Temperature-Dependent Amplified Spontaneous Emission in CsPbBr3 Thin Films Deposited by Single-Step RF-Magnetron Sputtering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin Film Fabrication
2.2. Morphological and Structural Characterization: SEM, and XRD
2.3. Chemical Characterization: XPS
2.4. Absorption and ASE Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoeckel, M.A.; Gobbi, M.; Bonacchi, S.; Liscio, F.; Ferlauto, L.; Orgiu, E.; Samorì, P. Reversible, Fast, and Wide-Range Oxygen Sensor Based on Nanostructured Organometal Halide Perovskite. Adv. Mater. 2017, 29, 1702469–1702475. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Petryk, M.; Liu, Z.; Chica, D.G.; Hadar, I.; Leak, C.; Ke, W.; Spanopoulos, I.; Lin, W.; Chung, D.Y.; et al. CsPbBr3 perovskite detectors with 1.4% energy resolution for high-energy γ-rays. Nat. Photonics 2021, 15, 36–42. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; Pelayo García de Arquer, F.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Bhaunit, S.; Raj Kar, M.; Thorat, B.N.; Bruno, A.; Mhaisalkar, S.G. Vacuum-Processed Metal Halide Perovskite Light-Emitting Diodes: Prospects and Challenges. ChemPlusChem 2021, 86, 558–573. [Google Scholar]
- Du, P.; Li, J.; Wang, L.; Liu, J.; Li, S.; Liu, N.; Li, Y.; Zhang, M.; Gao, L.; Ma, Y.; et al. Vacuum-Deposited Blue Inorganic Perovskite Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2019, 11, 47083–47090. [Google Scholar] [CrossRef]
- Du, P.; Li, J.; Wang, L.; Sun, L.; Wang, X.; Xu, X.; Yang, L.; Pang, J.; Liang, W.; Luo, J.; et al. Efficient and large-area all vacuum-deposited perovskite light-emitting diodes via spatial confinement. Nat. Commun. 2021, 12, 4751–4760. [Google Scholar] [CrossRef]
- Li, J.; Du, P.; Li, S.; Liu, J.; Zhu, M.; Tan, Z.; Hu, M.; Luo, J.; Guo, D.; Ma, L.; et al. High-Throughput Combinatorial Optimizations of Perovskite Light-Emitting Diodes Based on All-Vacuum Deposition. Adv. Funct. Mater. 2019, 29, 1903607–1903614. [Google Scholar] [CrossRef]
- Mi, Y.; Zhong, Y.; Zhang, Q.; Liu, X. Continuous-Wave Pumped Perovskite Lasers. Adv. Opt. Mater. 2019, 7, 1900544–1900550. [Google Scholar] [CrossRef]
- Zhong, Y.; Liao, K.; Du, W.; Zhu, J.; Shang, Q.; Zhou, F.; Wu, X.; Sui, X.; Shi, J.; Yue, S.; et al. Large-Scale Thin CsPbBr3 Single-Crystal Film Grown on Sapphire via Chemical Vapor Deposition: Toward Laser Array Application. ACS Nano 2020, 14, 15605–15615. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Mi, Y.; Sui, X.; Zhang, S.; Du, W.; Wang, R.; Shi, J.; Wu, X.; Qiu, X.; et al. All-Inorganic CsPbBr3 Nanowire Based Plasmonic Lasers. Adv. Opt. Mater. 2018, 6, 1800674–1800681. [Google Scholar] [CrossRef]
- Yen, M.; Lee, C.-J.; Liu, K.-H.; Peng, Y.; Leng, J.; Chang, T.-H.; Chang, C.-C.; Tamada, K.; Lee, Y.-J. All-inorganic perovskite quantum dot light-emitting memories. Nat. Commun. 2021, 12, 4460. [Google Scholar] [CrossRef] [PubMed]
- Park, N.G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Kumar, N.; Rani, J.; Kurchania, R. Advancement in CsPbBr3 inorganic perovskite solar cells: Fabrication, efficiency and stability. Sol. Energy 2021, 221, 197–205. [Google Scholar] [CrossRef]
- Tong, G.; Ono, L.K.; Qi, Y. Recent Progress of All-Bromide Inorganic Perovskite Solar Cells. Energy Technol. 2020, 8, 1900961. [Google Scholar] [CrossRef]
- Calisi, N.; Caporali, S.; Milanesi, A.; Innocenti, M.; Salvietti, E.; Bardi, U. Composition-Dependent Degradation of Hybrid and Inorganic Lead Perovskites in Ambient Conditions. Top. Catal. 2018, 61, 1201–1208. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; Yang, X.; Wang, Y.; He, B.; Tang, Q. Lanthanide Ions Doped CsPbBr3 Halides for HTM-Free 10.14%-Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V. Adv. Energy Mater. 2018, 8, 1802346–1802354. [Google Scholar] [CrossRef]
- Maafa, I.M. All-Inorganic Perovskite Solar Cells: Recent Advancements and Challenges. Nanomaterials 2022, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Zhou, Y.; Liu, H.; Xue, Q.; Li, X.; Chueh, C.-C.; Yip, H.-L.; Zhu, Z.; Jen, A.K.Y. Highly efficient all-inorganic perovskite solar cells with suppressed non-radiative recombination by a Lewis base. Nat. Commun. 2020, 11, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Huang, S.; Du, J.; Wang, C.; Leng, Y. Advances in inorganic and hybrid perovskites for miniaturized lasers. Nanophotonics 2020, 9, 2251–2272. [Google Scholar] [CrossRef] [Green Version]
- Calisi, N.; Galvanetto, E.; Borgioli, F.; Martinuzzi, S.M.; Bacci, T.; Caporali, S. Thin films deposition of fully inorganic metal halide perovskites: A review. Mater. Sci. Semicond. Process. 2022, 147, 106721–106730. [Google Scholar] [CrossRef]
- Borri, C.; Calisi, N.; Galvanetto, E.; Falsini, N.; Biccari, F.; Vinattieri, A.; Cucinotta, G.; Caporali, S. First Proof-of-Principle of Inorganic Lead Halide Perovskites Deposition by Magnetron-Sputtering. Nanomaterials 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Falsini, N.; Calisi, N.; Roini, G.; Ristori, A.; Biccari, F.; Scardi, P.; Barri, C.; Bollani, M.; Caporali, S.; Vinattieri, A. Large-Area Nanocrystalline Caesium Lead Chloride Thin Films: A Focus on the Exciton Recombination Dynamics. Nanomaterials 2021, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Calisi, N.; Caporali, S. Investigation of Open Air Stability of CsPbBr3 Thin-Film Growth on Different Substrates. Appl. Sci. 2020, 10, 7775. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Yuan, B.; Zhang, Y.; Wei, H.; Wu, Y.; Cao, B. Large-area CsPbBr3 perovskite films grown with effective one-step RF-magnetron sputtering. J. Appl. Phys. 2021, 129, 245303–245310. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Perulli, A.; Yantara, N.; Boix, P.P.; Anni, A. Amplified Spontaneous Emission Properties of Solution Processed CsPbBr3 Perovskite Thin Films. J. Phys. Chem. C 2017, 121, 14772–14778. [Google Scholar] [CrossRef]
- Li, M.; Shang, Q.; Li, C.; Li, S.; Liang, Y.; Yu, W.; Wu, C.; Zhao, L.; Zhong, Y.; Du, W.; et al. High Optical Gain of Solution-Processed Mixed-Cation CsPbBr3 Thin Films towards Enhanced Amplified Spontaneous Emission. Adv. Funct. Mater. 2021, 31, 2102210–2102217. [Google Scholar] [CrossRef]
- Liang, Y.; Shang, Q.; Li, M.; Zhang, S.; Liu, X.; Zhang, Q. Solvent Recrystallization-Enabled Green Amplified Spontaneous Emissions with an Ultra-Low Threshold from Pinhole-Free Perovskite Films. Adv. Funct. Mater. 2021, 31, 2106108–2106115. [Google Scholar] [CrossRef]
- Roh, K.; Zhao, L.; Rand, B.P. Tuning Laser Threshold within the Large Optical Gain Bandwidth of Halide Perovskite Thin Films. ACS Photonics 2021, 8, 2548–2554. [Google Scholar] [CrossRef]
- Pourdavoud, N.; Haeger, T.; Mayer, A.; Cegielski, P.J.; Giesecke, A.L.; Heiderhoff, R.; Olthof, S.; Zaefferer, S.; Shutsko, I.; Henkel, A.; et al. Room-Temperature Stimulated Emission and Lasing in Recrystallized Cesium Lead Bromide Perovskite Thin Films. Adv. Mater. 2019, 31, 1903717–1903726. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhanga, S.; Lia, L.; Chen, W. Research Progress On Large-Area Perovskite Thin Films and Solar Modules. J. Mater. 2017, 3, 231–244. [Google Scholar] [CrossRef]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8056. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Krieg, F.; Kovalenko, M.V.; Anni, M. Amplified Spontaneous Emission Threshold Reduction and Operational Stability Improvement in CsPbBr3 Nanocrystal Films by Hydrophobic Functionalization of the Substrate. Sci. Rep. 2019, 9, 17964. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.; Palatnik, A.; Sudzius, M.; Grodofzig, R.; Nehm, F.; Leo, K. Controlling and Optimizing Amplified Spontaneous Emission in Perovskites. ACS Appl. Mater. Interfaces 2020, 12, 35242–35249. [Google Scholar] [CrossRef]
- Cheng, S.; Chang, Q.; Wang, Z.; Xiao, L.; Chia, E.E.M.; Sun, H. Observation of Net Stimulated Emission in CsPbBr3 Thin Films Prepared by Pulsed Laser Deposition. Adv. Opt. Mater. 2021, 9, 2100564–2100572. [Google Scholar] [CrossRef]
- Reyes-Banda, M.; Fernandez-Izquierdo, L.; Krishnan, S.S.N.; Caraveo-Frescas, J.A.; Mathew, X.; Quevedo-Lopez, M. Material Properties Modulation in Inorganic Perovskite Films via Solution-Free Solid-State Reactions. ACS Appl. Electron. Mater. 2021, 3, 1468–1476. [Google Scholar] [CrossRef]
- Milanese, S.; De Giorgi, M.L.; Cerdán, L.; La-Placa, M.-G.; Jamaludin, N.F.; Bruno, A.; Bolink, H.J.; Kovalenko, M.V.; Anni, M. Amplified Spontaneous Emission Threshold Dependence on Determination Method in Dye-Doped Polymer and Lead Halide Perovskite Waveguides. Molecules 2022, 27, 4261. [Google Scholar] [CrossRef]
- Cha, J.H.; Han, J.H.; Yin, W.; Park, C.; Park, Y.; Ahn, T.K.; Cho, J.H.; Jung, D.Y. Photoresponse of CsPbBr3 and Cs4PbBr6 Perovskite Single Crystals. J. Phys. Chem. Lett. 2017, 8, 565–570. [Google Scholar] [CrossRef]
- Caicedo-Davila, S.; Gunder, R.; Marquez, J.A.; Levcenko, S.; Schwartzburg, K.; Unold, T.; Abou-Ras, D. Effects of Postdeposition Annealing on the Luminescence of Mixed Phase CsPb2Br5/CsPbBr3 Thin Films. J. Phys. Chem. C 2020, 124, 19514–19521. [Google Scholar] [CrossRef]
- Maity, G.; Pradhan, S.K. Composition related structural transition between mechanosynthesized CsPbBr3 and CsPb2Br5 perovskites and their optical properties. J. Alloys Compd. 2020, 816, 152612–152621. [Google Scholar] [CrossRef]
- Zhao, F.; Ren, A.; Li, P.; Li, Y.; Wu, J.; Wang, Z.M. Toward Continuous-Wave Pumped Metal Halide Perovskite Lasers: Strategies and Challenges. ACS Nano 2022, 16, 7116–7143. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and technology of magnetron sputtering discharges. Plasma Sources Sci. Technol. 2020, 29, 113001–113052. [Google Scholar] [CrossRef]
- Mahne, N.; Čekada, M.; Panjan, M. Total and Differential Sputtering Yields Explored by SRIM Simulations. Coatings 2022, 12, 1541. [Google Scholar] [CrossRef]
- Gabelloni, F.; Biccari, F.; Andreotti, G.; Balestri, D.; Checcucci, S.; Milanesi, A.; Calisi, N.; Caporali, S.; Vinattieri, A. Recombination dynamics in CsPbBr3 nanocrystals: Role of surface states. Opt. Mater. Express 2017, 7, 4367–4373. [Google Scholar] [CrossRef] [Green Version]
- Balena, A.; Perulli, A.; Fernandez, M.; De Giorgi, M.L.; Nedelcu, G.; Kovalenko, M.V.; Anni, M. Temperature Dependence of the Amplified Spontaneous Emission from CsPbBr3 Nanocrystal Thin Films. J. Phys. Chem. C 2018, 122, 5813–5819. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Pang, G.T.; Xing, G.C.; Chen, R. Temperature dependent optical characteristics of all-inorganic CsPbBr3 nanocrystals film. Mater. Today Phys. 2020, 15, 100259–100266. [Google Scholar] [CrossRef]
- Dey, A.; Rathod, P.; Kabra, D. Role of Localized States in Photoluminescence Dynamics of High Optical Gain CsPbBr3 Nanocrystals. Adv. Opt. Mater. 2018, 6, 1800109–1800117. [Google Scholar] [CrossRef]
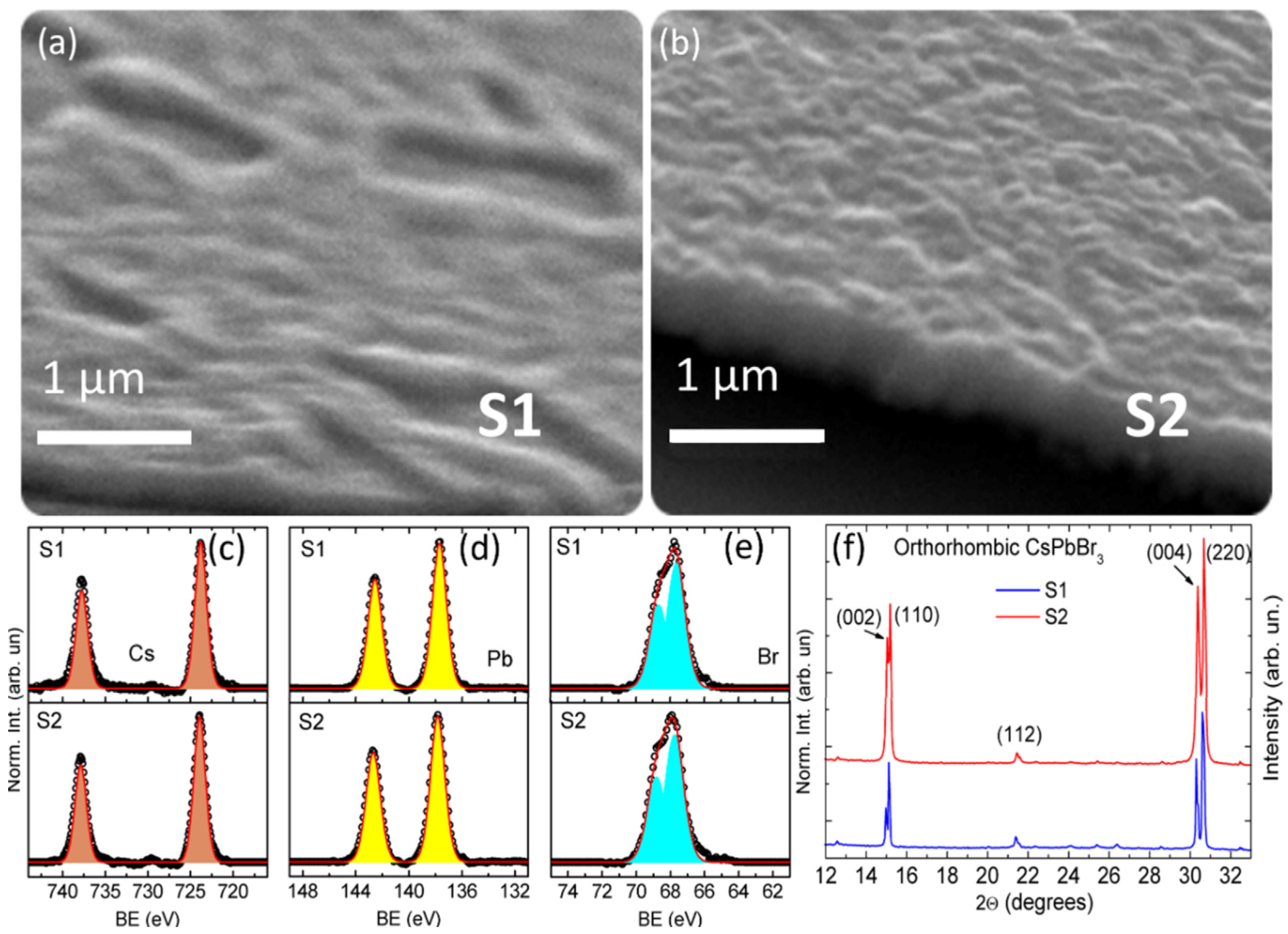
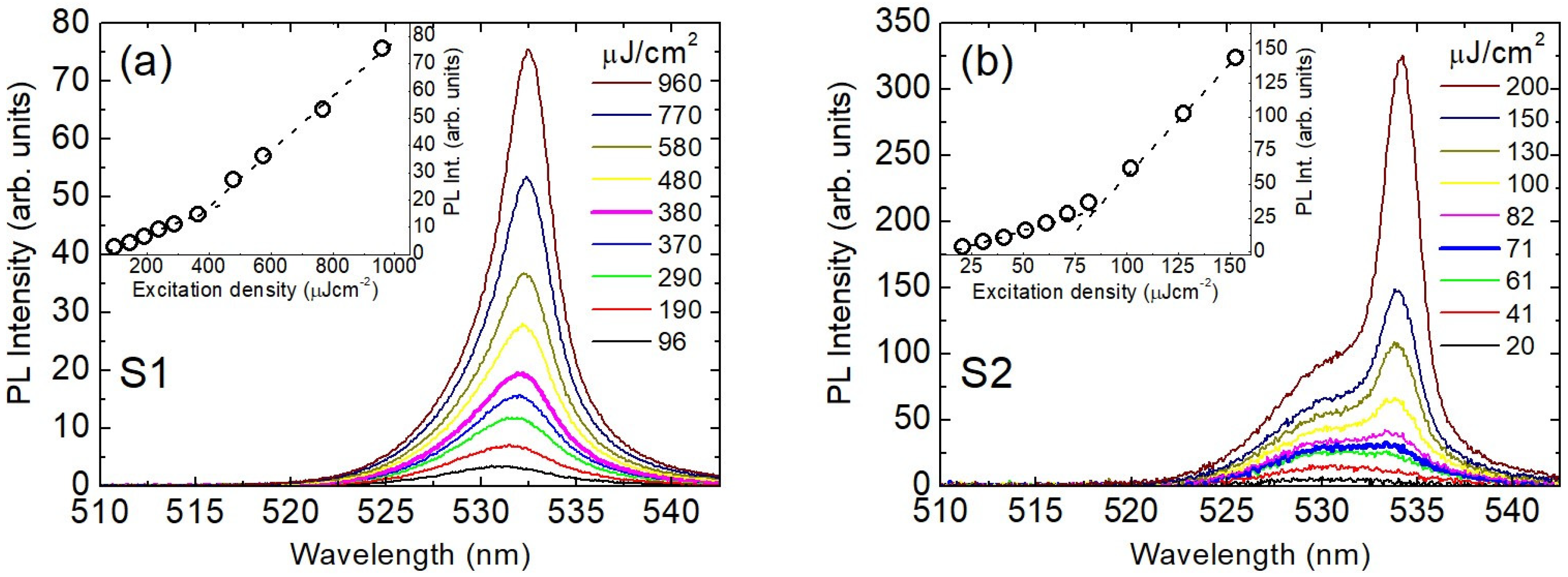
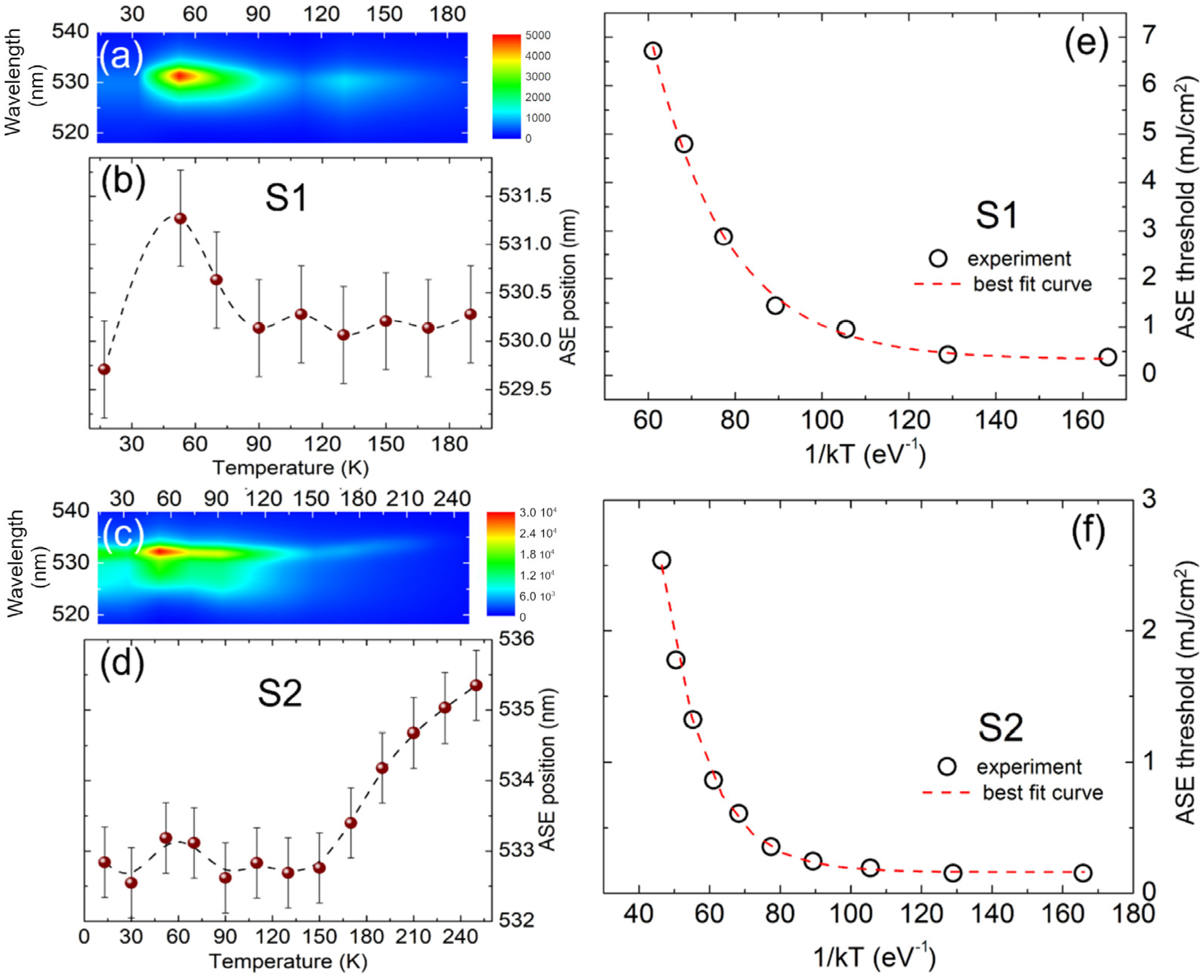
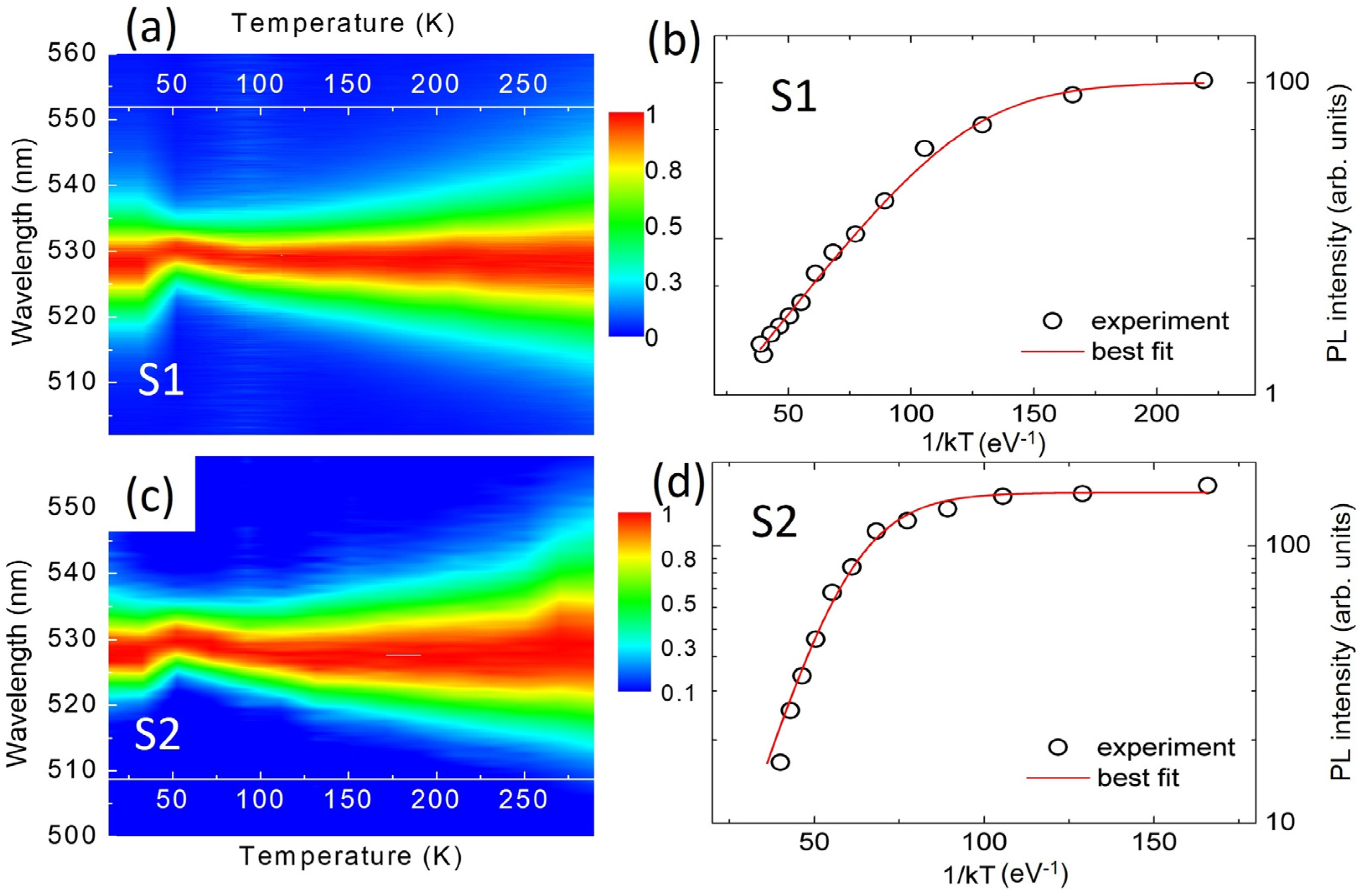
| Element | Cs | Pb | Br |
|---|---|---|---|
| S1 composition 1 | 15% | 29% | 56% |
| S2 composition 1 | 23% | 30% | 47% |
| Expected composition 1 | 20% | 20% | 60% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morello, G.; Milanese, S.; De Giorgi, M.L.; Calisi, N.; Caporali, S.; Biccari, F.; Falsini, N.; Vinattieri, A.; Anni, M. Temperature-Dependent Amplified Spontaneous Emission in CsPbBr3 Thin Films Deposited by Single-Step RF-Magnetron Sputtering. Nanomaterials 2023, 13, 306. https://doi.org/10.3390/nano13020306
Morello G, Milanese S, De Giorgi ML, Calisi N, Caporali S, Biccari F, Falsini N, Vinattieri A, Anni M. Temperature-Dependent Amplified Spontaneous Emission in CsPbBr3 Thin Films Deposited by Single-Step RF-Magnetron Sputtering. Nanomaterials. 2023; 13(2):306. https://doi.org/10.3390/nano13020306
Chicago/Turabian StyleMorello, Giovanni, Stefania Milanese, Maria Luisa De Giorgi, Nicola Calisi, Stefano Caporali, Francesco Biccari, Naomi Falsini, Anna Vinattieri, and Marco Anni. 2023. "Temperature-Dependent Amplified Spontaneous Emission in CsPbBr3 Thin Films Deposited by Single-Step RF-Magnetron Sputtering" Nanomaterials 13, no. 2: 306. https://doi.org/10.3390/nano13020306







