Thermokinetic Study of Aluminum-Induced Crystallization of a-Si: The Effect of Al Layer Thickness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electron Microscopy
2.3. X-ray Diffraction
2.4. Resistivity Measurements
2.5. Simultaneous Thermal Analysis
3. Results
3.1. TEM and XRD
3.2. Electrical Resistivity
3.3. Optical Microscopy
3.4. Simultaneous Thermal Analysis
4. Discussion
4.1. Resistivity
4.2. Estimation of the Kinetic Parameters Using Non-Isothermal Model-Free Methods
4.2.1. Kissinger Analysis
4.2.2. Friedman Analysis
4.3. Determination of the Most Appropriate Kinetic Model
4.4. Summary
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Jeurgens, L.P.H.; Mittemeijer, E.J. Metal-Induced Crystallization: Fundamentals and Applications; Jenny Stanford Publishing: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Boyce, J.B.; Mei, P.; Fulks, R.T.; Ho, J. Laser Processing of Polysilicon Thin-Film Transistors: Grain Growth and Device Fabrication. Phys. Status Solidi A 1998, 166, 729–741. [Google Scholar] [CrossRef]
- Magdy, S.; Ghazala, A.; Othman, H.A.; Sharaf El-Deen, L.M.; Nawwar, M.A.; Kashyout, A.E.B. Fabrication of Nanocrystalline Silicon Thin Films Utilized for Optoelectronic Devices Prepared by Thermal Vacuum Evaporation. ACS Omega 2020, 5, 27633–27644. [Google Scholar] [CrossRef]
- Murray, J.L.; McAlister, A.J. The Al-Si (aluminum-silicon) system. Bull. Alloy Phase Diagr. 1984, 5, 74–84. [Google Scholar] [CrossRef]
- Olesinski, R.W.; Abbaschian, G.J. The Cu−Si (Copper-Silicon) system. Bull. Alloy Phase Diagr. 1986, 7, 170–178. [Google Scholar] [CrossRef]
- Radnoczi, G.; Robertsson, A.; Hentzell, H.T.G.; Gong, S.F.; Hasan, M.A. Al induced crystallization of a-Si. J. Appl. Phys. 1991, 69, 6394–6399. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wang, J.Y.; Jeurgens, L.P.H.; Mittemeijer, E.J. Thermodynamics and mechanism of metal-induced crystallization in immiscible alloy systems: Experiments and calculations on Al/a-Ge and Al/a-Si bilayers. Phys. Rev. B 2008, 77, 045424. [Google Scholar] [CrossRef]
- Maity, G.; Dubey, S.; El-Azab, A.; Singhal, R.; Ojha, S.; Kulriya, P.K.; Dhar, S.; Som, T.; Kanjilale, D.; Patel, S.P. An assessment on crystallization phenomena of Si in Al/a-Si thin films via thermal annealing and ion irradiation. RSC Adv. 2020, 10, 4414–4426. [Google Scholar] [CrossRef]
- Nast, O.; Brehme, S.; Pritchard, S.; Aberle, A.G.; Wenham, S.R. Aluminium-induced crystallisation of silicon on glass for thin-film solar cells. Sol. Energy Mater. Sol. Cells 2001, 65, 385–392. [Google Scholar] [CrossRef]
- Shekoofa, O.; Wang, J.; Li, D.; Luo, Y.; Sun, C.; Hao, Z.; Han, Y.; Xiong, B.; Wang, L.; Li, H. Nano-crystalline thin films fabricated by Si-Al co-sputtering and metal induced crystallization for photovoltaic applications. Sol. Energy 2018, 173, 539–550. [Google Scholar] [CrossRef]
- Gestel, V.D.; Gordon, I.; Poortmans, J. Aluminum-induced crystallization for thin-film polycrystalline silicon solar cells: Achievements and perspective. Sol. Energy Mater. Sol. Cells 2013, 119, 261–270. [Google Scholar] [CrossRef]
- Nast, O.; Wenham, S.R. Elucidation of the layer exchange mechanism in the formation of polycrystalline silicon by aluminum-induced crystallization. J. Appl. Phys. 2000, 88, 124–132. [Google Scholar] [CrossRef]
- Gall, S.; Muske, M.; Sieber, I.; Nast, O.; Fuhs, W. Aluminum-induced crystallization of amorphous silicon. J. Non-Cryst. Sol. 2002, 299–302, 741–745. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, Z.M.; Mittemeijer, E.J. Mechanism of aluminum-induced layer exchange upon low-temperature annealing of amorphous Si/polycrystalline Al bilayers. J. Appl. Phys. 2007, 102, 113523. [Google Scholar] [CrossRef]
- Nast, O.; Puzzer, T.; Koschier, L.M.; Sproul, A.B.; Wenham, S.R. Aluminum-induced crystallization of amorphous silicon on glass substrates above and below the eutectic temperature. Appl. Phys. Lett. 1998, 73, 3214–3216. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Chen, I.-F.; Yu, M.-C. Stress Effect on Aluminum-Induced Crystallization of Sputtered Amorphous Silicon Thin Films. Jpn. J. Appl. Phys. 2003, 42, 4928–4934. [Google Scholar] [CrossRef]
- Qi, G.J.; Zhang, S.; Tang, T.T.; Li, J.F.; Sun, X.W.; Zeng, X.T. Experimental study of aluminum-induced crystallization of amorphous silicon thin films. Surf. Coat. Technol. 2005, 198, 300–303. [Google Scholar] [CrossRef]
- Chu, H.-Y.; Weng, M.-H.; Yang, R.-Y.; Huang, C.-W.; Li, C.-H. Effect of Al Thickness on the Al Induced Low Temperature Poly-Si Film Crystallization Process. In Proceedings of the 2009 4th IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Shenzhen, China, 5–8 January 2009; pp. 641–644. [Google Scholar] [CrossRef]
- Peng, C.C.; Chung, C.K.; Lin, J.F. Effects of Al film thickness and annealing temperature on the aluminum-induced crystallization of amorphous silicon and carrier mobility. Acta Mater. 2011, 59, 6093–6102. [Google Scholar] [CrossRef]
- Tutashkonko, S.; Usami, N. Effects of the Si/Al layer thickness on the continuity, crystalline orientation and the growth kinetics of the poly-Si thin films formed by aluminum-induced crystallization. Thin Solid Films 2016, 616, 213–219. [Google Scholar] [CrossRef]
- Her, Y.-C.; Chen, C.-W. Crystallization kinetics of ultrathin amorphous Si film induced by Al metal layer under thermal annealing and pulsed laser irradiation. J. Appl. Phys. 2007, 101, 043518. [Google Scholar] [CrossRef]
- Knaepen, W.; Detavernier, C.; Van Meirhaeghe, R.L.; Sweet, J.J.; Lavoie, C. In-situ X-ray Diffraction study of Metal Induced Crystallization of amorphous silicon. Thin Solid Films 2008, 516, 4946–4952. [Google Scholar] [CrossRef]
- Konno, T.J.; Sinclair, R. Crystallization of Amorphous Silicon-Aluminum thin Films: IN-SITU Observation and Thermal Analysis. MRS Online Proc. Libr. 1991, 237, 609–614. [Google Scholar] [CrossRef]
- Konno, T.J.; Sinclair, R. Crystallization of silicon in aluminium/amorphous-silicon multilayers. Phil. Mag. B 1992, 66, 749–765. [Google Scholar] [CrossRef]
- Klinger, M.; Jäger, A. Crystallographic Tool Box (CrysTBox): Automated tools for transmission electron microscopists and crystallographers. J. Appl. Crystallogr. 2015, 48, 2012–2018. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K. Pearson’s Crystal Data: Crystal Structure Database for Inorganic Compounds (on CD-ROM), Release 2011/12; ASM International®: Materials Park, OH, USA, 2011. [Google Scholar]
- Moiseenko, E.T.; Altunin, R.R.; Zharkov, S.M. In situ electron diffraction and resistivity characterization of solid state reaction process in Al/Cu bilayer thin films. Metall. Mater. Trans. A 2020, 51, 1428–1436. [Google Scholar] [CrossRef]
- Moiseenko, E.T.; Yumashev, V.V.; Altunin, R.R.; Solovyov, L.A.; Volochaev, M.N.; Belousov, O.V.; Zharkov, S.M. Thermokinetic study of intermetallic phase formation in an Al/Cu multilayer thin film system. Materialia 2023, 28, 101747. [Google Scholar] [CrossRef]
- Altunin, R.R.; Moiseenko, E.T.; Zharkov, S.M. Structural phase transformations in Al/Pt bilayer thin films during the solid-state reaction. Phys. Solid State 2018, 60, 1413–1418. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Altunin, R.R.; Yumashev, V.V.; Moiseenko, E.T.; Belousov, O.V.; Solovyov, L.A.; Volochaev, M.N.; Zeer, G.M. Kinetic study of solid-state reaction in Ag/Al multilayer thin films by in situ electron diffraction and simultaneous thermal analysis. J. Alloys Compd. 2021, 871, 159474. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Moiseenko, E.T.; Altunin, R.R. L10 ordered phase formation at solid state reactions in Cu/Au and Fe/Pd thin films. J. Solid State Chem. 2019, 269, 36–42. [Google Scholar] [CrossRef]
- Altunin, R.R.; Moiseenko, E.T.; Zharkov, S.M. Structural phase transformations during a solid-state reaction in a bilayer Al/Fe thin-film nanosystem. Phys. Solid State 2020, 62, 200–205. [Google Scholar] [CrossRef]
- Moiseenko, E.T.; Yumashev, V.V.; Altunin, R.R.; Zeer, G.M.; Nikolaeva, N.S.; Belousov, O.V.; Zharkov, S.M. Solid-state reaction in Cu/a-Si nanolayers: A comparative study of STA and electron diffraction data. Materials 2022, 15, 8457. [Google Scholar] [CrossRef] [PubMed]
- Zharkov, S.M.; Altunin, R.R.; Moiseenko, E.T.; Zeer, G.M.; Varnakov, S.N.; Ovchinnikov, S.G. Solid-state reactions in Fe/Si multilayer nanofilms. Solid State Phenom. 2014, 215, 144–149. [Google Scholar] [CrossRef]
- Zharkov, S.M.; Moiseenko, E.T.; Altunin, R.R.; Nikolaeva, N.S.; Zhigalov, V.S.; Myagkov, V.G. Study of solid-state reactions and order-disorder transitions in Pd/α-Fe(001) thin films. JETP Lett. 2014, 99, 405–409. [Google Scholar] [CrossRef]
- Moiseenko, E.T.; Altunin, R.R.; Zharkov, S.M. Formation of the atomically ordered L10 structure with the [001] orientation during the solid-state reaction in Fe/Pd bilayer thin films. Phys. Solid State 2017, 59, 1233–1237. [Google Scholar] [CrossRef]
- Myagkov, V.G.; Zhigalov, V.S.; Bykova, L.E.; Zharkov, S.M.; Matsynin, A.A.; Volochaev, M.N.; Tambasov, I.A.; Bondarenko, G.N. Thermite synthesis and characterization of Co–ZrO2 ferromagnetic nanocomposite thin films. J. Alloys Compd. 2016, 665, 197–203. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Solovyov, L.A. Full-profile refinement by derivative difference minimization. J. Appl. Crystallogr. 2004, 37, 743–749. [Google Scholar] [CrossRef]
- Hsu, T.-R. MEMS & Microsystems: Design and Manufacture; McGraw-Hill Science: New Delhi, India, 2002; ISBN 0-07-239391-2. [Google Scholar]
- Mahesh, R.; Sander, D.; Zharkov, S.M.; Kirschner, J. Stress and growth of Ag monolayers on a Fe(100) whisker. Phys. Rev. B 2003, 68, 45416. [Google Scholar] [CrossRef]
- He, D.; Wang, J.Y.; Mittemeijer, E.J. Reaction between a-Si and crystalline Al in Al/Si and Si/Al bilayers microstructural and thermodynamic analysis of layer exchange. Appl. Phys. A 2005, 80, 501–509. [Google Scholar] [CrossRef]
- Sain, T.; Singh, C.K.; Ilango, S.; Mathews, T. Crystallization kinetics and role of stress in Al induced layer exchange crystallization process of amorphous SiGe thin film on glass. J. Appl. Phys. 2019, 126, 125303. [Google Scholar] [CrossRef]
- Opfermann, J.R.; Kaisersberger, E.; Flammersheim, H.J. Model-free analysis of thermoanalytical data-advantages and limitations. Thermochim. Acta 2002, 391, 119–127. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Nonisothermal Crystallization Kinetics by DSC: Practical Overview. Processes 2023, 11, 1438. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Donovan, E.P.; Spaepen, F.; Turnbull, D.; Poate, J.M.; Jacobson, D.C. Calorimetric studies of crystallization and relaxation of amorphous Si and Ge prepared by ion implantation. J. Appl. Phys. 1985, 57, 1795–1804. [Google Scholar] [CrossRef]
- Donovan, E.P.; Spaepen, F.; Poate, J.M.; Jacobson, D.C. Homogeneous and interfacial heat releases in amorphous silicon. Appl. Phys. Lett. 1989, 55, 1516–1518. [Google Scholar] [CrossRef]
- Camacho, J.M.; Oliva, A.I. Surface and grain boundary contributions in the electrical resistivity of metallic nanofilms. Thin Solid Film. 2006, 515, 1881–1885. [Google Scholar] [CrossRef]
- Serway, R.A.; Jewett, J.W. Physics for Scientists and Engineers; Brooks Cole: Pacific Grove, CA, USA, 2004; p. 837. ISBN 0534408427. [Google Scholar]
- Martienssen, W.; Warlimont, H. Springer Handbook of Condensed Matter and Materials Data; Springer: Berlin/Heidelberg, Germany, 2005; p. 101. ISBN 3540335552. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Opfermann, J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. J. Therm. Anal. Calorim. 2000, 60, 641–658. [Google Scholar] [CrossRef]
- Tiwari, A.; Raj, B. Reactions and Mechanisms in Thermal Analysis of Advanced Materials; Scrivener Publishing: Beverly, MA, USA, 2015; pp. 551–553. ISBN 978-1-119-11757-5. [Google Scholar]
- Wang, J.Y.; Mittemeijer, E.J. A new method for the determination of the diffusion-induced concentration profile and the interdiffusion coefficient for thin film systems by Auger electron spectroscopical sputter depth profiling. J. Mat. Res. 2004, 19, 3389–3397. [Google Scholar] [CrossRef]
- Moukhina, E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J. Therm. Anal. Calorim. 2012, 109, 1203–1214. [Google Scholar] [CrossRef]
- Garn, P.D. An examination of the kinetic compensation effect. J. Therm. Anal. 1975, 7, 475–478. [Google Scholar] [CrossRef]
- L’vov, B.V.; Galwey, A.K. Interpretation of the kinetic compensation effect in heterogeneous reactions: Thermochemical approach. Int. Rev. Phys. Chem. 2013, 32, 515–557. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Blázquez, J.S.; Romero, F.J.; Conde, C.F.; Conde, A. A Review of Different Models Derived from Classical Kolmogorov, Johnson and Mehl, and Avrami (KJMA) Theory to Recover Physical Meaning in Solid-State Transformations. Phys. Status Solidi B 2022, 259, 2100524. [Google Scholar] [CrossRef]
- Safarik, D.J.; Mullins, C.B. Surface phase transformation kinetics: A geometrical model for thin films of nonvolatile and volatile solids. J. Chem. Phys. 2002, 117, 8110–8123. [Google Scholar] [CrossRef]
- Olson, G.L.; Roth, J.A. Kinetics of solid phase crystallization in amorphous silicon. Mater. Sci. Rep. 1988, 3, 1–77. [Google Scholar] [CrossRef]
- Her, Y.-C.; Chen, C.-W.; Wu, C.-L. Comparison of crystallization kinetics in a-Si∕Cu and a-Si∕Al bilayer recording films under thermal annealing and pulsed laser irradiation. J. Appl. Phys. 2006, 99, 113512. [Google Scholar] [CrossRef]
- Moghadam, M.M.; Voorhees, P.W. Thin film phase transformation kinetics: From theory to experiment. Scr. Mater. 2016, 124, 164–168. [Google Scholar] [CrossRef]
- Köster, U. Crystallization of amorphous silicon films. Phys. Stat. Sol. A 1978, 48, 313–321. [Google Scholar] [CrossRef]
- He, D.; Wang, J.Y.; Mittemeijer, E.J. The initial stage of the reaction between amorphous silicon and crystalline aluminum. J. Appl. Phys. 2005, 97, 093524. [Google Scholar] [CrossRef]
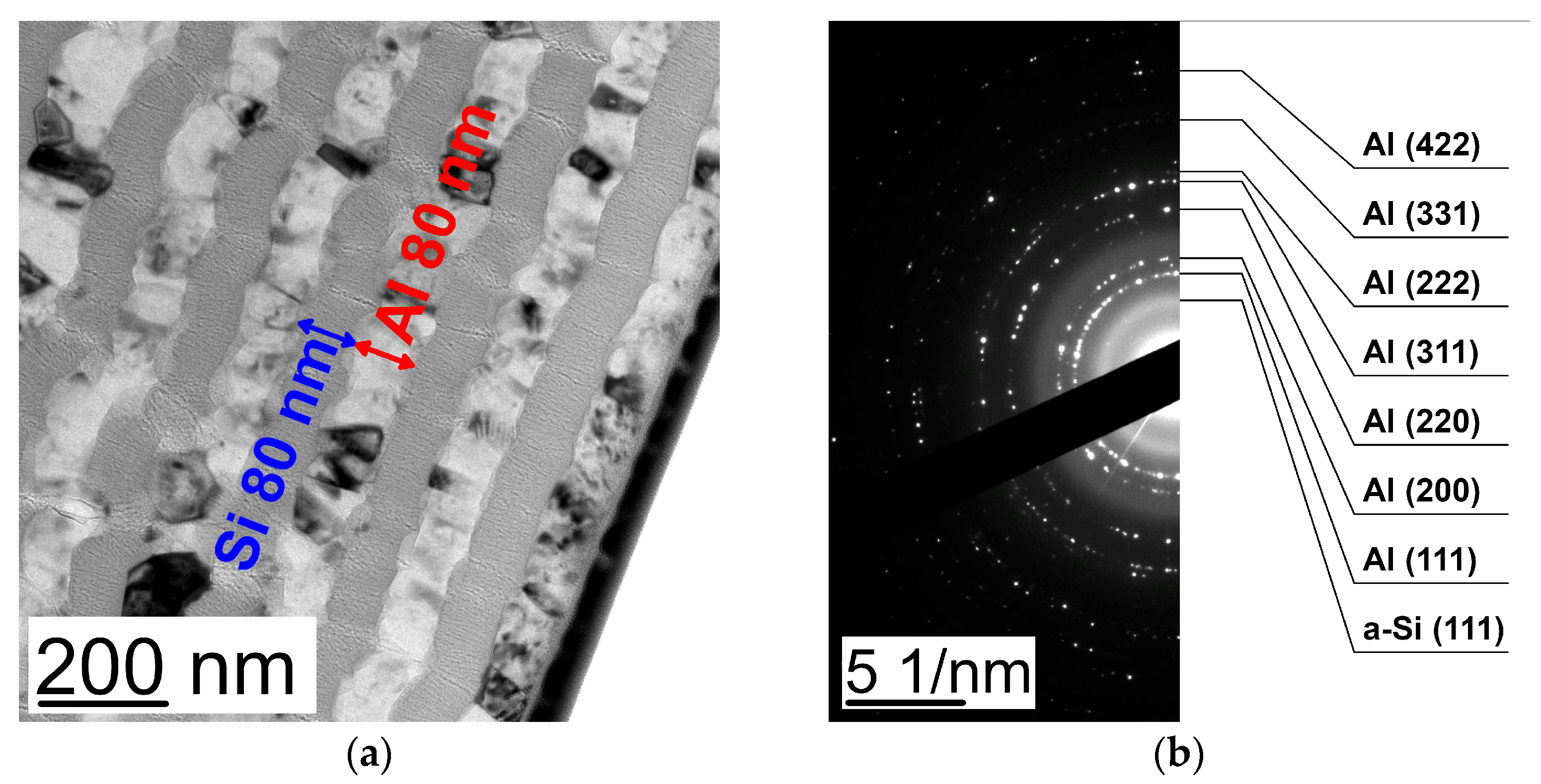



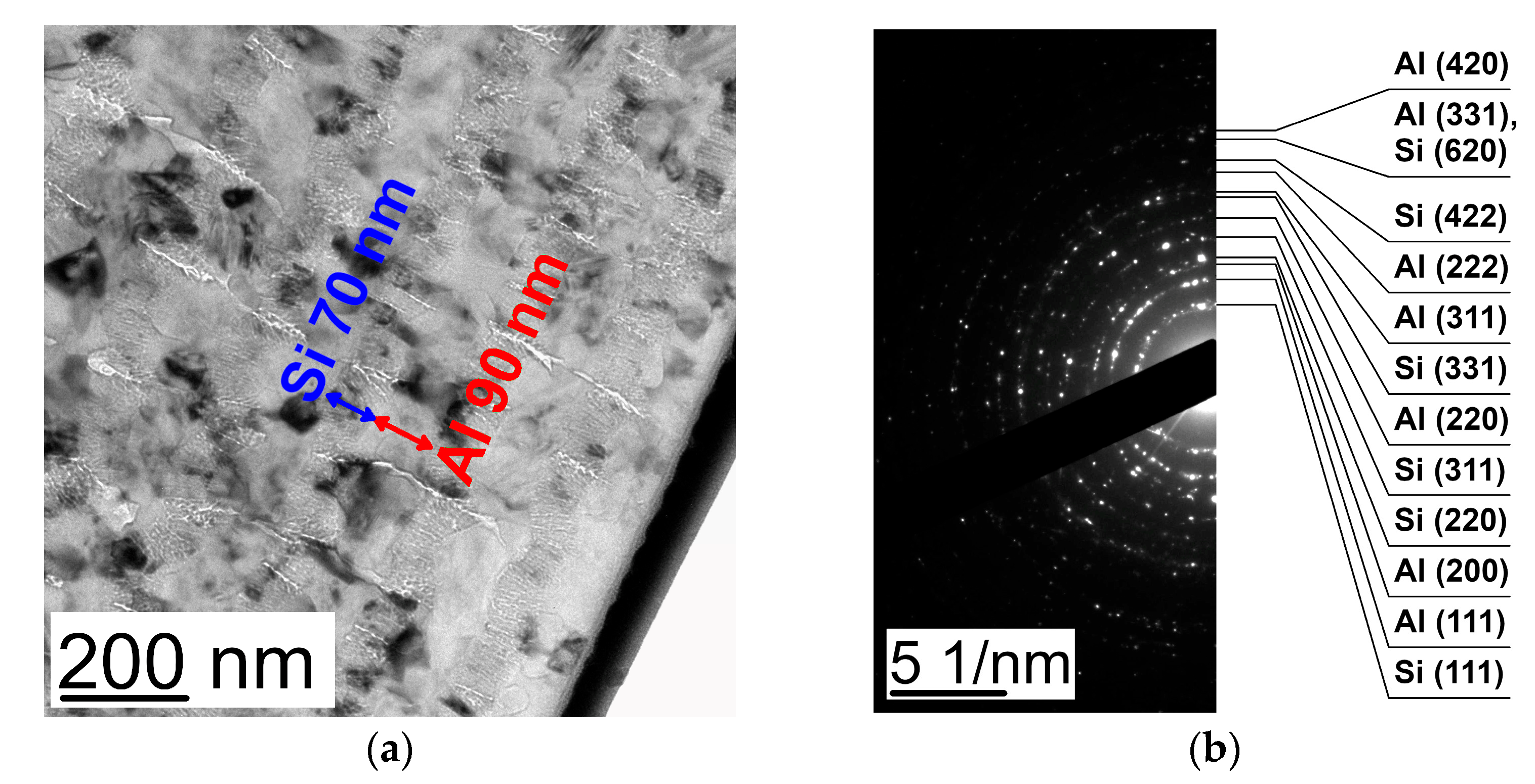












| Al 10 nm/Si 80 nm | Al 20 nm/Si 80 nm | Al 40 nm/Si 80 nm | Al 80 nm/Si 80 nm | |
|---|---|---|---|---|
| Al, wt.% | 14.3 | 24.0 | 36.6 | 53.5 |
| Si, wt.% | 85.7 | 76.0 | 63.4 | 46.5 |
| Al Layer Thickness, nm | a, Å | ❬D❭, nm | δ❬D❭, nm | ❬εAl❭111 | σAl, *,** GPa |
|---|---|---|---|---|---|
| 10 | 4.0488(9) | 11.4 | 2.5 | 0.0069 | −0.009 |
| 20 | 4.0503(3) | 19.3 | 6.0 | 0.0038 | 0.016 |
| 40 | 4.0480(3) | 35.6 | 12.3 | 0.0024 | −0.024 |
| 80 | 4.0485(3) | 61.3 | 21.3 | 0.0018 | −0.015 |
| Sample | aAl | σAl *, GPa | aSi | αSi | σSi **, GPa |
|---|---|---|---|---|---|
| (Al-10/Si-80) | 4.0699(5) | 0.355 | 5.399(3) | 89.62(5) | −0.879 |
| (Al-20/Si-80) | 4.0606(5) | 0.194 | 5.408(4) | 89.70(6) | −0.619 |
| (Al-40/Si-80) | 4.0550(4) | 0.097 | 5.418(2) | 89.81(3) | −0.340 |
| (Al-80/Si-80) | 4.0514(2) | 0.035 | 5.421(3) | 89.90(3) | −0.251 |
| Sample | Number of Bilayers | Layer Thickness, nm | Heating Rate β, °C/min | Characteristic Temperatures, °C | −ΔH **, kJ/(mol Si) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Tconversion-1% * | Tonset | Tmax | Tend | ||||
| (Al-10/Si-80) | 60 | 10 | 80 | 2.5 | 152 | 157 | 169 | 180 | 12.3 ± 0.4 |
| 5.0 | 160 | 167 | 177 | 188 | |||||
| 7.5 | 165 | 173 | 182 | 193 | |||||
| 10.0 | 170 | 179 | 185 | 196 | |||||
| (Al-20/Si-80) | 55 | 20 | 80 | 2.5 | 146 | 155 | 169 | 184 | 13.3 ± 0.9 |
| 5.0 | 155 | 165 | 178 | 190 | |||||
| 7.5 | 160 | 171 | 183 | 195 | |||||
| 10.0 | 163 | 176 | 186 | 199 | |||||
| (Al-40/Si-80) | 40 | 40 | 80 | 2.5 | 138 | 148 | 170 | 184 | 15.8 ± 0.8 |
| 5.0 | 148 | 160 | 179 | 193 | |||||
| 7.5 | 151 | 168 | 185 | 198 | |||||
| 10.0 | 152 | 172 | 188 | 202 | |||||
| (Al-80/Si-80) | 35 | 80 | 80 | 2.5 | 133 | 145 | 171 | 187 | 16.0 ± 1.0 |
| 5.0 | 139 | 155 | 180 | 196 | |||||
| 7.5 | 141 | 162 | 186 | 202 | |||||
| 10.0 | 147 | 168 | 190 | 208 | |||||
| Sample | Ea, kJ/mol | log(A, s−1) | R2 |
|---|---|---|---|
| (Al-10/Si-80) | 137 ± 3 | 14 ± 3 | 0.9993 |
| (Al-20/Si-80) | 129 ± 5 | 13 ± 3 | 0.9974 |
| (Al-40/Si-80) | 121 ± 4 | 12 ± 3 | 0.9975 |
| (Al-80/Si-80) | 117 ± 2 | 11 ± 3 | 0.9995 |
| Sample | Kinetic Parameters of the Reaction Models | F-Test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| One-step reaction: A–(1)→B | ||||||||||
| Al thickness, nm | One step (Cn-X) | R2 | Fexp | Fcrit. (0.05) | ||||||
| Ea, kJ/mol | log(A,s−1) | log(kcat) | n (reaction order) | |||||||
| 10 | 131.5 | 11.42 | 2.48 | 2.14 | 0.9898 | 2.26 | 1.09 | |||
| 20 | 130.6 | 11.81 | 1.84 | 1.88 | 0.9950 | 3.85 | 1.10 | |||
| 40 | 119.7 | 10.97 | 1.18 | 1.48 | 0.9972 | 2.09 | 1.09 | |||
| 80 | 112.5 | 10.34 | 0.77 | 1.36 | 0.9980 | 1.68 | 1.08 | |||
| Concurrent routes of the reaction: A–(I)→B A–(II)→B′ | ||||||||||
| Al thickness, nm | Reaction route I (Cn-X) | Reaction route II (An) | R2 | Fexp | Fcrit. (0.05) | |||||
| Ea1, kJ/mol | log(A1,s−1) | log(kcat1) | n1 (reaction order) | Ea2, kJ/mol | log(A2,s−1) | Avrami exponent m | ||||
| 10 | 176.4 | 13.59 | 5.50 | 2.38 | 45.6 | 2.13 | 2.07 | 0.9954 | 1.00 | 1.09 |
| 20 | 187.0 | 15.25 | 4.88 | 2.32 | 73.7 | 5.78 | 2.16 | 0.9986 | 1.00 | 1.10 |
| 40 | For the (Al/a-Si)n samples with the aluminum layer thickness of 40 and 80 nm, the obtained kinetic parameters have no physical or chemical meaning in terms of the given kinetic model since Ea > 500 kJ/mol, log(A) are the negative values and π > 50. | |||||||||
| 80 | ||||||||||
| Consecutive route of the reaction: A–(1)→B–(2)→C | ||||||||||
| Al thickness, nm | Step 1 (Cn-X) | Step 2 (Fn) | R2 | Fexp | Fcrit. (0.05) | |||||
| Ea1, kJ/mol | log(A1,s−1) | log(kcat1) | n1 (reaction order) | Ea2, kJ/mol | log(A2,s−1) | n2 (reaction order) | ||||
| 10 | 127.8 | 10.88 | 2.56 | 1.26 | 67.2 | 6.99 | 2.36 | 0.9926 | 1.63 | 1.09 |
| 20 | 127.2 | 11.43 | 1.78 | 1.14 | 38.1 | 3.68 | 3.35 | 0.9966 | 1.68 | 1.10 |
| 40 | 101.7 | 8.78 | 1.46 | 0.78 | 46.7 | 3.60 | 1.18 | 0.9986 | 1.00 | 1.09 |
| 80 | 97.4 | 8.60 | 1.03 | 0.69 | 30.1 | 1.78 | 1.21 | 0.9988 | 1.00 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zharkov, S.M.; Yumashev, V.V.; Moiseenko, E.T.; Altunin, R.R.; Solovyov, L.A.; Volochaev, M.N.; Zeer, G.M.; Nikolaeva, N.S.; Belousov, O.V. Thermokinetic Study of Aluminum-Induced Crystallization of a-Si: The Effect of Al Layer Thickness. Nanomaterials 2023, 13, 2925. https://doi.org/10.3390/nano13222925
Zharkov SM, Yumashev VV, Moiseenko ET, Altunin RR, Solovyov LA, Volochaev MN, Zeer GM, Nikolaeva NS, Belousov OV. Thermokinetic Study of Aluminum-Induced Crystallization of a-Si: The Effect of Al Layer Thickness. Nanomaterials. 2023; 13(22):2925. https://doi.org/10.3390/nano13222925
Chicago/Turabian StyleZharkov, Sergey M., Vladimir V. Yumashev, Evgeny T. Moiseenko, Roman R. Altunin, Leonid A. Solovyov, Mikhail N. Volochaev, Galina M. Zeer, Nataliya S. Nikolaeva, and Oleg V. Belousov. 2023. "Thermokinetic Study of Aluminum-Induced Crystallization of a-Si: The Effect of Al Layer Thickness" Nanomaterials 13, no. 22: 2925. https://doi.org/10.3390/nano13222925
APA StyleZharkov, S. M., Yumashev, V. V., Moiseenko, E. T., Altunin, R. R., Solovyov, L. A., Volochaev, M. N., Zeer, G. M., Nikolaeva, N. S., & Belousov, O. V. (2023). Thermokinetic Study of Aluminum-Induced Crystallization of a-Si: The Effect of Al Layer Thickness. Nanomaterials, 13(22), 2925. https://doi.org/10.3390/nano13222925







