A Novel Strategy for the Synthesis of High Stability of Luminescent Zero Dimensional–Two Dimensional CsPbBr3 Quantum Dot/1,4-bis(4-methylstyryl)benzene Nanoplate Heterostructures at an Atmospheric Condition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
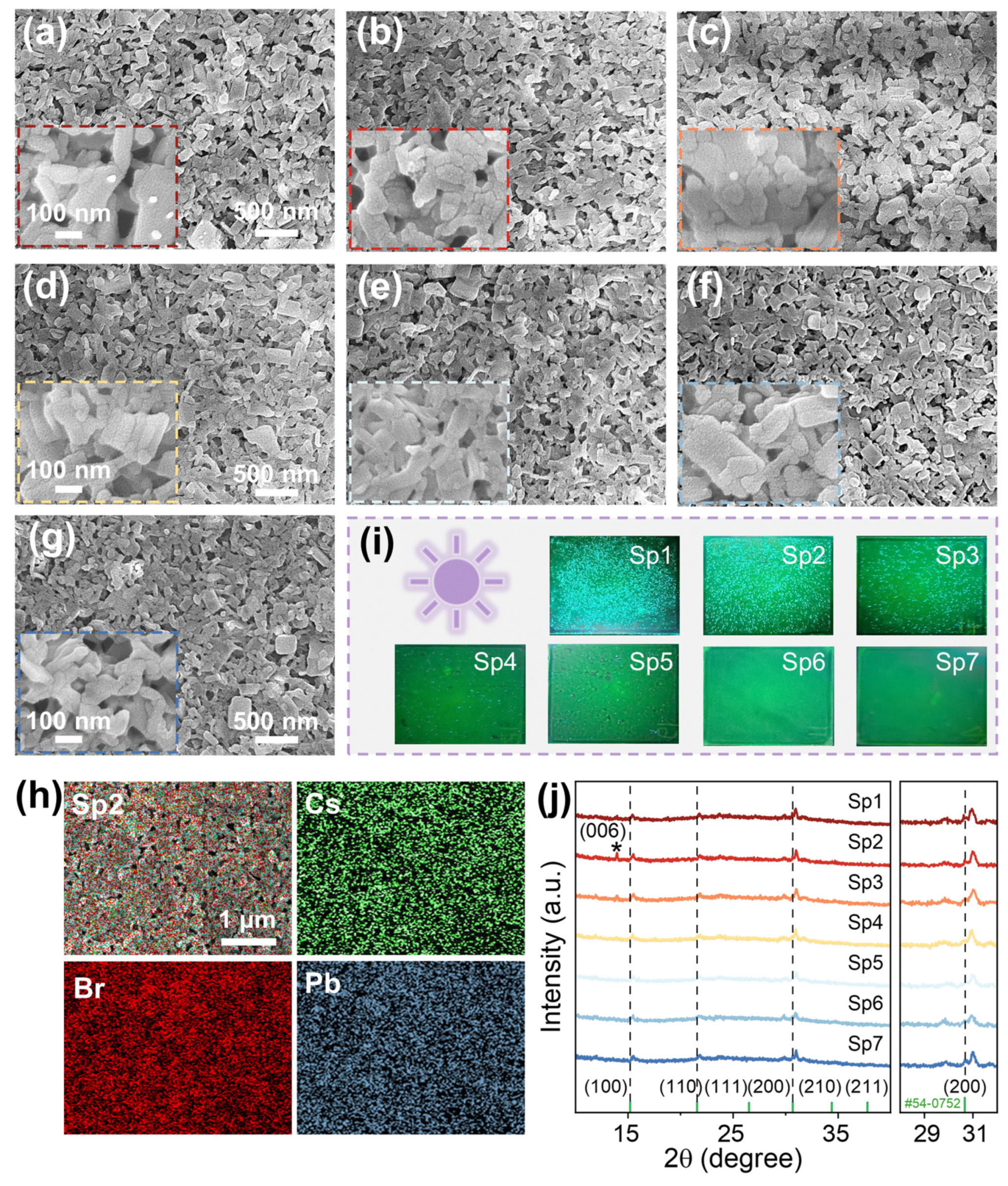
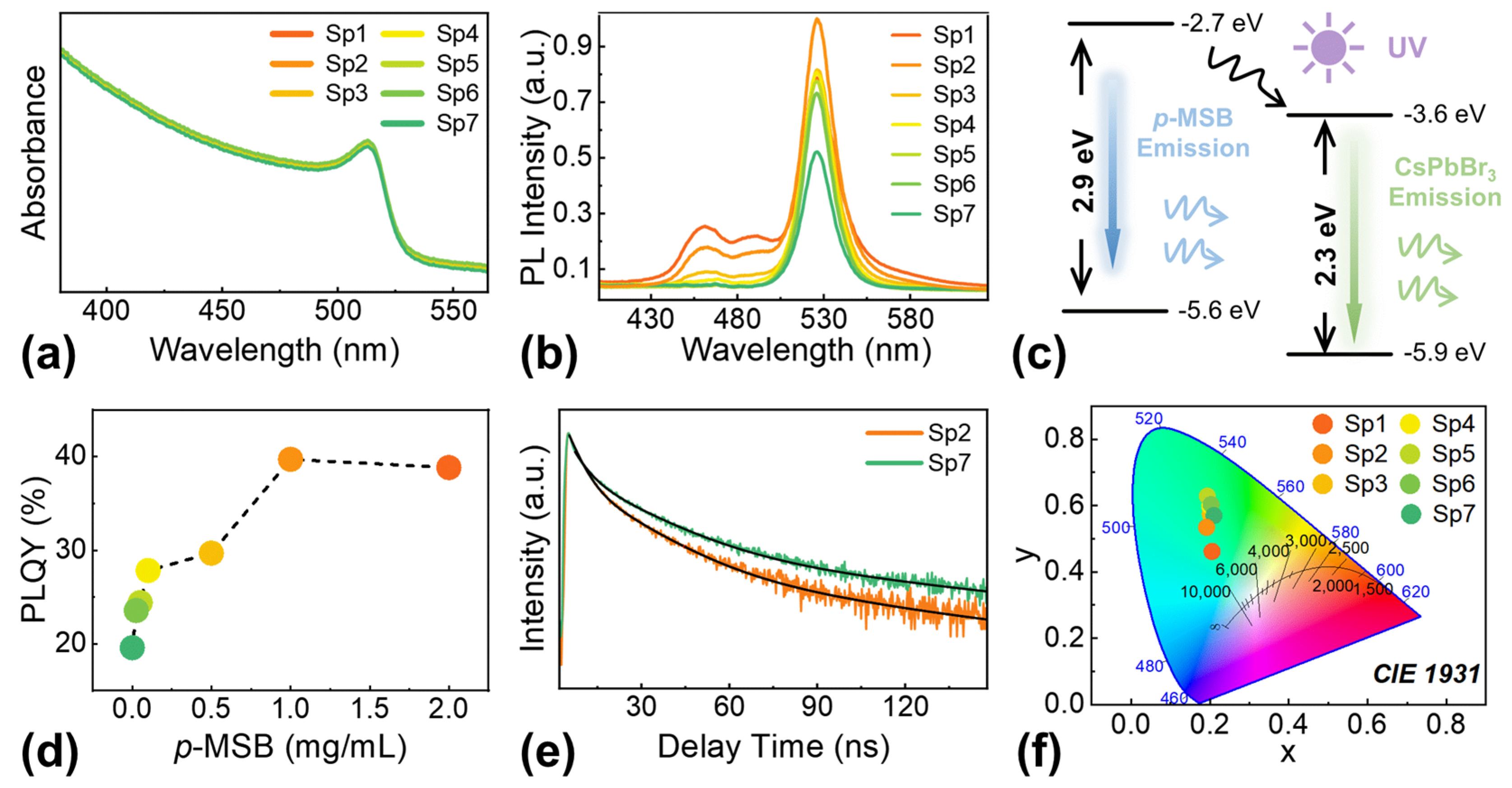

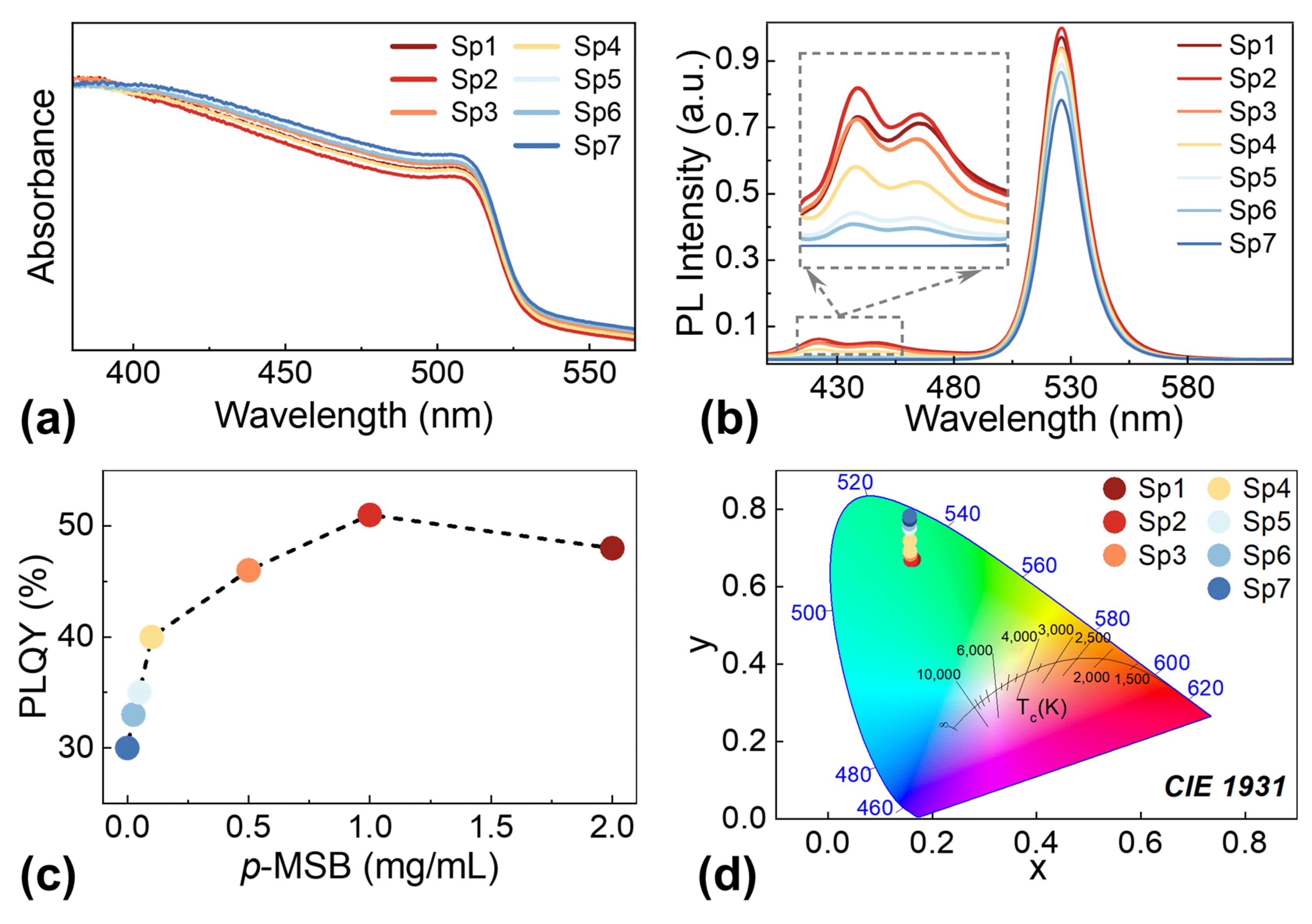
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, J.; Wang, J.; Yu, C.; Chen, X.; Wu, J.; Zhao, M.; Qu, F.; Xu, Z.; Han, J.; Xu, J. Single LED-based 46-m underwater wireless optical communication enabled by a multi-pixel photon counter with digital output. Opt. Commun. 2019, 438, 78–82. [Google Scholar] [CrossRef]
- Gussen, C.M.G.; Diniz, P.S.R.; Campos, M.L.R.; Martins, W.A.; Costa, F.M.; Gois, J.N. A Survey of Underwater Wireless Communication Technologies. J. Commun. Inf. Syst. 2016, 31, 242–255. [Google Scholar] [CrossRef]
- Zeng, Z.; Fu, S.; Zhang, H.; Dong, Y.; Cheng, J. A Survey of Underwater Optical Wireless Communications. IEEE Commun. Surv. Tutor. 2017, 19, 204–238. [Google Scholar] [CrossRef]
- Saeed, N.; Celik, A.; Al-Naffouri, T.Y.; Alouini, M.-S. Underwater optical wireless communications, networking, and localization: A survey. Ad Hoc Netw. 2019, 94, 101935. [Google Scholar] [CrossRef]
- Veldhuis, S.A.; Boix, P.P.; Yantara, N.; Li, M.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. Perovskite Materials for Light-Emitting Diodes and Lasers. Adv. Mater. 2016, 28, 6804–6834. [Google Scholar] [CrossRef]
- Ijaz, P.; Imran, M.; Soares, M.M.; Tolentino, H.C.N.; Martin-Garcia, B.; Giannini, C.; Moreels, I.; Manna, L.; Krahne, R. Composition-, Size-, and Surface Functionalization-Dependent Optical Properties of Lead Bromide Perovskite Nanocrystals. J. Phys. Chem. Lett. 2020, 11, 2079–2085. [Google Scholar] [CrossRef]
- Maddalena, F.; Witkowski, M.E.; Makowski, M.; Bachiri, A.; Mahler, B.; Wong, Y.-C.; Chua, C.Y.E.; Lee, J.X.; Drozdowski, W.; Springham, S.V.; et al. Stable and Bright Commercial CsPbBr3 Quantum Dot-Resin Layers for Apparent X-ray Imaging Screen. ACS Appl. Mater. Interfaces 2021, 13, 59450–59459. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Ou, X.; Huang, B.; Almutlaq, J.; Zhumekenov, A.A.; Guan, X.; Han, S.; Liang, L.; Yi, Z.; et al. All-inorganic perovskite nanocrystal scintillators. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef]
- Akhil, S.; Palabathuni, M.; Biswas, S.; Singh, R.; Mishra, N. Highly Stable Amine-Free CsPbBr3 Perovskite Nanocrystals for Perovskite-Based Display Applications. ACS Appl. Nano Mater. 2022, 5, 13561–13572. [Google Scholar] [CrossRef]
- Koscher, B.A.; Swabeck, J.K.; Bronstein, N.D.; Alivisatos, A.P. Essentially Trap-Free CsPbBr3 Colloidal Nanocrystals by Postsynthetic Thiocyanate Surface Treatment. J. Am. Chem. Soc. 2017, 139, 6566–6569. [Google Scholar] [CrossRef]
- Kim, Y.; Yassitepe, E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X.; Kanjanaboos, P.; Nogueira, A.F.; Sargent, E.H. Efficient Luminescence from Perovskite Quantum Dot Solids. ACS Appl. Mater. Interfaces 2015, 7, 25007–25013. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zou, Y.; Wu, L.; Huang, Q.; Yang, D.; Chen, M.; Ban, M.; Wu, C.; Wu, T.; Bai, S.; et al. Highly Luminescent and Stable Perovskite Nanocrystals with Octylphosphonic Acid as a Ligand for Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 3784–3792. [Google Scholar] [CrossRef]
- Tan, Y.; Li, R.; Xu, H.; Qin, Y.; Song, T.; Sun, B. Ultrastable and Reversible Fluorescent Perovskite Films Used for Flexible Instantaneous Display. Adv. Funct. Mater. 2019, 29, 1900730. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, P.; Pan, A.; Yan, K.; Zhu, Y.; Yang, M.; He, L. Unusual Stability and Temperature-Dependent Properties of Highly Emissive CsPbBr3 Perovskite Nanocrystals Obtained from in Situ Crystallization in Poly(vinylidene difluoride). ACS Appl. Mater. Interfaces 2019, 11, 22786–22793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Wu, D.; Xue, J.; Qiu, Y.; Liao, M.; Pei, Q.; Goorsky, M.S.; He, X. Homogeneous Freestanding Luminescent Perovskite Organogel with Superior Water Stability. Adv. Mater. 2019, 31, e1902928. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, P.; Wang, R.; Yuan, H.; Xin, Y.; Ren, X.; Chen, Q.; Yin, H. Stable MAPbBr3@PbBr(OH) composites with high photoluminescence quantum yield: Synthesis, optical properties, formation mechanism, and catalytic application. Appl. Surf. Sci. 2023, 616, 156442. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef]
- Tang, X.; Yang, J.; Li, S.; Liu, Z.; Hu, Z.; Hao, J.; Du, J.; Leng, Y.; Qin, H.; Lin, X.; et al. Single Halide Perovskite/Semiconductor Core/Shell Quantum Dots with Ultrastability and Nonblinking Properties. Adv. Sci. 2019, 6, 1900412. [Google Scholar] [CrossRef]
- Shao, H.; Bai, X.; Pan, G.; Cui, H.; Zhu, J.; Zhai, Y.; Liu, J.; Dong, B.; Xu, L.; Song, H. Highly efficient and stable blue-emitting CsPbBr3@SiO2 nanospheres through low temperature synthesis for nanoprinting and WLED. Nanotechnology 2018, 29, 285706. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, J.; Zou, X.; Pang, X.; Yang, R.; Chen, S.; Fang, Y.; Shao, T.; Luo, X.; Zhang, L. Three-dimensional TiO2/SiO2 composite aerogel films via atomic layer deposition with enhanced H2S gas sensing performance. Ceram. Int. 2018, 44, 1078–1085. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, B.; Zheng, W.; Kong, L.; Wan, Q.; Zhang, C.; Li, Z.; Cao, X.; Liu, M.; Li, L. Ceramic-like stable CsPbBr3 nanocrystals encapsulated in silica derived from molecular sieve templates. Nat. Commun. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bi, C.; Yuan, J.; Zhang, L.; Tian, J. Original Core–Shell Structure of Cubic CsPbBr3@Amorphous CsPbBrx Perovskite Quantum Dots with a High Blue Photoluminescence Quantum Yield of over 80%. ACS Energy Lett. 2017, 3, 245–251. [Google Scholar] [CrossRef]
- Xia, M.; Zhu, S.; Luo, J.; Xu, Y.; Tian, P.; Niu, G.; Tang, J. Ultrastable Perovskite Nanocrystals in All-Inorganic Transparent Matrix for High-Speed Underwater Wireless Optical Communication. Adv. Opt. Mater. 2021, 9, 2002239. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, X.; Xie, Z.; Cai, X.; Liang, S.; Ma, P.A.; Hou, Z.; Cheng, Z.; Lin, J. Enhancing the Stability of Perovskite Quantum Dots by Encapsulation in Crosslinked Polystyrene Beads via a Swelling-Shrinking Strategy toward Superior Water Resistance. Adv. Funct. Mater. 2017, 27, 1703535. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, J. Thermal, physical and chemical stability of porous polystyrene-type beads with different degrees of crosslinking. Polym. Degrad. Stab. 2001, 73, 163–167. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, H.; Xie, L.; Xuan, T.; Sun, Y.; Si, S.; Jiang, B.; Chen, W.; Zhuang, J.; Hu, C.; et al. Precipitating CsPbBr3 quantum dots in boro-germanate glass with a dense structure and inert environment toward highly stable and efficient narrow-band green emitters for wide-color-gamut liquid crystal displays. J. Mater. Chem. C 2019, 7, 13139–13148. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, L.; Li, J.; Liu, X.; Wang, R.; Wang, L.; Tu, G. Growth mechanism of CsPbBr3 perovskite nanocrystals by a co-precipitation method in a CSTR system. Nano Res. 2018, 12, 121–127. [Google Scholar] [CrossRef]
- Lou, Y.; Niu, Y.; Yang, D.; Xu, Q.; Hu, Y.; Shen, Y.; Ming, J.; Chen, J.; Zhang, L.; Zhao, Y. Rod-shaped thiocyanate-induced abnormal band gap broadening in SCN− doped CsPbBr3 perovskite nanocrystals. Nano Res. 2018, 11, 2715–2723. [Google Scholar] [CrossRef]
- Joshi, H.; Thapa, R.K.; Laref, A.; Sukkabot, W.; Pachuau, L.; Vanchhawng, L.; Grima-Gallardo, P.; Musa Saad, H.-E.M.; Rai, D.P. Electronic and optical properties of cubic bulk and ultrathin surface [001] slab of CsPbBr3. Surf. Interfaces 2022, 30, 101829. [Google Scholar] [CrossRef]
- Long, N.; Fu, Y.; Xu, T.; Ding, D.; Zhang, S.; Sun, S.; Kang, S.; Xu, T.; Dai, S.; Nie, Q.; et al. Nanocrystallization and optical properties of CsPbBr3−I perovskites in chalcogenide glasses. J. Eur. Ceram. Soc. 2021, 41, 4584–4589. [Google Scholar] [CrossRef]
- Bera, K.P.; Haider, G.; Huang, Y.T.; Roy, P.K.; Paul Inbaraj, C.R.; Liao, Y.M.; Lin, H.I.; Lu, C.H.; Shen, C.; Shih, W.Y.; et al. Graphene Sandwich Stable Perovskite Quantum-Dot Light-Emissive Ultrasensitive and Ultrafast Broadband Vertical Phototransistors. ACS Nano 2019, 13, 12540–12552. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Qin, W.; Liu, M.; Ren, X.; Wu, X.; Yang, L.; Yin, S. Scalable room-temperature synthesis of plum-pudding-like Cs4PbBr6/CsPbBr3 microcrystals exhibiting excellent photoluminescence. J. Mater. Chem. C 2019, 7, 4733–4739. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; Zhu, K.; Johnson, J.C.; Berry, J.J.; van de Lagemaat, J.; Beard, M.C. Large polarization-dependent exciton optical Stark effect in lead iodide perovskites. Nat. Commun. 2016, 7, 12613. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, J.; Shang, X.; Ma, E.; Lin, F.; Zheng, W.; Tu, D.; Li, R.; Chen, X. Unusual Temperature Dependence of Bandgap in 2D Inorganic Lead-Halide Perovskite Nanoplatelets. Adv. Sci. 2021, 8, 2100084. [Google Scholar] [CrossRef]
- Nakanotani, H.; Kabe, R.; Yahiro, M.; Takenobu, T.; Iwasa, Y.; Adachi, C. Blue-Light-Emitting Ambipolar Field-Effect Transistors Using an Organic Single Crystal of 1,4-Bis(4-methylstyryl)benzene. Appl. Phys. Express 2008, 1, 091801. [Google Scholar] [CrossRef]
- Song, Y.H.; Park, S.-Y.; Yoo, J.S.; Park, W.K.; Kim, H.S.; Choi, S.H.; Kwon, S.B.; Kang, B.K.; Kim, J.P.; Jung, H.S.; et al. Efficient and stable green-emitting CsPbBr3 perovskite nanocrystals in a microcapsule for light emitting diodes. Chem. Eng. J. 2018, 352, 957–963. [Google Scholar] [CrossRef]
- Ruan, L.J.; Tang, B.; Ma, Y. Improving the Stability of CsPbBr3 Nanocrystals in Ethanol by Capping with PbBr2-Adlayers. J. Phys. Chem. C 2019, 123, 11959–11967. [Google Scholar] [CrossRef]
- Zhihai, W.; Jiao, W.; Yanni, S.; Jun, W.; Yafei, H.; Pan, W.; Nengping, W.; Zhenfu, Z. Air-stable all-inorganic perovskite quantum dot inks for multicolor patterns and white LEDs. J. Mater. Sci. 2019, 54, 6917–6929. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, C.; Cai, Z.; Xiao, C.; Chen, X.; Yi, K.; Yang, Y.; Lu, Y.; Wei, D. Enhanced photoelectrical response of thermodynamically epitaxial organic crystals at the two-dimensional limit. Nat. Commun. 2019, 10, 756. [Google Scholar] [CrossRef]
- Baba, K.; Nishida, K. Preparation of 1,4-bis(4-methylstyryl)benzene nanocrystals by a wet process and evaluation of their optical properties. Nanoscale Res. Lett. 2014, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Bhardwaj, K.; Basel, S.; Pradhan, S.; Eling, C.J.; Adawi, A.M.; Bouillard, J.-S.G.; Stasiuk, G.J.; Reiss, P.; Pariyar, A.; et al. Long-term ambient air-stable cubic CsPbBr3 perovskite quantum dots using molecular bromine. Nanoscale Adv. 2019, 1, 3388–3391. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Fu, Y.; Zhou, Y.; Han, J.; Mao, Y.; Li, M.; Liu, J.; Huang, M. The surface-enhanced Raman scattering of all-inorganic perovskite quantum dots of CsPbBr3 encapsulated in a ZIF-8 metal–organic framework. New J. Chem. 2020, 44, 17570–17576. [Google Scholar] [CrossRef]
- Cai, Z.; Cao, M.; Jin, Z.; Yi, K.; Chen, X.; Wei, D. Large photoelectric-gating effect of two-dimensional van-der-Waals organic/tungsten diselenide heterointerface. NPJ 2D Mater. Appl. 2018, 2, 21. [Google Scholar] [CrossRef]
- Xu, J.; Yu, S.; Shang, X.; Chen, X. Temperature Dependence of Bandgap in Lead-Halide Perovskites with Corner-Sharing Octahedra. Adv. Photonics Res. 2022, 4, 2200193. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Cen, G.; Liu, Y.; Zhao, C.; Wang, G.; Fu, Y.; Yan, G.; Yuan, Y.; Su, C.; Zhao, Z.; Mai, W. Atomic-Layer Deposition-Assisted Double-Side Interfacial Engineering for High-Performance Flexible and Stable CsPbBr3 Perovskite Photodetectors toward Visible Light Communication Applications. Small 2019, 15, e1902135. [Google Scholar] [CrossRef]
- Yasuda, T.; Saito, M.; Nakamura, H.; Tsutsui, T. Organic Field-Effect Transistors Based on Oligo-p-Phenylenevinylene Derivatives. Jpn. J. Appl. Phys. 2006, 45, L313–L315. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.K.; Yuan, F.; Johnston, A.; Liu, Y.; Ma, D.; Choi, M.J.; Chen, B.; Chekini, M.; Baek, S.W.; et al. Bipolar-shell resurfacing for blue LEDs based on strongly confined perovskite quantum dots. Nat. Nanotechnol. 2020, 15, 668–674. [Google Scholar] [CrossRef]
- Yang, H.S.; Noh, S.H.; Suh, E.H.; Jung, J.; Oh, J.G.; Lee, K.H.; Jang, J. Enhanced Stabilities and Production Yields of MAPbBr3 Quantum Dots and Their Applications as Stretchable and Self-Healable Color Filters. ACS Appl. Mater. Interfaces 2021, 13, 4374–4384. [Google Scholar] [CrossRef]
- Qaid, S.M.H.; Ghaithan, H.M.; Al-Asbahi, B.A.; Aldwayyan, A.S. Ultra-Stable Polycrystalline CsPbBr3 Perovskite-Polymer Composite Thin Disk for Light-Emitting Applications. Nanomaterials 2020, 10, 2382. [Google Scholar] [CrossRef]
- Yang, L.; Fu, B.; Li, X.; Chen, H.; Li, L. Poly(vinylidene fluoride)-passivated CsPbBr3 perovskite quantum dots with near-unity photoluminescence quantum yield and superior stability. J. Mater. Chem. C 2021, 9, 1983–1991. [Google Scholar] [CrossRef]
- Li, P.; Cheng, Y.; Zhou, L.; Yu, X.; Jiang, J.; He, M.; Liang, X.; Xiang, W. Photoluminescence properties and device application of CsPb2Br5 quantum dots in glasses. Mater. Res. Bull. 2018, 105, 63–67. [Google Scholar] [CrossRef]
- Wang, L.; Ma, D.; Guo, C.; Jiang, X.; Li, M.; Xu, T.; Zhu, J.; Fan, B.; Liu, W.; Shao, G.; et al. CsPbBr3 nanocrystals prepared by high energy ball milling in one-step and structural transformation from CsPbBr3 to CsPb2Br5. Appl. Surf. Sci. 2021, 543, 148782. [Google Scholar] [CrossRef]
- Lei, J.; Gao, F.; Wang, H.; Li, J.; Jiang, J.; Wu, X.; Gao, R.; Yang, Z.; Liu, S.F. Efficient planar CsPbBr3 perovskite solar cells by dual-source vacuum evaporation. Sol. Energy Mater. Sol. Cells 2018, 187, 1–8. [Google Scholar] [CrossRef]
- Tong, G.; Chen, T.; Li, H.; Qiu, L.; Liu, Z.; Dang, Y.; Song, W.; Ono, L.K.; Jiang, Y.; Qi, Y. Phase transition induced recrystallization and low surface potential barrier leading to 10.91%-efficient CsPbBr3 perovskite solar cells. Nano Energy 2019, 65, 104015. [Google Scholar] [CrossRef]
- Yu, M.; He, X.; Li, D.; Lin, J.; Yu, C.; Fang, Y.; Liu, Z.; Guo, Z.; Huang, Y.; Tang, C. Anchoring of CsPbBr3 perovskite quantum dots on BN nanostructures for enhanced efficiency and stability: A comparative study. J. Mater. Chem. C 2021, 9, 842–850. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, e1800764. [Google Scholar] [CrossRef]
- Liu, J.; Peng, Y.; Zhu, B.; Li, Y.; Zhang, L.; Yu, J. ZIF-8 derived ZnO-CsPbBr3 polyhedrons for efficient triethylamine detection. Sens. Actuators B Chem. 2022, 357, 131366. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.; Kong, L.; Wang, B.; Zhang, C.; Yuan, Q.; Huang, S.; Liu, Y.; Li, L. Enhancing the stability of CsPbBr3 nanocrystals by sequential surface adsorption of S2− and metal ions. Chem. Commun. 2018, 54, 9345–9348. [Google Scholar] [CrossRef]
- Barbé, J.; Kumar, V.; Newman, M.J.; Lee, H.K.H.; Jain, S.M.; Chen, H.; Charbonneau, C.; Rodenburg, C.; Tsoi, W.C. Dark electrical bias effects on moisture-induced degradation in inverted lead halide perovskite solar cells measured by using advanced chemical probes. Sustain. Energy Fuels 2018, 2, 905–914. [Google Scholar] [CrossRef]
- Di Girolamo, D.; Dar, M.I.; Dini, D.; Gontrani, L.; Caminiti, R.; Mattoni, A.; Graetzel, M.; Meloni, S. Dual effect of humidity on cesium lead bromide: Enhancement and degradation of perovskite films. J. Mater. Chem. A 2019, 7, 12292–12302. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; He, B.; Tang, Q. High-Purity Inorganic Perovskite Films for Solar Cells with 9.72 % Efficiency. Angew. Chem. Int. Ed. 2018, 57, 3787–3791. [Google Scholar] [CrossRef]
- Sandeep, K. CsPbBr3 perovskite nanocrystals coated paper substrate as atmospheric humidity sensor. Mater. Today Proc. 2021, 41, 610–612. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Li, H.; Tong, G.; Chen, T.; Zhu, H.; Li, G.; Chang, Y.; Wang, L.; Jiang, Y. Interface engineering using a perovskite derivative phase for efficient and stable CsPbBr3 solar cells. J. Mater. Chem. A 2018, 6, 14255–14261. [Google Scholar] [CrossRef]
- Shan, Q.; Li, J.; Song, J.; Zou, Y.; Xu, L.; Xue, J.; Dong, Y.; Huo, C.; Chen, J.; Han, B.; et al. All-inorganic quantum-dot light-emitting diodes based on perovskite emitters with low turn-on voltage and high humidity stability. J. Mater. Chem. C 2017, 5, 4565–4570. [Google Scholar] [CrossRef]
- Yang, H.; Yin, W.; Dong, W.; Gao, L.; Tan, C.-H.; Li, W.; Zhang, X.; Zhang, J. Enhancing the light-emitting performance and stability in CsPbBr3 perovskite quantum dots via simultaneous doping and surface passivation. J. Mater. Chem. C 2020, 8, 14439–14445. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, M.-y.; Liu, S.; Ma, Y.; Sun, B.; Wang, L.; Lu, H.; Wen, X.; Liu, S.; Ding, X. A Novel Strategy for the Synthesis of High Stability of Luminescent Zero Dimensional–Two Dimensional CsPbBr3 Quantum Dot/1,4-bis(4-methylstyryl)benzene Nanoplate Heterostructures at an Atmospheric Condition. Nanomaterials 2023, 13, 2723. https://doi.org/10.3390/nano13192723
Wang Y, Li M-y, Liu S, Ma Y, Sun B, Wang L, Lu H, Wen X, Liu S, Ding X. A Novel Strategy for the Synthesis of High Stability of Luminescent Zero Dimensional–Two Dimensional CsPbBr3 Quantum Dot/1,4-bis(4-methylstyryl)benzene Nanoplate Heterostructures at an Atmospheric Condition. Nanomaterials. 2023; 13(19):2723. https://doi.org/10.3390/nano13192723
Chicago/Turabian StyleWang, Yanran, Ming-yu Li, Shijie Liu, Yuan Ma, Bo Sun, Liangyu Wang, Haifei Lu, Xiaoyan Wen, Sisi Liu, and Xumin Ding. 2023. "A Novel Strategy for the Synthesis of High Stability of Luminescent Zero Dimensional–Two Dimensional CsPbBr3 Quantum Dot/1,4-bis(4-methylstyryl)benzene Nanoplate Heterostructures at an Atmospheric Condition" Nanomaterials 13, no. 19: 2723. https://doi.org/10.3390/nano13192723
APA StyleWang, Y., Li, M.-y., Liu, S., Ma, Y., Sun, B., Wang, L., Lu, H., Wen, X., Liu, S., & Ding, X. (2023). A Novel Strategy for the Synthesis of High Stability of Luminescent Zero Dimensional–Two Dimensional CsPbBr3 Quantum Dot/1,4-bis(4-methylstyryl)benzene Nanoplate Heterostructures at an Atmospheric Condition. Nanomaterials, 13(19), 2723. https://doi.org/10.3390/nano13192723








