Effect of Laser-Textured Cu Foil with Deep Ablation on Si Anode Performance in Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser Texturing Process
2.2. Characterization
2.3. Fabrication of the Si Anode
2.4. Electrochemical Tests
3. Results and Discussion
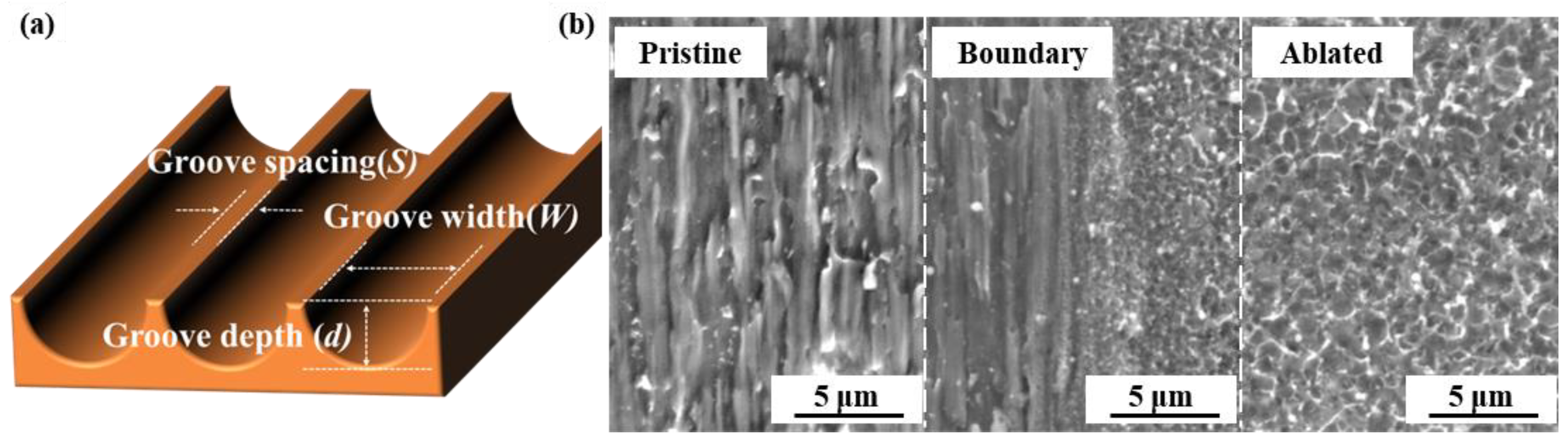
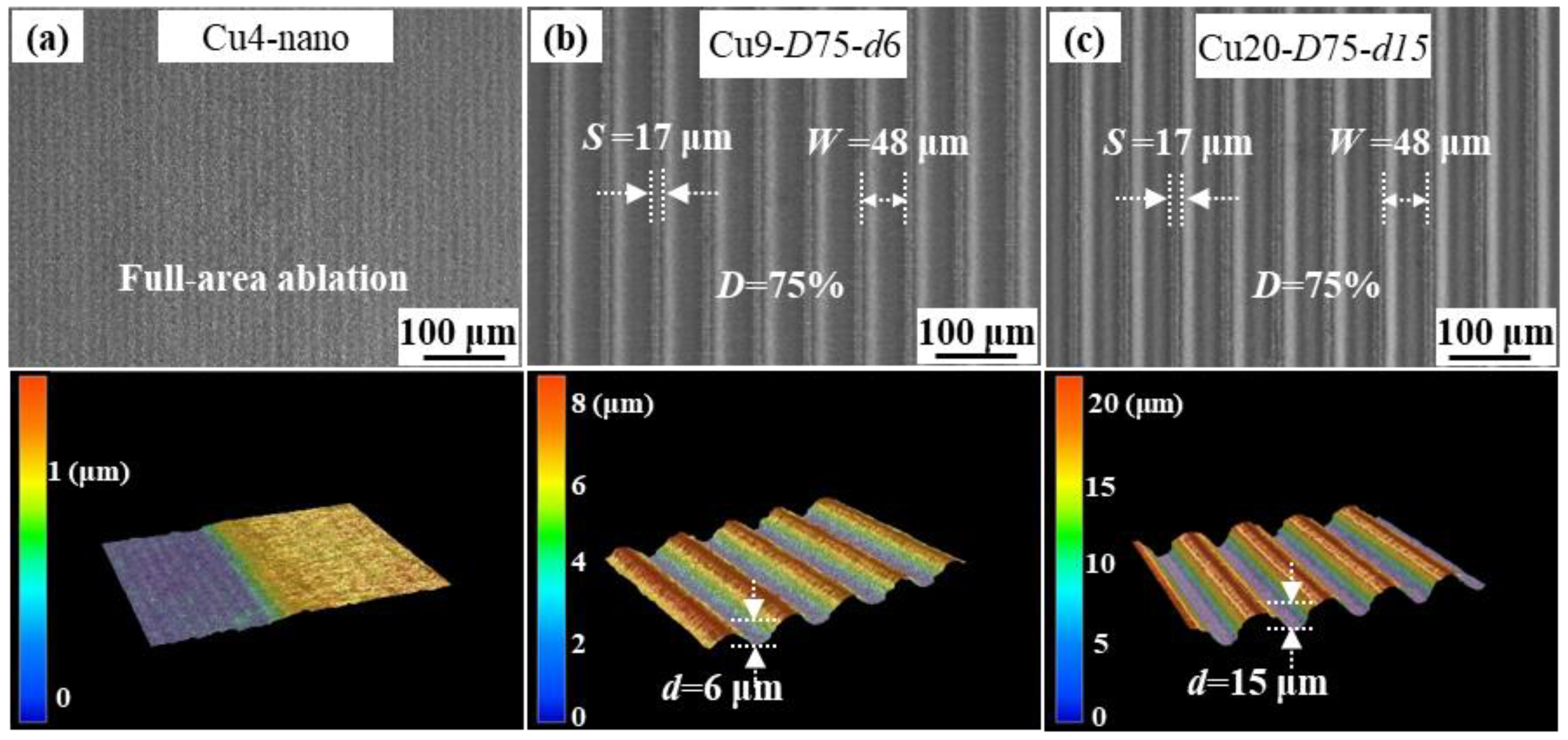
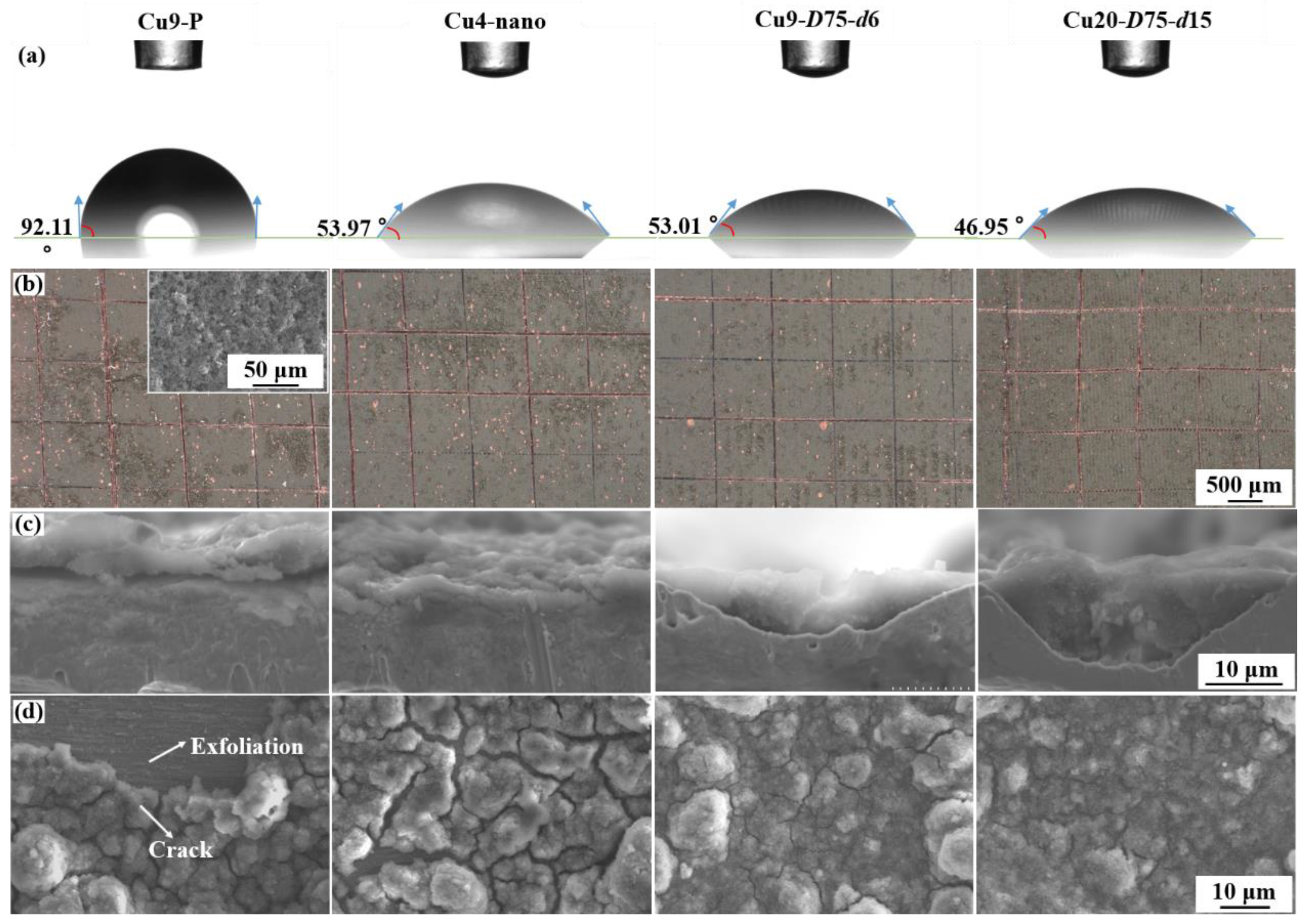
3.1. Characterization of Textured Cu Foil
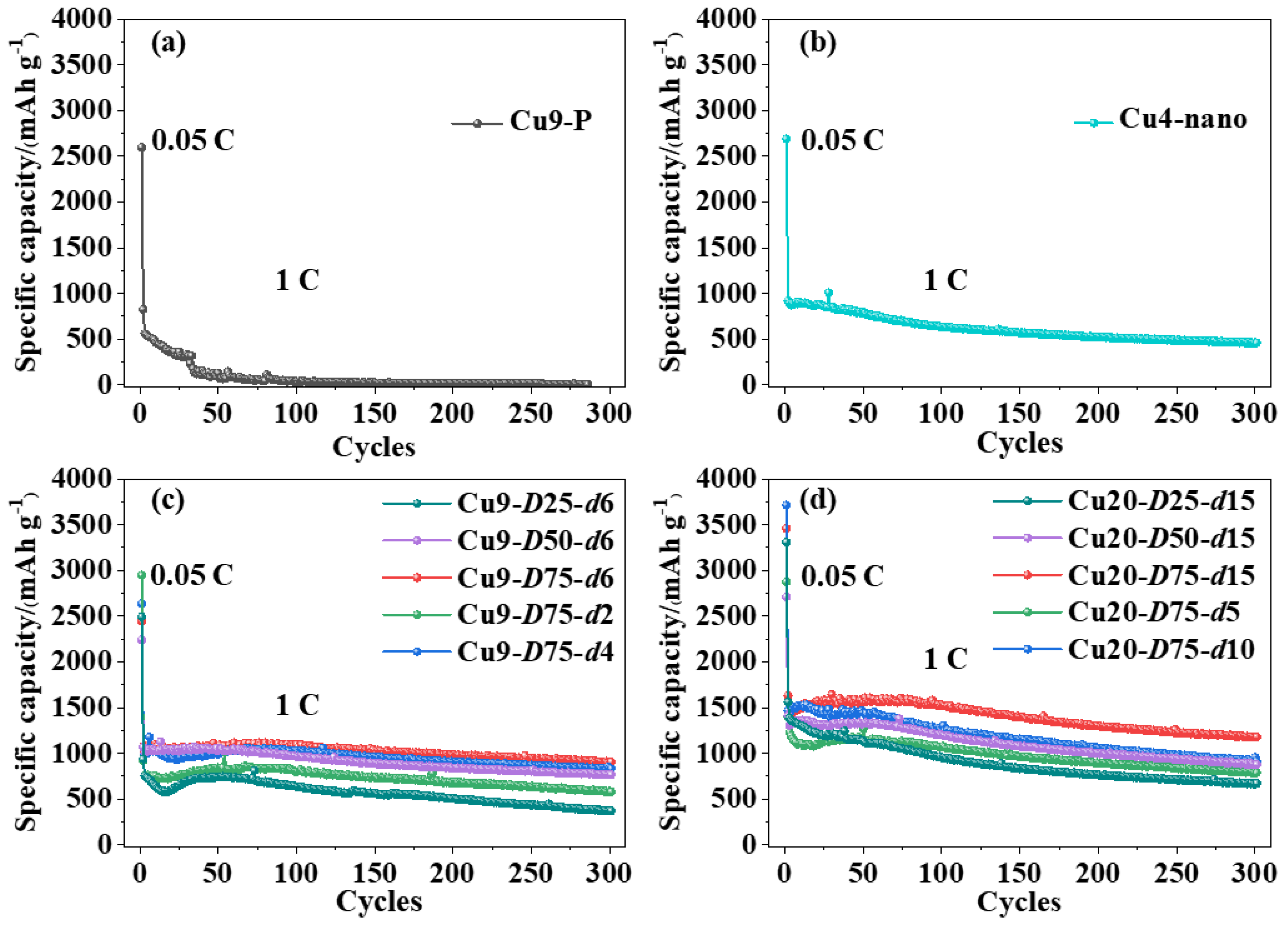
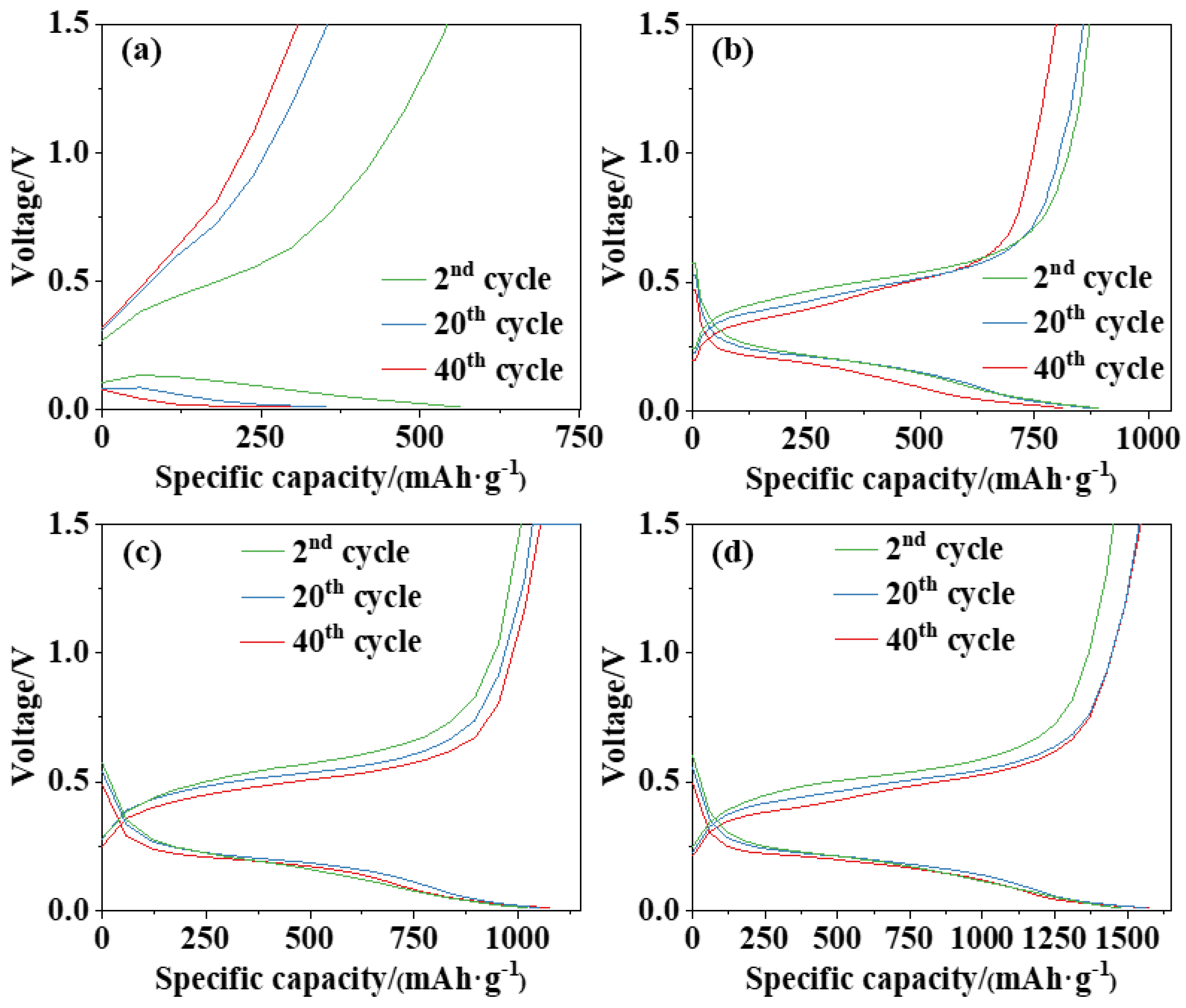
3.2. Electrochemical Performance of Si Anodes
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, L.; Zheng, M.; Wang, J.; Li, S.; Xu, J.; Xiao, R.; Huang, T. Alloy-type lithium anode prepared by laser microcladding and dealloying for improved cycling/rate performance. ACS Nano 2022, 16, 17220–17228. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yue, M.; Wang, S.; Zhao, Y.; Zhang, J. A review of performance attenuation and mitigation strategies of lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2107769. [Google Scholar] [CrossRef]
- Cao, L.; Huang, T.; Zhang, Q.; Cui, M.; Xu, J.; Xiao, R. Porous Si/Cu anode with high initial coulombic efficiency and volumetric capacity by comprehensive utilization of laser additive manufacturing-chemical dealloying. ACS Appl. Mater. Interfaces 2020, 12, 57071–57078. [Google Scholar] [CrossRef]
- Cao, L.; Xiao, R.; Wang, J.; Li, S.; Xu, J.; Huang, T. Recycling waste Al-Si alloy for micrometer-sized spongy Si with high areal/volumetric capacity and stability in lithium-ion batteries. ACS Sustain. Chem. Eng. 2022, 10, 8143–8150. [Google Scholar] [CrossRef]
- Reyter, D.; Rousselot, S.; Mazouzi, D.; Gauthier, M.; Moreau, P.; Lestriez, B.; Guyomard, D.; Roué, L. An electrochemically roughened Cu current collector for Si-based electrode in Li-ion batteries. J. Power Sources 2013, 239, 308–314. [Google Scholar] [CrossRef]
- Wang, S.E.; Kim, D.; Kim, M.J.; Kim, J.H.; Kang, Y.C.; Roh, K.C.; Choi, J.; Lee, H.W.; Jung, D.S. Achieving cycling stability in anode of lithium-ion batteries with silicon-embedded titanium oxynitride microsphere. Nanomaterials 2023, 13, 132. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Yue, H.; Wang, L.; Yin, Y.; Zhang, X.; Zhang, C.; Yang, S. Anti-cognition in lithium-ion battery electrolytes: Comparable performance with degraded electrolyte. J. Power Sources 2020, 464, 228241. [Google Scholar] [CrossRef]
- Tsao, C.H.; Yang, T.K.; Chen, K.Y.; Fang, C.E.; Ueda, M.; Richter, F.H.; Janek, J.; Chiu, C.C.; Kuo, P.L. Comparing the ion-conducting polymers with sulfonate and ether moieties as cathode binders for high-power lithium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 9846–9855. [Google Scholar] [CrossRef]
- Zheng, Y.; Yin, D.; Seifert, H.J.; Pfleging, W. Investigation of fast-charging and degradation processes in 3d silicon-graphite anodes. Nanomaterials 2022, 12, 140. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Zhang, X.; Ke, Y.; Qiu, Z.; Luo, J.; Tang, Y.; Wang, C.; Yuan, Y.; Huang, Y. A review on structuralized current collectors for high-performance lithium-ion battery anodes. Appl. Energy 2020, 276, 115464. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, S.H.; Kim, M.C.; So, J.Y.; Han, S.B.; Kwak, D.H.; Bae, W.G.; Park, K.W. Micro-patterned 3D Si electrodes fabricated using an imprinting process for high-performance lithium-ion batteries. J. Appl. Electrochem. 2018, 48, 1057–1068. [Google Scholar] [CrossRef]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2021, 485, 229321. [Google Scholar] [CrossRef]
- Kumar, V.; Verma, R.; Kango, S.; Sharma, V.S. Recent progresses and applications in laser-based surface texturing systems. Mater. Today Commun. 2021, 26, 101736. [Google Scholar] [CrossRef]
- Petare, A.C.; Mishra, A.; Palani, I.A.; Jain, N.K. Study of laser texturing assisted abrasive flow finishing for enhancing surface quality and microgeometry of spur gears. Int. J. Adv. Manuf. Technol. 2019, 101, 785–799. [Google Scholar] [CrossRef]
- Allahyari, E.; Nivas, J.J.; Oscurato, S.L.; Salvatore, M.; Ausanio, G.; Vecchione, A.; Fittipaldi, R.; Maddalena, P.; Bruzzese, R.; Amoruso, S. Laser surface texturing of copper and variation of the wetting response with the laser pulse fluence. Appl. Surf. Sci. 2019, 470, 817–824. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Z.; Zhong, J.; Wang, T.; Wang, L.; Xu, H.; Cao, J.; Li, J.; Zhang, G.; Fei, H.; et al. Hierarchically micro/nanostructured current collectors induced by ultrafast femtosecond laser strategy for high-performance lithium-ion batteries. Energy Environ. Mater. 2022, 5, 969–976. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, Y.; Trifonova, A.; Pfleging, W. Laser structured Cu foil for high-performance lithium-ion battery anodes. J. Appl. Electrochem. 2017, 47, 829–837. [Google Scholar] [CrossRef]
- Li, Q.; Sun, X.; Zhao, W.; Hou, X.; Zhang, Y.; Zhao, F.; Li, X.; Mei, X. Processing of a large-scale microporous group on copper foil current collectors for lithium batteries using femtosecond laser. Adv. Eng. Mater. 2020, 22, 2000710. [Google Scholar] [CrossRef]
- Anoop, K.K.; Fittipaldi, R.; Rubano, A.; Wang, X.; Paparo, D.; Vecchione, A.; Marrucci, L.; Bruzzese, R.; Amoruso, S. Direct femtosecond laser ablation of copper with an optical vortex beam. J. Appl. Phys. 2014, 116, 113102. [Google Scholar] [CrossRef]
- Choudhury, R.; Wild, J.; Yang, Y. Engineering current collectors for batteries with high specific energy. Joule 2021, 5, 1301–1305. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational design on materials for developing next generation lithium-ion secondary battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef]
- Li, S.; He, Q.; Chen, K.; Huang, S.; Wu, F.; Wang, G.; Sun, W.; Fu, S.; Feng, X.; Zhou, Y.; et al. Facile chemical fabrication of a three-dimensional copper current collector for stable lithium metal anodes. J. Electrochem. Soc. 2021, 168, 070502. [Google Scholar] [CrossRef]
- Cao, F.F.; Deng, J.W.; Xin, S.; Ji, H.X.; Schmidt, O.G.; Wan, L.J.; Guo, Y.G. Cu-Si nanocable arrays as high-rate anode materials for lithium-ion batteries. Adv. Mater. 2011, 23, 4415–4420. [Google Scholar] [CrossRef] [PubMed]
- Baldan, A. Adhesion phenomena in bonded joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- Pfleging, W. Recent progress in laser texturing of battery materials: A review of tuning electrochemical performances, related material development, and prospects for large-scale manufacturing. Int. J. Extrem. Manuf. 2021, 3, 012002. [Google Scholar] [CrossRef]
- Jeon, H.; Cho, I.; Jo, H.; Kim, K.; Ryou, M.H.; Lee, Y.M. Highly rough copper current collector: Improving adhesion property between a silicon electrode and current collector for flexible lithium-ion batteries. RSC Adv. 2017, 7, 35681–35686. [Google Scholar] [CrossRef]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]
- So, J.Y.; Moon, S.H.; Kim, M.C.; Kim, S.J.; Han, S.B.; Lee, C.H.; Kim, J.E.; Kim, H.J.; Jun, J.; Song, K.Y.; et al. Stress dispersed cu metal anode by laser multiscale patterning for lithium-ion batteries with high capacity. Metals 2018, 8, 410. [Google Scholar] [CrossRef]


| Thickness of Cu Foil (μm) | Current Collector | D (%) | d (μm) |
|---|---|---|---|
| 4 | Cu4-nano | - | - |
| 9 | Cu9-P | - | - |
| Cu9-D25-d6 | 25% | 6 | |
| Cu9-D50-d6 | 50% | 6 | |
| Cu9-D75-d6 | 75% | 6 | |
| Cu9-D75-d2 | 75% | 2 | |
| Cu9-D75-d4 | 75% | 4 | |
| 20 | Cu20-D25-d15 | 25% | 15 |
| Cu20-D50-d15 | 50% | 15 | |
| Cu20-D75-d15 | 75% | 15 | |
| Cu20-D75-d5 | 75% | 5 | |
| Cu20-D75-d10 | 75% | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Cao, L.; Li, S.; Xu, J.; Xiao, R.; Huang, T. Effect of Laser-Textured Cu Foil with Deep Ablation on Si Anode Performance in Li-Ion Batteries. Nanomaterials 2023, 13, 2534. https://doi.org/10.3390/nano13182534
Wang J, Cao L, Li S, Xu J, Xiao R, Huang T. Effect of Laser-Textured Cu Foil with Deep Ablation on Si Anode Performance in Li-Ion Batteries. Nanomaterials. 2023; 13(18):2534. https://doi.org/10.3390/nano13182534
Chicago/Turabian StyleWang, Jingbo, Li Cao, Songyuan Li, Jiejie Xu, Rongshi Xiao, and Ting Huang. 2023. "Effect of Laser-Textured Cu Foil with Deep Ablation on Si Anode Performance in Li-Ion Batteries" Nanomaterials 13, no. 18: 2534. https://doi.org/10.3390/nano13182534









