MOF-Derived CeO2 and CeZrOx Solid Solutions: Exploring Ce Reduction through FTIR and NEXAFS Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Thermogravimetric (TG) Analysis
2.3. Powder X-ray Diffraction (PXRD)
2.4. Specific Surface Area (SSA)
2.5. Transmission Electron Microscopy (TEM)
2.6. In Situ Fourier Transform-Infrared (FT-IR)
2.7. Ambient Pressure Near-Edge X-ray Absorption Spectra (AP-NEXAFS)
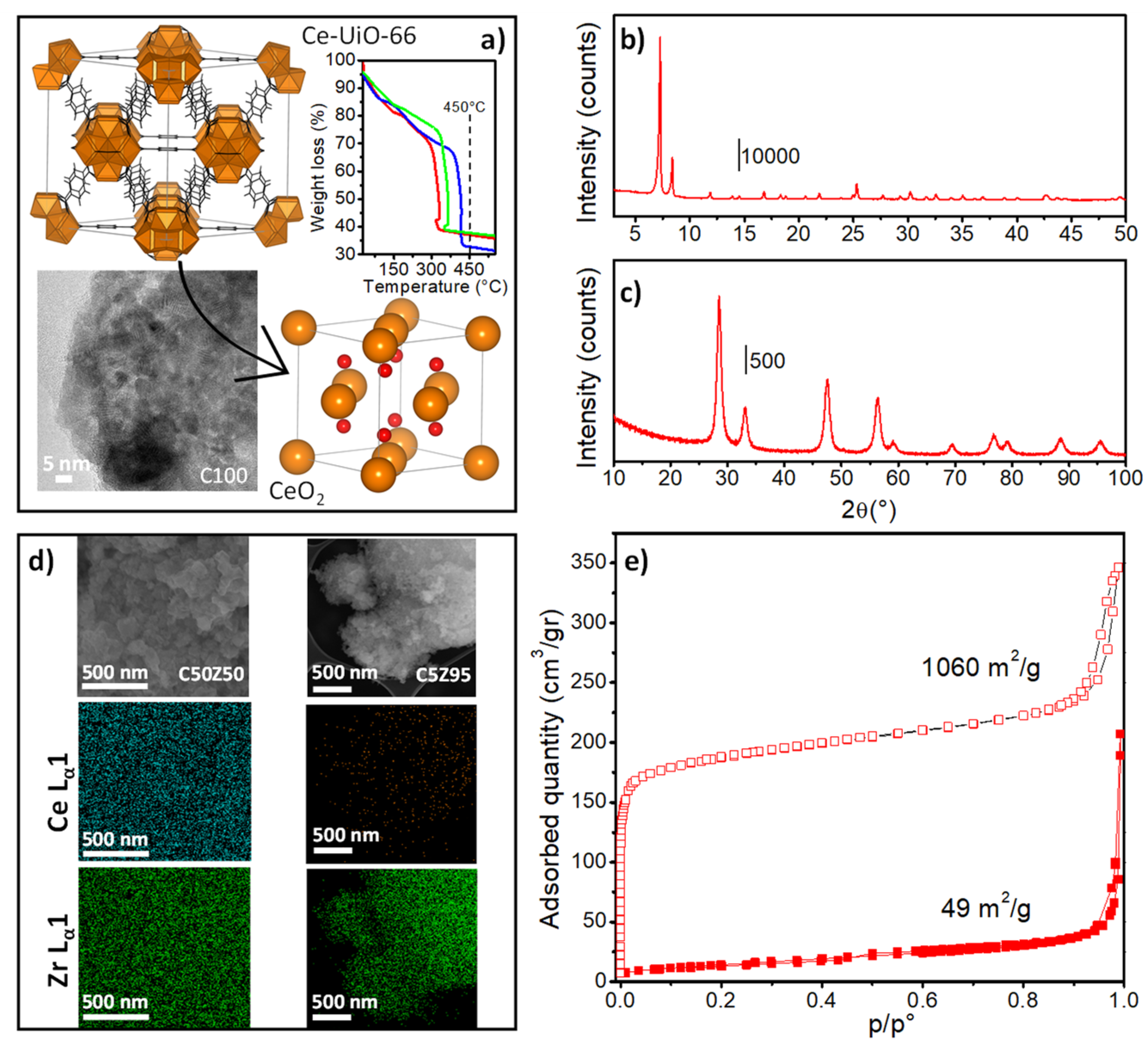
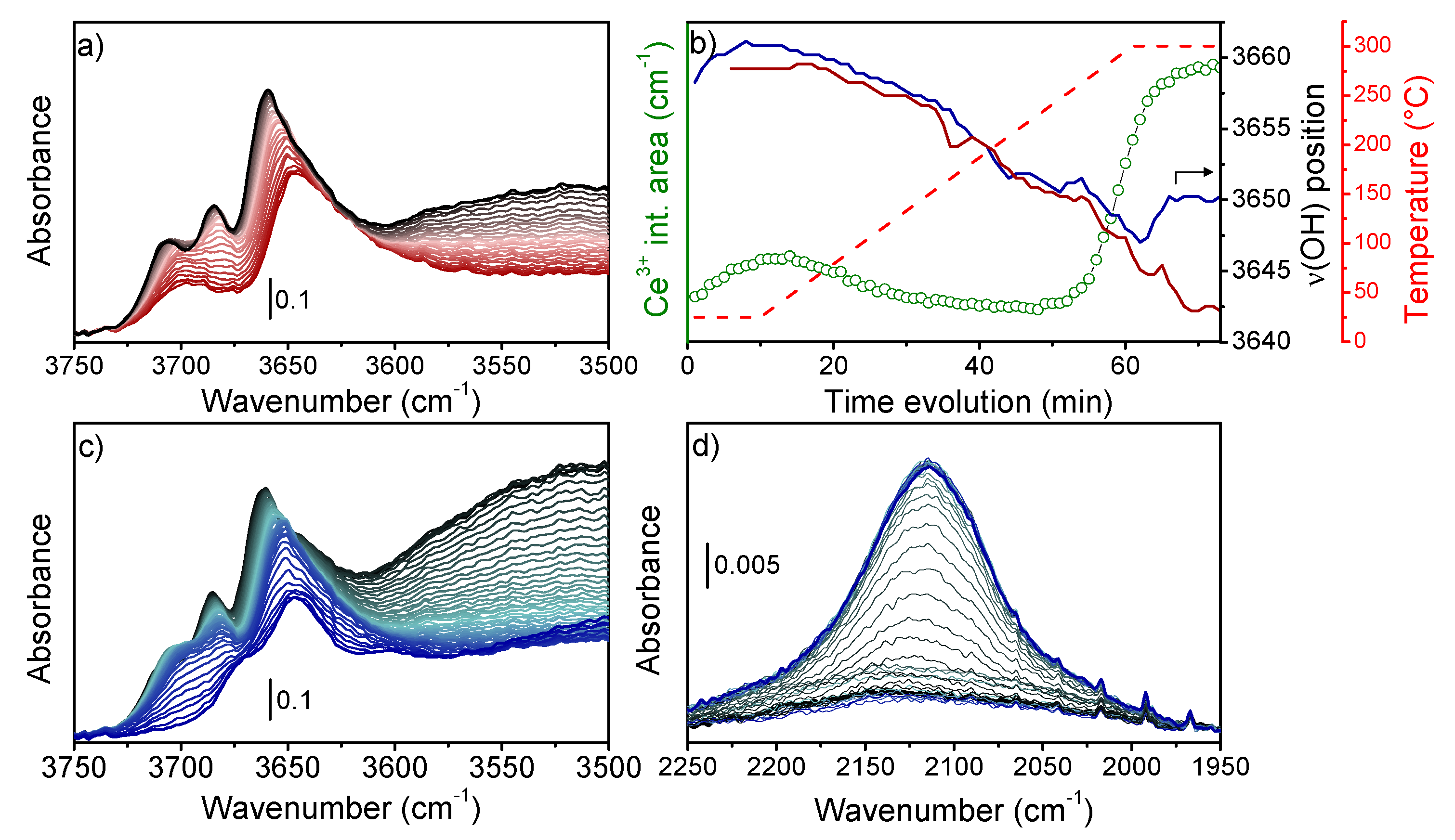
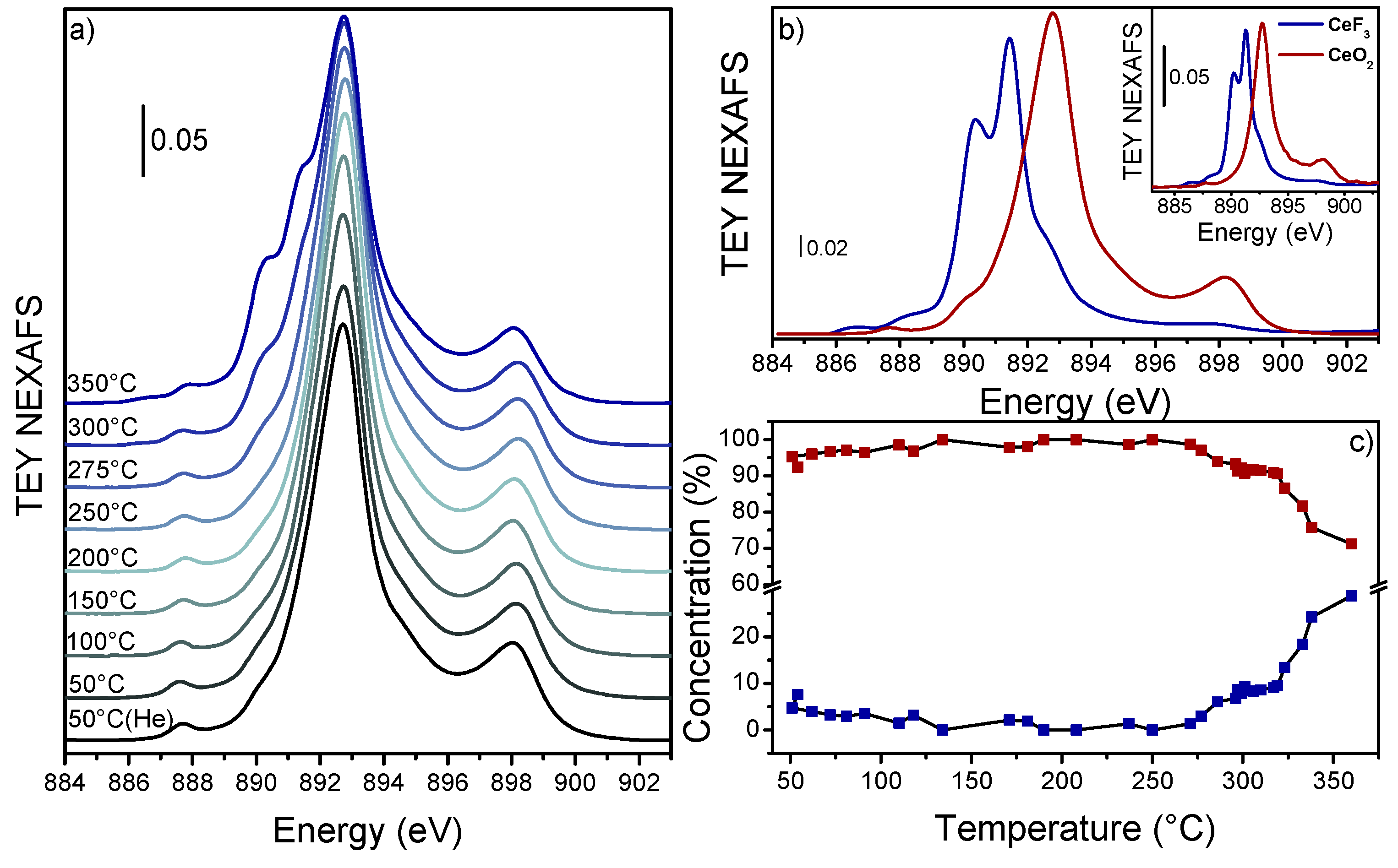
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wei, M.; Evans, D.G.; Duan, X. CeO2-Based Heterogeneous Catalysts toward Catalytic Conversion of CO2. J. Mater. Chem. A 2016, 4, 5773–5783. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Kumar, A.; Khraisheh, M. A Review of CeO2 Supported Catalysts for CO2 Reduction to CO through the Reverse Water Gas Shift Reaction. Catalysts 2022, 12, 1101. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Song, S.; Zhang, H. Syntheses and Applications of Noble-Metal-Free CeO2-Based Mixed-Oxide Nanocatalysts. Chem 2019, 5, 1743–1774. [Google Scholar] [CrossRef]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen Defects and Surface Chemistry of Ceria: Quantum Chemical Studies Compared to Experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Boaro, M.; Colussi, S.; Trovarelli, A. Ceria-Based Materials in Hydrogenation and Reforming Reactions for CO2 Valorization. Front. Chem. 2019, 7, 28. [Google Scholar] [CrossRef]
- Chang, K.; Zhang, H.; Cheng, M.J.; Lu, Q. Application of Ceria in CO2 Conversion Catalysis. ACS Catal. 2020, 10, 613–631. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, M.G.; Shin, D.; Han, J.W. Design of Ceria Catalysts for Low-Temperature CO Oxidation. ChemCatChem 2020, 12, 11–26. [Google Scholar] [CrossRef]
- Chen, D.; Niakolas, D.K.; Papaefthimiou, V.; Ioannidou, E.; Neophytides, S.G.; Zafeiratos, S. How the Surface State of Nickel/Gadolinium-Doped Ceria Cathodes Influences the Electrochemical Performance in Direct CO2 Electrolysis. J. Catal. 2021, 404, 518–528. [Google Scholar] [CrossRef]
- Can, F.; Berland, S.; Royer, S.; Courtois, X.; Duprez, D. Composition-Dependent Performance of CexZr1-XO2 Mixed-Oxide-Supported WO3 Catalysts for the NOx Storage Reduction-Selective Catalytic Reduction Coupled Process. ACS Catal. 2013, 3, 1120–1132. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lu, Y.; Zhang, L.; Kong, Z.; Yang, T.; Tao, L.; Zou, Y.; Wang, S. Defect Engineering on CeO2—Based Catalysts for Heterogeneous Catalytic Applications. Small Struct. 2021, 2, 2100058. [Google Scholar] [CrossRef]
- Konsolakis, M. Facet-Dependent Reactivity of Ceria Nanoparticles Exemplified. Catalysts 2021, 11, 452. [Google Scholar] [CrossRef]
- Bozon-Verduraz, F.; Bensalem, A. IR Studies of Cerium Dioxide: Influence of Impurities and Defects. J. Chem. Soc. Faraday Trans. 1994, 90, 653–657. [Google Scholar] [CrossRef]
- Kurajica, S.; Mužina, K.; Dražić, G.; Matijašić, G.; Duplančić, M.; Mandić, V.; Župančić, M.; Munda, I.K. A Comparative Study of Hydrothermally Derived Mn, Fe, Co, Ni, Cu and Zn Doped Ceria Nanocatalysts. Mater. Chem. Phys. 2020, 244, 122689. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, L.; Zhang, L.; Cao, Y.; Yu, S.; Tang, C.; Dong, L. Migration of Copper Species in CexCu1−XO2 Catalyst Driven by Thermal Treatment and the Effect on CO Oxidation. Phys. Chem. Chem. Phys. 2017, 19, 21840–21847. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction Property and Catalytic Activity of Ce1−XNiXO2 Mixed Oxide Catalysts for CH4 Oxidation. Appl. Catal. A Gen. 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Barreau, M.; Chen, D.; Zhang, J.; Papaefthimiou, V.; Petit, C.; Salusso, D.; Borfecchia, E.; Turczyniak-Surdacka, S.; Sobczak, K.; Mauri, S.; et al. Synthesis of Ni-Doped Ceria Nanoparticles and Their Unusual Surface Reduction in Hydrogen. Mater. Today Chem. 2022, 26, 101011. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.A.; Iqbal, S.; AL-Anazy, M.M.; Baghdadi, H.B. Designing of Highly Active G-C3N4/Sn Doped ZnO Heterostructure as a Photocatalyst for the Disinfection and Degradation of the Organic Pollutants under Visible Light Irradiation. J. Photochem. Photobiol. A Chem. 2021, 418, 113393. [Google Scholar] [CrossRef]
- Li, Y.; Han, W.; Wang, R.; Weng, L.T.; Serrano-Lotina, A.; Bañares, M.A.; Wang, Q.; Yeung, K.L. Performance of an Aliovalent-Substituted CoCeOx Catalyst from Bimetallic MOF for VOC Oxidation in Air. Appl. Catal. B Environ. 2020, 275, 119121. [Google Scholar] [CrossRef]
- Nanoparticles, T.C.; Elias, J.S.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-horn, Y. Structure, Bonding, and Catalytic Activity of Monodisperse, Transition-Metal-Substituted CeO2 Nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. [Google Scholar]
- Derafa, W.; Paloukis, F.; Mewafy, B.; Baaziz, W.; Ersen, O.; Petit, C.; Corbel, G.; Zafeiratos, S. Synthesis and Characterization of Nickel-Doped Ceria Nanoparticles with Improved Surface Reducibility. RSC Adv. 2018, 8, 40712–40719. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Wang, K.; Huang, J.; Liu, X.; Fu, D.; Li, Q.; Zhan, G. M XO Y-ZrO2 (M = Zn, Co, Cu) Solid Solutions Derived from Schiff Base-Bridged UiO-66 Composites as High-Performance Catalysts for CO2 Hydrogenation. ACS Appl. Mater. Interfaces 2019, 11, 33263–33272. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Q.; Fang, H.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Ce-UiO-66 Derived CeO2 Octahedron Catalysts for Efficient Ketonization of Propionic Acid. Ind. Eng. Chem. Res. 2020, 59, 17269–17278. [Google Scholar] [CrossRef]
- Fang, R.; Luque, R.; Li, Y. Efficient One-Pot Fructose to DFF Conversion Using Sulfonated Magnetically Separable MOF-Derived Fe3O4 (111) Catalysts. Green Chem. 2017, 19, 647–655. [Google Scholar] [CrossRef]
- More, G.S.; Srivastava, R. Efficient Activation of CO2 over Ce-MOF-Derived CeO2for the Synthesis of Cyclic Urea, Urethane, and Carbamate. Ind. Eng. Chem. Res. 2021, 60, 12492–12504. [Google Scholar] [CrossRef]
- Mei, J.; Shen, Y.; Wang, Q.; Shen, Y.; Li, W.; Zhao, J.; Chen, J.; Zhang, S. Roles of Oxygen Species in Low-Temperature Catalytic o-Xylene Oxidation on MOF-Derived Bouquetlike CeO2. ACS Appl. Mater. Interfaces 2022, 14, 35694–35703. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, F.; Wang, Y.; Jia, M.; Tao, X.; Jin, Y.; Zhang, X. MOF-Derived CeO2 Supported Ag Catalysts for Toluene Oxidation: The Effect of Synthesis Method. Mol. Catal. 2021, 515, 111922. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Ma, X.; Yang, X.; Lin, M.; Ge, M. MnOx-CeO2 Catalyst Derived from Metal-Organic Frameworks for Toluene Oxidation. Catal. Today 2020, 355, 580–586. [Google Scholar] [CrossRef]
- Bagi, S.; Yuan, S.; Rojas-Buzo, S.; Shao-Horn, Y.; Román-Leshkov, Y. A Continuous Flow Chemistry Approach for the Ultrafast and Low-Cost Synthesis of MOF-808. Green Chem. 2021, 23, 9982–9991. [Google Scholar] [CrossRef]
- Wei, Y.S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.-C.; Jiang, H.-L. Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Buzo, S.; Concepción, P.; Olloqui-Sariego, J.L.; Moliner, M.; Corma, A. Metalloenzyme-Inspired Ce-MOF Catalyst for Oxidative Halogenation Reactions. ACS Appl. Mater. Interfaces 2021, 13, 31021–31030. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Cai, X.; Jiang, H.L. Improving MOF Stability: Approaches and Applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal–Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Garvie, L.A.J.; Buseck, P.R. Determination of Ce4+/Ce3+ in Electron-Beam-Damaged CeO2 by Electron Energy-Loss Spectroscopy. J. Phys. Chem. Solids 1999, 60, 1943–1947. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Di Liberto, G.; Tosoni, S.; Pacchioni, G. Formation of Reversible Adducts by Adsorption of Oxygen on Ce-ZrO2: An Unusual H2 Ionic Superoxide. J. Phys. Chem. C 2019, 123, 27088–27096. [Google Scholar] [CrossRef]
- Karnatak, R.C.; Esteva, J.M.; Dexpert, H.; Gasgnier, M.; Caro, P.E.; Albert, L. X-Ray Absorption Studies of CeO2, PrO2, and TbO2. I. Manifestation of Localized and Extended f States in the 3d Absorption Spectra. Phys. Rev. B 1987, 36, 1745–1749. [Google Scholar] [CrossRef]
- Paparazzo, E. Use and Mis-Use of x-Ray Photoemission Spectroscopy Ce3d Spectra of Ce2O3 and CeO2. J. Phys. Condens. Matter 2018, 30, 343003. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Salusso, D.; Bonino, F.; Paganini, M.C.; Bordiga, S. Unraveling the Reversible Formation of Defective Ce3+ Sites in the UiO-66(Ce) Material: A Multi-Technique Study. Mater. Today Chem. 2023, 27, 101337. [Google Scholar] [CrossRef]
- Laachir, A.; Perrichon, V.; Badri, A.; Lamotte, J.; Catherine, E.; Lavalley, J.C.; El Fallah, J.; Hilaire, L.; Le Normand, F.; Quéméré, E.; et al. Reduction of CeO2 by Hydrogen. Magnetic Susceptibility and Fourier-Transform Infrared, Ultraviolet and X-Ray Photoelectron Spectroscopy Measurements. J. Chem. Soc., Faraday Trans. 1991, 87, 1601–1609. [Google Scholar] [CrossRef]
- Vindigni, F.; Manzoli, M.; Tabakova, T.; Idakiev, V.; Boccuzzi, F.; Chiorino, A. Effect of Ceria Structural Properties on the Catalytic Activity of Au-CeO2 Catalysts for WGS Reaction. Phys. Chem. Chem. Phys. 2013, 15, 13400–13408. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Duan, X.; Liu, S.; Wang, Z. Enhanced Broadband Excitable Near-Infrared Luminescence in Ce3+/Yb3+ Codoped Oxyapatite Based Glass Ceramics. Phys. B Condens. Matter 2020, 582, 411898. [Google Scholar] [CrossRef]
- Lin, J.; Su, Q. Luminescence and Energy Transfer of Rare-Earth-Metal Ions in Mg2Y8(SiO4)6O2. J. Mater. Chem. 1995, 5, 1151–1154. [Google Scholar] [CrossRef]
- Herrmann, A.; Othman, H.A.; Assadi, A.A.; Tiegel, M.; Kuhn, S.; Rüssel, C. Spectroscopic Properties of Cerium-Doped Aluminosilicate Glasses. Opt. Mater. Express 2015, 5, 720. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Hao, Z.; Zhang, X.; Zhang, L.; Luo, Y.; Zhang, J. Efficient Near-Infrared Downconversion and Energy Transfer Mechanism of Ce3+/Yb3+ Codoped Calcium Scandate Phosphor. Inorg. Chem. 2015, 54, 4806–4810. [Google Scholar] [CrossRef]
- Binet, C.; Badri, A.; Lavalley, J.C. A Spectroscopic Characterization of the Reduction of Ceria from Electronic Transitions of Intrinsic Point Defects. J. Phys. Chem. 1994, 98, 6392–6398. [Google Scholar] [CrossRef]
- Thomas, S.; Marie, O.; Bazin, P.; Lietti, L.; Visconti, C.G.; Corbetta, M.; Manenti, F.; Daturi, M. Modelling a Reactor Cell for Operando IR Studies: From Qualitative to Fully Quantitative Kinetic Investigations. Catal. Today 2017, 283, 176–184. [Google Scholar] [CrossRef]
- Meunier, F.C.; Goguet, A.; Shekhtman, S.; Rooney, D.; Daly, H. A Modified Commercial DRIFTS Cell for Kinetically Relevant Operando Studies of Heterogeneous Catalytic Reactions. Appl. Catal. A Gen. 2008, 340, 196–202. [Google Scholar] [CrossRef]
- Melián-Cabrera, I. Temperature Control in DRIFT Cells Used for: In Situ and Operando Studies: Where Do We Stand Today? Phys. Chem. Chem. Phys. 2020, 22, 26088–26092. [Google Scholar] [CrossRef]
- Lammert, M.; Glißmann, C.; Stock, N. Tuning the Stability of Bimetallic Ce(IV)/Zr(IV)-Based MOFs with UiO-66 and MOF-808 Structures. Dalt. Trans. 2017, 46, 2425–2429. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Developments of the Program Fullprof. Newsl. Comm. Powder Diffr. IUCr 2001, 26, 12–19. [Google Scholar]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld Refinement of Debye-Scherrer Synchrotron X-Ray Data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Simonne, D.; Martini, A.; Signorile, M.; Piovano, A.; Braglia, L.; Torelli, P.; Borfecchia, E.; Ricchiardi, G. THORONDOR: Software for Fast Treatment and Analysis of Low-Energy XAS Data. J. Synchrotron Radiat. 2020, 27, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Ruckebusch, C.; Blanchet, L. Multivariate Curve Resolution: A Review of Advanced and Tailored Applications and Challenges. Anal. Chim. Acta 2013, 765, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; De Vos, D.; Stock, N. Cerium-Based Metal Organic Frameworks with UiO-66 Architecture: Synthesis, Properties and Redox Catalytic Activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomie Distances in Halides and Chaleogenides. Acta Crystallogr. Sect. A Found. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Pellicer-Carreño, I.; Grau-Atienza, A.; Boada, R.; Diaz-Moreno, S.; Narciso-Romero, J.; Serrano-Ruiz, J.C.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. Mixed-Valence Ce/Zr Metal-Organic Frameworks: Controlling the Oxidation State of Cerium in One-Pot Synthesis Approach. Adv. Funct. Mater. 2021, 31, 2102582. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H Stretching Frequencies and O-H O Hydrogen Bond Lengths in Minerals. Hydrog. Bond Res. 1999, 1059, 103–115. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Q.; Feng, M.; Zhang, P. Temperature Dependence of IR Absorption of OH Species in Clinopyroxene. Am. Mineral. 2010, 95, 1439–1443. [Google Scholar] [CrossRef]
- Mayerhöfer, T.G.; Pipa, A.V.; Popp, J. Beer’s Law-Why Integrated Absorbance Depends Linearly on Concentration. ChemPhysChem 2019, 20, 2748–2753. [Google Scholar] [CrossRef] [PubMed]
- Badri, A.; Binet, C.; Lavalley, J.C. Use of Methanol as an IR Molecular Probe to Study the Surface of Polycrystalline Ceria. J. Chem. Soc. Faraday Trans. 1997, 93, 1159–1168. [Google Scholar] [CrossRef]
- Deplano, G.; Signorile, M.; Crocellà, V.; Porcaro, N.G.; Atzori, C.; Solemsli, B.G.; Svelle, S.; Bordiga, S. Titration of Cu(I) Sites in Cu-ZSM-5 by Volumetric CO Adsorption. ACS Appl. Mater. Interfaces 2022, 14, 21059–21068. [Google Scholar] [CrossRef]
- Menegazzo, F.; Manzoli, M.; Chiorino, A.; Boccuzzi, F.; Tabakova, T.; Signoretto, M.; Pinna, F.; Pernicone, N. Quantitative Determination of Gold Active Sites by Chemisorption and by Infrared Measurements of Adsorbed CO. J. Catal. 2006, 237, 431–434. [Google Scholar] [CrossRef]
- Daturi, M.; Binet, C.; Lavalley, J.; Sporken, R. Surface Investigation on CexZr1−xO2 Compounds. Phys. Chem. Chem. Phys. 1999, 1, 5717–5724. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-Based Oxides in the Three-Way Catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data Analysis for X-ray Absorption Spectroscopy Using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salusso, D.; Mauri, S.; Deplano, G.; Torelli, P.; Bordiga, S.; Rojas-Buzo, S. MOF-Derived CeO2 and CeZrOx Solid Solutions: Exploring Ce Reduction through FTIR and NEXAFS Spectroscopy. Nanomaterials 2023, 13, 272. https://doi.org/10.3390/nano13020272
Salusso D, Mauri S, Deplano G, Torelli P, Bordiga S, Rojas-Buzo S. MOF-Derived CeO2 and CeZrOx Solid Solutions: Exploring Ce Reduction through FTIR and NEXAFS Spectroscopy. Nanomaterials. 2023; 13(2):272. https://doi.org/10.3390/nano13020272
Chicago/Turabian StyleSalusso, Davide, Silvia Mauri, Gabriele Deplano, Piero Torelli, Silvia Bordiga, and Sergio Rojas-Buzo. 2023. "MOF-Derived CeO2 and CeZrOx Solid Solutions: Exploring Ce Reduction through FTIR and NEXAFS Spectroscopy" Nanomaterials 13, no. 2: 272. https://doi.org/10.3390/nano13020272
APA StyleSalusso, D., Mauri, S., Deplano, G., Torelli, P., Bordiga, S., & Rojas-Buzo, S. (2023). MOF-Derived CeO2 and CeZrOx Solid Solutions: Exploring Ce Reduction through FTIR and NEXAFS Spectroscopy. Nanomaterials, 13(2), 272. https://doi.org/10.3390/nano13020272





