Sculpting Windows onto AuAg Hollow Cubic Nanocrystals
Abstract
:1. Introduction
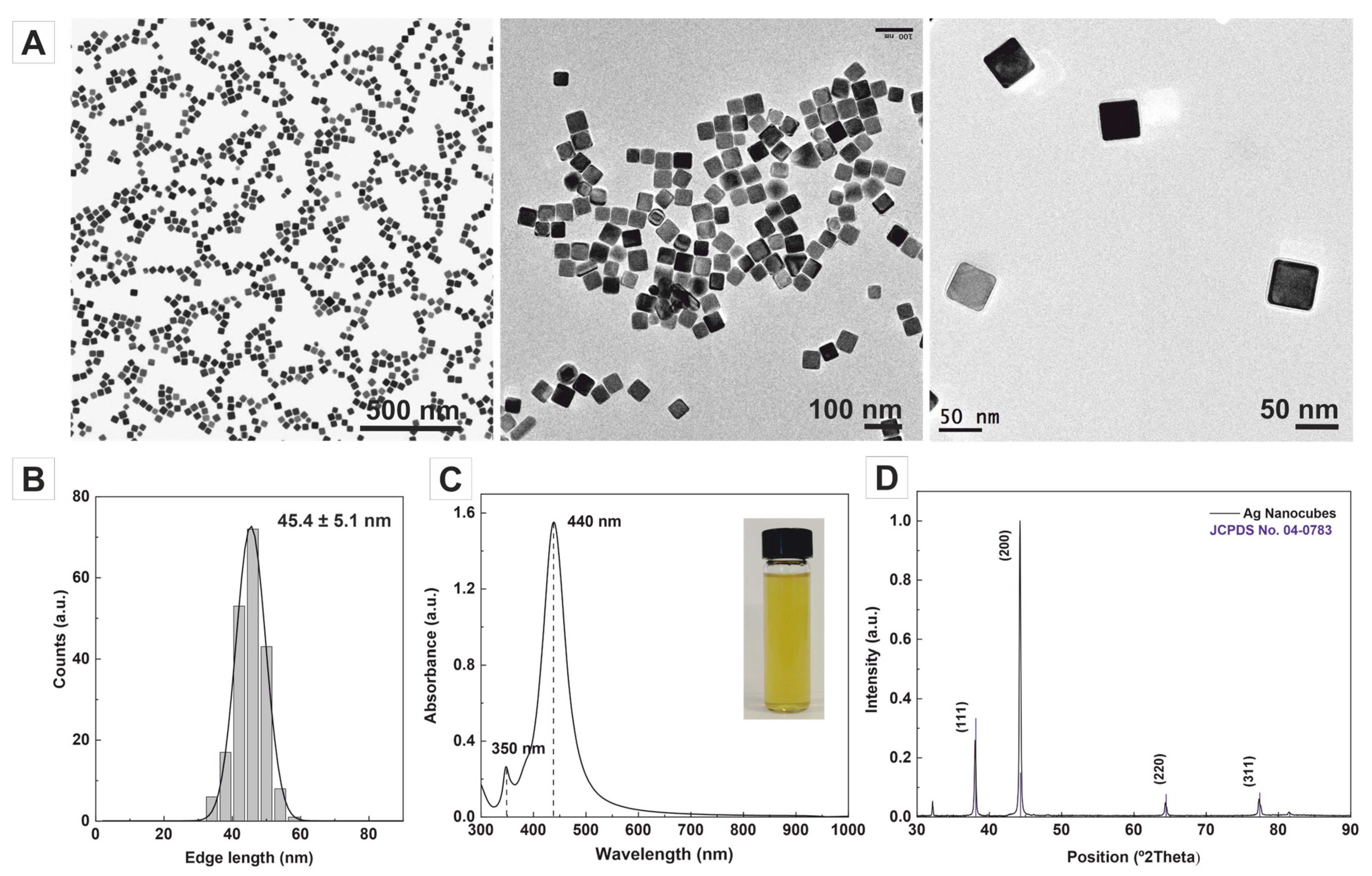
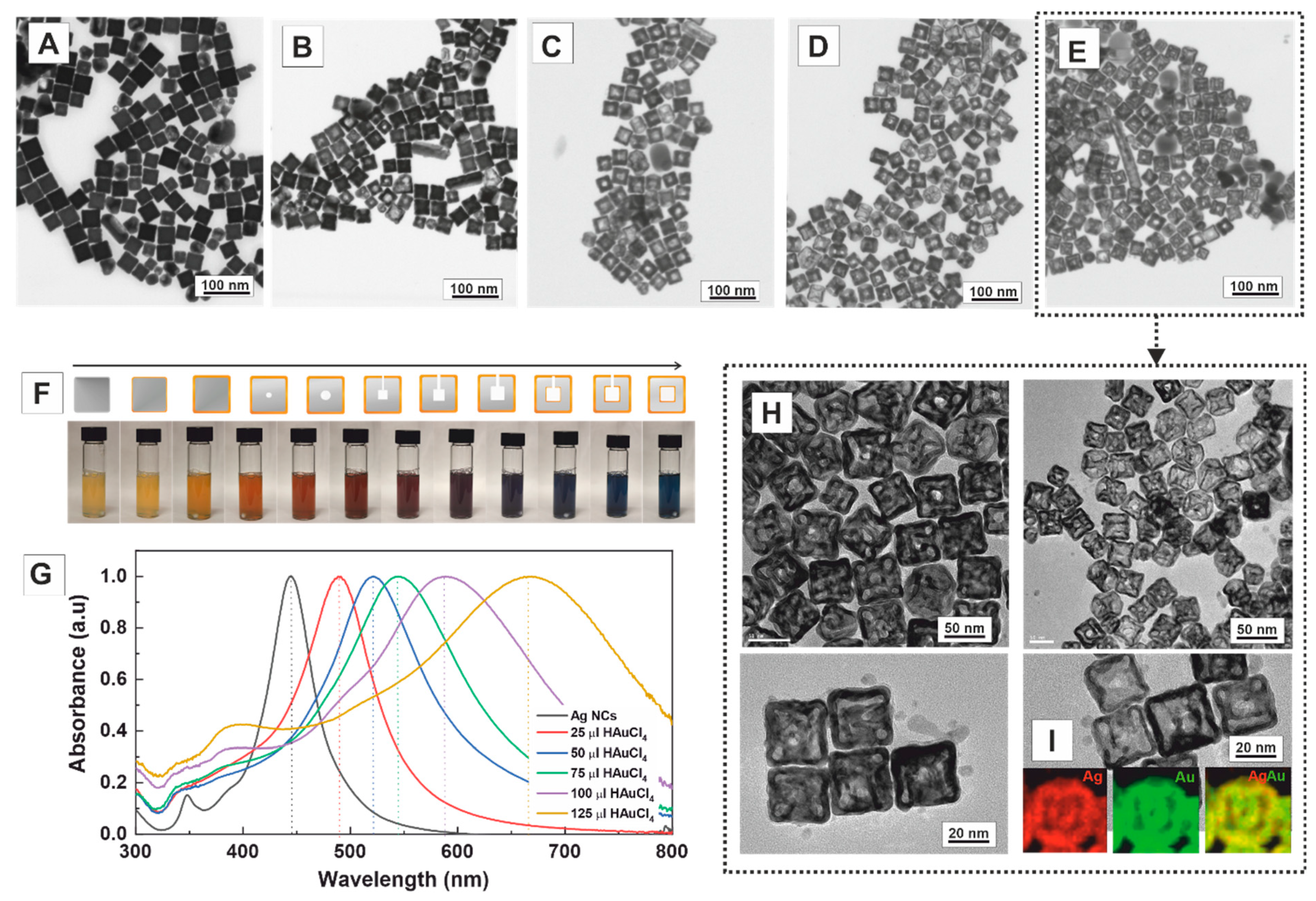
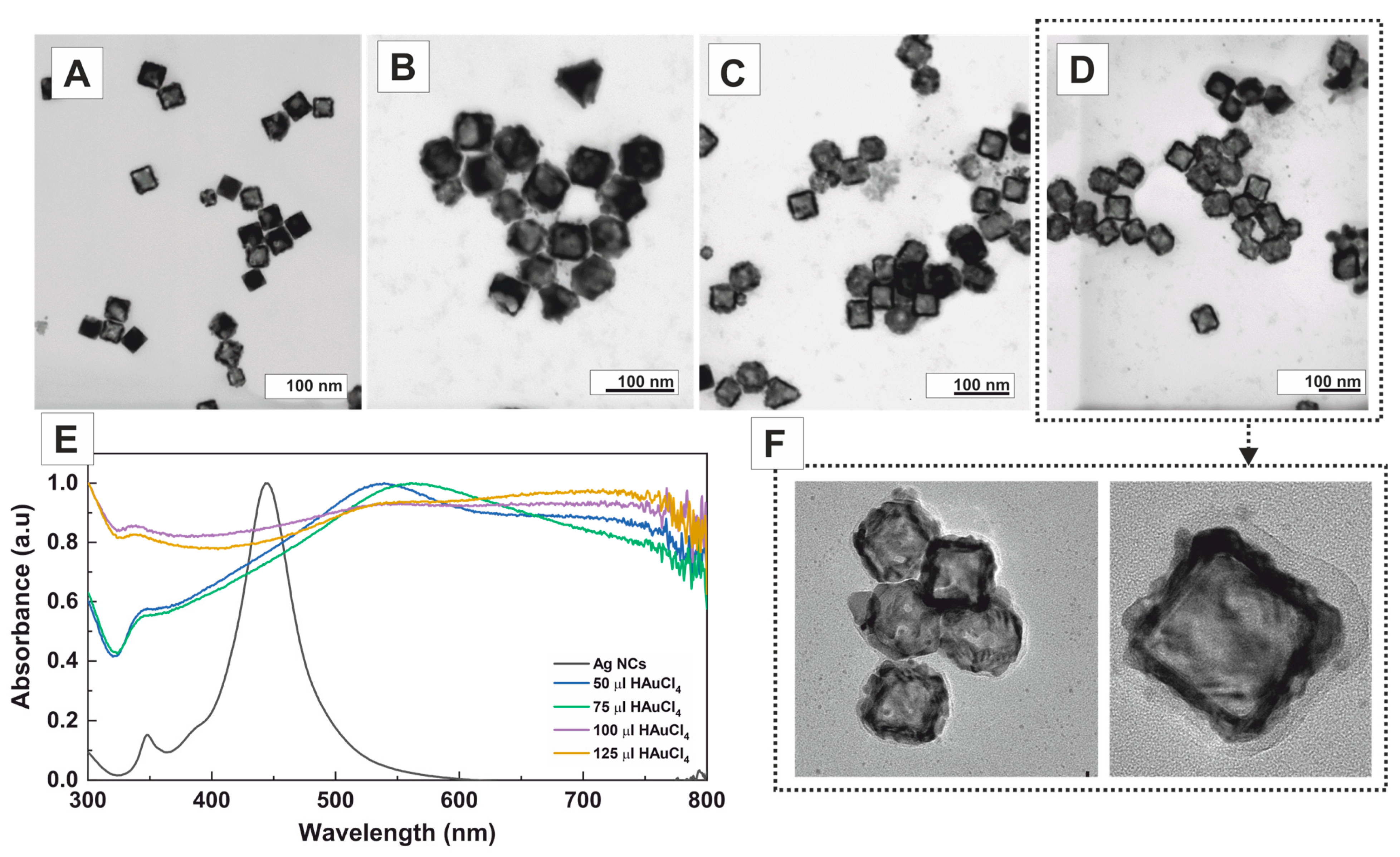
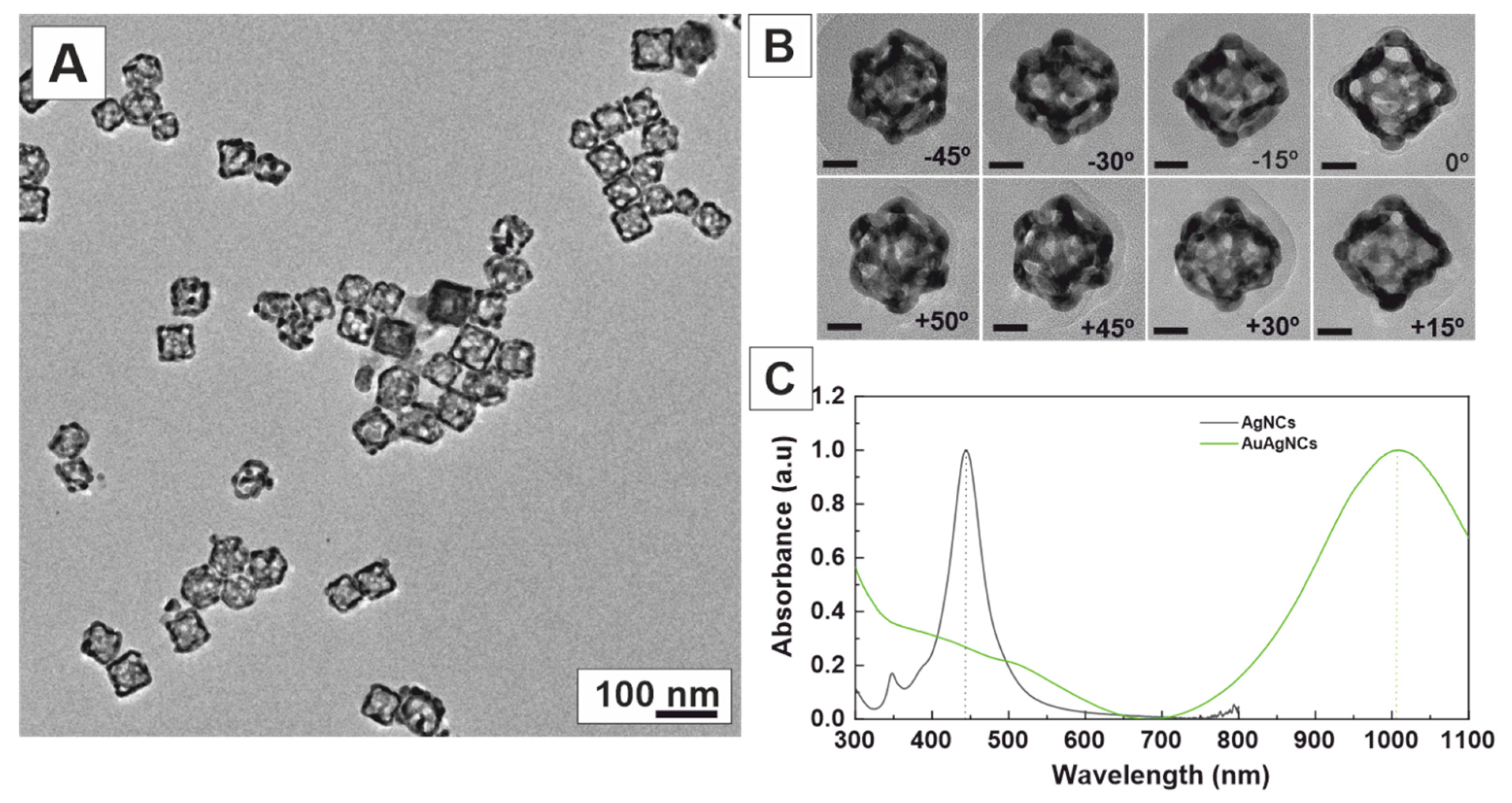
2. Results
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Yang, J.; Lu, X. Tailoring galvanic replacement reaction for the preparation of Pt/Ag bimetallic hollow nanostructures with controlled number of voids. ACS Nano 2012, 6, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Saira, F.; El-Sayed, M.A. Experimental evidence for the nanocage effect in catalysis with hollow nanoparticles. Nano Lett. 2010, 10, 3764–3769. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Garlyyev, B.; El-Sayed, M.A. Controlling the Catalytic Efficiency on the Surface of Hollow Gold Nanoparticles by Introducing an Inner Thin Layer of Platinum or Palladium. J. Phys. Chem. Lett. 2014, 5, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Roling, L.T.; Wang, X.; Vara, M.; Chi, M.; Liu, J.; Choi, S.I.; Park, J.; Herron, J.A.; Xie, Z.; et al. NANOCATALYSTS: Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416. [Google Scholar] [CrossRef]
- Skrabalak, S.E.; Chen, J.; Sun, Y.; Lu, X.; Au, L.; Cobley, C.M.; Xia, Y. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Luehmann, H.; Xia, X.; Wan, D.; Cutler, C.; Xia, Y. Radioluminescent gold nanocages with controlled radioactivity for real-time in vivo imaging. Nano Lett. 2013, 13, 581–585. [Google Scholar] [CrossRef]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G.; et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; O’Neil, D.; El-Sayed, M.A. Hollow and Solid Metallic Nanoparticles in Sensing and in Nanocatalysis. Chem. Mater. 2013, 26, 44–58. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Lin, H.Y.; Hou, Y.T.; Yang, L.C.; Sun, A.Y.; Liu, J.Y.; Chang, C.W.; Wan, D. Near-IR-Absorbing Gold Nanoframes with Enhanced Physiological Stability and Improved Biocompatibility for In Vivo Biomedical Applications. ACS Appl. Mater. Interfaces 2017, 9, 3873–3884. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Arbiol, J.; Puntes, V.F. Carving at the nanoscale: Sequential galvanic exchange and Kirkendall growth at room temperature. Science 2011, 334, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Lu, X.; Xia, Y. A Comparative Study of Galvanic Replacement Reactions Involving Ag Nanocubes and AuCl2 or AuCl4. Adv. Mater. 2008, 20, 2517–2522. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium. J. Am. Chem. Soc. 2004, 126, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Cobley, C.M.; Xia, Y. Engineering the Properties of Metal Nanostructures via Galvanic Replacement Reactions. Mater. Sci. Eng. R Rep. 2010, 70, 44–62. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Alloying and Dealloying Processes Involved in the Preparation of Metal Nanoshells through a Galvanic Replacement Reaction. Nano Lett. 2003, 3, 1569–1572. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th anniversary article: Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef]
- Hubert, C.; Chomette, C.; Desert, A.; Madeira, A.; Perro, A.; Florea, I.; Ihiawakrim, D.; Ersen, O.; Lombardi, A.; Pertreux, E.; et al. Versatile template-directed synthesis of gold nanocages with a predefined number of windows. Nanoscale Horiz. 2021, 6, 311–318. [Google Scholar] [CrossRef]
- Parak, W.J. Materials science. Complex colloidal assembly. Science 2011, 334, 1359–1360. [Google Scholar] [CrossRef]
- Khalavka, Y.; Becker, J.; Sonnichsen, C. Synthesis of rod-shaped gold nanorattles with improved plasmon sensitivity and catalytic activity. J. Am. Chem. Soc. 2009, 131, 1871–1875. [Google Scholar] [CrossRef]
- Xiong, W.; Sikdar, D.; Walsh, M.; Si, K.J.; Tang, Y.; Chen, Y.; Mazid, R.; Weyland, M.; Rukhlenko, I.D.; Etheridge, J.; et al. Single-crystal caged gold nanorods with tunable broadband plasmon resonances. Chem. Commun. (Camb.) 2013, 49, 9630–9632. [Google Scholar] [CrossRef]
- González, E.; Merkoçi, F.; Arenal, R.; Arbiol, J.; Esteve, J.; Bastús, N.G.; Puntes, V. Enhanced reactivity of high-index surface platinum hollow nanocrystals. J. Mater. Chem. A 2016, 4, 200–208. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Fu, Z.W.; Qin, D. Transformation of Ag nanocubes into Ag-Au hollow nanostructures with enriched Ag contents to improve SERS activity and chemical stability. ACS Appl. Mater. Interfaces 2014, 6, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- McEachran, M.; Keogh, D.; Pietrobon, B.; Cathcart, N.; Gourevich, I.; Coombs, N.; Kitaev, V. Ultrathin gold nanoframes through surfactant-free templating of faceted pentagonal silver nanoparticles. J. Am. Chem. Soc. 2011, 133, 8066–8069. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Gandra, N.; Singamaneni, S. Monitoring controlled release of payload from gold nanocages using surface enhanced Raman scattering. ACS Nano 2013, 7, 4252–4260. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; McLellan, J.M.; Siekkinen, A.; Xiong, Y.; Li, Z.Y.; Xia, Y. Facile synthesis of gold-silver nanocages with controllable pores on the surface. J. Am. Chem. Soc. 2006, 128, 14776–14777. [Google Scholar] [CrossRef]
- Sun, X.; Kim, J.; Gilroy, K.D.; Liu, J.; Konig, T.A.; Qin, D. Gold-Based Cubic Nanoboxes with Well-Defined Openings at the Corners and Ultrathin Walls Less Than Two Nanometers Thick. ACS Nano 2016, 10, 8019–8025. [Google Scholar] [CrossRef]
- Lu, F.; Xin, H.; Xia, W.; Liu, M.; Zhang, Y.; Cai, W.; Gang, O. Tailoring Surface Opening of Hollow Nanocubes and Their Application as Nanocargo Carriers. ACS Cent. Sci. 2018, 4, 1742–1750. [Google Scholar] [CrossRef]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef]
- Merkoçi, F.; Patarroyo, J.; Russo, L.; Piella, J.; Genç, A.; Arbiol, J.; Bastús, N.G.; Puntes, V. Understanding galvanic replacement reactions: The case of Pt and Ag. Mater. Today Adv. 2020, 5, 100037. [Google Scholar] [CrossRef]
- Peng, X.; Manna, L.; Yang, W.; Wickham, J.; Scher, E.; Kadavanich, A.; Alivisatos, A.P. Shape control of CdSe nanocrystals. Nature 2000, 404, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Korgel, B.A. The importance of the CTAB surfactant on the colloidal seed-mediated synthesis of gold nanorods. Langmuir 2008, 24, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Moriones, O.H.; Bastus, N.G.; Puntes, V. Dynamic Equilibrium in the Cetyltrimethylammonium Bromide-Au Nanoparticle Bilayer, and the Consequent Impact on the Formation of the Nanoparticle Protein Corona. Bioconjugate Chem. 2019, 30, 2917–2930. [Google Scholar] [CrossRef]
- Patarroyo, J.; Genc, A.; Arbiol, J.; Bastus, N.G.; Puntes, V. One-pot polyol synthesis of highly monodisperse short green silver nanorods. Chem. Commun. 2016, 52, 10960–10963. [Google Scholar] [CrossRef] [PubMed]
- Near, R.; Hayden, S.; El-Sayed, M. Extinction vs. Absorption: Which Is the Indicator of Plasmonic Field Strength for Silver Nanocubes? J. Phys. Chem. C 2012, 116, 23019–23026. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Z.-Y.; Liu, Y.; Xia, Y. Quantitative Analysis of Dipole and Quadrupole Excitation in the Surface Plasmon Resonance of Metal Nanoparticles. J. Phys. Chem. C 2008, 112, 20233–20240. [Google Scholar] [CrossRef]
- Zhou, S.; Mesina, D.S.; Organt, M.A.; Yang, T.-H.; Yang, X.; Huo, D.; Zhao, M.; Xia, Y. Site-selective growth of Ag nanocubes for sharpening their corners and edges, followed by elongation into nanobars through symmetry reduction. J. Mater. Chem. C 2018, 6, 1384–1392. [Google Scholar] [CrossRef]
- Bansal, V.; Li, V.; O’Mullane, A.P.; Bhargava, S.K. Shape dependent electrocatalytic behaviour of silver nanoparticles. CrystEngComm 2010, 12, 4280–4286. [Google Scholar] [CrossRef]
- Park, T.-H.; Lee, H.; Lee, J.; Jang, D.-J. Morphology evolution of Ag/Au nanocomposites via temperature-controlled galvanic exchange to enhance catalytic activity. RSC Adv. 2017, 7, 7718–7724. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Fu, Z.W.; Qin, D. Galvanic replacement-free deposition of Au on Ag for core-shell nanocubes with enhanced chemical stability and SERS activity. J. Am. Chem. Soc. 2014, 136, 8153–8156. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, J.; Lee, J.Y.; Zhang, J.; Boothroyd, C. Synthesis of Ag@AgAu metal core/alloy shell bimetallic nanoparticles with tunable shell compositions by a galvanic replacement reaction. Small 2008, 4, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Bastus, N.G.; Piella, J.; Puntes, V. Quantifying the Sensitivity of Multipolar (Dipolar, Quadrupolar, and Octapolar) Surface Plasmon Resonances in Silver Nanoparticles: The Effect of Size, Composition, and Surface Coating. Langmuir 2016, 32, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Genç, A.; Patarroyo, J.; Sancho-Parramon, J.; Bastús Neus, G.; Puntes, V.; Arbiol, J. Hollow metal nanostructures for enhanced plasmonics: Synthesis, local plasmonic properties and applications. Nanophotonics 2017, 6, 193. [Google Scholar] [CrossRef]
- Jing, H.; Wang, H. Structural Evolution of Ag–Pd Bimetallic Nanoparticles through Controlled Galvanic Replacement: Effects of Mild Reducing Agents. Chem. Mater. 2015, 27, 2172–2180. [Google Scholar] [CrossRef]
- Xiong, Y.; Washio, I.; Chen, J.; Cai, H.; Li, Z.Y.; Xia, Y. Poly(vinyl pyrrolidone): A dual functional reductant and stabilizer for the facile synthesis of noble metal nanoplates in aqueous solutions. Langmuir 2006, 22, 8563–8570. [Google Scholar] [CrossRef]
- Russo, L.; Merkoçi, F.; Patarroyo, J.; Piella, J.; Merkoçi, A.; Bastús, N.G.; Puntes, V. Time- and Size-Resolved Plasmonic Evolution with nm Resolution of Galvanic Replacement Reaction in AuAg Nanoshells Synthesis. Chem. Mater. 2018, 30, 5098–5107. [Google Scholar] [CrossRef]
- Chen, J.; Wiley, B.; Li, Z.Y.; Campbell, D.; Saeki, F.; Cang, H.; Au, L.; Lee, J.; Li, X.; Xia, Y. Gold Nanocages: Engineering Their Structure for Biomedical Applications. Adv. Mater. 2005, 17, 2255–2261. [Google Scholar] [CrossRef]
- Yang, X.; Skrabalak, S.E.; Li, Z.Y.; Xia, Y.; Wang, L.V. Photoacoustic tomography of a rat cerebral cortex in vivo with au nanocages as an optical contrast agent. Nano Lett. 2007, 7, 3798–3802. [Google Scholar] [CrossRef]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.Y.; Zhang, H.; Xia, Y.; Li, X. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007, 7, 1318–1322. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patarroyo, J.; Bastús, N.G.; Puntes, V. Sculpting Windows onto AuAg Hollow Cubic Nanocrystals. Nanomaterials 2023, 13, 2590. https://doi.org/10.3390/nano13182590
Patarroyo J, Bastús NG, Puntes V. Sculpting Windows onto AuAg Hollow Cubic Nanocrystals. Nanomaterials. 2023; 13(18):2590. https://doi.org/10.3390/nano13182590
Chicago/Turabian StylePatarroyo, Javier, Neus G. Bastús, and Victor Puntes. 2023. "Sculpting Windows onto AuAg Hollow Cubic Nanocrystals" Nanomaterials 13, no. 18: 2590. https://doi.org/10.3390/nano13182590




