Rational Construction of C@Sn/NSGr Composites as Enhanced Performance Anodes for Lithium Ion Batteries
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Graphene
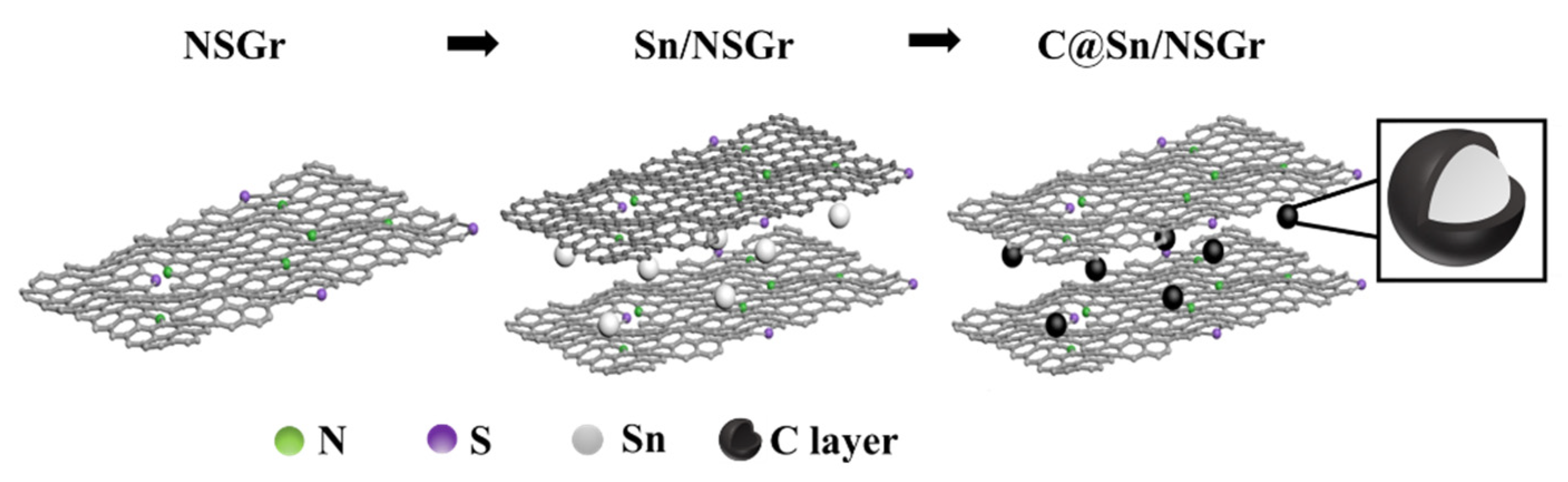
2.2. Preparation of the C@Sn/NSGr Composites
2.3. Characterization of the Materials
2.4. Electrochemical Tests
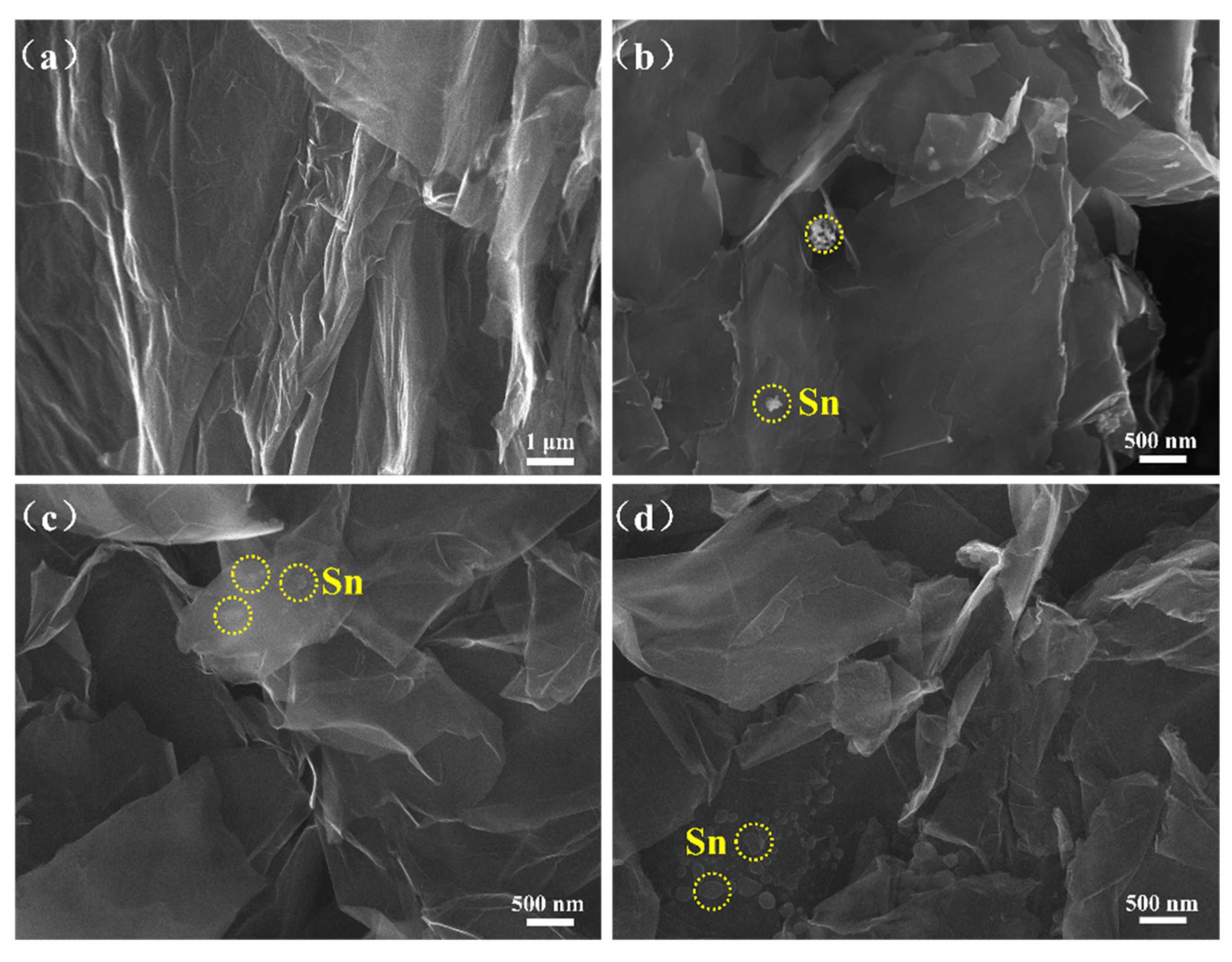
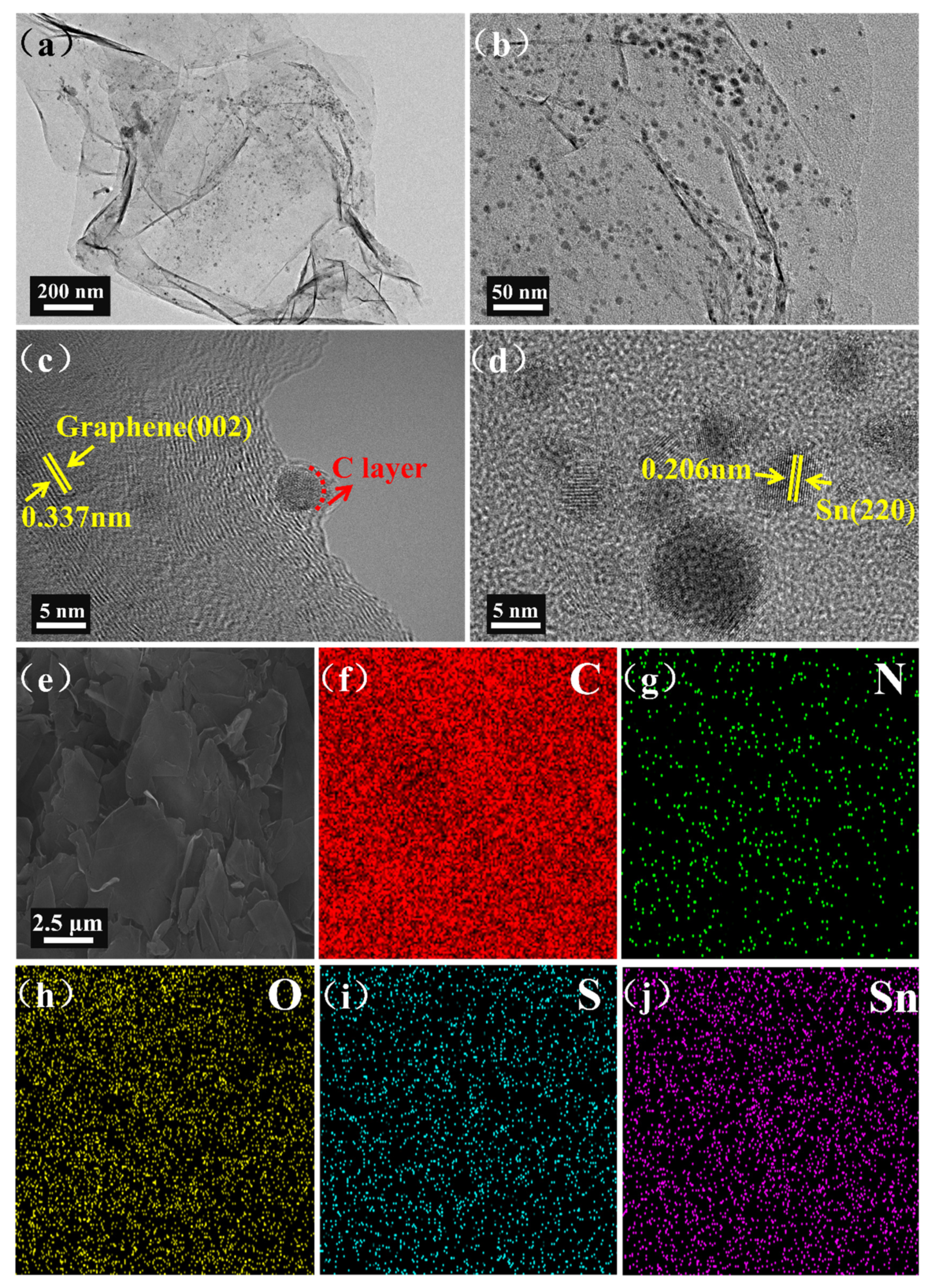
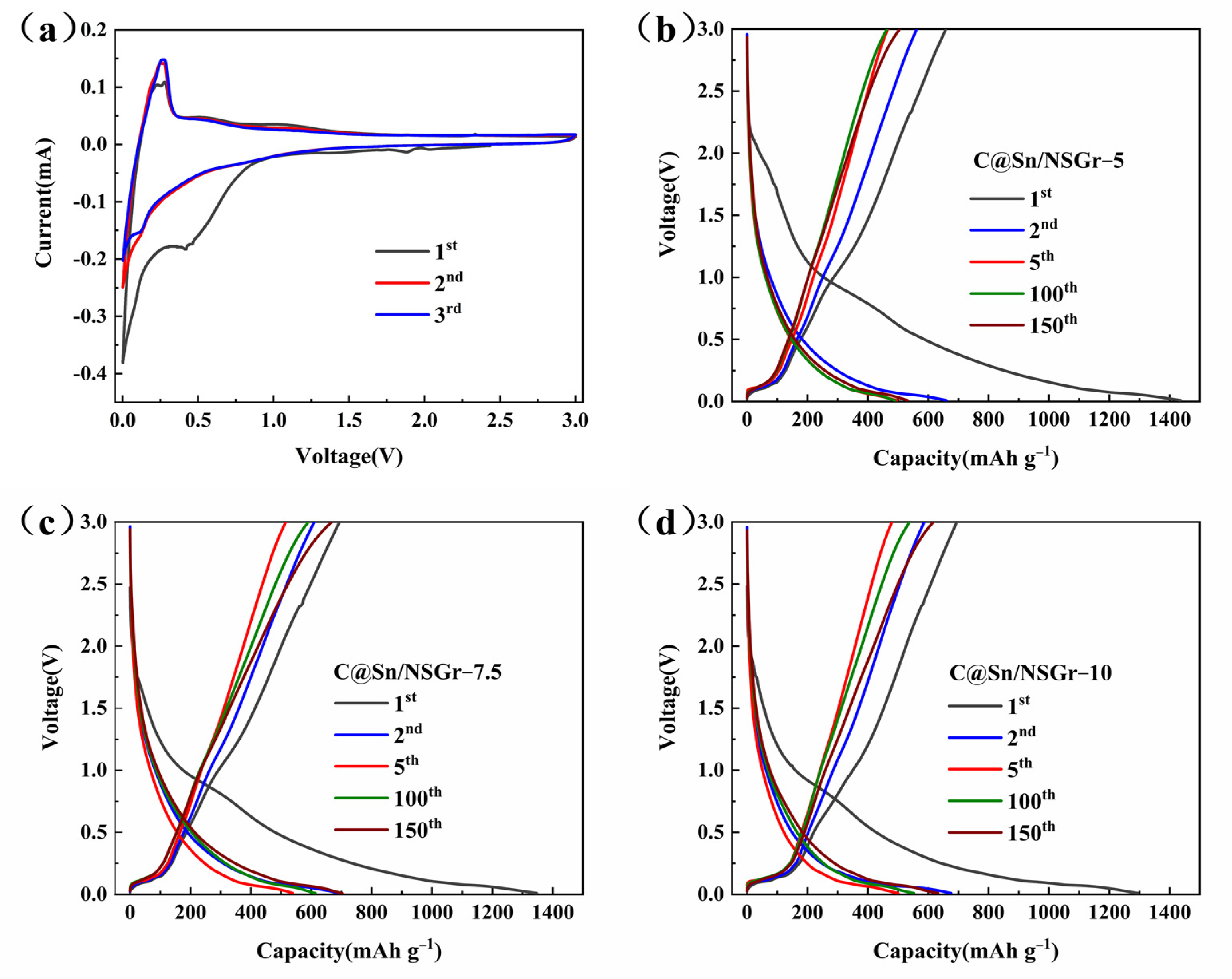
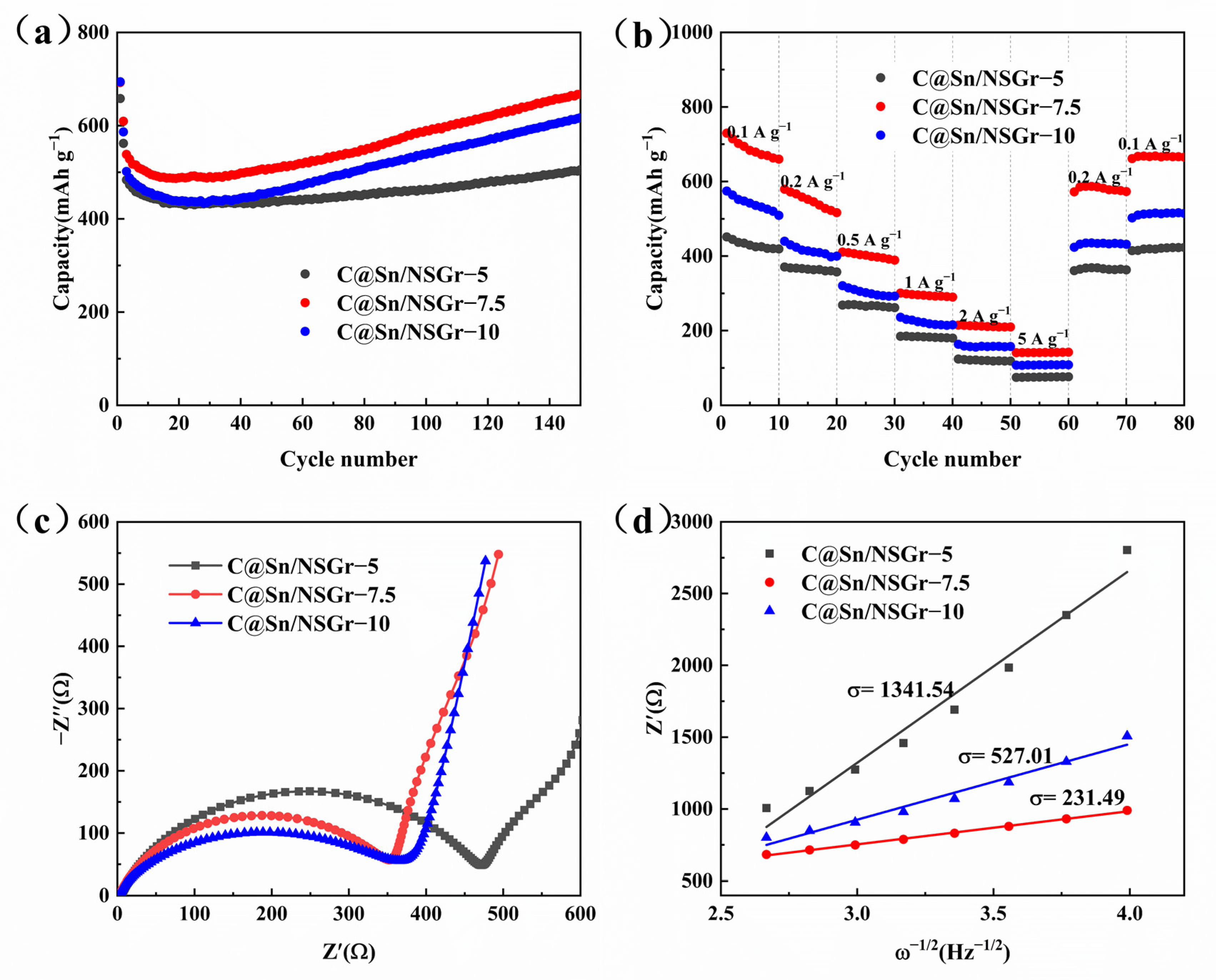
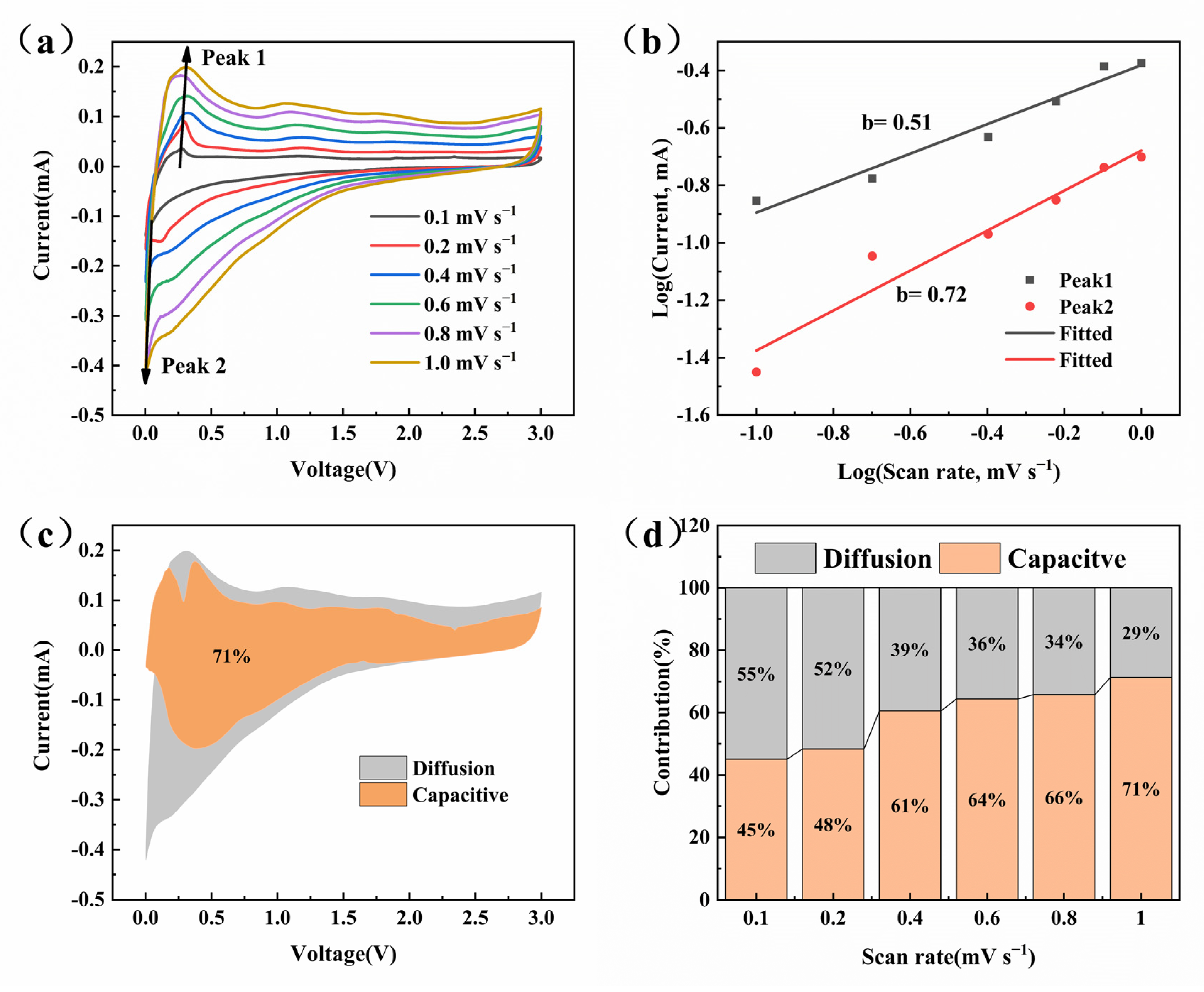
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef]
- Ding, S.; Cheng, W.; Du, G.; Su, Q.; Guo, L.; Chen, X.; Zhang, S.; Shang, L.; Hao, X.; Xu, B.; et al. Millimeter-scale laminar graphene matrix by organic molecule confinement reaction. Carbon 2020, 161, 277–286. [Google Scholar] [CrossRef]
- Cui, R.; Lin, J.; Cao, X.; Hao, P.; Xie, X.; Zhou, S.; Wang, Y.; Liang, S.; Pan, A. In situ formation of porous LiCuVO4/LiVO3/C nanotubes as a high-capacity anode material for lithium ion batteries. Inorg. Chem. Front. 2020, 7, 340–346. [Google Scholar] [CrossRef]
- Xia, J.; Liu, L.; Xie, J.; Yan, H.; Yuan, Y.; Chen, M.; Huang, C.; Zhang, Y.; Nie, S.; Wang, X. Layer-by-layered SnS2/graphene hybrid nanosheets via ball-milling as promising anode materials for lithium ion batteries. Electrochim. Acta 2018, 269, 452–461. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.-H.; Lu, W.; Dai, L. Recent Advances in Fiber-Shaped Supercapacitors and Lithium-Ion Batteries. Adv. Mater. 2020, 32, e1902779. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Abraham, K.M. Prospects and Limits of Energy Storage in Batteries. J. Phys. Chem. Lett. 2015, 6, 830–844. [Google Scholar] [CrossRef]
- Wu, S.; Xu, R.; Lu, M.; Ge, R.; Iocozzia, J.; Han, C.; Jiang, B.; Lin, Z. Graphene-Containing Nanomaterials for Lithium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1500400. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, R.; Jiang, D.; Qian, Z.; Li, Y.; Yang, K.; Sun, Y.; Zeng, Z.; Wu, F. Recent Developments of Two-Dimensional Anode Materials and Their Composites in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 7440–7461. [Google Scholar] [CrossRef]
- Xu, H.; Chen, H.; Gao, C. Advanced Graphene Materials for Sodium/Potassium/Aluminum-Ion Batteries. ACS Mater. Lett. 2021, 3, 1221–1237. [Google Scholar] [CrossRef]
- Yi, L.; Liu, L.; Guo, G.; Chen, X.; Zhang, Y.; Yu, S.; Wang, X. Expanded graphite@SnO2@ polyaniline Composite with Enhanced Performance as Anode Materials for Lithium Ion Batteries. Electrochim. Acta 2017, 240, 63–71. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Zhang, H.; Jiang, H.; Li, C. Confined Synthesis of FeS2 Nanoparticles Encapsulated in Carbon Nanotube Hybrids for Ultrastable Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 4251–4255. [Google Scholar] [CrossRef]
- Wang, B.-P.; Lv, R.; Lan, D.-S. Preparation and electrochemical properties of Sn/C composites. Rare Met. 2019, 38, 996–1002. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, M.; Zhao, W.; Xu, R.; Liu, Y.; Shen, H. SnO2 Nanosheets Anchored on a 3D, Bicontinuous Electron and Ion Transport Carbon Network for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38006–38014. [Google Scholar] [CrossRef]
- Jin, X.; Liang, M.; Bai, X.; He, C.; Zhao, N. NaCl Pinning Induced Ultrafine Sn Nanoparticles Anchored on Three-Dimensional Porous Carbon for Na Storage. ACS Appl. Energy Mater. 2022, 5, 7382–7391. [Google Scholar] [CrossRef]
- Wu, X.L.; Guo, Y.; Wan, L. Rational design of anode materials based on Group IVA elements (Si, Ge, and Sn) for lithium-ion batteries. Chem. Asian J. 2013, 8, 1948–1958. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Cui, L.; Qu, Y.; Li, Q.; Li, X.; Wang, Y.; Wang, H. Electroless plating of a Sn-Ni/graphite sheet composite with improved cyclability as an anode material for lithium ion batteries. RSC. Adv. 2018, 8, 15427–15435. [Google Scholar] [CrossRef]
- Riedel, O.; Düttmann, A.; Dühnen, S.; Kolny-Olesiak, J.; Gutsche, C.; Parisi, J.; Winter, M.; Knipper, M.; Placke, T. Surface-Modified Tin Nanoparticles and Their Electrochemical Performance in Lithium Ion Battery Cells. ACS Appl. Nano Mater. 2019, 2, 3577–3589. [Google Scholar] [CrossRef]
- Meng, F.; Zhai, Y.; Ma, Y.; Zou, X.; Guo, X.; Xu, J.; Zhu, Y.; Wang, J.; Gong, L. Stabilization of Ultra-Small Stannic Oxide Nanoparticles in Optimizing the Lithium Storage Kinetics. Energy Fuels 2022, 36, 4034–4041. [Google Scholar] [CrossRef]
- Nowak, A.P.; Trzciński, K.; Szkoda, M.; Trykowski, G.; Gazda, M.; Karczewski, J.; Łapiński, M.; Maskowicz, D.; Sawczak, M.; Lisowska-Oleksiak, A. Nano Tin/Tin Oxide Attached onto Graphene Oxide Skeleton as a Fluorine Free Anode Material for Lithium-Ion Batteries. Inorg. Chem. 2020, 59, 4150–4159. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Q.; Han, X.; Yang, J.; Chen, J. Pitaya-like Sn@C nanocomposites as high-rate and long-life anode for lithium-ion batteries. Nanoscale 2014, 6, 2827–2832. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y. Sn@CNT nanostructures rooted in graphene with high and fast Li-storage capacities. ACS Nano 2011, 5, 8108–8114. [Google Scholar] [CrossRef]
- Ding, S.; Cheng, W.; Zhang, L.; Du, G.; Hao, X.; Nie, G.; Xu, B.; Zhang, M.; Su, Q.; Serra, C.A. Organic molecule confinement reaction for preparation of the Sn nanoparticles@graphene anode materials in Lithium-ion battery. J. Colloid Interface Sci. 2021, 589, 308–317. [Google Scholar] [CrossRef]
- Zhao, Z.; Das, S.; Xing, G.; Fayon, P.; Heasman, P.; Jay, M.; Bailey, S.; Lambert, C.; Yamada, H.; Wakihara, T.; et al. A 3D Organically Synthesized Porous Carbon Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 11952–11956. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in Photocatalysis: A Review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef]
- Qin, J.; He, C.; Zhao, N.; Wang, Z.; Shi, C.; Liu, E.-Z.; Li, J. Graphene networks anchored with sn@graphene as lithium ion battery anode. ACS Nano 2014, 8, 1728–1738. [Google Scholar]
- Li, N.; Song, H.; Cui, H.; Wang, C. Sn@graphene grown on vertically aligned graphene for high-capacity, high-rate, and long-life lithium storage. Nano Energy 2014, 3, 102–112. [Google Scholar] [CrossRef]
- Jarulertwathana, N.; Laokawee, V.; Susingrat, W.; Hwang, S.-J.; Sarakonsri, T. Nano-structure tin/nitrogen-doped reduced graphene oxide composites as high capacity lithium-ion batteries anodes. J. Mater. Sci. Mater. Electron. 2017, 28, 18994–19002. [Google Scholar]
- Sun, Q.; Huang, Y.; Wu, S.; Gao, Z.; Liu, H.; Hu, P.; Qie, L. Facile Synthesis of Sn/Nitrogen-Doped Reduced Graphene Oxide Nanocomposites with Superb Lithium Storage Properties. Nanomaterials 2019, 9, 1084. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Xiao, Q.; Jia, L.; Lin, H.; Zhang, Y. Hierarchical Sulfur-Doped Graphene Foam Embedded with Sn Nanoparticles for Superior Lithium Storage in LiFSI-Based Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 30500–30507. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, S.; Du, J.; Jin, Q.; Zhang, T.; Cheng, F.; Chen, J. Ultrasmall Sn nanoparticles embedded in nitrogen-doped porous carbon as high-performance anode for lithium-ion batteries. Nano Lett. 2014, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhu, J.; Zeng, W.; Hou, S.; Gong, F.; Li, F.; Li, C.C.; Duan, H. Tin quantum dots embedded in nitrogen-doped carbon nanofibers as excellent anode for lithium-ion batteries. Nano Energy 2014, 9, 61–70. [Google Scholar] [CrossRef]
- Heller, E.J.; Yang, Y.; Kocia, L.; Chen, W.; Fang, S.; Borunda, M.; Kaxiras, E. Theory of Graphene Raman Scattering. ACS Nano 2016, 10, 2803–2818. [Google Scholar] [CrossRef] [PubMed]
- Passe-Coutrin, N.; Altenor, S.; Cossement, D.; Jean-Marius, C.; Gaspard., S. Comparison of parameters calculated from the BET and Freundlich isotherms obtained by nitrogen adsorption on activated carbons: A new method for calculating the specific surface area. Microporous Mesoporous Mater. 2008, 111, 517–522. [Google Scholar] [CrossRef]
- Hayati-Ashtiani, M. Characterization of Nano-Porous Bentonite (Montmorillonite) Particles using FTIR and BET-BJH Analyses. Part. Part. Syst. Charact. 2011, 28, 71–76. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous Carbons: Structure-Oriented Design and Versatile Applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Weng, B.; Wang, B.; Li, C. Facile synthesis of nitrogen and sulfur co-doped carbon dots and application for Fe(III) ions detection and cell imaging. Sens. Actuators B Chem. 2016, 223, 689–696. [Google Scholar] [CrossRef]
- Paparazzo, E. On the interpretation of XPS spectra of metal (Pt, Pt–Sn) nanoparticle/graphene systems. Carbon 2013, 63, 578–581. [Google Scholar] [CrossRef]
- Ye, X.; Lin, Z.; Liang, S.; Huang, X.; Qiu, X.; Qiu, Y.; Liu, X.; Xie, D.; Deng, H.; Xiong, X.; et al. Upcycling of Electroplating Sludge into Ultrafine Sn@C Nanorods with Highly Stable Lithium Storage Performance. Nano Lett. 2019, 19, 1860–1866. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Ye, H.; Chen, J.; Zhang, L.; Wei, H.; Liu, Z.; Qian, Y. Controlled Tin Oxide Nanoparticles Encapsulated in N-Doped Carbon Nanofibers for Superior Lithium-Ion Storage. ACS Appl. Energy Mater. 2022, 5, 1840–1848. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, M.; Feng, J.; Ma, Y.; Xiong, S. Enhancing the cycling stability of Na-ion batteries by bonding SnS2ultrafine nanocrystals on amino-functionalized graphene hybrid nanosheets. Energy Environ. Sci. 2016, 9, 1430–1438. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Zhao, H.; Yu, H. Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: The enhanced performance by sulfur doping. Electrochim. Acta 2016, 204, 169–175. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, S.; Meng, Z.; Sun, Z.; Han, W.Q. Ultrasmall Sn nanodots embedded inside N-doped carbon microcages as high-performance lithium and sodium ion battery anodes. J. Mater. Chem. A 2017, 5, 8334–8342. [Google Scholar] [CrossRef]
- Hwang, J.; Nam, D.; Kim, J. Carbon-coated Sn-reduced graphene oxide composite synthesized using supercritical methanol and high-pressure free meniscus coating for Na-ion batteries. J. Supercrit. Fluids 2022, 189, 105720. [Google Scholar] [CrossRef]
- Mao, Y.; Duan, H.; Xu, B.; Zhang, L.; Hu, Y.; Zhao, C.; Wang, Z.; Chen, L.; Yang, Y. Lithium storage in nitrogen-rich mesoporous carbon materials. Energy Environ. Sci. 2012, 5, 7950–7955. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Tan, X.; Wang, H.; Holt, C.M.B.; Stephenson, T.; Olsena, B.C.; Mitlin, D. Mesoporous nitrogen-rich carbons derived from protein for ultra-high capacity battery anodes and supercapacitors. Energy Environ. Sci. 2013, 6, 871–878. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Li, S.; Yang, L.; Ji, X. Tailoring Rod-Like FeSe2 Coated with Nitrogen-Doped Carbon for High-Performance Sodium Storage. Adv. Funct. Mater. 2018, 28, 1801765. [Google Scholar] [CrossRef]
- Xu, W.; Tang, C.; Huang, N.; Du, A.; Wu, M.; Zhang, J.; Zhang, H. Adina Rubella-Like Microsized SiO@N-Doped Carbon Grafted with N-Doped Carbon Nanotubes as Anodes for High-Performance Lithium Storage. Small Sci. 2022, 2, 2100105. [Google Scholar] [CrossRef]
- Lu, P.; Sun, Y.; Xiang, H.; Liang, X.; Yu, Y. 3D Amorphous Carbon with Controlled Porous and Disordered Structures as a High-Rate Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702434. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, T.; Cheng, Y.-J.; Yin, S.; Xia, Y.; Ji, Q.; Xu, Z.; Liang, S.; Ma, L.; Zuo, X.; et al. SnO2/Sn/Carbon nanohybrid lithium-ion battery anode with high reversible capacity and excellent cyclic stability. Nano Select 2021, 2, 642–653. [Google Scholar] [CrossRef]
- Fang, Y.; Yu, X.Y.; Lou, X.W.D. Formation of Hierarchical Cu-Doped CoSe2 Microboxes via Sequential Ion Exchange for High-Performance Sodium-Ion Batteries. Adv. Mater. 2018, 30, e1706668. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Li, Y.; Wang, X.; Zhang, Z.; Huang, J.; Zhang, J.; Liang, X.; Su, J.; Ouyang, L.; Huang, J. Rational Construction of C@Sn/NSGr Composites as Enhanced Performance Anodes for Lithium Ion Batteries. Nanomaterials 2023, 13, 271. https://doi.org/10.3390/nano13020271
Yang G, Li Y, Wang X, Zhang Z, Huang J, Zhang J, Liang X, Su J, Ouyang L, Huang J. Rational Construction of C@Sn/NSGr Composites as Enhanced Performance Anodes for Lithium Ion Batteries. Nanomaterials. 2023; 13(2):271. https://doi.org/10.3390/nano13020271
Chicago/Turabian StyleYang, Guanhua, Yihong Li, Xu Wang, Zhiguo Zhang, Jiayu Huang, Jie Zhang, Xinghua Liang, Jian Su, Linhui Ouyang, and Jianling Huang. 2023. "Rational Construction of C@Sn/NSGr Composites as Enhanced Performance Anodes for Lithium Ion Batteries" Nanomaterials 13, no. 2: 271. https://doi.org/10.3390/nano13020271
APA StyleYang, G., Li, Y., Wang, X., Zhang, Z., Huang, J., Zhang, J., Liang, X., Su, J., Ouyang, L., & Huang, J. (2023). Rational Construction of C@Sn/NSGr Composites as Enhanced Performance Anodes for Lithium Ion Batteries. Nanomaterials, 13(2), 271. https://doi.org/10.3390/nano13020271





