Blue Laser for Polymerization of Bulk-Fill Composites: Influence on Polymerization Kinetics
Abstract
:1. Introduction
- (I)
- there is no difference between the laser light and commercial LED-light-curing device in the tested parameters;
- (II)
- there is no difference between different curing protocols with the same radiant exposure;
- (III)
- there is no difference in polymerization kinetics among materials.
2. Materials and Methods
- (I)
- 3300 mW/cm2 for 3 s;
- (II)
- 2000 mW/cm2 for 5 s;
- (III)
- 1000 mW/cm2 for 10 s.
2.1. Polymerization Kinetics
2.2. Post-Cure DC Evaluation
2.3. Statistical Analysis
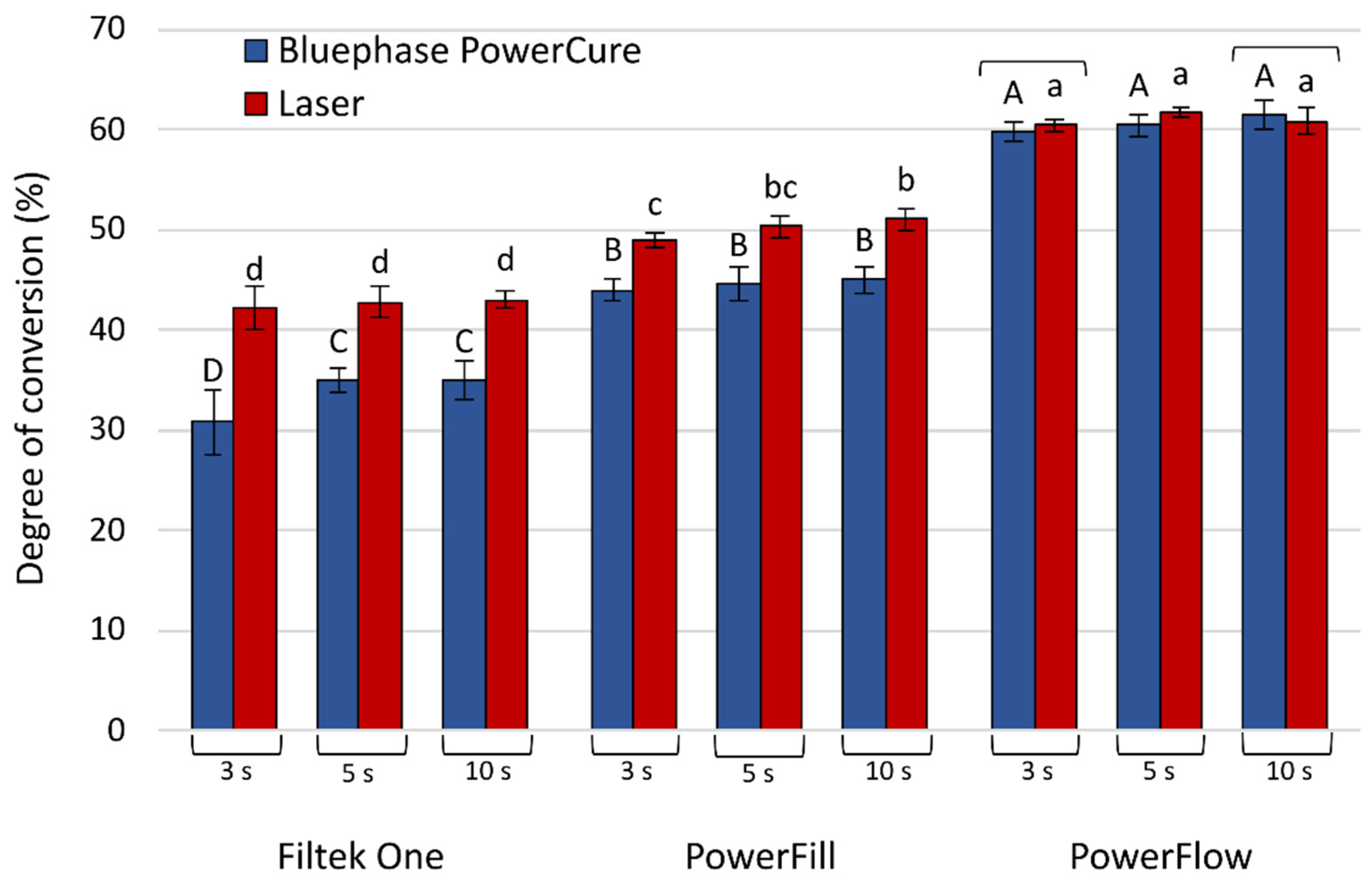
3. Results
3.1. Polymerization Kinetics
3.2. Post-Cure DC Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldman, L.; Hornby, P.; Meyer, R.; Goldman, B. Impact of the Laser on Dental Caries. Nature 1964, 203, 417. [Google Scholar] [CrossRef] [PubMed]
- Pick, R.M.; Pecaro, B.C. Use of the CO2 laser in soft tissue dental surgery. Lasers Surg. Med. 1987, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.A.; Fallon, S.D. Lasers in contemporary oral and maxillofacial surgery. Dent. Clin. N. Am. 2004, 48, 861–888. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Stahli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 176–198. [Google Scholar] [CrossRef] [Green Version]
- Matosevic, D.; Tarle, Z.; Miljanić, S.; Meic, Z.; Pichler, L.; Pichler, G. Laser induced Fluorescence of Carious lesion Porphyrins. Acta Stomatol. Croat. 2010, 44, 82–84. [Google Scholar]
- Montedori, A.; Abraha, I.; Orso, M.; D’Errico, P.G.; Pagano, S.; Lombardo, G. Lasers for caries removal in deciduous and permanent teeth. Cochrane Database Syst. Rev. 2016, 9, CD010229. [Google Scholar] [CrossRef]
- Tarle, Z.; Meniga, A.; Ristic, M.; Sutalo, J.; Pichler, G. Polymerization of composites using pulsed laser. Eur. J. Oral Sci. 1995, 103, 394–398. [Google Scholar] [CrossRef]
- Meniga, A.; Tarle, Z.; Ristic, M.; Sutalo, J.; Pichler, G. Pulsed blue laser curing of hybrid composite resins. Biomaterials 1997, 18, 1349–1354. [Google Scholar] [CrossRef]
- Severin, C.; Macchi-Grisot, F. Polymerization of Bowen’s resins by ultraviolet laser beam. Ligament 1980, 136, 101–102. [Google Scholar]
- Santini, A. Current status of visible light activation units and the curing of light-activated resin-based composite materials. Dent. Update 2010, 37, 214–217. [Google Scholar] [CrossRef]
- Dederich, D.N.; Bushick, R.D. Lasers in dentistry: Separating science from hype. J. Am. Dent. Assoc. 2004, 135, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Matošević, D.; Pandurić, V.; Janković, B.; Knežević, A.; Klarić, E.; Tarle, Z. Light Intensity of Curing Units in Dental Offices in Zagreb, Croatia. Acta Stomatol. Croat. 2011, 45, 31–40. [Google Scholar]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Marovic, D.; Par, M.; Thieu, M.K.L.; Reseland, J.E.; Johnsen, G.F. Bulk Fill Composites Have Similar Performance to Conventional Dental Composites. Int. J. Mol. Sci. 2020, 21, 5136. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Macro-, micro- and nano-mechanical investigations on silorane and methacrylate-based composites. Dent. Mater. 2009, 25, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Marovic, D.; Panduric, V.; Tarle, Z.; Ristic, M.; Sariri, K.; Demoli, N.; Klaric, E.; Jankovic, B.; Prskalo, K. Degree of conversion and microhardness of dental composite resin materials. J. Mol. Struct. 2013, 1044, 299–302. [Google Scholar] [CrossRef]
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907. [Google Scholar] [CrossRef]
- Ilie, N. Resin-Based Bulk-Fill Composites: Tried and Tested, New Trends, and Evaluation Compared to Human Dentin. Materials 2022, 15, 8095. [Google Scholar] [CrossRef]
- Todd, J. Scientific Documentation 3s PowerCure; Ivoclar Vivadent: Schaan, Liechtenstein, 2019. [Google Scholar]
- Carek, A.; Dukaric, K.; Miler, H.; Marovic, D.; Tarle, Z.; Par, M. Post-Cure Development of the Degree of Conversion and Mechanical Properties of Dual-Curing Resin Cements. Polymers 2022, 14, 3649. [Google Scholar] [CrossRef]
- Braga, S.S.L.; Price, R.B.; Juckes, S.M.; Sullivan, B.; Soares, C.J. Effect of the violet light from polywave light-polymerizing units on two resin cements that use different photoinitiators. J. Prosthet. Dent. 2022. [Google Scholar] [CrossRef]
- Ilie, N.; Watts, D.C. Outcomes of ultra-fast (3 s) photo-cure in a RAFT-modified resin-composite. Dent. Mater. 2020, 36, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Marovic, D.; Attin, T.; Tarle, Z.; Taubock, T.T. Effect of rapid high-intensity light-curing on polymerization shrinkage properties of conventional and bulk-fill composites. J. Dent. 2020, 101, 103448. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Burrer, P.; Prskalo, K.; Schmid, S.; Schubiger, A.L.; Marovic, D.; Tarle, Z.; Attin, T.; Taubock, T.T. Polymerization Kinetics and Development of Polymerization Shrinkage Stress in Rapid High-Intensity Light-Curing. Polymers 2022, 14, 3296. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Spanovic, N.; Marovic, D.; Attin, T.; Tarle, Z.; Taubock, T.T. Rapid high-intensity light-curing of bulk-fill composites: A quantitative analysis of marginal integrity. J. Dent. 2021, 111, 103708. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Marovic, D.; Attin, T.; Tarle, Z.; Taubock, T.T. The effect of rapid high-intensity light-curing on micromechanical properties of bulk-fill and conventional resin composites. Sci. Rep. 2020, 10, 10560. [Google Scholar] [CrossRef]
- Marovic, D.; Par, M.; Crnadak, A.; Sekelja, A.; Negovetic Mandic, V.; Gamulin, O.; Rakic, M.; Tarle, Z. Rapid 3 s Curing: What Happens in Deep Layers of New Bulk-Fill Composites? Materials 2021, 14, 515. [Google Scholar] [CrossRef]
- Trevisanello, L.; Meneghini, M.; Mura, G.; Sanna, C.; Buso, S.; Spiazzi, G.; Vanzi, M.; Meneghesso, G.; Zanoni, E. Thermal stability analysis of High Brightness LED during high temperature and electrical aging. Proc. SPIE-Int. Soc. Opt. Eng. 2007, 6669, 231–240. [Google Scholar] [CrossRef]
- Li, C.; Liu, P.; Shao, P.; Pei, J.; Li, Y.; Pawlik, T.M.; Martin, E.W.; Xu, R.X. Handheld projective imaging device for near-infrared fluorescence imaging and intraoperative guidance of sentinel lymph node resection. J. Biomed. Opt. 2019, 24, 080503. [Google Scholar] [CrossRef]
- Arrizabalaga-Larrañaga, A.; Nielen, M.W.F.; Blokland, M.H. Hand-Held Diode Laser for On-Site Analysis Using Transportable Mass Spectrometry. Anal. Chem. 2021, 93, 8122–8127. [Google Scholar] [CrossRef]
- Wheeland, R.G. Simulated consumer use of a battery-powered, hand-held, portable diode laser (810 nm) for hair removal: A safety, efficacy and ease-of-use study. Lasers Surg. Med. 2007, 39, 476–493. [Google Scholar] [CrossRef]
- Ilie, N. Impact of light transmittance mode on polymerisation kinetics in bulk-fill resin-based composites. J. Dent. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Par, M.; Lapas-Barisic, M.; Gamulin, O.; Panduric, V.; Spanovic, N.; Tarle, Z. Long Term Degree of Conversion of two Bulk-Fill Composites. Acta Stomatol. Croat. 2016, 50, 292–300. [Google Scholar] [CrossRef] [PubMed]
- 3M. Filtek One Bulk Fill Restaurative. Shrinkage, Stress and Bulk Fill Restoratives. 2020. Available online: https://multimedia.3m.com/mws/media/1317665O/1317663m-filtek-one-bulk-fill-restorative-shrink-stressand-bulk-fill-restoratives.pdf (accessed on 10 November 2022).
- Arikawa, H.; Kanie, T.; Fujii, K.; Takahashi, H.; Ban, S. Effect of filler properties in composite resins on light transmittance characteristics and color. Dent. Mater. J. 2007, 26, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klarić, N.; Macan, M.; Par, M.; Tarle, Z.; Marović, D. Effect of Rapid Polymerization on Water Sorption and Solubility of Bulk-fill Composites. Acta Stomatol. Croat. 2022, 56, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Delfino, C.; Pfeifer, C.; Braga, R.; Youssef, M.; Turbino, M. Shrinkage stress and mechanical properties of photoactivated composite resin using the argon ion laser. Appl. Phys. B 2009, 96, 79–84. [Google Scholar] [CrossRef]
- Svelto, O. Principles of Lasers, 5th ed.; Springer Science + Business Media: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Fleming, M.G.; Maillet, W.A. Photopolymerization of composite resin using the argon laser. J. Can. Dent. Assoc. 1999, 65, 447–450. [Google Scholar]
- Rocha, M.G.; Maucoski, C.; Roulet, J.F.; Price, R.B. Depth of cure of 10 resin-based composites light-activated using a laser diode, multi-peak, and single-peak light-emitting diode curing lights. J. Dent. 2022, 122, 104141. [Google Scholar] [CrossRef]
- Harlow, J.E.; Rueggeberg, F.A.; Labrie, D.; Sullivan, B.; Price, R.B. Transmission of violet and blue light through conventional (layered) and bulk cured resin-based composites. J. Dent. 2016, 53, 44–50. [Google Scholar] [CrossRef]
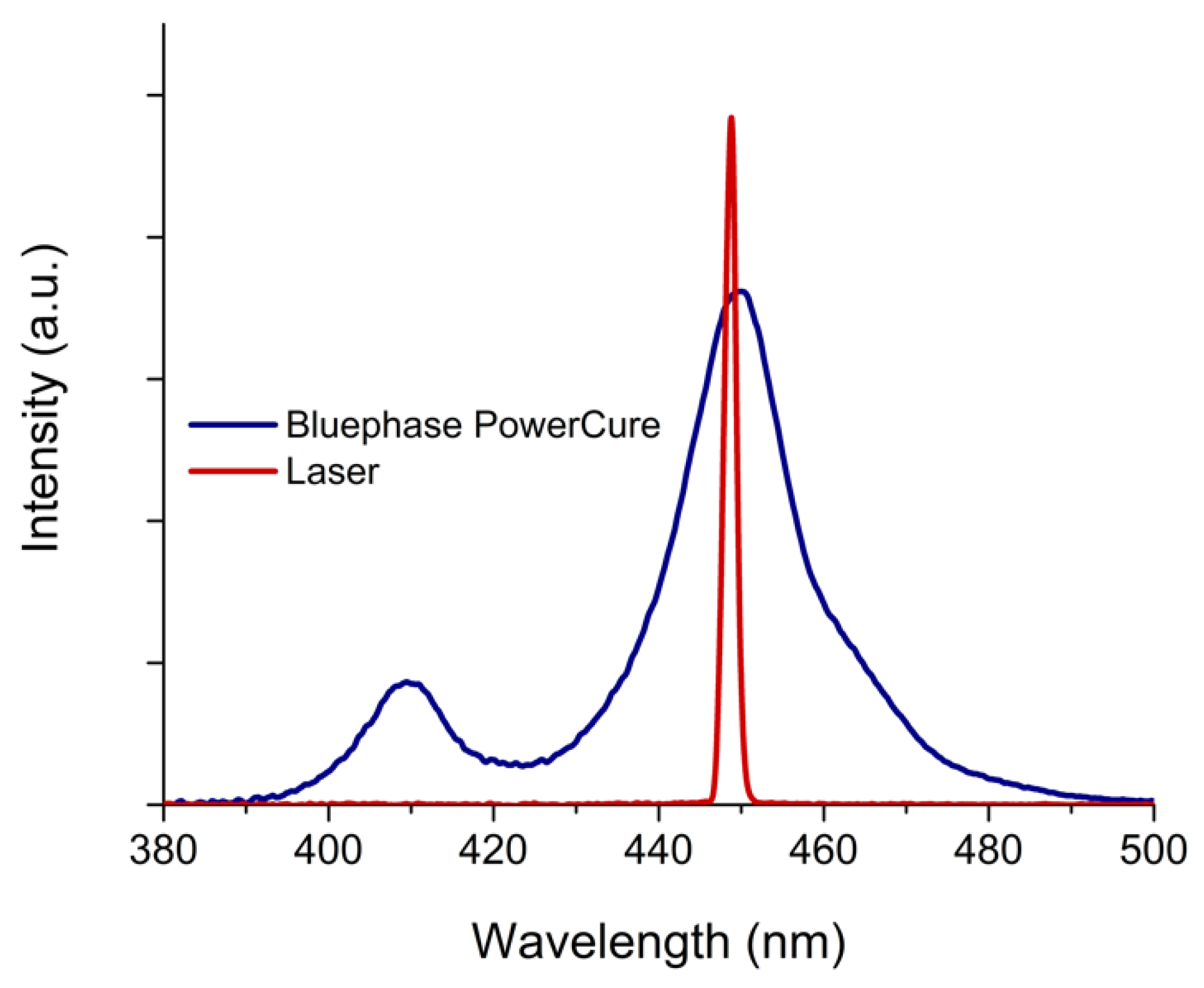





| Material | Manufacturer | Resin | Filler wt%/vol% | Photoinitiator |
|---|---|---|---|---|
| Filtek One Bulk Fill Restorative | 3M ESPE, St. Paul, MN, USA | AUDMA, DDDMA, proprietary AFM | 76.5/58.5 | CQ/amine |
| Tetric PowerFill | Ivoclar Vivadent, Schaan, Liechtenstein | Bis-GMA, Bis-EMA, UDMA, PBPA, DCP, β-allyl sulfone | 76/53 | CQ/amine Ivocerin |
| Tetric PowerFlow | Ivoclar Vivadent, Schaan, Liechtenstein | Bis-GMA, Bis-EMA, UDMA, DCP | 68/46 | CQ/amine Ivocerin |
| Bluephase PowerCure | Laser | ||||||
|---|---|---|---|---|---|---|---|
| Filtek One | PowerFill | PowerFlow | Filtek One | PowerFill | PowerFlow | ||
| a | 3 s @ 3000 mW/cm2 | 15.63 (15.11–16.15) | 35.90 (34.77–37.03) | 49.28 (47.73–50.82) | 30.37 (29.81–30.93) | 40.96 (39.80–42.12) | 54.53 (52.89–56.18) |
| 5 s @ 2000 mW/cm2 | 19.87 (19.34–20.41) | 36.87 (35.90–37.85) | 51.87 (50.55–53.20) | 32.35 (31.64–33.07) | 41.74 (40.77–42.71) | 54.18 (52.93–55.43) | |
| 10 s @ 1000 mW/cm2 | 22.38 (21.92–22.85) | 36.89 (36.24–37.54) | 52.69 (51.77–53.60) | 33.89 (33.26–34.53) | 44.14 (43.37–44.91) | 56.86 (55.81–57.90) | |
| b | 3 s @ 3000 mW/cm2 | 0.12 (0.11–0.13) | 0.43 (0.41–0.46) | 0.51 (0.48–0.54) | 0.16 (0.15–0.16) | 0.49 (0.47–0.51) | 0.51 (0.48–0.54) |
| 5 s @ 2000 mW/cm2 | 0.13 (0.12–0.13) | 0.32 (0.31–0.34) | 0.41 (0.39–0.43) | 0.14 (0.13–0.15) | 0.44 (0.42–0.46) | 0.46 (0.44–0.48) | |
| 10 s @ 1000 mW/cm2 | 0.11 (0.10–0.11) | 0.23 (0.22–0.24) | 0.29 (0.28–0.29) | 0.12 (0.12–0.13) | 0.28 (0.27–0.29) | 0.24 (0.24–0.25) | |
| c | 3 s @ 3000 mW/cm2 | 14.32 (14.06–14.57) | 10.05 (9.75–10.35) | 12.72 (12.36–13.08) | 14.38 (14.14–14.61) | 10.10 (9.83–10.36) | 11.36 (11.01–11.71) |
| 5 s @ 2000 mW/cm2 | 15.45 (15.19–15.71) | 10.47 (10.20–10.75) | 12.26 (11.93–12.60) | 14.52 (14.20–14.84) | 10.05 (9.81–10.28) | 11.28 (10.99–11.57) | |
| 10 s @ 1000 mW/cm2 | 14.68 (14.42–14.94) | 10.18 (9.96–10.40) | 11.65 (11.38–11.93) | 13.12 (12.82–13.42) | 9.54 (9.30–9.77) | 9.99 (9.67–10.31) | |
| d | 3 s @ 3000 mW/cm2 | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) |
| 5 s @ 2000 mW/cm2 | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | |
| 10 s @ 1000 mW/cm2 | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negovetic Mandic, V.; Par, M.; Marovic, D.; Rakić, M.; Tarle, Z.; Klarić Sever, E. Blue Laser for Polymerization of Bulk-Fill Composites: Influence on Polymerization Kinetics. Nanomaterials 2023, 13, 303. https://doi.org/10.3390/nano13020303
Negovetic Mandic V, Par M, Marovic D, Rakić M, Tarle Z, Klarić Sever E. Blue Laser for Polymerization of Bulk-Fill Composites: Influence on Polymerization Kinetics. Nanomaterials. 2023; 13(2):303. https://doi.org/10.3390/nano13020303
Chicago/Turabian StyleNegovetic Mandic, Visnja, Matej Par, Danijela Marovic, Mario Rakić, Zrinka Tarle, and Eva Klarić Sever. 2023. "Blue Laser for Polymerization of Bulk-Fill Composites: Influence on Polymerization Kinetics" Nanomaterials 13, no. 2: 303. https://doi.org/10.3390/nano13020303






