635–6643. [Google Scholar] [CrossRef]
- Gao, Z.; Han, L.; Gao, H.; Chen, J.; Sun, Z.; Zhu, C.; Zhang, Y.; Shi, J.; Chen, S.; Wang, H. Coupling core-shell Bi@Void@TiO2 heterostructures into carbon nanofibers for achieving fast potassium storage and long cycling stability. J. Mater. Chem. A 2022, 10, 12908–12920. [Google Scholar] [CrossRef]
- Dong, S.; Li, Z.; Xing, Z.; Wu, X.; Ji, X.; Zhang, X. Novel Potassium-Ion Hybrid Capacitor Based on an Anode of K2Ti6O13 Microscaffolds. ACS Appl. Mater. Interfaces 2018, 10, 15542–15547. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, H.; Zhang, S.; Han, M.; Cao, Y.; Liu, S.; Yang, Z.; Chen, A.; Sun, J. K2Ti6O13/carbon core-shell nanorods as a superior anode material for high-rate potassium-ion batteries. Nanoscale 2020, 12, 11427–11434. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Liu, H.; Li, W.; Xu, C.; Han, Q.; Zhang, Z.; Jiao, L. K2Ti6O13 nanorods for potassium-ion battery anodes. J. Electroanal. Chem. 2019, 841, 51–55. [Google Scholar] [CrossRef]
- Xu, S.-M.; Ding, Y.-C.; Liu, X.; Zhang, Q.; Wang, K.-X.; Chen, J.-S. Boosting Potassium Storage Capacity Based on Stress-Induced Size-Dependent Solid-Solution Behavior. Adv. Energy Mater. 2018, 8, 1802175. [Google Scholar] [CrossRef]
- Kishore, B.; Venkatesh, G.; Munichandraiah, N. K2Ti4O9: A Promising Anode Material for Potassium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2551–A2554. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Z.-S.; Zheng, S.; Wang, X.; Qin, J.; Wang, S.; Shi, X.; Bao, X. Ti3C2 MXene-Derived Sodium/Potassium Titanate Nanoribbons for High-Performance Sodium/Potassium Ion Batteries with Enhanced Capacities. ACS Nano 2017, 11, 4792–4800. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, Q.; Pang, H.; Yu, Z.; Yan, D. Ti3C2Tx@K2Ti4O9 composite materials by controlled oxidation and alkalization strategy for potassium ion batteries. Ceram. Int. 2022, 48, 16418–16424. [Google Scholar] [CrossRef]
- Zheng, X.; Li, P.; Zhu, H.; Zhao, G.; Rui, K.; Shu, J.; Xu, X.; Wang, X.; Sun, W.; Dou, S.X. Understanding the structural and chemical evolution of layered potassium titanates for sodium ion batteries. Energy Storage Mater. 2020, 25, 502–509. [Google Scholar] [CrossRef]
- Han, J.; Xu, M.; Niu, Y.; Li, G.-N.; Wang, M.; Zhang, Y.; Jia, M.; Li, C.M. Exploration of K2Ti8O17 as an anode material for potassium-ion batteries. Chem. Commun. 2016, 52, 11274–11276. [Google Scholar] [CrossRef]
- Pan, H.; Lu, X.; Yu, X.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L. Sodium Storage and Transport Properties in Layered Na2Ti3O7 for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2013, 3, 1186–1194. [Google Scholar] [CrossRef]
- Fu, S.; Ni, J.; Xu, Y.; Zhang, Q.; Li, L. Hydrogenation Driven Conductive Na2Ti3O7 Nanoarrays as Robust Binder-Free Anodes for Sodium-Ion Batteries. Nano Lett. 2016, 16, 4544–4551. [Google Scholar] [CrossRef]
- Li, P.; Wang, W.; Gong, S.; Lv, F.; Huang, H.; Luo, M.; Yang, Y.; Yang, C.; Zhou, J.; Qian, C.; et al. Hydrogenated Na2Ti3O7 Epitaxially Grown on Flexible N-Doped Carbon Sponge for Potassium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 37974–37980. [Google Scholar] [CrossRef]
- Yi, T.-F.; Yang, S.-Y.; Xie, Y. Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J. Mater. Chem. A 2015, 3, 5750–5777. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Huang, T.; Wang, B.; Zheng, H.; Luo, Q.; Peng, D.-L.; Wei, Q. Surface-controlled sodium-ion storage mechanism of Li4Ti5O12 anode. Energy Storage Mater. 2023, 54, 724–731. [Google Scholar] [CrossRef]
- Natarajan, S.; Subramanyan, K.; Aravindan, V. Focus on Spinel Li4Ti5O12 as Insertion Type Anode for High-Performance Na-Ion Batteries. Small 2019, 15, 1904484. [Google Scholar] [CrossRef]
- Kim, H.J.; Jo, J.H.; Choi, J.U.; Voronina, N.; Ahn, D.; Jeon, T.-Y.; Yashiro, H.; Aniskevich, Y.; Ragoisha, G.; Streltsov, E.; et al. Long Life Anode Material for Potassium Ion Batteries with High-Rate Potassium Storage. Energy Storage Mater. 2021, 40, 197–208. [Google Scholar] [CrossRef]
- Zeng, C.; Xie, F.; Yang, X.; Jaroniec, M.; Zhang, L.; Qiao, S.-Z. Ultrathin Titanate Nanosheets/Graphene Films Derived from Confined Transformation for Excellent Na/K Ion Storage. Angew. Chem. Int. Edit. 2018, 57, 8540–8544. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Dong, L.; Sun, B.; Yan, K.; Zhang, J.; Wan, S.; He, F.; Munroe, P.; Notten, P.H.L.; Wang, G. K2Ti2O5@C Microspheres with Enhanced K+ Intercalation Pseudocapacitance Ensuring Fast Potassium Storage and Long-Term Cycling Stability. Small 2020, 16, 1906131. [Google Scholar] [CrossRef] [PubMed]

| Materials | Potassium Storage Performances | References |
|---|---|---|
| TiO2/NC-HN | 277.2, 244.7, 210.2, 185.5, 165.7, 126.9 and 79.1 mA h g−1 at 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 A g−1, respectively. | [45] |
| HeTiO2eC MTs | 208.5, 180.1, 114.6 and 97.3 mA h g−1 at 0.2, 0.4, 1 and 2 A g−1, respectively. | [40] |
| TO/C | 176.2, 155.0, 131.4, 115.8, 93.1, 62.3, 36.3 and 27.9 mA h g−1 at 0.1, 0.2, 0.5, 1, 2, 5, 10 and 20 C rate, respectively. | [47] |
| G-TiO2 NTs | 271.6, 258.7, 217.3, 189.3, 166.8, 133.4 and 129.2 mA h g−1 at 0.05, 0.1, 0.2, 0.5, 1, 2 and 5 A g−1, respectively. | [41] |
| TiO2-5-Ta/CNF | 187.9, 170.9, 154.1, 140.6, 137.6, 126.3, 118.5 and 102.8 mA h g−1 at 0.1, 0.2, 0.4, 0.8, 1, 2, 3 and 5 A g−1, respectively. | [55] |
| TiO2-RP/CN | 248.9, 221.8, 199.8, 178.8, 158.7 and 151.4 mA h g−1 at 0.05, 0.1, 0.2, 0.4, 0.8 and 1 A g−1, respectively | [74] |
| TiSe2/TiO2/C | 245, 191, 142, 105, 68 and 43 mA h g−1 at 0.1, 0.2, 0.5, 1, 2 and 5 A g−1, respectively | [62] |
| Ti3C2/TiO2/rGO | 592.5, 413.9, 349.1, 293.5 and 221.7 mA h g−1 at 0.1, 0.2, 0.3, 0.5, 1.0 A g−1, respectively | [60] |
| TiO2/RGO | 354.3, 282.2, 220.9, 151.7 and 107.1 mA h g−1 at 0.05, 0.1, 0.2, 0.5 and 1 A g−1, respectively. | [61] |
| MOF-NOC@L-TiO2 | 555, 355, 253, 179, 130, 87 and 73 mA h g−1 at 0.05, 0.1, 0.2, 0.5, 1, 2 and 3 A g−1, respectively | [63] |
| y-Bi2O3@TiO2 | 373.9, 347.7, 299.6, 252.8, 207.7 and 139.1 mA h g−1 at 0.1, 0.2, 0.3, 0.5, 1.0 and 2.0 A g−1, respectively | [69] |
| Bi@Void@TiO2 CNF | 388.8, 301.9, 281.4, 230.5, 191.5, 159.7, 90 and 64.9 mA h g−1 at 0.05, 0.1, 0.2, 0.5, 1, 2, 5 and 10 A g−1, respectively | [75] |
| TiO2@A-MoS3 @NC | 463, 398, 333, 268, 189 and 104 mA h g−1 at 0.1, 0.2, 0.5, 1, 2 and 5 A g−1, respectively. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Zhao, Y.; Zhu, X.; Yang, K.; Li, M. Recent Advances and Challenges in Ti-Based Oxide Anodes for Superior Potassium Storage. Nanomaterials 2023, 13, 2539. https://doi.org/10.3390/nano13182539
Deng Q, Zhao Y, Zhu X, Yang K, Li M. Recent Advances and Challenges in Ti-Based Oxide Anodes for Superior Potassium Storage. Nanomaterials. 2023; 13(18):2539. https://doi.org/10.3390/nano13182539
Chicago/Turabian StyleDeng, Qinglin, Yang Zhao, Xuhui Zhu, Kaishuai Yang, and Mai Li. 2023. "Recent Advances and Challenges in Ti-Based Oxide Anodes for Superior Potassium Storage" Nanomaterials 13, no. 18: 2539. https://doi.org/10.3390/nano13182539
APA StyleDeng, Q., Zhao, Y., Zhu, X., Yang, K., & Li, M. (2023). Recent Advances and Challenges in Ti-Based Oxide Anodes for Superior Potassium Storage. Nanomaterials, 13(18), 2539. https://doi.org/10.3390/nano13182539








