Photoluminescence of Cesium-Doped Sodium Iodide Films Irradiated by UV LED
Abstract
:1. Introduction
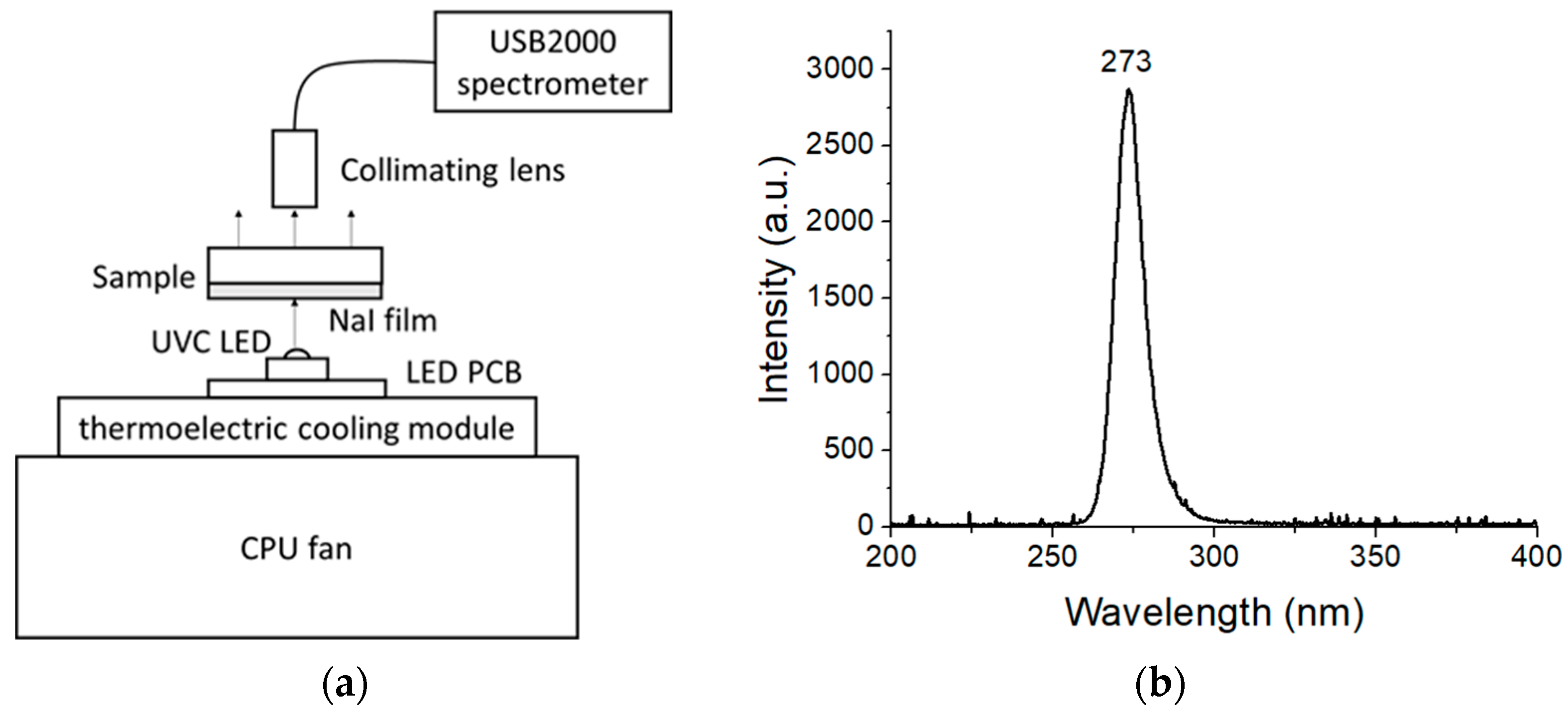
2. Materials and Methods
3. Results and Discussion
3.1. XRD Characteristic of Pure NaI and NaI Cs-Dopped Films
3.1.1. XRD Characteristic of NaI Films on the Glass Substrate
3.1.2. XRD Characteristic of NaI Films on Fused Silica Substrate
3.1.3. XRD Characteristic Variation under Different Deposition Treatment
3.2. Photoluminescence of NaI and NaI:Cs Films
3.2.1. Photoluminescence of NaI Films on a Glass Substrate
3.2.2. Photoluminescence of NaI Films on Fused Silica Substrate
3.3. PL Affected by Fused Silica and B270 Substrate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Min, S.; Kang, H.; Seo, B.; Cheong, J.; Roh, C.; Hong, S. A review of nanomaterial based scintillators. Energies 2021, 14, 7701. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Lv, S.C.; Yang, Z.M.; Qiu, J.R.; Zhou, S.F. Structured scintillators for efficient radiation detection. Adv. Sci. 2022, 9, 2102439. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.R.; Dietrich, H.B.; Murray, R.B.; Rock, T.J. Scintillation response of NaI(T1) and KI(T1) to channeled ions. Phys. Rev. B 1973, 7, 1743. [Google Scholar] [CrossRef]
- Popov, A.I.; Chernov, S.A.; Trinkler, L.E. Time-resolved luminescence of CsI Tl crystals excited by pulsed electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 122, 602–605. [Google Scholar] [CrossRef]
- Shen, F.Z.; Fu, Q.B.; Huang, T.C.; Wang, W. A compact dual gamma neutron detector based on NaI(Tl plus Li) scintillator readout with sipm. Crystals 2022, 12, 1077. [Google Scholar] [CrossRef]
- Hsu, J.C.; Ma, Y.S. Luminescence of CsI and CsI:Na films under LED and X-ray excitation. Coatings 2019, 9, 751. [Google Scholar] [CrossRef]
- Gundacker, S.; Turtos, R.M.; Auffray, E.; Lecoq, P. Precise rise and decay time measurements of inorganic scintillators by means of X-ray and 511 keV excitation. Nucl. Instrum. Methods A 2018, 891, 42–52. [Google Scholar] [CrossRef]
- Kumar, V.; Luo, Z.P. A review on X-ray excited emission decay dynamics in inorganic scintillator materials. Photonics 2021, 8, 71. [Google Scholar] [CrossRef]
- Miyata, E.; Miki, M.; Tawa, N.; Miyaguchi, K. X-ray responsivities of direct-scintillator-deposited charge-coupled device. Jpn. J. Appl. Phys. 2005, 44, 1476–1484. [Google Scholar] [CrossRef]
- Kurosawa, S.; Shoji, Y.; Pejchal, J.; Yokota, Y.; Yoshikawa, A. Radiation imaging with a new scintillator and a CMOS camera. J. Instrum. 2014, 9, C07015. [Google Scholar] [CrossRef]
- Lecoq, P. Development of new scintillators for medical applications. Nucl. Instrum. Meth. A 2016, 809, 130–139. [Google Scholar] [CrossRef]
- Xie, S.W.; Zhang, X.; Zhang, Y.B.; Ying, G.Y.; Huang, Q.; Xu, J.F.; Peng, Q.Y. Evaluation of various scintillator materials in radiation detector design for positron emission tomography (PET). Crystals 2020, 10, 869. [Google Scholar] [CrossRef]
- Makek, M.; Bosnar, D.; Kozuljevic, A.M.; Pavelic, L. Investigation of GaGG:Ce with TOFPET2 ASIC readout for applications in gamma imaging systems. Crystals 2020, 10, 1073. [Google Scholar] [CrossRef]
- Beaulieu, L.; Beddar, S. Review of plastic and liquid scintillation dosimetry for photon, electron, and proton therapy. Phys. Med. Biol. 2016, 61, R305–R343. [Google Scholar] [CrossRef] [PubMed]
- Dilillo, G.; Zampa, N.; Campana, R.; Fuschino, F.; Pauletta, G.; Rashevskaya, I.; Ambrosino, F.; Baruzzo, M.; Cauz, D.; Cirrincione, D.; et al. Space applications of GAGG:Ce scintillators: A study of afterglow emission by proton irradiation. Nucl. Instrum. Meth. B 2022, 513, 33–43. [Google Scholar] [CrossRef]
- Rizzo, A.; Narici, L.; Messi, R.; Cipollone, P.; De Donato, C.; Di Fino, L.; Iannilli, M.; La Tessa, C.; Manea, C.; Masciantonio, G.; et al. A compact Time-Of-Flight detector for space applications: The LIDAL system. Nucl. Instrum. Meth. A 2018, 898, 98–104. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kataoka, J.; Arimoto, M.; Masuda, T.; Yoshino, M.; Kamada, K.; Yoshikawa, A.; Sato, H.; Usuki, Y. Evaluation of GAGG: Ce scintillators for future space applications. J. Instrum. 2018, 13, P02023. [Google Scholar] [CrossRef]
- Hu, J.; Liu, J.; Chen, L.; Zhang, Z.; Ouyang, X.; He, S.; Xu, M.; Zhou, L.; Yan, W.; Yao, Z. Identification of heavy ions by imaging individual particle tracks in CF4-filled gas scintillation detectors. J. Instrum. 2018, 13, P09025. [Google Scholar] [CrossRef]
- Koshimizu, M. Recent progress of organic scintillators. Jpn. J. Appl. Phys. 2023, 62, 010503. [Google Scholar] [CrossRef]
- Jansky, J.; Janda, J.; Mazankova, V.; Cvachovec, F. Optimization of composition of liquid organic scintillators for fast neutron spectrometry. Nucl. Instrum. Meth. A 2021, 1010, 165523. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.Y.; Nikl, M.; Hamel, M.; Wu, H.S.; Qian, S.; Kucerkova, R.; Babin, V.; Ren, G.H.; Wu, Y.T. Preparation and performance of plastic scintillators with copper iodide complex-loaded for radiation detection. Polymer 2022, 249, 124832. [Google Scholar] [CrossRef]
- Kim, C.; Lee, W.; Melis, A.; Elmughrabi, A.; Lee, K.; Park, C.; Yeom, J.Y. A review of inorganic scintillation crystals for extreme environments. Crystals 2021, 11, 669. [Google Scholar] [CrossRef]
- Weber, M.J. Inorganic scintillators: Today and tomorrow. J. Lumin. 2002, 100, 35–45. [Google Scholar] [CrossRef]
- Bourret-Courchesne, E.D.; Bizarri, G.A.; Borade, R.; Gundiah, G.; Samulon, E.C.; Yan, Z.; Derenzo, S.E. Crystal growth and characterization of alkali-earth halide scintillators. J. Cryst. Growth 2012, 352, 78–83. [Google Scholar] [CrossRef]
- Ma, Y.S.; Hsu, J.C.; Liu, C.P.; Chen, H.L. Fabrication of columnar CsI and CsI:Na scintillation films deposited by vacuum thermal evaporation at high deposition rates. Vacuum 2015, 122, 96–102. [Google Scholar] [CrossRef]
- Canning, A.; Chaudhry, A.; Boutchko, R.; Gronbech-Jensen, N. First-principles study of luminescence in Ce-doped inorganic scintillators. Phys. Rev. B 2011, 83, 125115. [Google Scholar] [CrossRef]
- Boutboul, T.; Akkerman, A.; Breskin, A.; Chechik, R. Electron inelastic mean free path and stopping power modeling in alkali halides in the 50 eV–10 keV energy range. J. Appl. Phys. 1996, 79, 6714–6721. [Google Scholar] [CrossRef]
- Nishimura, H.; Sakata, M.; Tsujimoto, T.; Nakayama, M. Origin of the 4.1-eV luminescence in pure CsI scintillator. Phys. Rev. B Condens. Matter. 1995, 51, 2167–2172. [Google Scholar] [CrossRef]
- Khodadoost, E.; Araghi, M.E.A. Scintillation response of europium and indium-co-doped CsI(Na) single crystal under the exposure of gamma-ray. Nucl. Instrum. Meth. A 2019, 942, 162351. [Google Scholar] [CrossRef]
- Davi, F. A brief overview of existence results and decay time estimates for a mathematical modeling of scintillating crystals. Math. Method. Appl. Sci. 2021, 44, 13833–13854. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kapustyanyk, V.; Tsybulskyi, V.; Rudyk, V.; Kraus, H. Luminescence and scintillation properties of CsI: A potential cryogenic scintillator. Phys. Status Solidi B 2015, 252, 804–810. [Google Scholar] [CrossRef]
- Moszynski, M.; Balcerzyk, M.; Czarnacki, W.; Kapusta, M.; Klamra, W.; Schotanus, P.; Syntfeld, A.; Szawlowski, M. Study of pure NaI at room and liquid nitrogen temperatures. In Proceedings of the 2002 IEEE Nuclear Science Symposium Conference Record, Norfolk, VA, USA, 10–16 November 2022; IEEE: Piscataway, NJ, USA, 2002; pp. 346–351. [Google Scholar]
- Keszthel, S.; Hrehuss, G. Scintillation response function and decay time of CsI(Na) to charged particles. Nucl. Instrum. Methods 1969, 68, 9–12. [Google Scholar] [CrossRef]
- Mouhti, I.; Elanique, A.; Messous, M.Y.; Benahmed, A.; McFee, J.E.; Elgoub, Y.; Griffith, P. Characterization of CsI(Tl) and LYSO(Ce) scintillator detectors by measurements and Monte Carlo simulations. Appl. Radiat. Isot. 2019, 154, 108878. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, L.; Moszynski, M.; Syntfeld-Kazuch, A.; Szawlowski, M.; Szczesniak, T. Measuring the scintillation decay time for different energy depositions in NaI:Tl, LSO:Ce and CeBr3 scintillators. Nucl. Instrum. Meth. A 2014, 749, 68–73. [Google Scholar] [CrossRef]
- McGregor, D.S. Materials for Gamma-Ray Spectrometers: Inorganic Scintillators. Annu. Rev. Mater. Res. 2018, 48, 245–277. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Sobottka, S.E.; Williams, M.B. Performance and fabrication of thin-film NaI(Tl) scintillators for use on imaging photomultiplier Tubes. IEEE Trans. Nucl. Sci. 1993, 40, 413–416. [Google Scholar] [CrossRef]
- Taranyuk, V.; Gektin, A.; Kisil, I.; Kolesnikov, A. NaI(Tl) and CsI(Tl) scintillation crystal growth by skull method. J. Cryst. Growth 2011, 318, 820–822. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, Y.; Joo, K.S.; Sihn, B.S.; Chong, J.W.; Kim, S.E.; Lee, K.H.; Choe, Y.S.; Kim, B.T. Development of a miniature scintillation camera using an NaI(Tl) scintillator and PSPMT for scintimammography. Phys. Med. Biol. 2000, 45, 3481–3488. [Google Scholar] [CrossRef]
- Siciliano, E.R.; Ely, J.H.; Kouzes, R.T.; Milbrath, B.D.; Schweppe, J.E.; Stromswold, D.C. Comparison of PVT and NaI(Tl) scintillators for vehicle portal monitor applications. Nucl. Instrum. Meth. A 2005, 550, 647–674. [Google Scholar] [CrossRef]
- Milbrath, B.D.; Choate, B.J.; Fast, J.E.; Hensley, W.K.; Kouzes, R.T.; Schweppe, J.E. Comparison of LaBr3: Ce and NAI(Tl) scintillators for radio-isotope identification devices. Nucl. Instrum. Meth. A 2007, 572, 774–784. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Farsoni, A.; Szydel, D.; Sabet, H. Growth and evaluation of improved CsI:Tl and NaI:Tl scintillators. Crystals 2022, 12, 1517. [Google Scholar] [CrossRef]
- Chuong, H.D.; Le, N.T.M.; Tam, H.D. Semi-empirical method for determining the density of liquids using a NaI(Tl) scintillation detector. Appl. Radiat. Isot. 2019, 152, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, V.; Trefilova, L.; Meleshko, A.; Ganja, Y. Short-living absorption and emission of CsI(Na). J. Lumin. 2011, 131, 2579–2581. [Google Scholar] [CrossRef]
- Guguschev, C.; Calvert, G.; Podowitz, S.; Vailionis, A.; Yeckel, A.; Feigelson, R.S. The application of floating dies for high speed growth of CsI single crystals by edge-defined film-fed growth (EFG). J. Cryst. Growth 2014, 404, 231–240. [Google Scholar] [CrossRef]
- Lee, J.Y.; Adhikari, G.; Ha, C.; Kim, H.J.; Kim, N.Y.; Kim, S.K.; Kim, Y.D.; Lee, H.S. A Study of NaI(Tl) crystal encapsulation using organic scintillators for the dark matter search. Nucl. Instrum. Meth. A 2020, 953, 163141. [Google Scholar] [CrossRef]
- Shpilinskaya, A.L.; Kudin, A.M.; Andryushchenko, L.A.; Didenko, A.V.; Zelenskaya, O.V. A protective hydrophobic coating for CsI(Tl) Crystals. Instrum. Exp. Tech. 2020, 63, 30–33. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of apparatus geometry and deposition conditions on the structure and topography of thick sputtered coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Thornton, J.A. High-rate thick-film growth. Annu. Rev. Mater. Res. 1977, 7, 239–260. [Google Scholar] [CrossRef]
- Hsu, J.C.; Lu, C.C.; Wang, P.W.; Chen, H.L.; Yu, J.C. The luminescence-energy efficiency and anti-deliquesce of CsI, NaI and the mixture films. In Proceedings of the 10th International Symposium on Sputtering Plasma Processes, Kanagawa, Japan, 8–10 July 2009. [Google Scholar]
- Li, J.; Wu, X.L.; Hu, D.S.; Yang, Y.M.; Qiu, T.; Shen, J.C. Splitting of X-ray diffraction peak in (Ge:SiO2)/SiO2 multilayers. Solid State Commun. 2004, 131, 21–25. [Google Scholar] [CrossRef]
- Wang, W.K.; Liu, K.F.; Tsai, P.C.; Xu, Y.J.; Huang, S.Y. Influence of annealing temperature on the properties of ZnGa2O4 thin films by magnetron sputtering. Coatings 2019, 9, 859. [Google Scholar] [CrossRef]
- Mohamedkhair, A.K.; Drmosh, Q.A.; Qamar, M.; Yamani, Z.H. Nanostructured magneli-phase W18O49 thin films for photoelectrochemical water splitting. Catalysts 2020, 10, 526. [Google Scholar] [CrossRef]
- Kumar, G.R.; Savariraj, A.D.; Karthick, S.N.; Selvam, S.; Balamuralitharan, B.; Kim, H.J.; Viswanathan, K.K.; Vijaykumar, M.; Prabakar, K. Phase transition kinetics and surface binding states of methylammonium lead iodide perovskite. Phys. Chem. Chem. Phys. 2016, 18, 7284–7292. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Correia, M.R.; Pereira, E.; O’Donnell, K.P.; Alves, E.; Sequeira, A.D.; Franco, N. Interpretation of double X-ray diffraction peaks from InGaN layers. Appl. Phys. Lett. 2001, 79, 1432–1434. [Google Scholar] [CrossRef]
- Pereira, S.; Correia, M.R.; Pereira, E.; O’Donnell, K.P.; Martin, R.W.; White, M.E.; Alves, E.; Sequeira, A.D.; Franco, N. Splitting of X-ray diffraction and photoluminescence peaks in InGaN/GaN layers. Mater. Sci. Eng. B 2002, 93, 163–167. [Google Scholar] [CrossRef]
- Lebedynskiy, A.M.; Shiran, N.V.; Gektin, A.V.; Fedorov, A.G.; Vasyukov, S.A.; Mateychenko, P.V. Structure and luminescence of CsI:Eu columnar films. J. Appl. Spectrosc. 2012, 79, 583–588. [Google Scholar] [CrossRef]
- Cha, B.K.; Bae, J.H.; Lee, C.H.; Chang, S.; Cho, G. The sensitivity and spatial resolution dependence on the microstructures of CsI:Tl scintillation layer for X-ray imaging detectors. Nucl. Instrum. Meth. A 2011, 633, S297–S299. [Google Scholar] [CrossRef]
- Yao, D.L.; Gu, M.; Liu, X.L.; Huang, S.M.; Liu, B.; Ni, C. Fabrication and performance of columnar CsI(Tl) scintillation films with single preferred orientation. IEEE Trans. Nucl. Sci. 2013, 60, 1632–1636. [Google Scholar] [CrossRef]
- Ahmed, S.N. Physics and Engineering of Radiation Detection, 1st ed.; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2007. [Google Scholar]
- Sawant, A.; Zeman, H.; Samant, S.; Lovhoiden, G.; Weinberg, B.; DiBianca, F. Theoretical analysis and experimental evaluation of a CsI(Tl) based electronic portal imaging system. Med. Phys. 2002, 29, 1042–1053. [Google Scholar] [CrossRef]
- Nakayama, M.; Ando, N.; Hirai, J.; Nishimura, H. Scintillation activated by nanoparticle formation in CsI: Na thin films. J. Lumin. 2004, 108, 359–363. [Google Scholar] [CrossRef]
- Leblans, P.; Struye, L.; Elen, S.; Mans, I.; Vrielinck, H.; Callens, F. X-ray enhancement of CsI:Eu2+ radioluminescence. J. Lumin. 2015, 165, 68–76. [Google Scholar] [CrossRef]
- Babin, V.; Fabeni, P.; Nikl, M.; Nitsch, K.; Pazzi, G.P.; Polak, K.; Phani, A.R.; Santucci, S.; Zazubovich, S. Luminescence of Pb2+-based aggregates in CsI matrix. Radiat. Eff. Defects Solids 1999, 149, 119–123. [Google Scholar] [CrossRef]
- Nikl, M.; Babin, V.; Mares, J.A.; Kamada, K.; Kurosawa, S.; Yoshikawa, A.; Tous, J.; Houzvicka, J.; Blazek, K. The role of cerium variable charge state in the luminescence and scintillation mechanism in complex oxide scintillators: The effect of air annealing. J. Lumin. 2016, 169, 539–543. [Google Scholar] [CrossRef]
- Zhong, J.P.; Liang, H.B.; Su, Q.; Zhang, G.B.; Dorenbos, P.; Birowosuto, M.D. Effects of annealing treatments on luminescence and scintillation properties of Ce: Lu3Al5O12 crystal grown by Czochralski method. J. Rare Earths 2007, 25, 568–572. [Google Scholar]
- Wang, C.; Ding, D.Z.; Wu, Y.T.; Li, H.Y.; Chen, X.F.; Shi, J.; Wang, Q.Q.; Ye, L.; Ren, G.H. Effect of thermal annealing on scintillation properties of Ce:Gd2Y1Ga2.7Al2.3O12 under different atmosphere. Appl. Phys. A 2017, 123, 384. [Google Scholar] [CrossRef]
- Moszynski, M.; Syntfeld-Kazuch, A.; Swiderski, L.; Sibczynski, P.; Grodzicka, M.; Szczesniak, T.; Gektin, A.V.; Schotanus, P.; Shiran, N.; Williams, R.T. Energy resolution and slow components in undoped CsI crystals. IEEE Trans. Nucl. Sci. 2016, 63, 459–466. [Google Scholar] [CrossRef]
- Balamurugan, N.; Arulchakkaravarthi, A.; Selvakumar, S.; Lenin, M.; Kumar, R.; Muralithar, S.; Sivaji, K.; Ramasamy, P. Growth and characterization of undoped and thallium doped cesium iodide single crystals. J. Cryst. Growth 2006, 286, 294–299. [Google Scholar] [CrossRef]
- Babin, V.; Krasnikov, A.; Wieczorek, H.; Zazubovich, S. Luminescence of complicated thallium centres in CsI:Tl. Nucl. Instrum. Methods Phys. Res. A 2002, 486, 486–489. [Google Scholar] [CrossRef]






| Sample | Substrate | Annealing | Dominant Peak(s) | Other Observable Peak | Peak Splitting |
|---|---|---|---|---|---|
| NaI powder | - | before | (002) | (111), (002), (022), (113), (222), (004), (133), (024), (224), (115) | No |
| pure NaI | B270 | before | (002) | (022), (113), (024), (115), | (022), (113), (115) |
| after | (002) | (022), (113), (133), (024), (115) | (113) | ||
| NaI:Cs(1%) | B270 | before | (002) | (022), (113), (024), (004), (115) | (002), (022) |
| after | (022), (113) | (024), (115) | No | ||
| NaI:Cs(2%) | B270 | before | (002) | (022), (004) | (002) |
| after | (022), (113) | (024), (115) | No | ||
| pure NaI | Fused silica | before | (022), (113) | (111), (024), (224), (115), | (022) |
| after | (022), (113) | (024), (115) | All | ||
| NaI:Cs(1%) | Fused silica | before | (002) | (022), (113), (024), (115), | (022), (113) |
| after | (022), (113) | (024), (115) | All | ||
| NaI:Cs(2%) | Fused silica | before | (022), (113) | (024), (115) | All |
| after | (022), (113) | (024), (115) | All |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-Y.; Kuan, Y.-H.; Yu, G.; Sun, Y.-S.; Hsu, J.-C. Photoluminescence of Cesium-Doped Sodium Iodide Films Irradiated by UV LED. Nanomaterials 2023, 13, 2747. https://doi.org/10.3390/nano13202747
Wu H-Y, Kuan Y-H, Yu G, Sun Y-S, Hsu J-C. Photoluminescence of Cesium-Doped Sodium Iodide Films Irradiated by UV LED. Nanomaterials. 2023; 13(20):2747. https://doi.org/10.3390/nano13202747
Chicago/Turabian StyleWu, Hsing-Yu, Yu-Hung Kuan, Guoyu Yu, Yung-Shin Sun, and Jin-Cherng Hsu. 2023. "Photoluminescence of Cesium-Doped Sodium Iodide Films Irradiated by UV LED" Nanomaterials 13, no. 20: 2747. https://doi.org/10.3390/nano13202747
APA StyleWu, H.-Y., Kuan, Y.-H., Yu, G., Sun, Y.-S., & Hsu, J.-C. (2023). Photoluminescence of Cesium-Doped Sodium Iodide Films Irradiated by UV LED. Nanomaterials, 13(20), 2747. https://doi.org/10.3390/nano13202747









