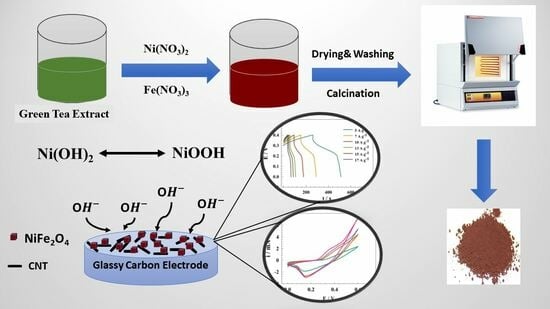
Green Synthesis of NiFe2O4 Nano-Spinel Oxide-Decorated Carbon Nanotubes for Efficient Capacitive Performance—Effect of Electrolyte Concentration
Abstract
:1. Introduction
2. Experimental Section
2.1. Green Synthesis of NiFe2O4
2.2. Electrode Preparation and Electrochemical Measurements
3. Results and Discussion
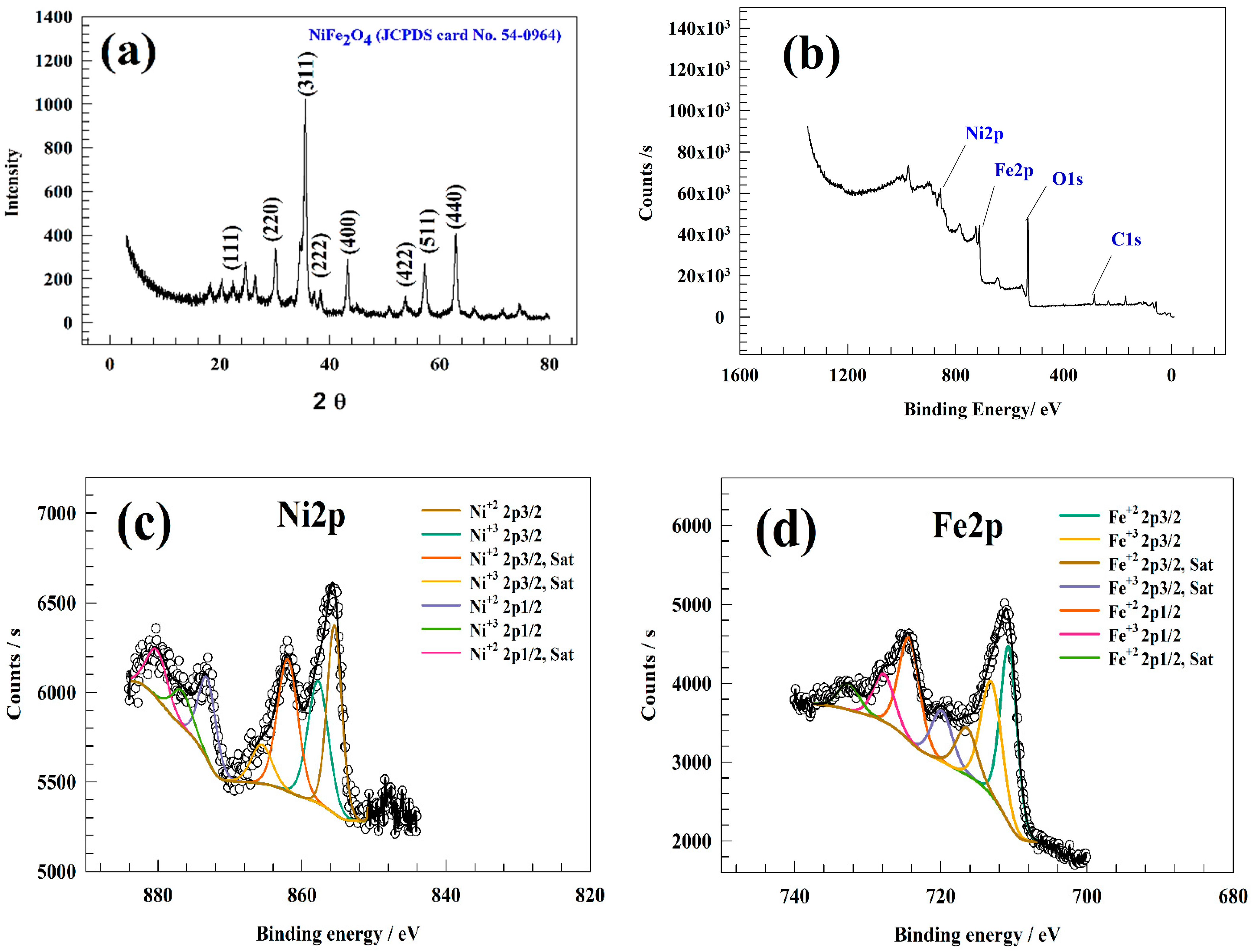
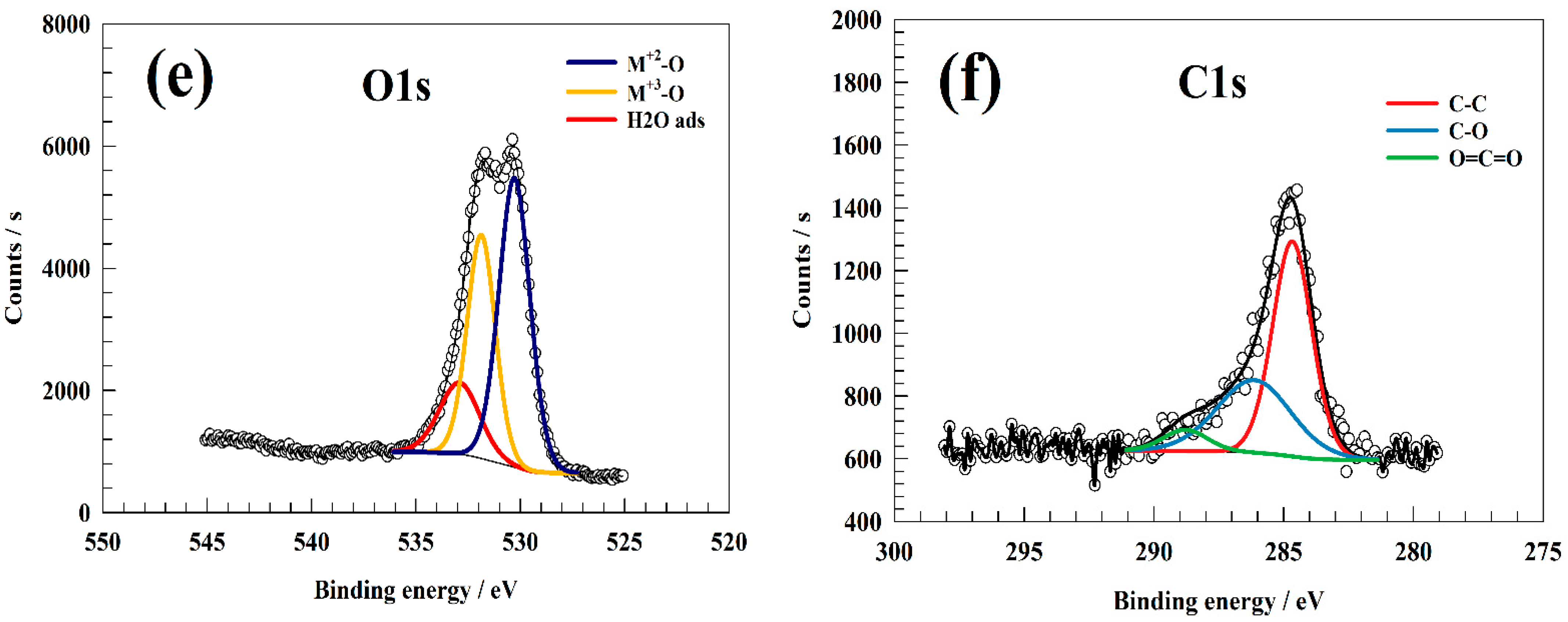
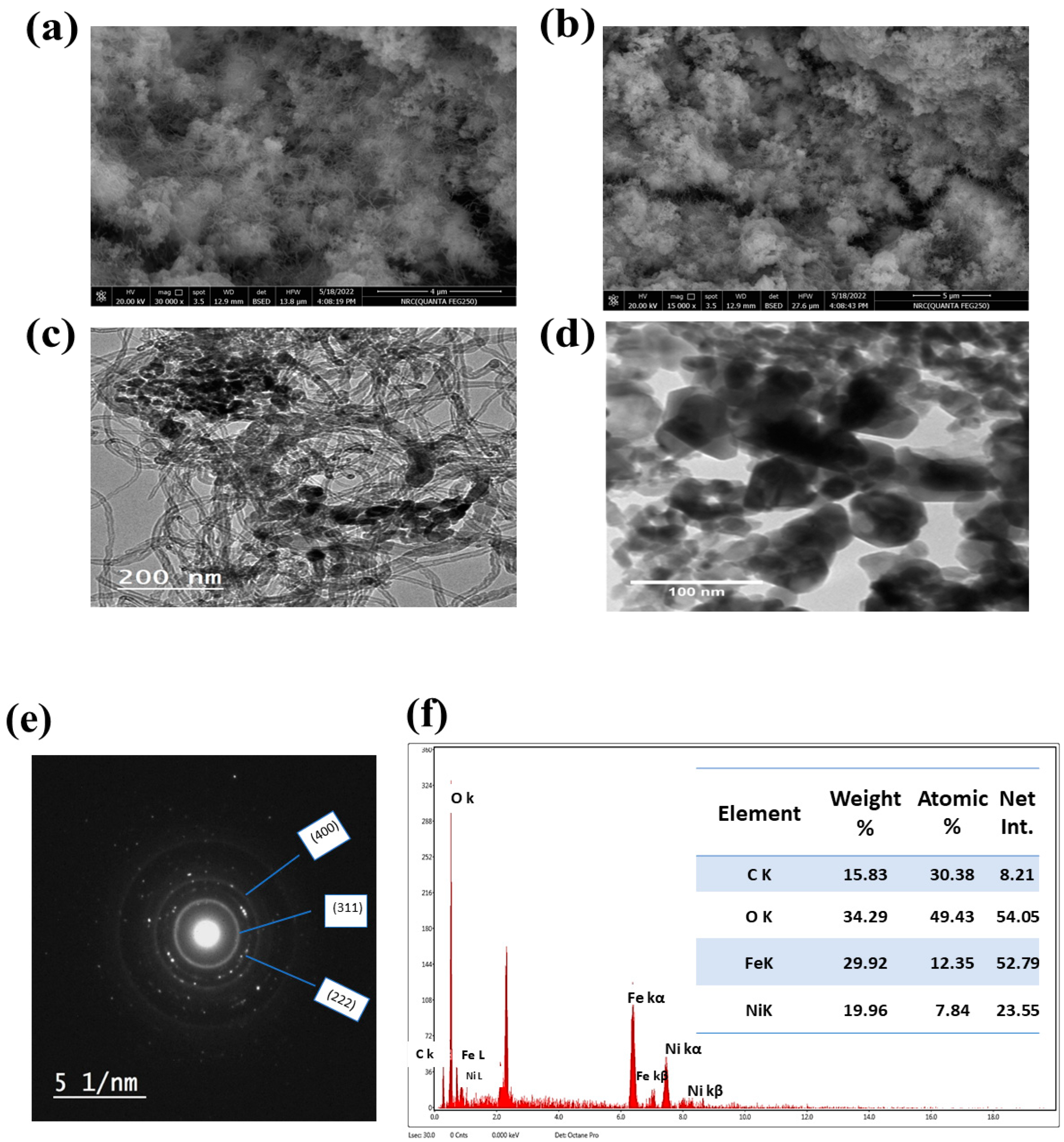
3.1. Material Characterization
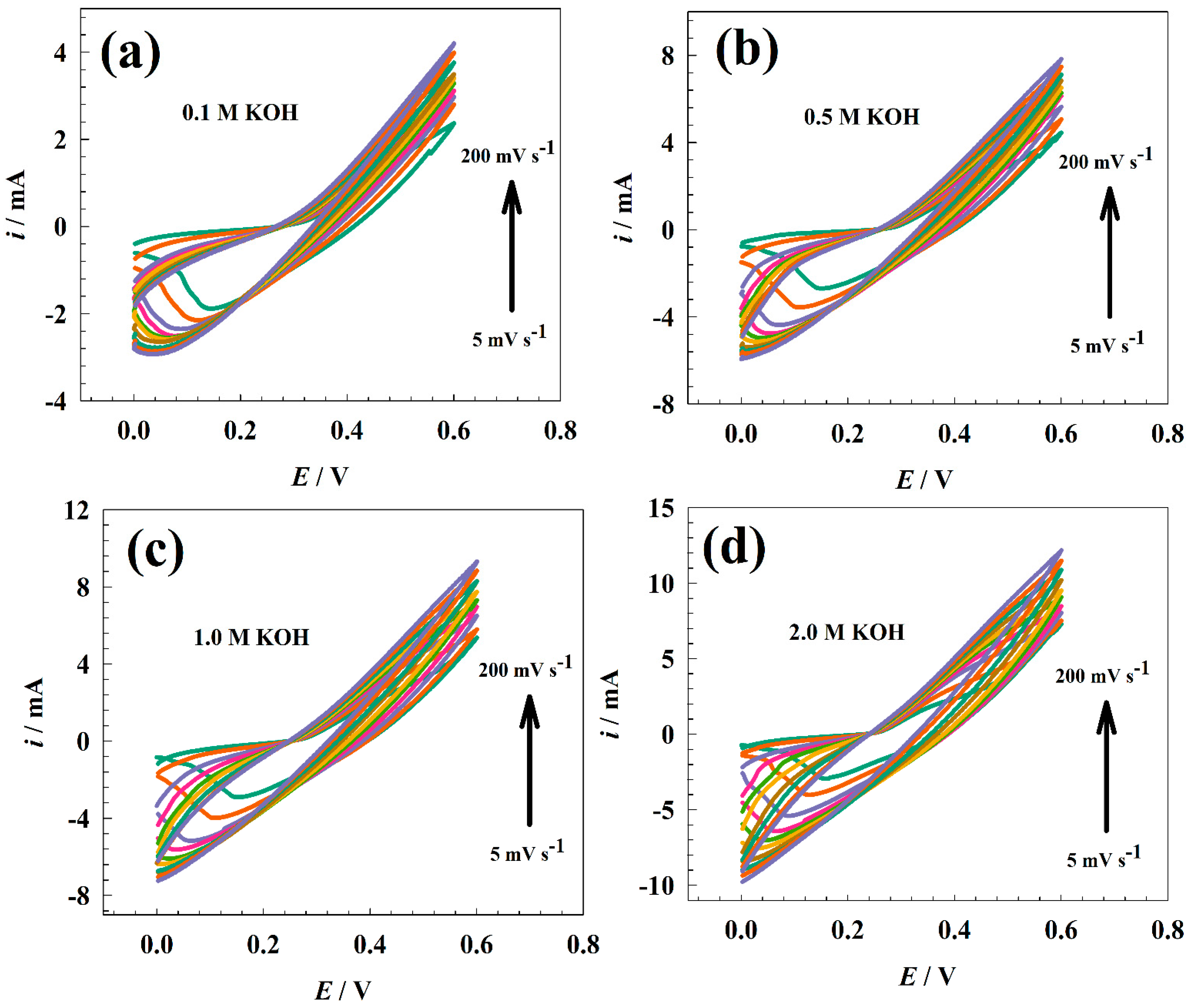
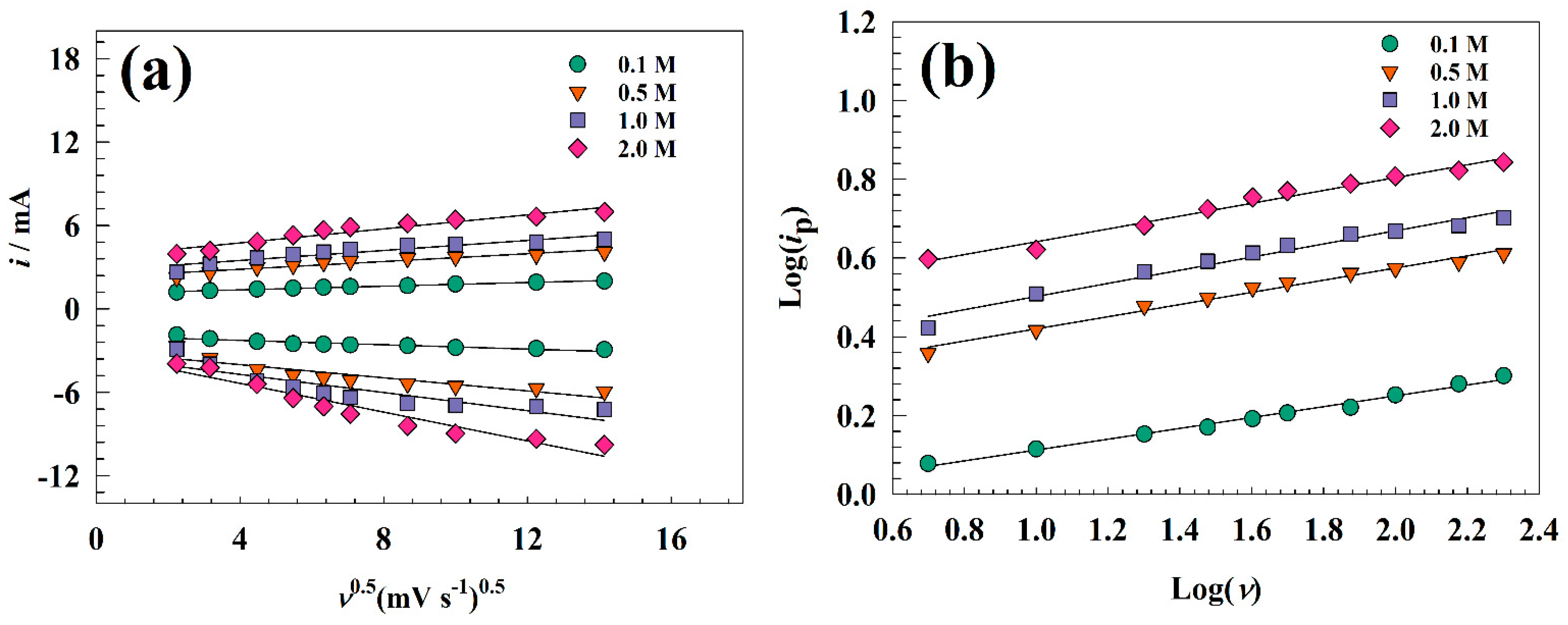
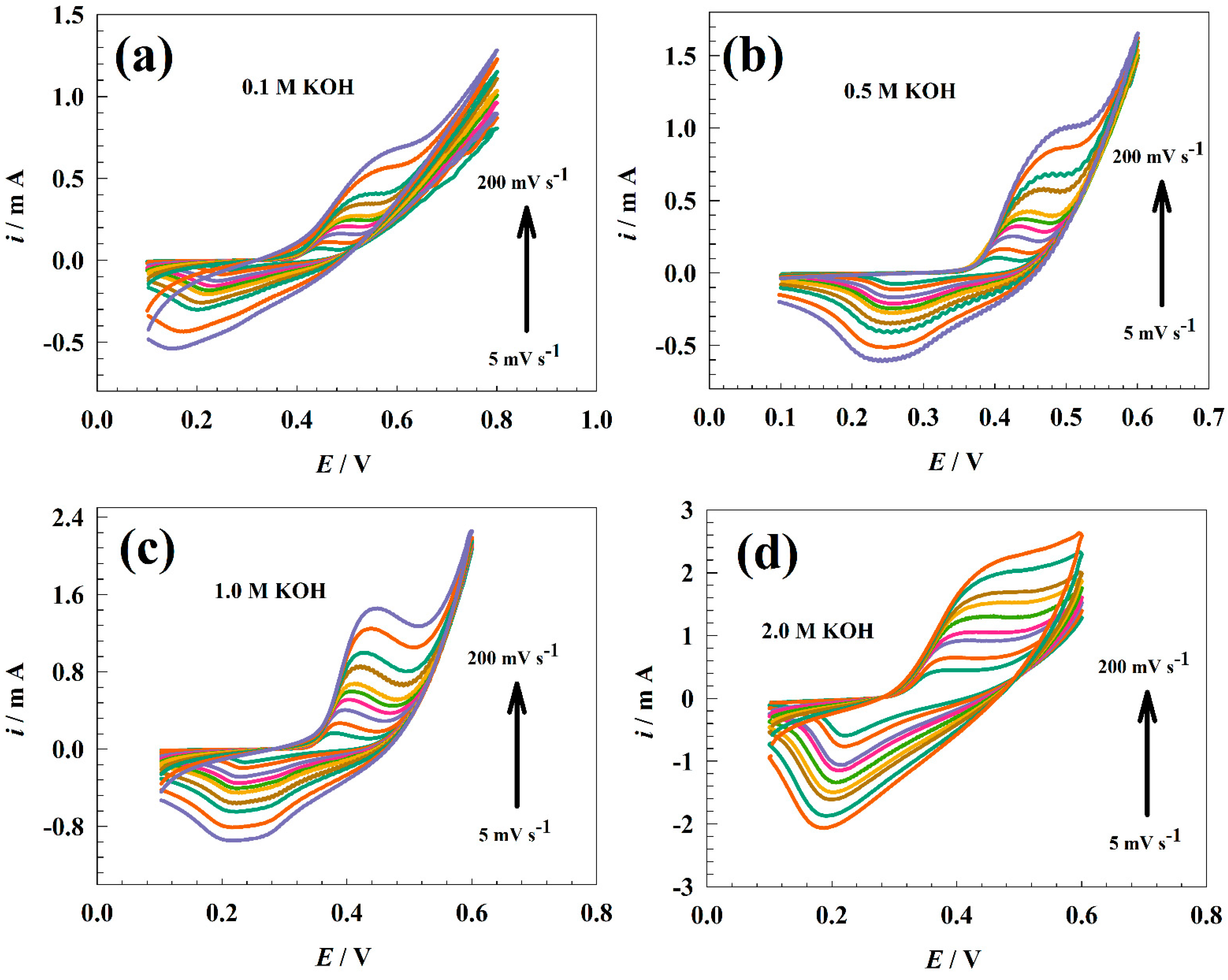
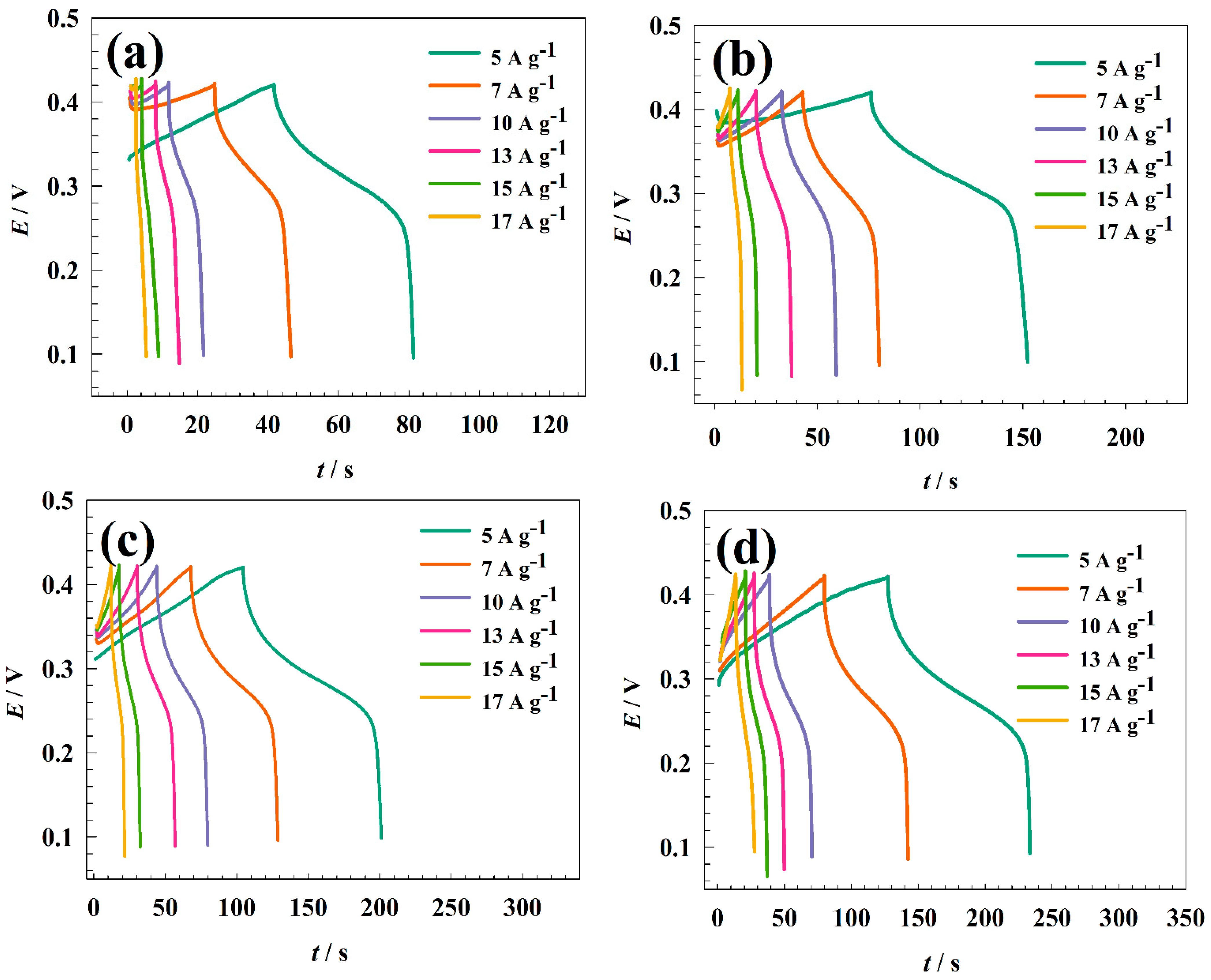
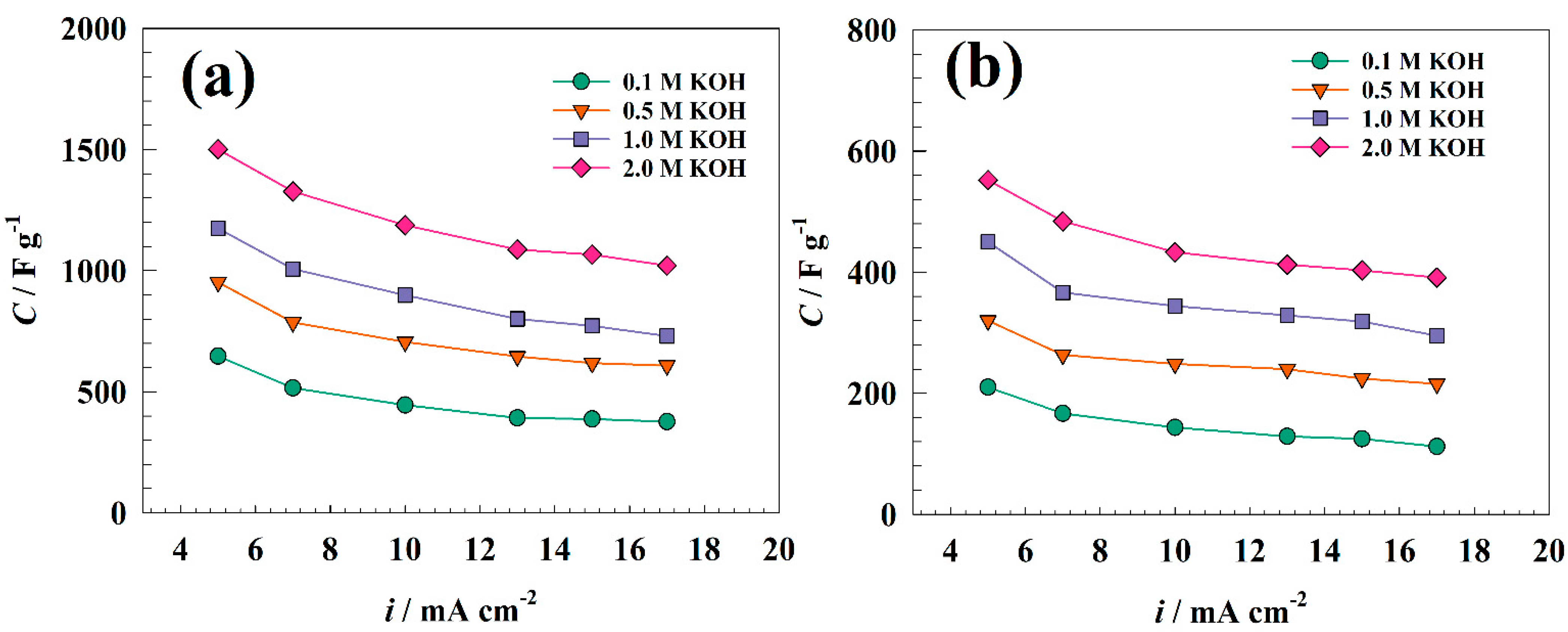
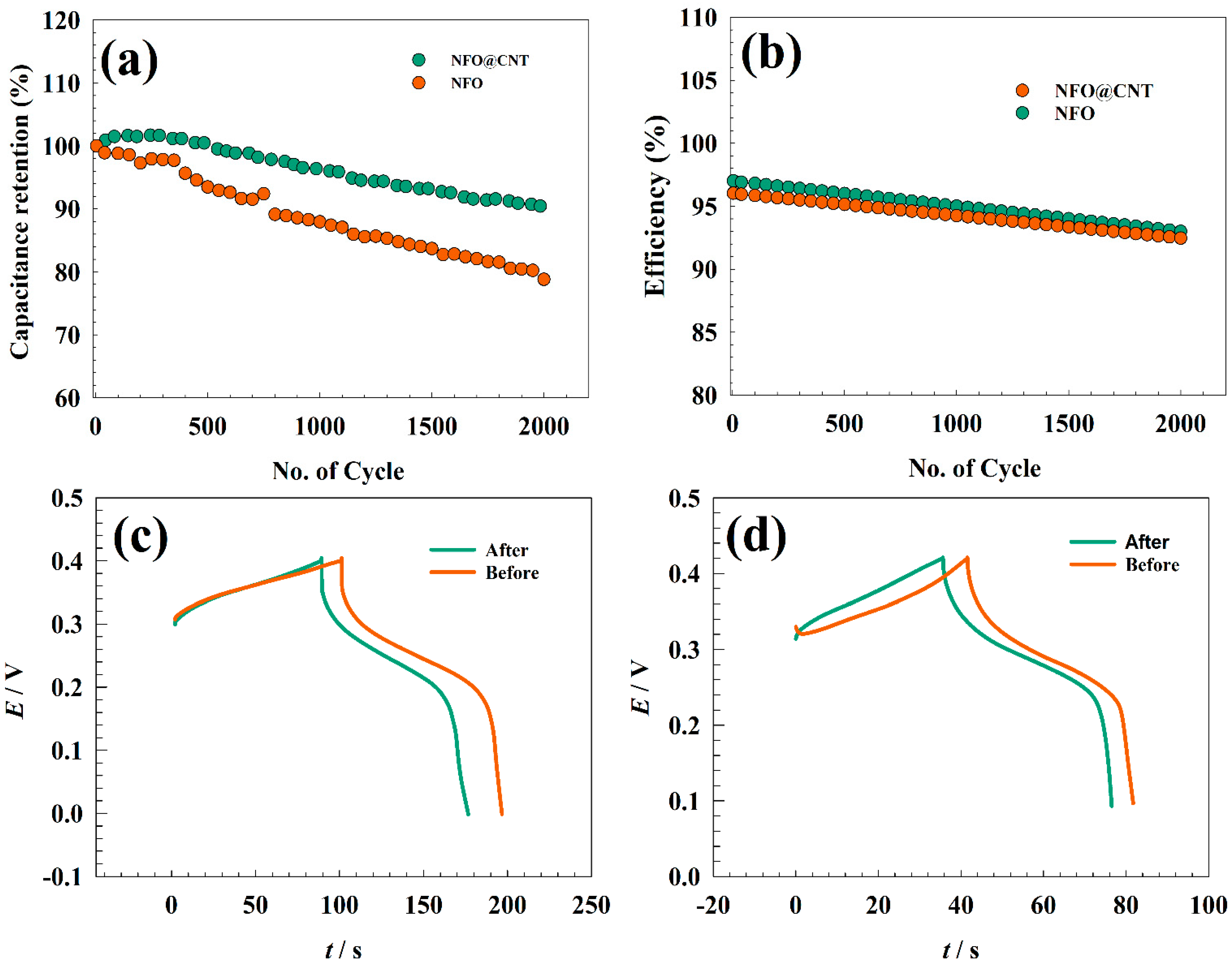
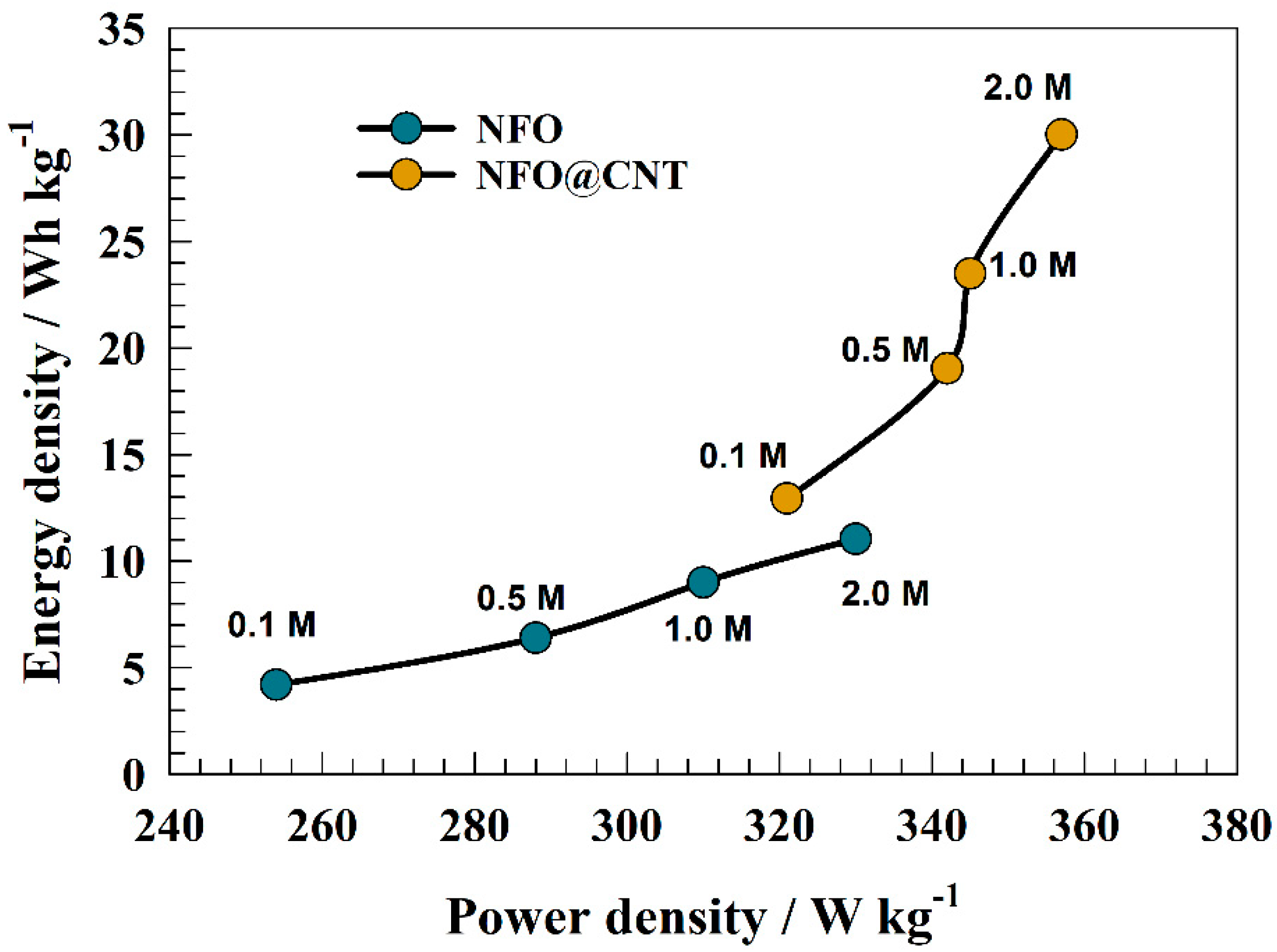
3.2. Electrochemical Characterization
| Electrode | Preparation | Electrolytes | Potential Window (V) | Cs/F g−1 | Rate Capability | Stability (Cycle/Current) | Ref. |
|---|---|---|---|---|---|---|---|
| GC/NFO | Green method | 1.0 M KOH | 0.32 | 450 (5 A g−1) | 65.1% (5 to 17 A g−1) | 78.8% (2000 cycles at 10 A g−1) | This work |
| GC/NFO@CNT | Green method | 1.0 M KOH | 0.4 | 1169 (at 5 A g−1) | 62.4% (5 to 17 A g−1) | 90.1% (2000 cycles at 10 A g−1) | This work |
| Fe-MnO2 | Hydrothermal | 0.5 M Na2SO4 | 1.0 | 145 F g−1 (1 A g−1) | 71.4% (1 to 10 A g−1) | 95.9% (5000 cycles at 3 A g−1) | [68] |
| Ni–Co double hydroxide | Electrodeposition | 6.0 M KOH | 0.45 | 1246 F g−1 (1 A g−1) | 91.8% (1 to 10 A g−1) | 80.1% (1000 cycles at 10 A g−1) | [69] |
| CoNiFe-layered double hydroxide | In situ growth method | 6.0 M KOH | 0.4 | 1203 F g−1 (1 A g−1) | 77.1% (1 to 10 A g−1) | 94% (1000 cycles at 20 A g−1) | [70] |
| MnO2@CNTs/CNTs | Vacuum filtration | 1 M Na2SO4 | 1.0 | 149 (0.2 A g−1) | 85% (0.2 to 5 A g−1) | 90% (5000 cycles at 50 mV s−1 | [71] |
| Fe-MnO2@ CNF | Chemical | 1 M Na2SO4 | 1.0 | 210 (0.3 A g−1) | 83% (0.3 to 10 A g−1) | 94% (4500 cycles at 2 A g−1) | [72] |
| RGO/Fe2O3 | Chemical | 2.0 M KOH | 1.1 | 469.5 (4 A g−1) | 49% (4 to 8 A g−1) | 88% (5000 cycles at 8 A g−1) | [73] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, Q.; Ma, X.; Zhang, D.; Zhou, H.; Liao, Y.; Zhang, H.; Xu, S.; Levchenko, I.; Bazaka, K. Interfacial modification of titanium dioxide to enhance photocatalytic efficiency towards H2 production. J. Colloid Interface Sci. 2019, 556, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, X.; Li, X.; Liu, J.; Li, C.; Feng, X.; Shu, C.; Yu, Z.-Z. An environmental energy-enhanced solar steam evaporator derived from MXene-decorated cellulose acetate cigarette filter with ultrahigh solar steam generation efficiency. J. Colloid Interface Sci. 2022, 606, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Eliwa, A.S.; Hefnawy, M.A.; Medany, S.S.; Deghadi, R.G.; Hosny, W.M.; Mohamed, G.G. Synthesis and characterization of lead-based metal–organic framework nano-needles for effective water splitting application. Sci. Rep. 2023, 13, 12531. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Luo, S.; Feng, J.; Yang, L.; Li, P.; Wang, Q.; Zhang, Y.; Liu, X.; Chang, L. Asymmetric, Flexible Supercapacitor Based on Fe–Co Alloy@Sulfide with High Energy and Power Density. ACS Appl. Mater. Interfaces 2021, 13, 49952–49963. [Google Scholar] [CrossRef]
- Deng, B.; Yang, Y.; Liu, Y.; Yin, B.; Yang, M. A hierarchically combined reduced graphene oxide/Nickel oxide hybrid supercapacitor device demonstrating compliable flexibility and high energy density. J. Colloid Interface Sci. 2022, 618, 399–410. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.; Hou, D.; Yin, J.; Liu, D.; He, Y.; Lin, H. Hierarchical porous carbon prepared from biomass through a facile method for supercapacitor applications. J. Colloid Interface Sci. 2018, 530, 338–344. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Xiao, Y.; Wang, T.; Wang, J.; Romero, C.E.; Pan, W. High performance aqueous supercapacitor based on nitrogen-doped coal-based activated carbon electrode materials. J. Colloid Interface Sci. 2020, 580, 77–87. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Q.; Luo, S.; Zhang, Y.; Liu, X.; Liu, Y.; Wang, Z.; Hao, A.; Yi, T. Coal-based S hybrid self-doped porous carbon for high-performance supercapacitors and potassium-ion batteries. J. Power Sources 2020, 461, 228151. [Google Scholar] [CrossRef]
- Yan, S.; Luo, S.; Liu, H.; Yang, L.; Wang, Q.; Zhang, Y.; Liu, X. In-situ partial reduction-sulfurized Fe3O4@ FeS based on pickling iron red as a versatile electrode for high-performance lithium ion batteries and supercapacitor devices. Surf. Coat. Technol. 2022, 429, 127980. [Google Scholar] [CrossRef]
- Sagadevan, S.; Marlinda, A.R.; Johan, M.R.; Umar, A.; Fouad, H.; Alothman, O.Y.; Khaled, U.; Akhtar, M.S.; Shahid, M.M. Reduced graphene/nanostructured cobalt oxide nanocomposite for enhanced electrochemical performance of supercapacitor applications. J. Colloid Interface Sci. 2020, 558, 68–77. [Google Scholar] [CrossRef]
- Maruthamani, D.; Vadivel, S.; Kumaravel, M.; Saravanakumar, B.; Paul, B.; Dhar, S.S.; Habibi-Yangjeh, A.; Manikandan, A.; Ramadoss, G. Fine cutting edge shaped Bi2O3rods/reduced graphene oxide (RGO) composite for supercapacitor and visible-light photocatalytic applications. J. Colloid Interface Sci. 2017, 498, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, H.; Lu, Y.; Yang, J.; Zhu, X.; Zheng, Y.; Lou, G.; Wu, Y.; Wu, Q.; Shen, Z. Rational design of cobalt–nickel double hydroxides for flexible asymmetric supercapacitor with improved electrochemical performance. J. Colloid Interface Sci. 2021, 581, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Younas, W.; Naveed, M.; Cao, C.; Zhu, Y.; Du, C.; Ma, X.; Mushtaq, N.; Tahir, M.; Naeem, M. Facile one-step microwave-assisted method to synthesize nickel selenide nanosheets for high-performance hybrid supercapacitor. J. Colloid Interface Sci. 2022, 608, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Sui, D.; Yao, M.; Si, L.; Yan, K.; Shi, J.; Wang, J.; Xu, C.C.; Zhang, Y. Biomass-derived carbon coated SiO2 nanotubes as superior anode for lithium-ion batteries. Carbon 2023, 205, 510–518. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; El-Bagoury, N.; Fadlallah, S.A. High-performance IN738 superalloy derived from turbine blade waste for efficient ethanol, ethylene glycol, and urea electrooxidation. J. Appl. Electrochem. 2023, 53, 1337–1348. [Google Scholar] [CrossRef]
- Eliwa, A.S.; Hefnawy, M.A.; Medany, S.S.; Deghadi, R.G.; Hosny, W.M.; Mohamed, G.G. Ultrasonic-assisted synthesis of nickel metal-organic framework for efficient urea removal and water splitting applications. Synth. Met. 2023, 294, 117309. [Google Scholar] [CrossRef]
- Moraveji, S.; Fotouhi, L.; Zirak, M.; Shahrokhian, S. Bimetallic nickel-cobalt nanospheres electrodeposited on nickel foam as a battery-type electrode material for fabrication of asymmetric supercapacitors. J. Alloys Compd. 2023, 946, 169409. [Google Scholar] [CrossRef]
- Lorenzo, C.; Bouquain, D.; Hibon, S.; Hissel, D. Synthesis of degradation mechanisms and of their impacts on degradation rates on proton-exchange membrane fuel cells and lithium-ion nickel–manganese–cobalt batteries in hybrid transport applications. Reliab. Eng. Syst. Saf. 2021, 212, 107369. [Google Scholar] [CrossRef]
- Samant, P.V.; Fernandes, J.B. Nickel-modified manganese oxide as an active electrocatalyst for oxidation of methanol in fuel cells. J. Power Sources 1999, 79, 114–118. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Ma, H.; Zhang, H.; Ma, C.; Duan, W.; Zhao, Y.; Miao, Z. Defect-rich Ni3S4−x as a robust electrode material for supercapacitor and aqueous Ni-Zn battery applications. J. Alloys Compd. 2023, 933, 167733. [Google Scholar] [CrossRef]
- Karuppiah, K.; Vaidyanathan, R.; Lakshmanan, S.P.; Rajendran, K.; Dhandapani, P.; Venkatesan, S.; Suresh, K.; Rajaraman, V. Synthesis, characterization and electrochemical investigation on nickel manganese oxide-Polybutylene Sebacate composite electrode of biodegradable nature for micro capacitor applications. J. Indian Chem. Soc. 2023, 100, 100896. [Google Scholar] [CrossRef]
- Yan, S.; Luo, S.; Sun, M.; Wang, Q.; Zhang, Y.; Liu, X. Facile hydrothermal synthesis of urchin-like NiCo2O4 as advanced electrochemical pseudocapacitor materials. Int. J. Energy Res. 2021, 45, 20186–20198. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Competition between enzymatic and non-enzymatic electrochemical determination of cholesterol. J. Electroanal. Chem. 2023, 930, 117169. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Fadlallah, S.A.; El-Sherif, R.M.; Medany, S.S. Systematic DFT studies of CO-Tolerance and CO oxidation on Cu-doped Ni surfaces. J. Mol. Graph. Model. 2023, 118, 108343. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Polyaniline-Supported Nickel Oxide Flower for Efficient Nitrite Electrochemical Detection in Water. Polymers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Liu, B.; Zhong, C.; Hu, W. Facile synthesis of uniformly coated ZnO@ Bi2O3 composites anode for long-cycle-life zinc–nickel battery. J. Energy Storage 2023, 58, 106350. [Google Scholar] [CrossRef]
- Cox, P.A. Transition Metal Oxides: An Introduction to Their Electronic Structure and Properties; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Medany, S.S.; Hefnawy, M.A. Nickel–cobalt oxide decorated Chitosan electrocatalyst for ethylene glycol oxidation. Surf. Interfaces 2023, 40, 103077. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T. Research progress in preparation and application of spinel-type metallic oxides (M ≥ 2). J. Alloys Compd. 2023, 962, 171117. [Google Scholar] [CrossRef]
- Van Hoang, N.; Hiep, N.T.; Hung, N.M.; Nguyen, C.V.; Hung, P.T.; Hoat, P.D.; Heo, Y.-W. Optimization of synthesis conditions and sensing performance of electrospun NiFe2O4 nanofibers for H2S and NO2 detection. J. Alloys Compd. 2023, 936, 168276. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Shailajha, S.; Seema, S.; Kairon Mubina, M.S. Enhanced Magneto-Electric Properties of ZnAl2O4@ NiFe2O4 Nanocomposites in Magnetic Sensor Applications. J. Supercond. Nov. Magn. 2023, 36, 693–709. [Google Scholar] [CrossRef]
- Tian, W.; Li, L.; Zhu, G. Interface Engineering of Oxygen-Vacancy-Rich VO-NiFe2O4@ Ni2P Heterostructure for Highly Efficient Oxygen Evolution Reaction. Catal. Lett. 2023, 36, 693–709. [Google Scholar] [CrossRef]
- Jia, L.; Du, G.; Han, D.; Wang, Y.; Zhao, W.; Su, Q.; Ding, S.; Xu, B. Magnetic electrode configuration with polypyrrole-wrapped Ni/NiFe2O4 core–shell nanospheres to boost electrocatalytic water splitting. Chem. Eng. J. 2023, 454, 140278. [Google Scholar] [CrossRef]
- Wang, X.; Luo, J.; Tuo, Y.; Gu, Y.; Liu, W.; Wang, S.; Zhou, Y.; Zhang, J. Hierarchical heterostructure of NiFe2O4 nanoflakes grown on the tip of NiCo2O4 nanoneedles with enhanced interfacial polarization effect to achieve highly efficient electrocatalytic oxygen evolution. Chem. Eng. J. 2023, 457, 141169. [Google Scholar] [CrossRef]
- Mao, J.; He, B.; Sui, H.; Cui, L.; Chen, H.; Duan, Y.; Yang, P.; Tang, Q. Interfacial modification of in-situ polymerized AMPS/NiFe2O4 quantum dots for efficient and air-stable CsPbBr3 perovskite solar cells. Chem. Eng. J. 2023, 461, 141943. [Google Scholar] [CrossRef]
- Kumar, S.; Hashmi, S.Z.; Srivastava, G.; Singh, J.; Quraishi, A.M.; Dalela, S.; Ahmed, F.; Alvi, P.A. Synthesis and investigations of structural, surface morphology, electrochemical, and electrical properties of NiFe2O4 nanoparticles for usage in supercapacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 868. [Google Scholar]
- Manzoor, S.; Alburaih, H.A.; Nisa, M.U.; Aman, S.; Abdullah, M.; Abid, A.G. A novel porous rod with nanosphere CuS2/NiFe2O4 nanocomposite for low-cost high-performance energy storage system. J. Mater. Sci. Mater. Electron. 2023, 34, 294. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Yue, S.; Zheng, H.; Liu, X.; Gao, P.; Qin, H.; Xiao, H. Effects of Alternating Magnetic Fields on the OER of Heterogeneous Core–Shell Structured NiFe2O4@(Ni, Fe) S/P. ACS Appl. Mater. Interfaces 2023, 15, 11631–11641. [Google Scholar] [CrossRef]
- Luo, C.; Xie, H.; Wang, Q.; Luo, G.; Liu, C. A review of the application and performance of carbon nanotubes in fuel cells. J. Nanomater. 2015, 2015, 4. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Nafady, A.; Mohamed, S.K.; Medany, S.S. Facile green synthesis of Ag/carbon nanotubes composite for efficient water splitting applications. Synth. Met. 2023, 294, 117310. [Google Scholar] [CrossRef]
- Wang, S.; Xu, P.; Tian, J.; Liu, Z.; Feng, L. Phase structure tuning of graphene supported Ni-NiO Nanoparticles for enhanced urea oxidation performance. Electrochim. Acta. 2021, 370, 137755. [Google Scholar] [CrossRef]
- Yan, S.; Luo, S.; Feng, J.; Li, P.; Guo, R.; Wang, Q.; Zhang, Y.; Liu, Y.; Bao, S. Rational design of flower-like FeCo2S4/reduced graphene oxide films: Novel binder-free electrodes with ultra-high conductivity flexible substrate for high-performance all-solid-state pseudocapacitor. Chem. Eng. J. 2020, 381, 122695. [Google Scholar] [CrossRef]
- Hu, T.; Sun, J.; Zhang, Y.; Liu, Y.; Jiang, H.; Dong, X.; Zheng, J.; Meng, C.; Huang, C. PVA-assisted hydrated vanadium pentoxide/reduced graphene oxide films for excellent Li+ and Zn2+ storage properties. J. Mater. Sci. Technol. 2021, 83, 7–17. [Google Scholar] [CrossRef]
- Munde, A.V.; Mulik, B.B.; Chavan, P.P.; Sathe, B.R. Enhanced electrocatalytic activity towards urea oxidation on Ni nanoparticle decorated graphene oxide nanocomposite. Electrochim. Acta. 2020, 349, 136386. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Medany, S.S.; El-Sherif, R.M.; Fadlallah, S.A. NiO-MnOx/Polyaniline/Graphite Electrodes for Urea Electrocatalysis: Synergetic Effect between Polymorphs of MnOx and NiO. ChemistrySelect 2022, 7, e202103735. [Google Scholar] [CrossRef]
- Alamro, F.S.; Hefnawy, M.A.; Nafee, S.S.; Al-Kadhi, N.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Chitosan Supports Boosting NiCo2O4 for Catalyzed Urea Electrochemical Removal Application. Polymers 2023, 15, 3058. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; Nafee, S.S.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Zinc Nanocomposite Supported Chitosan for Nitrite Sensing and Hydrogen Evolution Applications. Polymers 2023, 15, 2357. [Google Scholar] [CrossRef]
- Yan, S.; Luo, S.; Wang, Q.; Zhang, Y.; Liu, X. Rational design of hierarchically sulfide and MXene-reinforced porous carbon nanofibers as advanced electrode for high energy density flexible supercapacitors. Compos. Part B Eng. 2021, 224, 109246. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, F.; Wu, L.; Wang, J.; Dai, S.; Zhu, H.; Cheng, G.; Ding, J. Porous conductive electrode for highly sensitive flexible capacitive pressure sensor over a wide range. J. Alloys Compd. 2023, 934, 167919. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Z.; Li, Y.; Du, Y.; Liu, H.; Guo, Y. A novel hydrochar and nickel composite for the electrochemical supercapacitor electrode material. Mater. Lett. 2012, 74, 111–114. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Liu, L.; Wang, J.; Zhang, L.; Shi, M.; Chen, X. Ultra-stable sandwich shaped flexible MXene/CNT@Ni films for high performance supercapacitor. J. Alloys Compd. 2023, 941, 168963. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Cao, W.-Q.; Zhang, Y.-L.; He, P.; Shu, J.-C.; Cao, M.-S. Tailoring rGO-NiFe2O4 hybrids to tune transport of electrons and ions for supercapacitor electrodes. J. Alloys Compd. 2019, 811, 152011. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, L.; Yan, J.; Yan, W.; Wu, C.; Lian, J.; Huang, Y.; Bao, J.; Qiu, J.; Xu, L. Facile preparation of NiFe2O4/MoS2 composite material with synergistic effect for high performance supercapacitor. J. Alloys Compd. 2017, 726, 608–617. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Sankar, K.V.; Sanjeeviraja, C.; Selvan, R.K. Synthesis and physico-chemical property evaluation of PANI–NiFe2O4 nanocomposite as electrodes for supercapacitors. J. Alloys Compd. 2013, 553, 350–357. [Google Scholar] [CrossRef]
- Naik, M.M.; Vinuth, M.; Kumar, V.U.; Hemakumar, K.H.; Preethi, G.; Kumar, M.P.; Nagaraju, G. A facile green synthesis of nickel ferrite nanoparticles using Tamarindus Indica seeds for magnetic and photocatalytic studies. Nanotechnol. Environ. Eng. 2023, 8, 143–151. [Google Scholar] [CrossRef]
- Amiri, M.; Pardakhti, A.; Ahmadi-Zeidabadi, M.; Akbari, A.; Salavati-Niasari, M. Magnetic nickel ferrite nanoparticles: Green synthesis by Urtica and therapeutic effect of frequency magnetic field on creating cytotoxic response in neural cell lines. Colloids Surf. B Biointerfaces 2018, 172, 244–253. [Google Scholar] [CrossRef]
- Sarala, E.; Vinuth, M.; Naik, M.M.; Reddy, Y.V.R. Green synthesis of nickel ferrite nanoparticles using Terminalia catappa: Structural, magnetic and anticancer studies against MCF-7 cell lines. J. Hazard. Mater. Adv. 2022, 8, 100150. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, Q.; Srinivasan, G.; Takoudis, C.G. Cyclic chemical vapor deposition of nickel ferrite thin films using organometallic precursor combination. ECS J. Solid State Sci. Technol. 2014, 3, P345. [Google Scholar] [CrossRef]
- Ganesan, P.; Sivanantham, A.; Shanmugam, S. Inexpensive electrochemical synthesis of nickel iron sulphides on nickel foam: Super active and ultra-durable electrocatalysts for alkaline electrolyte membrane water electrolysis. J. Mater. Chem. A 2016, 4, 16394–16402. [Google Scholar] [CrossRef]
- Rameshan, C.; Ng, M.L.; Shavorskiy, A.; Newberg, J.T.; Bluhm, H. Water adsorption on polycrystalline vanadium from ultra-high vacuum to ambient relative humidity. Surf. Sci. 2015, 641, 141–147. [Google Scholar] [CrossRef]
- Joshi, N.; da Silva, L.F.; Shimizu, F.M.; Mastelaro, V.R.; M’Peko, J.-C.; Lin, L.; Oliveira, O.N. UV-assisted chemiresistors made with gold-modified ZnO nanorods to detect ozone gas at room temperature. Microchim. Acta 2019, 186, 418. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms that control the adsorption–desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry characteristics. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta. 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, J.; Ding, M.; Gu, C.; Vafakhah, S.; Zhang, W.; Li, D.; Valdivia y Alvarado, P.; Yang, H.Y. Hierarchical Co3O4/CNT decorated electrospun hollow nanofiber for efficient hybrid capacitive deionization. Sep. Purif. Technol. 2021, 266, 118593. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yao, M.; Yang, L.; Zhu, Y.; Liu, P.; Xing, O.; Zhou, J.; Wang, M.; Luo, H.; et al. A Low-Cost, Self-Standing NiCo2O4@CNT/CNT Multilayer Electrode for Flexible Asymmetric Solid-State Supercapacitors. Adv. Funct. Mater. 2017, 27, 1702160. [Google Scholar] [CrossRef]
- Tourchi Moghadam, M.T.; Seifi, M.; Jamali, F.; Azizi, S.; Askari, M.B. ZnWO4-CNT as a superior electrode material for ultra-high capacitance supercapacitor. Surf. Interfaces 2022, 32, 102134. [Google Scholar] [CrossRef]
- Cao, D.; Ren, M.; Xiong, J.; Pan, L.; Wang, Y.; Ji, X.; Qiu, T.; Yang, J.; Zhang, C. Self-assembly of hierarchical Ti3C2Tx-CNT/SiNPs resilient films for high performance lithium ion battery electrodes. Electrochim. Acta 2020, 348, 136211. [Google Scholar] [CrossRef]
- Yan, L.; Niu, L.; Shen, C.; Zhang, Z.; Lin, J.; Shen, F.; Gong, Y.; Li, C.; Liu, X.; Xu, S. Modulating the electronic structure and pseudocapacitance of δ-MnO2 through transitional metal M (M = Fe, Co and Ni) doping. Electrochim. Acta 2019, 306, 529–540. [Google Scholar] [CrossRef]
- Wei, Z.; Yuan, J.; Tang, S.; Wu, D.; Wu, L. Porous nanorods of nickel–cobalt double hydroxide prepared by electrochemical co-deposition for high-performance supercapacitors. J. Colloid Interface Sci. 2019, 542, 15–22. [Google Scholar] [CrossRef]
- Wang, F.; Sun, S.; Xu, Y.; Wang, T.; Yu, R.; Li, H. High performance asymmetric supercapacitor based on Cobalt Nickle Iron-layered double hydroxide/carbon nanofibres and activated carbon. Sci. Rep. 2017, 7, 4707. [Google Scholar] [CrossRef]
- Wu, P.; Cheng, S.; Yang, L.; Lin, Z.; Gui, X.; Ou, X.; Zhou, J.; Yao, M.; Wang, M.; Zhu, Y.; et al. Synthesis and Characterization of Self-Standing and Highly Flexible δ-MnO2@CNTs/CNTs Composite Films for Direct Use of Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 23721–23728. [Google Scholar] [CrossRef]
- Li, J.; Hu, B.; Nie, P.; Shang, X.; Jiang, W.; Xu, K.; Yang, J.; Liu, J. Fe-regulated δ-MnO2 nanosheet assembly on carbon nanofiber under acidic condition for high performance supercapacitor and capacitive deionization. Appl. Surf. Sci. 2021, 542, 148715. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Niu, L.; Li, R.; Guo, H.; Shi, Y.; Li, C.; Liu, X.; Gong, Y. Hydrothermal synthesis of CuCo2O4/CuO nanowire arrays and RGO/Fe2O3 composites for high-performance aqueous asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 9977–9985. [Google Scholar] [CrossRef]




| KOH (M) | Rs | R1 | Q1 | W | |
|---|---|---|---|---|---|
| R ( Ω cm2) | R (Ω cm2) | Y0 (Ω−1cm2s−n) | n | Y0 (Ω−1cm2s−n) | |
| 0.1 | 6.43 | 10.14 | 0.000656 | 0.1181 | 0.11318 |
| 0.5 | 3.71 | 6.14 | 0.012638 | 0.37549 | 0.16978 |
| 1.0 | 4.14 | 5.42 | 0.029051 | 0.23167 | 0.29914 |
| 2.0 | 2.28 | 3.43 | 0.019652 | 0.27925 | 0.54293 |
| KOH (M) | Rs | R1 | R2 | Q1 | Q2 | ||
|---|---|---|---|---|---|---|---|
| R (Ω cm2) | R (Ω cm2) | R (Ω cm2) | Y0 (Ω−1cm2s−n) | n | Y0 (Ω−1cm2s−n) | m | |
| 0.1 | 12.11 | 17.11 | 40.15 | 0.0013199 | 0.2482 | 0.0043451 | 0.75486 |
| 0.5 | 6.87 | 8.87 | 19.21 | 0.0025709 | 0.31794 | 0.00089369 | 0.76123 |
| 1.0 | 1.96 | 2.95 | 14.96 | 0.0026587 | 0.40007 | 0.00079411 | 0.73669 |
| 2.0 | 1.46 | 2.65 | 12.79 | 0.002747 | 0.63216 | 0.0009814 | 0.81583 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashal, A.H.; Hefnawy, M.A.; Ahmed, H.A.; El-Atawy, M.A.; Pashameah, R.A.; Medany, S.S. Green Synthesis of NiFe2O4 Nano-Spinel Oxide-Decorated Carbon Nanotubes for Efficient Capacitive Performance—Effect of Electrolyte Concentration. Nanomaterials 2023, 13, 2643. https://doi.org/10.3390/nano13192643
Bashal AH, Hefnawy MA, Ahmed HA, El-Atawy MA, Pashameah RA, Medany SS. Green Synthesis of NiFe2O4 Nano-Spinel Oxide-Decorated Carbon Nanotubes for Efficient Capacitive Performance—Effect of Electrolyte Concentration. Nanomaterials. 2023; 13(19):2643. https://doi.org/10.3390/nano13192643
Chicago/Turabian StyleBashal, Ali H., Mahmoud A. Hefnawy, Hoda A. Ahmed, Mohamed A. El-Atawy, Rami Adel Pashameah, and Shymaa S. Medany. 2023. "Green Synthesis of NiFe2O4 Nano-Spinel Oxide-Decorated Carbon Nanotubes for Efficient Capacitive Performance—Effect of Electrolyte Concentration" Nanomaterials 13, no. 19: 2643. https://doi.org/10.3390/nano13192643
APA StyleBashal, A. H., Hefnawy, M. A., Ahmed, H. A., El-Atawy, M. A., Pashameah, R. A., & Medany, S. S. (2023). Green Synthesis of NiFe2O4 Nano-Spinel Oxide-Decorated Carbon Nanotubes for Efficient Capacitive Performance—Effect of Electrolyte Concentration. Nanomaterials, 13(19), 2643. https://doi.org/10.3390/nano13192643