Halogen-Doped Chevrel Phase Janus Monolayers for Photocatalytic Water Splitting
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Balan, A.P.; Puthirath, A.B.; Roy, S.; Costin, G.; Oliveira, E.F.; Saadi, M.; Sreepal, V.; Friedrich, R.; Serles, P.; Biswas, A.; et al. Non-Van Der Waals Quasi-2D Materials; Recent Advances in Synthesis, Emergent Properties and Applications. Mater. Today 2022, 58, 164–200. [Google Scholar] [CrossRef]
- Gibaja, C.; Rodríguez-San-Miguel, D.; Paz, W.S.; Torres, I.; Salagre, E.; Segovia, P.; Michel, E.G.; Assebban, M.; Ares, P.; Hernández-Maldonado, D. Exfoliation of Alpha-Germanium: A Covalent Diamond-Like Structure. Adv. Mater. 2021, 33, 2006826. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Felser, C.; Yan, B. Graphene-Like Dirac States and Quantum Spin Hall Insulators in Square-Octagonal MX2(M = Mo, W; X = S, Se, Te) Isomers. Phys. Rev. B 2015, 92, 165421. [Google Scholar] [CrossRef]
- Ma, Y.; Kou, L.; Li, X.; Dai, Y.; Heine, T. Two-Dimensional Transition Metal Dichalcogenides with a Hexagonal Lattice: Room-Temperature Quantum Spin Hall Insulators. Phys. Rev. B 2016, 93, 035442. [Google Scholar] [CrossRef]
- Gavryushkin, P.; Sagatov, N.; Sukhanova, E.; Medrish, I.; Popov, Z. Janus Structures of SMoSe and SVSe Compositions with Low Enthalpy and Unusual Crystal Chemistry. J. Appl. Crystallogr. 2022, 55, 1324–1335. [Google Scholar] [CrossRef]
- Sukhanova, E.V.; Bereznikova, L.A.; Manakhov, A.M.; Al Qahtani, H.S.; Popov, Z.I. A Novel Membrane-like 2D A’-MoS2 as Anode for Lithium- and Sodium-Ion Batteries. Membranes 2022, 12, 1156. [Google Scholar] [CrossRef]
- Sukhanova, E.; Kvashnin, A.; Bereznikova, L.; Zakaryan, H.; Aghamalyan, M.; Kvashnin, D.G.; Popov, Z. 2D-Mo3S4 Phase as Promising Contact for MoS2. Appl. Surf. Sci. 2022, 589, 152971. [Google Scholar] [CrossRef]
- Joseph, T.; Ghorbani-Asl, M.; Kvashnin, A.G.; Larionov, K.V.; Popov, Z.; Sorokin, P.B.; Krasheninnikov, A.V. Nonstoichiometric Phases of Two-Dimensional Transition-Metal Dichalcogenides: From Chalcogen Vacancies to Pure Metal Membranes. J. Phys. Chem. Lett. 2019, 10, 6492–6498. [Google Scholar] [CrossRef]
- Chepkasov, I.V.; Sukhanova, E.V.; Kvashnin, A.G.; Zakaryan, H.A.; Aghamalyan, M.A.; Mamasakhlisov, Y.S.; Manakhov, A.M.; Popov, Z.I.; Kvashnin, D.G. Computational Design of Gas Sensors Based on V3S4 Monolayer. Nanomaterials 2022, 12, 774. [Google Scholar] [CrossRef]
- Sukhanova, E.V.; Kvashnin, A.G.; Agamalyan, M.A.; Zakaryan, H.A.; Popov, Z.I. Map of Two-Dimensional Tungsten Chalcogenide Compounds (W–S, W–Se, W–Te) Based on USPEX Evolutionary Search. JETP Lett. 2022, 115, 292–296. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, Q.; Xu, W.; Cao, J. High Anisotropic Optoelectronics in Monolayer Binary M8X12 (M = Mo, W; X = S, Se, Te). ACS Appl. Mater. Interfaces 2022, 14, 27056–27062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guan, X.; Ren, X.; Liu, T.; Huang, W.; Cao, J.; Jin, C. Deriving 2D M2X3 (M = Mo, W, X = S, Se) by Periodic Assembly of Chalcogen Vacancy Lines in Their MX2 Counterparts. Nanoscale 2020, 12, 8285–8293. [Google Scholar] [CrossRef]
- Chevrel, R.; Sergent, M.; Prigent, J. Sur de Nouvelles Phases Sulfurées Ternaires Du Molybdène. J. Solid State Chem. 1971, 3, 515–519. [Google Scholar] [CrossRef]
- Singstock, N.R.; Ortiz-Rodríguez, J.C.; Perryman, J.T.; Sutton, C.; Velázquez, J.M.; Musgrave, C.B. Machine Learning Guided Synthesis of Multinary Chevrel Phase Chalcogenides. J. Am. Chem. Soc. 2021, 143, 9113–9122. [Google Scholar] [CrossRef]
- Peña, O. Chevrel Phases: Past, Present and Future. Phys. C Supercond. Its Appl. 2015, 514, 95–112. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Li, H.; Wang, K.; Jiang, K. Large-Scale Fabricating Carbon Coating Chevrel Phase in Molten Salts: Implications for High-Performance Magnesium-Ion Battery Cathode. J. Alloys Compd. 2022, 925, 166745. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype Systems for Rechargeable Magnesium Batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef]
- Geng, L.; Lv, G.; Xing, X.; Guo, J. Reversible Electrochemical Intercalation of Aluminum in Mo6S8. Chem. Mater. 2015, 27, 4926–4929. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, A.; Zhang, Q.; Gao, T.; Yue, J.; Meng, F.; Gong, Y.; Xi, S.; Lin, Z.; Mao, M.; et al. Cation-Synergy Stabilizing Anion Redox of Chevrel Phase Mo6S8 in Aluminum Ion Battery. Energy Storage Mater. 2021, 37, 87–93. [Google Scholar] [CrossRef]
- Elgendy, A.; Papaderakis, A.A.; Byrne, C.; Sun, Z.; Lauritsen, J.V.; Higgins, E.P.C.; Ejigu, A.; Cernik, R.; Walton, A.S.; Lewis, D.J.; et al. Nanoscale Chevrel-Phase Mo6S8 Prepared by a Molecular Precursor Approach for Highly Efficient Electrocatalysis of the Hydrogen Evolution Reaction in Acidic Media. ACS Appl. Energy Mater. 2021, 4, 13015–13026. [Google Scholar] [CrossRef]
- Masschelein, P.; Candolfi, C.; Dauscher, A.; Gendarme, C.; Rabih, A.R.A.O.; Gougeon, P.; Potel, M.; Gall, P.; Gautier, R.; Lenoir, B. Influence of S and Te Substitutions on the Thermoelectric Properties of the Cluster Compound Ag3.8Mo9Se11. J. Alloys Compd. 2018, 739, 360–367. [Google Scholar] [CrossRef]
- Marini, G.; Sanna, A.; Pellegrini, C.; Bersier, C.; Tosatti, E.; Profeta, G. Superconducting Chevrel Phase PbMo6S8 from First Principles. Phys. Rev. B 2021, 103, 144507. [Google Scholar] [CrossRef]
- Chen, J.; Millis, A.J.; Reichman, D.R. Intermolecular Coupling and Superconductivity in PbMo6S8 and Other Chevrel Phase Compounds. Phys. Rev. Mater. 2018, 2, 114801. [Google Scholar] [CrossRef]
- Mao, M.; Lin, Z.; Tong, Y.; Yue, J.; Zhao, C.; Lu, J.; Zhang, Q.; Gu, L.; Suo, L.; Hu, Y.-S.; et al. Iodine Vapor Transport-Triggered Preferential Growth of Chevrel Mo6S8 Nanosheets for Advanced Multivalent Batteries. ACS Nano 2020, 14, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Chevrel, R.; Sergent, M. Chemistry and Structure of Ternary Molybdenum Chalcogenides. In Superconductivity in Ternary Compounds I: Structural, Electronic, and Lattice Properties; Fischer, Ø., Maple, M.B., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 25–86. ISBN 978-3-642-81868-4. [Google Scholar]
- Chang, C.L.; Tao, Y.K.; Swinnea, J.S.; Steinfink, H. Oxygen Substitution in Sn and Ni Chevrel Phases. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 1461–1465. [Google Scholar] [CrossRef]
- Sergent, M.; Fischer, Ø.; Decroux, M.; Perrin, C.; Chevrel, R. Stabilization of Mo6S8 by Halogens; New Superconducting Compounds: Mo6S6Br2, Mo6S6I2. J. Solid State Chem. 1977, 22, 87–92. [Google Scholar] [CrossRef]
- Knöll, R.; Goren, S.; Korn, C.; Shames, A.; Perrin, C.; Privalov, A.; Vieth, H.-M. NMR Study of the Influence of Iodine Substitution in the Chevrel Compounds Mo6Te8−xIx and Mo6Se8−xIx. Phys. B Condens. Matter 2002, 324, 157–166. [Google Scholar] [CrossRef]
- Lin, F.; Fang, Y.; Che, X.; Zhang, S.; Huang, F. Superconductivity in the Electron-Doped Chevrel Phase Compound Mo6S6.8Te1.2. Inorg. Chem. 2020, 59, 6785–6789. [Google Scholar] [CrossRef]
- Zhong, Q.; Dai, Z.; Liu, J.; Zhao, Y.; Meng, S. Phonon Thermal Transport in Janus Single Layer M2XY (M = Ga; X, Y = S, Se, Te): A Study Based on First-Principles. Phys. E Low-Dimens. Syst. Nanostructures 2020, 115, 113683. [Google Scholar] [CrossRef]
- Yin, W.-J.; Tan, H.-J.; Ding, P.-J.; Wen, B.; Li, X.-B.; Teobaldi, G.; Liu, L.-M. Recent Advances in Low-Dimensional Janus Materials: Theoretical and Simulation Perspectives. Mater. Adv. 2021, 2, 7543–7558. [Google Scholar] [CrossRef]
- Lv, M.-H.; Li, C.-M.; Sun, W.-F. Spin-Orbit Coupling and Spin-Polarized Electronic Structures of Janus Vanadium-Dichalcogenide Monolayers: First-Principles Calculations. Nanomaterials 2022, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shang, J.; Kou, L.; Li, C.; Deng, Z. Mechanical Behaviors in Janus Transition-Metal Dichalcogenides: A Molecular Dynamics Simulation. Nanomaterials 2022, 12, 1910. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Ma, Y.; Huang, B.; Dai, Y. Two-Dimensional Janus PtSSe for Photocatalytic Water Splitting under the Visible or Infrared Light. J. Mater. Chem. A 2019, 7, 603–610. [Google Scholar] [CrossRef]
- Ju, L.; Bie, M.; Shang, J.; Tang, X.; Kou, L. Janus Transition Metal Dichalcogenides: A Superior Platform for Photocatalytic Water Splitting. J. Phys. Mater. 2020, 3, 022004. [Google Scholar] [CrossRef]
- Tang, X.; Kou, L. 2D Janus Transition Metal Dichalcogenides: Properties and Applications. Phys. Status Solidi (B) 2022, 259, 2100562. [Google Scholar] [CrossRef]
- Ju, L.; Qin, J.; Shi, L.; Yang, G.; Zhang, J.; Sun, L. Rolling the WSSe Bilayer into Double-Walled Nanotube for the Enhanced Photocatalytic Water-Splitting Performance. Nanomaterials 2021, 11, 705. [Google Scholar] [CrossRef]
- Lin, L.; Hisatomi, T.; Chen, S.; Takata, T.; Domen, K. Visible-Light-Driven Photocatalytic Water Splitting: Recent Progress and Challenges. Trends Chem. 2020, 2, 813–824. [Google Scholar] [CrossRef]
- Feliczak-Guzik, A. Nanomaterials as Photocatalysts—Synthesis and Their Potential Applications. Materials 2022, 16, 193. [Google Scholar] [CrossRef]
- Lu, A.-Y.; Zhu, H.; Xiao, J.; Chuu, C.-P.; Han, Y.; Chiu, M.-H.; Cheng, C.-C.; Yang, C.-W.; Wei, K.-H.; Yang, Y.Y.P.; et al. Janus Monolayers of Transition Metal Dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L.; et al. Janus Monolayer Transition-Metal Dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Yagmurcukardes, M.; Sevik, C.; Peeters, F.M. Electronic, Vibrational, Elastic, and Piezoelectric Properties of Monolayer Janus MoSTe phases: A First-Principles Study. Phys. Rev. B 2019, 100, 045415. [Google Scholar] [CrossRef]
- Wang, Z. 2H→1T′ Phase Transformation in Janus Monolayer MoSSe and MoSTe: An Efficient Hole Injection Contact for 2H-MoS2. J. Mater. Chem. C 2018, 6, 13000–13005. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, H.; Yu, Z.; Liu, Y.; Li, Y. First-Principles Study of Square Phase MX2 and Janus MXY (M = Mo, W; X, Y = S, Se, Te) Transition Metal Dichalcogenide Monolayers under Biaxial Strain. Phys. E Low-Dimens. Syst. Nanostructures 2019, 110, 134–139. [Google Scholar] [CrossRef]
- Tang, X.; Li, S.; Ma, Y.; Du, A.; Liao, T.; Gu, Y.; Kou, L. Distorted Janus Transition Metal Dichalcogenides: Stable Two-Dimensional Materials with Sizable Band Gap and Ultrahigh Carrier Mobility. J. Phys. Chem. C 2018, 122, 19153–19160. [Google Scholar] [CrossRef]
- Qin, Y.; Sayyad, M.; Montblanch, A.R.; Feuer, M.S.G.; Dey, D.; Blei, M.; Sailus, R.; Kara, D.M.; Shen, Y.; Yang, S.; et al. Reaching the Excitonic Limit in 2D Janus Monolayers by In Situ Deterministic Growth. Adv. Mater. 2021, 34, 2106222. [Google Scholar] [CrossRef]
- Wan, X.; Chen, E.; Yao, J.; Gao, M.; Miao, X.; Wang, S.; Gu, Y.; Xiao, S.; Zhan, R.; Chen, K.; et al. Synthesis and Characterization of Metallic Janus MoSH Monolayer. ACS Nano 2021, 15, 20319–20331. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal-Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1992, 46, 6671. [Google Scholar] [CrossRef]
- Blöchl, P.; Först, C.J.; Schimpl, J. Projector Augmented Wave Method: Ab Initio Molecular Dynamics with Full Wave Functions. Bull. Mater. Sci. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First Principles Phonon Calculations in Materials Science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Oreshonkov, A.S. SI: Advances in Density Functional Theory (DFT) Studies of Solids. Materials 2022, 15, 2099. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Für Krist. -Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational Density-Functional Perturbation Theory for Dielectrics and Lattice Dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef]
- Porezag, D.; Pederson, M.R. Infrared Intensities and Raman-Scattering Activities within Density-Functional Theory. Phys. Rev. B 1996, 54, 7830–7836. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Park, C.-H.; Ihm, J. A Rigorous Method of Calculating Exfoliation Energies from First Principles. Nano Lett. 2018, 18, 2759–2765. [Google Scholar] [CrossRef]
- Friedrich, R.; Ghorbani-Asl, M.; Curtarolo, S.; Krasheninnikov, A.V. Data-Driven Quest for Two-Dimensional Non-van der Waals Materials. Nano Lett. 2022, 22, 989–997. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, Z.; Huang, J.; Sumpter, B.G.; Chen, Z. MX Anti-MXenes from Non-Van Der Waals Bulks for Electrochemical Applications: The Merit of Metallicity and Active Basal Plane. ACS Nano 2021, 15, 6233–6242. [Google Scholar] [CrossRef]
- Mounet, N.; Gibertini, M.; Schwaller, P.; Campi, D.; Merkys, A.; Marrazzo, A.; Sohier, T.; Castelli, I.E.; Cepellotti, A.; Pizzi, G.; et al. Two-Dimensional Materials from High-Throughput Computational Exfoliation of Experimentally Known Compounds. Nat. Nanotechnol. 2018, 13, 246–252. [Google Scholar] [CrossRef]
- Padilha, A.C.M.; Soares, M.R.S.; Leite, E.R.; Fazzio, A. Theoretical and Experimental Investigation of 2D Hematite. J. Phys. Chem. C 2019, 123, 16359–16365. [Google Scholar] [CrossRef]
- Haga, T.; Fujimoto, Y.; Saito, S. Electronic Structure and Scanning Tunneling Microscopy Images of Heterostructures Consisting of Graphene and Carbon-Doped Hexagonal Boron Nitride Layers. Phys. Rev. B 2019, 100, 125403. [Google Scholar] [CrossRef]
- Vanpoucke, D.E.P.; Brocks, G. Formation of Pt-Induced Ge Atomic Nanowires on Pt/Ge(001): A Density Functional Theory Study. Phys. Rev. B 2008, 77, 241308. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, J. Proposed Photosynthesis Method for Producing Hydrogen from Dissociated Water Molecules Using Incident Near-Infrared Light. Phys. Rev. Lett. 2014, 112, 018301. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.; Angus, J.C.; Anderson, A.B.; Wolter, S.D.; Stoner, B.R.; Sumanasekera, G.U. Charge Transfer Equilibria between Diamond and an Aqueous Oxygen Electrochemical Redox Couple. Science 2007, 318, 1424–1430. [Google Scholar] [CrossRef]
- Ersan, F.; Ataca, C. Janus PtXnY2−n (X, Y = S, Se, Te ; 0 ≤ n ≤ 2) Monolayers for Enhanced Photocatalytic Water Splitting. Phys. Rev. Appl. 2020, 13, 064008. [Google Scholar] [CrossRef]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear Optical Properties in the Projector-Augmented Wave Methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Eberlein, T.; Bangert, U.; Nair, R.R.; Jones, R.; Gass, M.; Bleloch, A.L.; Novoselov, K.; Geim, A.; Briddon, P.R. Plasmon Spectroscopy of Free-Standing Graphene Films. Phys. Rev. B 2008, 77, 233406. [Google Scholar] [CrossRef]
- Lalitha, S.; Karazhanov, S.; Ravindran, P.; Senthilarasu, S.; Sathyamoorthy, R.; Janabergenov, J. Electronic Structure, Structural and Optical Properties of Thermally Evaporated CdTe Thin Films. Phys. B Condens. Matter 2007, 387, 227–238. [Google Scholar] [CrossRef]
- Potel, M.; Gougeon, P.; Chevrel, R.; Sergent, M. Labilité Des Cations Dans Les Chalcogénures Ternaires de Molybdène: Voies d’accès à de Nouvelles Synthèses. Rev. De Chim. Minérale 1984, 21, 509–536. [Google Scholar]
- Chu, H.; Pan, J.; Bai, S.; Ma, Y.; Feng, Y.; Wen, Y.; Yang, Y.; Luo, R.; Chen, A. Carbon Coated Chevrel Phase of Mo6S8 as Anode Material for Improving Electrochemical Properties of Aqueous Lithium-Ion Batteries. Electrochim. Acta 2017, 258, 236–240. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Von Lim, Y.; Huang, S.; Zhang, J.; Liu, B.; Chen, T.; Yang, H.Y. 3D Hierarchical Defect-Rich NiMo3S4 Nanosheet Arrays Grown on Carbon Textiles for High-Performance Sodium-Ion Batteries and Hydrogen Evolution Reaction. Nano Energy 2018, 49, 460–470. [Google Scholar] [CrossRef]
- Holmgren, D.J.; Demers, R.T.; Klein, M.V.; Ginsberg, D.M. Raman Study of Phonons in Chevrel-Phase Crystals. Phys. Rev. B 1987, 36, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-S.; Hu, Q.; Li, S.; Bhoraskar, S.V.; Yoo, J.-B. Synthesis of Novel 1—Dimensional Structure from Mo6S8 Chevrel Phase of Electrode for Mg Batteries. Mater. Res. Express 2022, 9, 085502. [Google Scholar] [CrossRef]
- Yao, Y.; Ao, K.; Lv, P.; Wei, Q. MoS2 Coexisting in 1T and 2H Phases Synthesized by Common Hydrothermal Method for Hydrogen Evolution Reaction. Nanomaterials 2019, 9, 844. [Google Scholar] [CrossRef] [PubMed]
- Oreshonkov, A.S.; Sukhanova, E.V.; Popov, Z.I. Raman Spectroscopy of Janus MoSSe Monolayer Polymorph Modifications Using Density Functional Theory. Materials 2022, 15, 3988. [Google Scholar] [CrossRef] [PubMed]
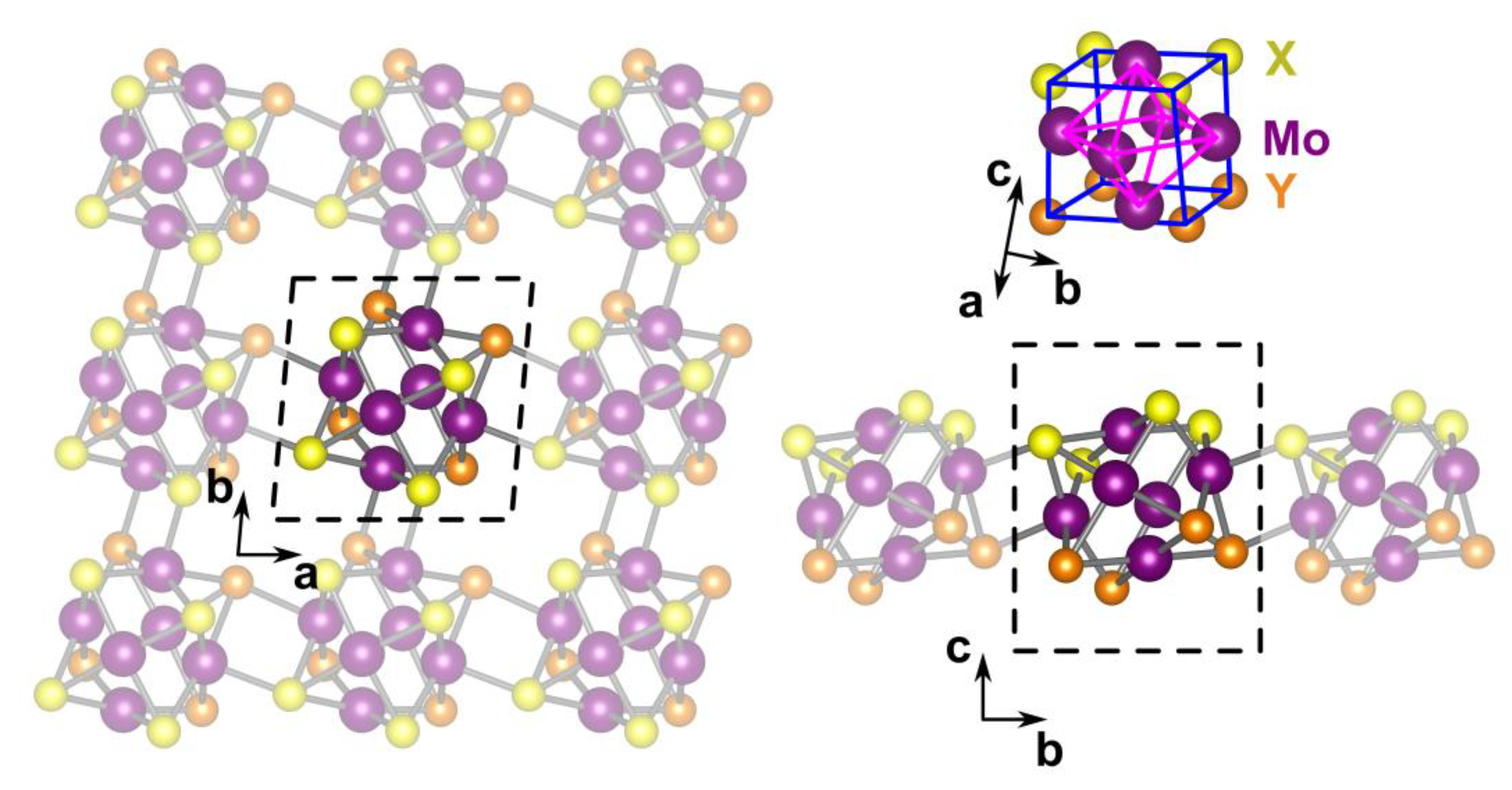
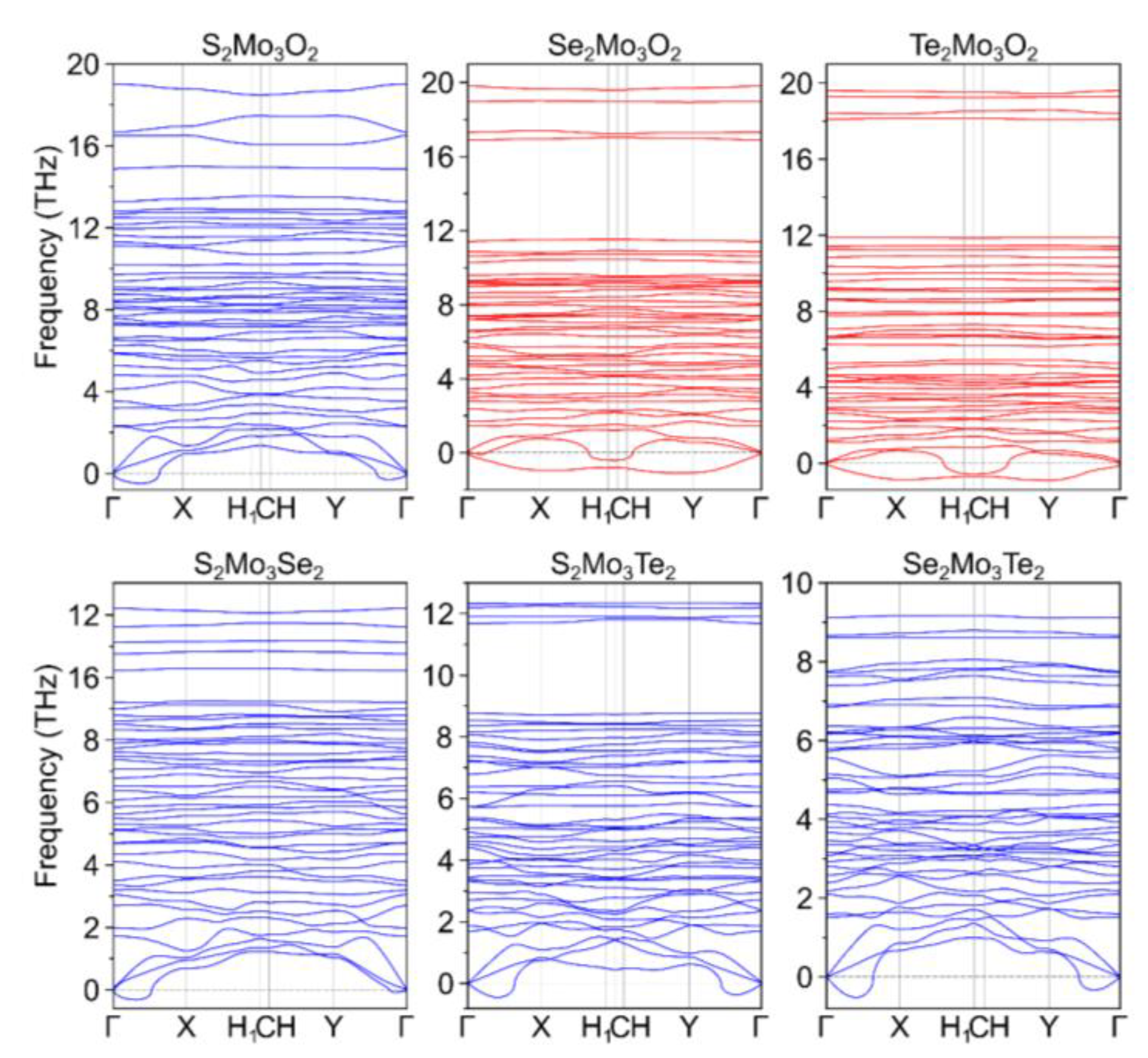
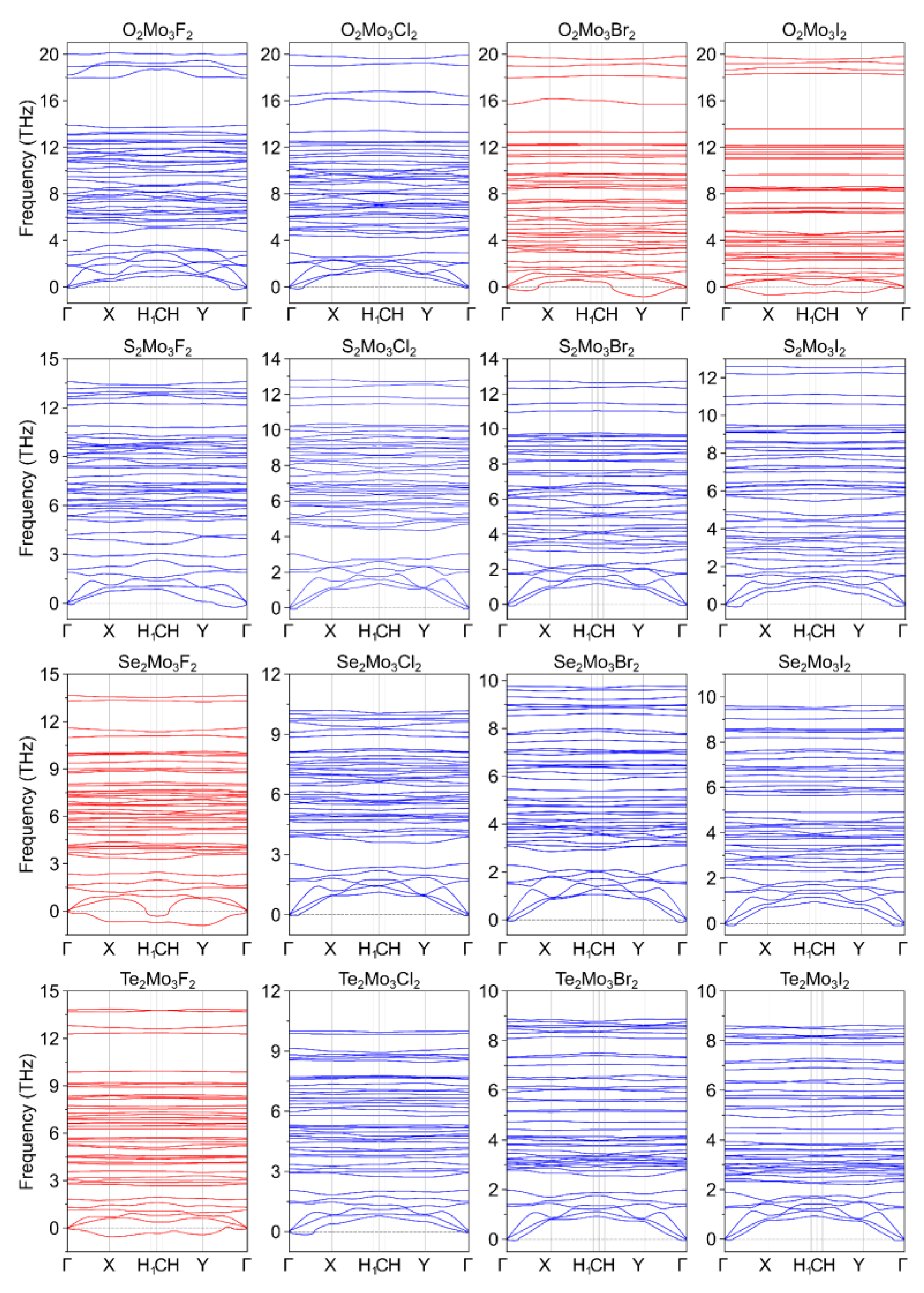

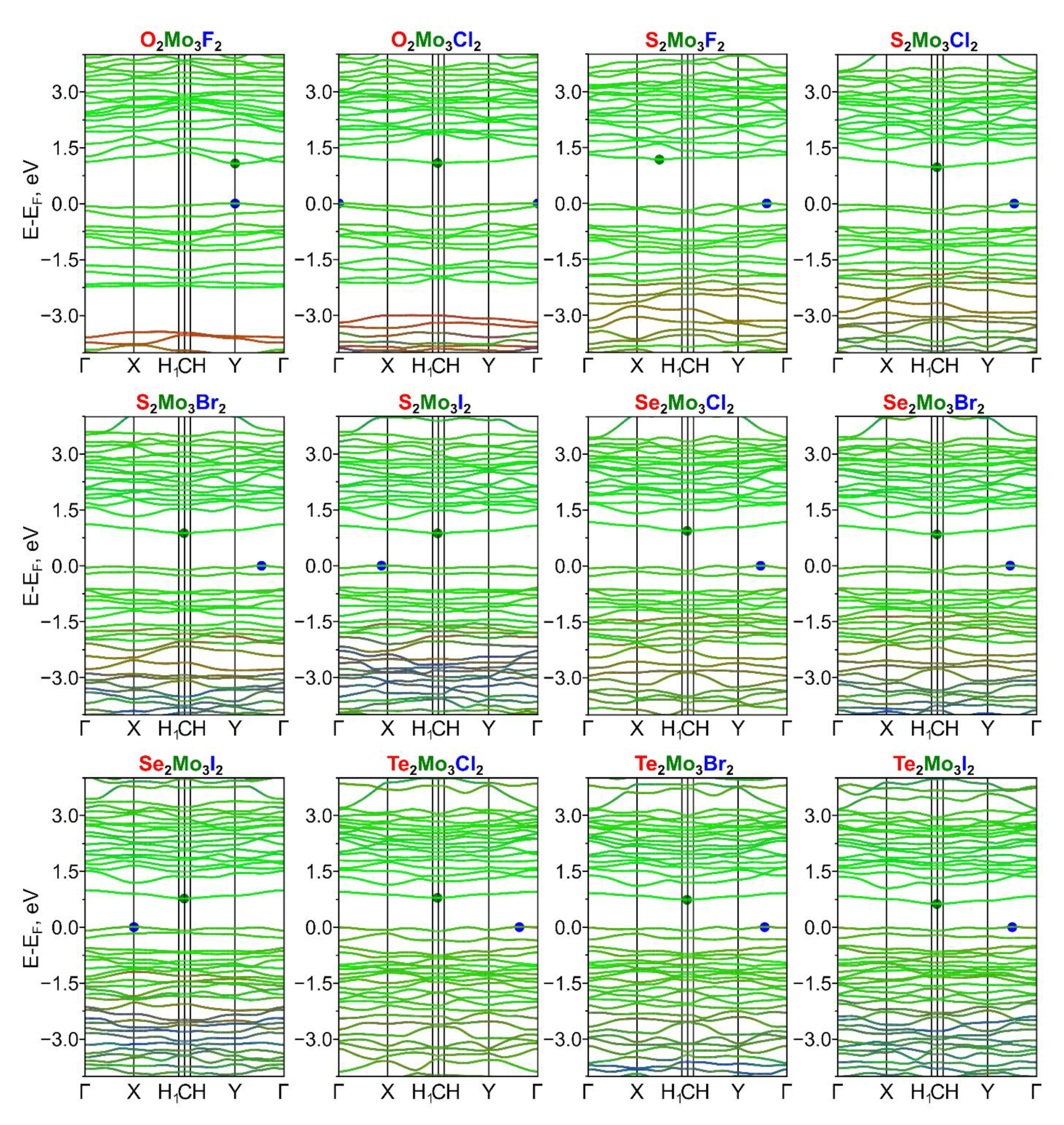
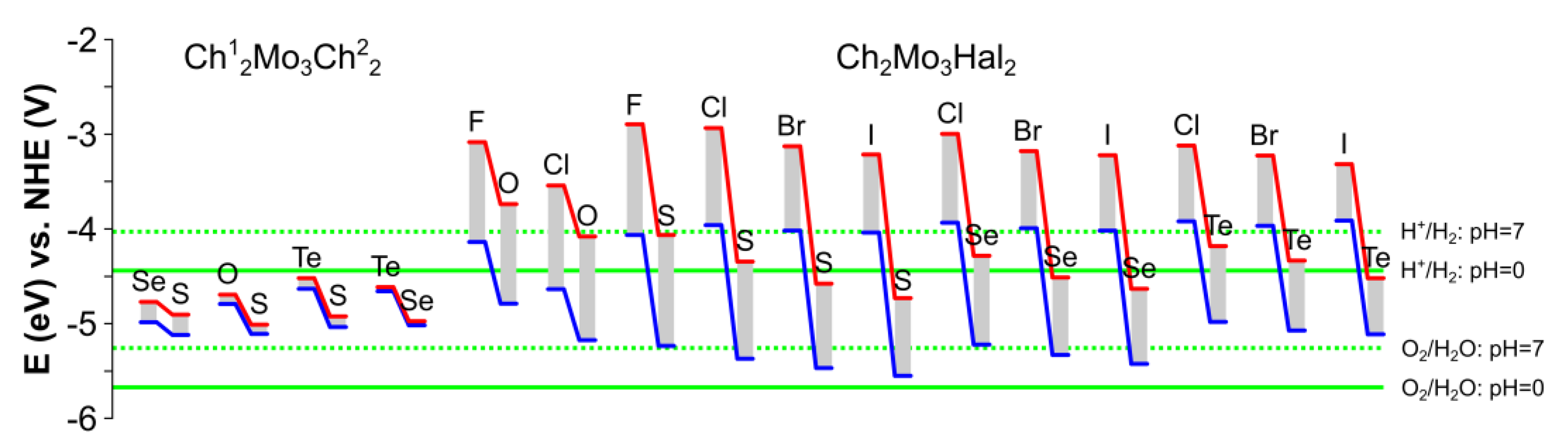
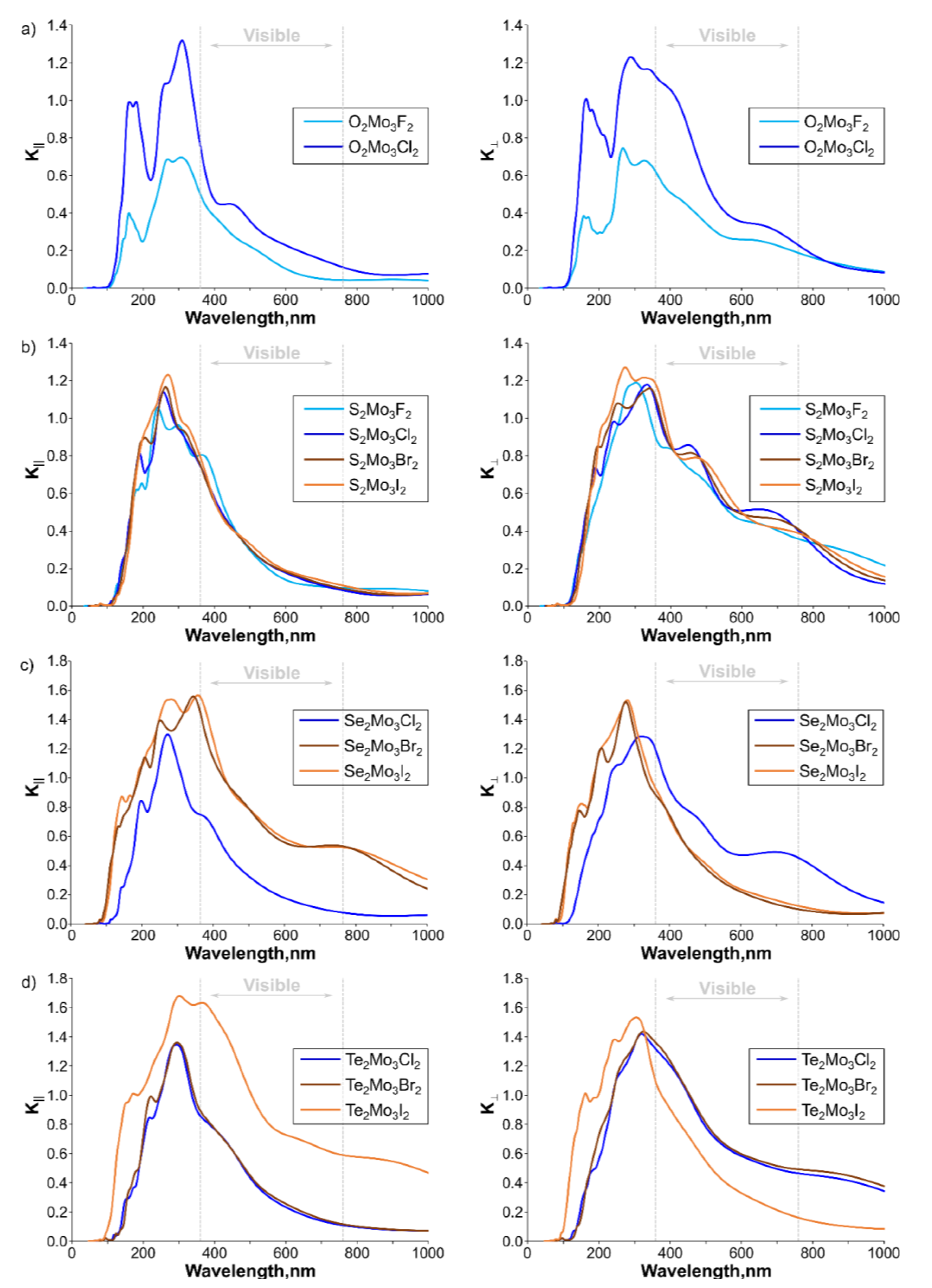
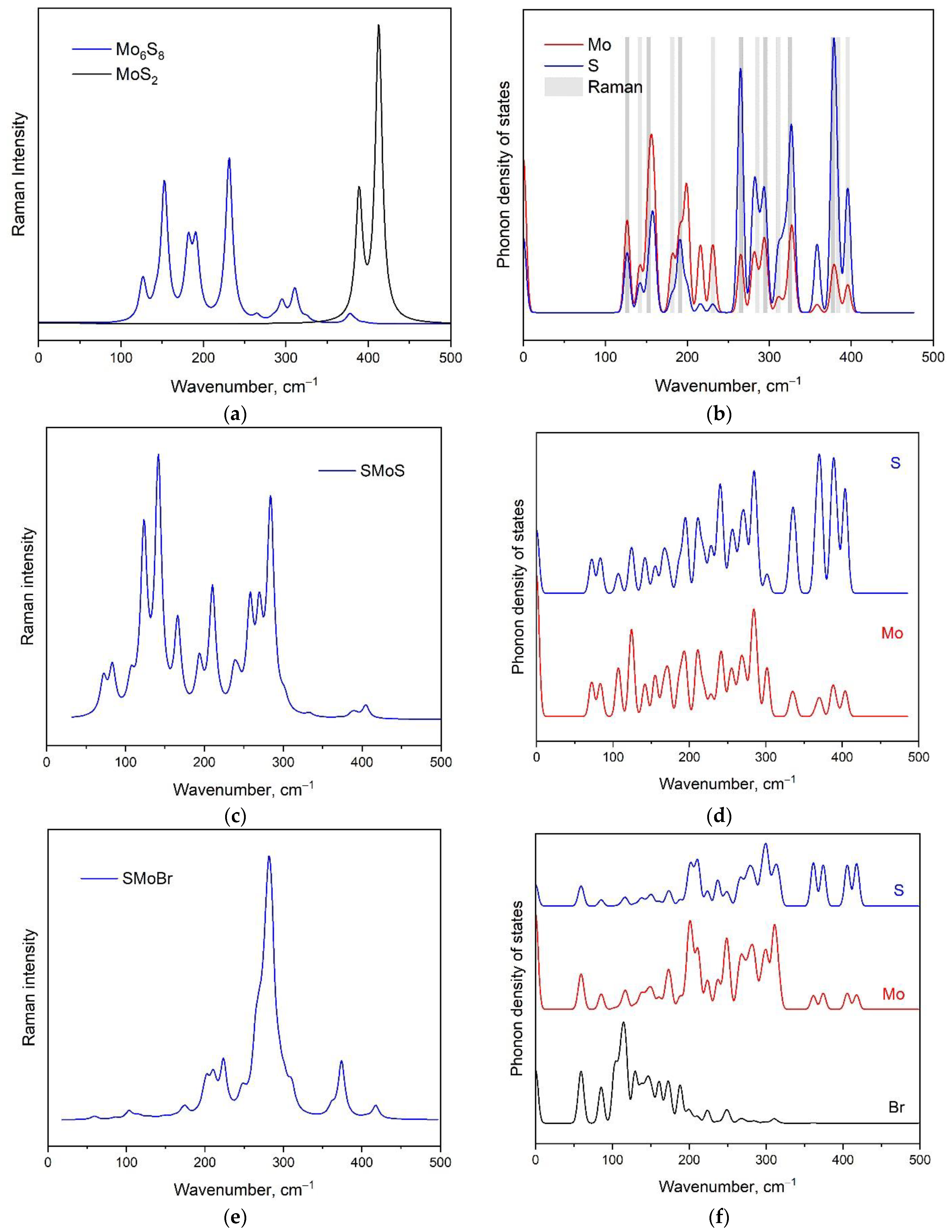
| Ch12Mo3Ch22 Monolayer | a, Å | b, Å | γ, ° |
|---|---|---|---|
| S2Mo3S2 | 6.518 | 6.623 | 84.89 |
| Se2Mo3Se2 | 6.644 | 6.738 | 85.10 |
| Te2Mo3Te2 | 6.834 | 6.902 | 89.73 |
| S2Mo3Se2 | 6.644 | 6.738 | 85.10 |
| S2Mo3O2 | 6.190 | 6.425 | 84.10 |
| S2Mo3Te2 | 6.834 | 6.902 | 89.73 |
| Se2Mo3O2 | 6.499 | 6.543 | 88.66 |
| Se2Mo3Te2 | 6.943 | 6.957 | 87.39 |
| Te2Mo3O2 | 6.861 | 7.074 | 90.92 |
| Ch2Mo3Hal2 Monolayer | a, Å | b, Å | γ, ° |
|---|---|---|---|
| O2Mo3F2 | 6.148 | 6.131 | 85.87 |
| O2Mo3Cl2 | 6.362 | 6.286 | 87.38 |
| O2Mo3Br2 | 6.864 | 6.436 | 87.31 |
| O2Mo3I2 | 7.337 | 7.328 | 84.04 |
| S2Mo3F2 | 6.517 | 6.521 | 86.01 |
| S2Mo3Cl2 | 6.656 | 6.637 | 86.49 |
| S2Mo3Br2 | 6.791 | 6.773 | 86.60 |
| S2Mo3I2 | 7.061 | 7.033 | 86.38 |
| Se2Mo3F2 | 6.720 | 6.741 | 85.78 |
| Se2Mo3Cl2 | 6.796 | 6.780 | 86.45 |
| Se2Mo3Br2 | 6.909 | 6.892 | 86.46 |
| Se2Mo3I2 | 7.133 | 7.116 | 86.29 |
| Te2Mo3F2 | 7.170 | 7.172 | 85.51 |
| Te2Mo3Cl2 | 7.087 | 7.089 | 86.33 |
| Te2Mo3Br2 | 7.142 | 7.135 | 86.34 |
| Te2Mo3I2 | 7.291 | 7.277 | 86.17 |
| Monolayer | (001) Surface | Surface | Eg, eV | ΔΦ, eV |
|---|---|---|---|---|
| S2Mo3Se2 | Se | S | 0.21 | 0.14 |
| S2Mo3O2 | O | S | 0.10 | 0.32 |
| S2Mo3Te2 | Te | S | 0.11 | 0.40 |
| Se2Mo3Te2 | Te | Se | 0.05 | 0.36 |
| O2Mo3F2 | F | O | 1.05 | 0.65 |
| O2Mo3Cl2 | O | Cl | 1.10 | 0.54 |
| S2Mo3F2 | F | S | 1.17 | 1.17 |
| S2Mo3Cl2 | Cl | S | 1.03 | 1.41 |
| S2Mo3Br2 | Br | S | 0.89 | 1.45 |
| S2Mo3I2 | I | S | 0.82 | 1.51 |
| Se2Mo3Cl2 | Cl | Se | 0.94 | 1.29 |
| Se2Mo3Br2 | Br | Se | 0.82 | 1.33 |
| Se2Mo3I2 | I | Se | 0.80 | 1.41 |
| Te2Mo3Cl2 | Cl | Te | 0.80 | 1.06 |
| Te2Mo3Br2 | Br | Te | 0.74 | 1.11 |
| Te2Mo3I2 | I | Te | 0.59 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhanova, E.V.; Sagatov, N.E.; Oreshonkov, A.S.; Gavryushkin, P.N.; Popov, Z.I. Halogen-Doped Chevrel Phase Janus Monolayers for Photocatalytic Water Splitting. Nanomaterials 2023, 13, 368. https://doi.org/10.3390/nano13020368
Sukhanova EV, Sagatov NE, Oreshonkov AS, Gavryushkin PN, Popov ZI. Halogen-Doped Chevrel Phase Janus Monolayers for Photocatalytic Water Splitting. Nanomaterials. 2023; 13(2):368. https://doi.org/10.3390/nano13020368
Chicago/Turabian StyleSukhanova, Ekaterina V., Nursultan E. Sagatov, Aleksandr S. Oreshonkov, Pavel N. Gavryushkin, and Zakhar I. Popov. 2023. "Halogen-Doped Chevrel Phase Janus Monolayers for Photocatalytic Water Splitting" Nanomaterials 13, no. 2: 368. https://doi.org/10.3390/nano13020368
APA StyleSukhanova, E. V., Sagatov, N. E., Oreshonkov, A. S., Gavryushkin, P. N., & Popov, Z. I. (2023). Halogen-Doped Chevrel Phase Janus Monolayers for Photocatalytic Water Splitting. Nanomaterials, 13(2), 368. https://doi.org/10.3390/nano13020368







