Formic Acid Decomposition Using Palladium-Zinc Preformed Colloidal Nanoparticles Supported on Carbon Nanofibre in Batch and Continuous Flow Reactors: Experimental and Computational Fluid Dynamics Modelling Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalytic Tests
3. Modelling Methodology
4. Results and Discussion
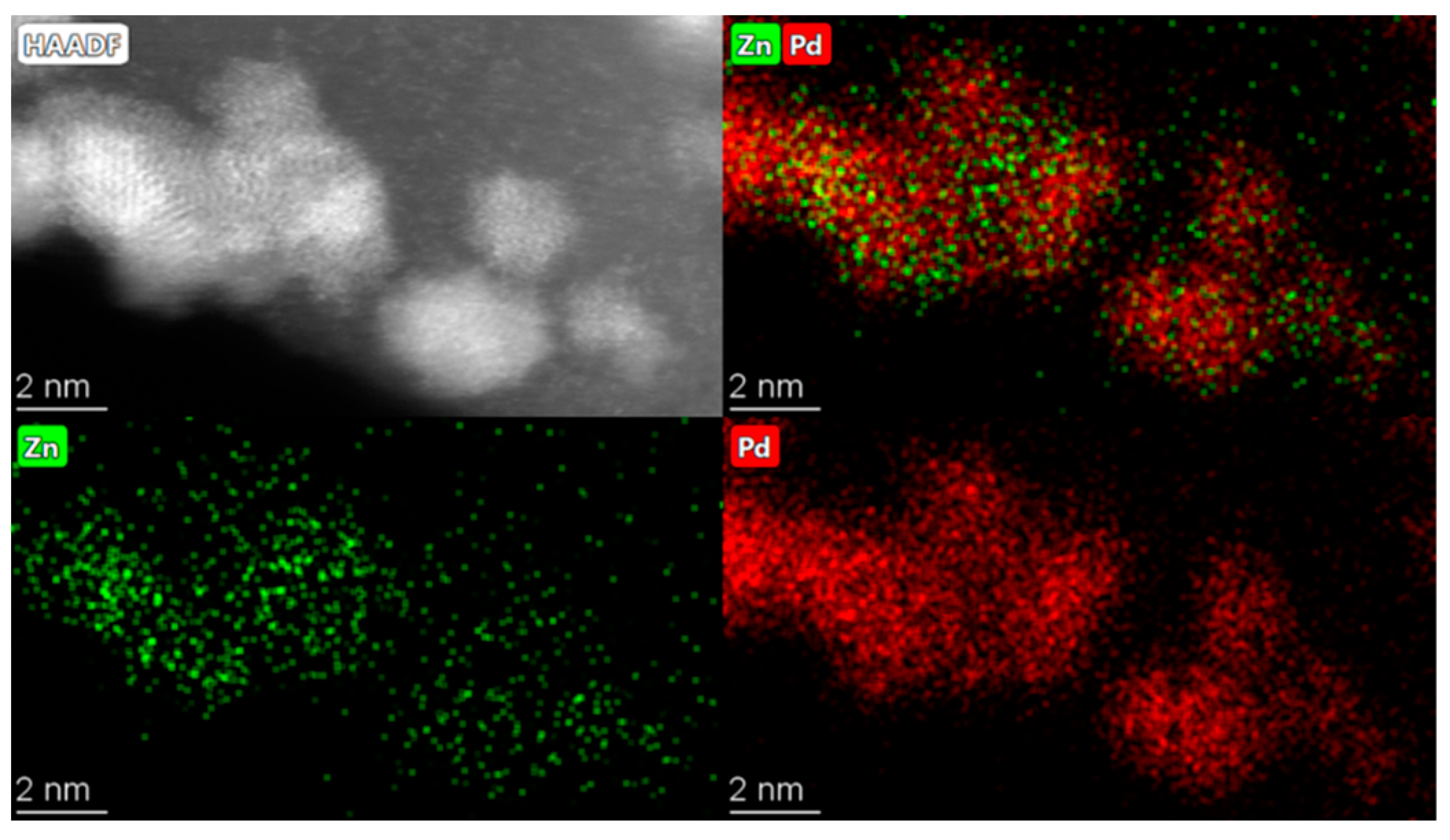
4.1. Catalyst Characterisation
4.2. Modelling Results
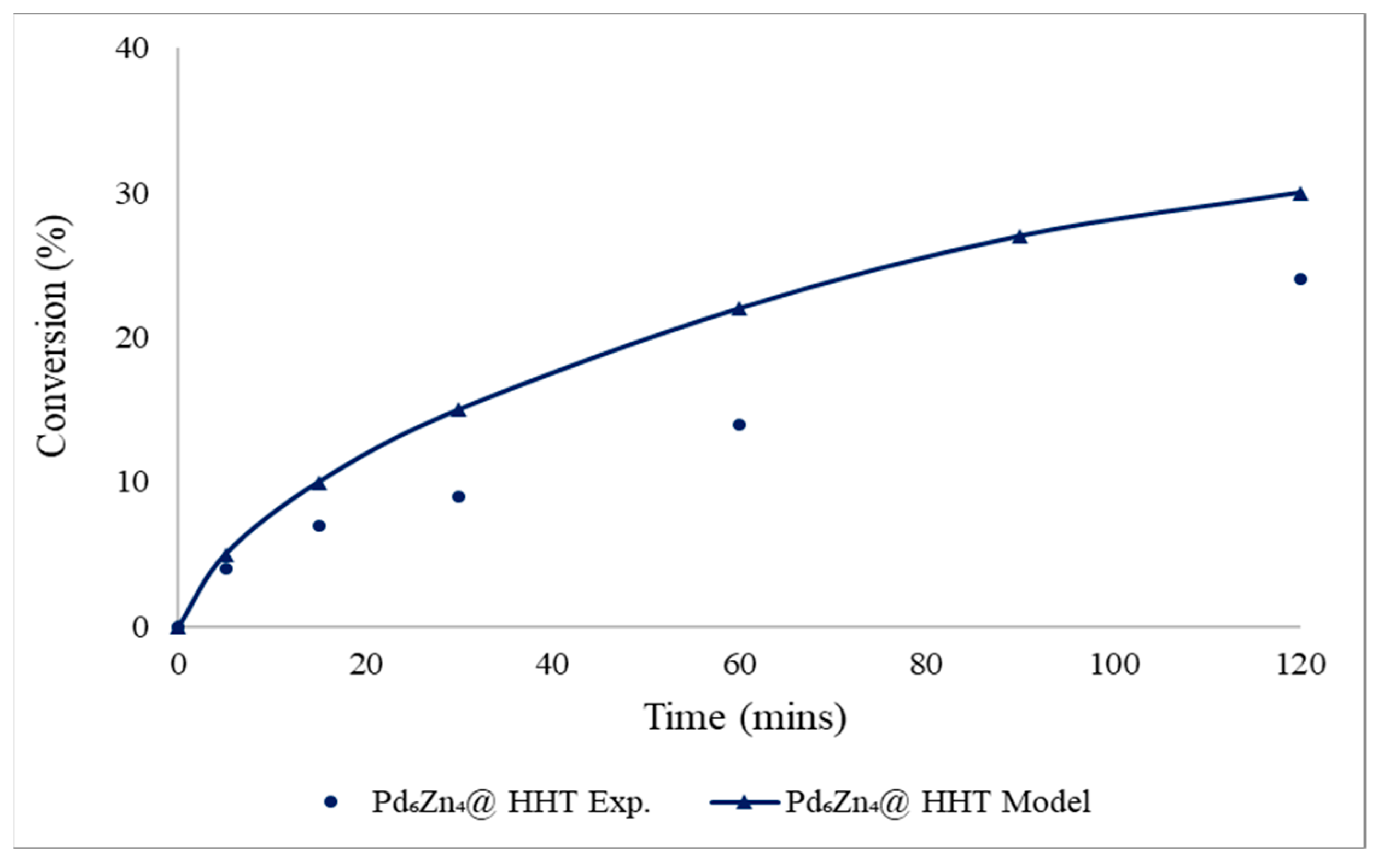
4.2.1. Batch Reactor Validation
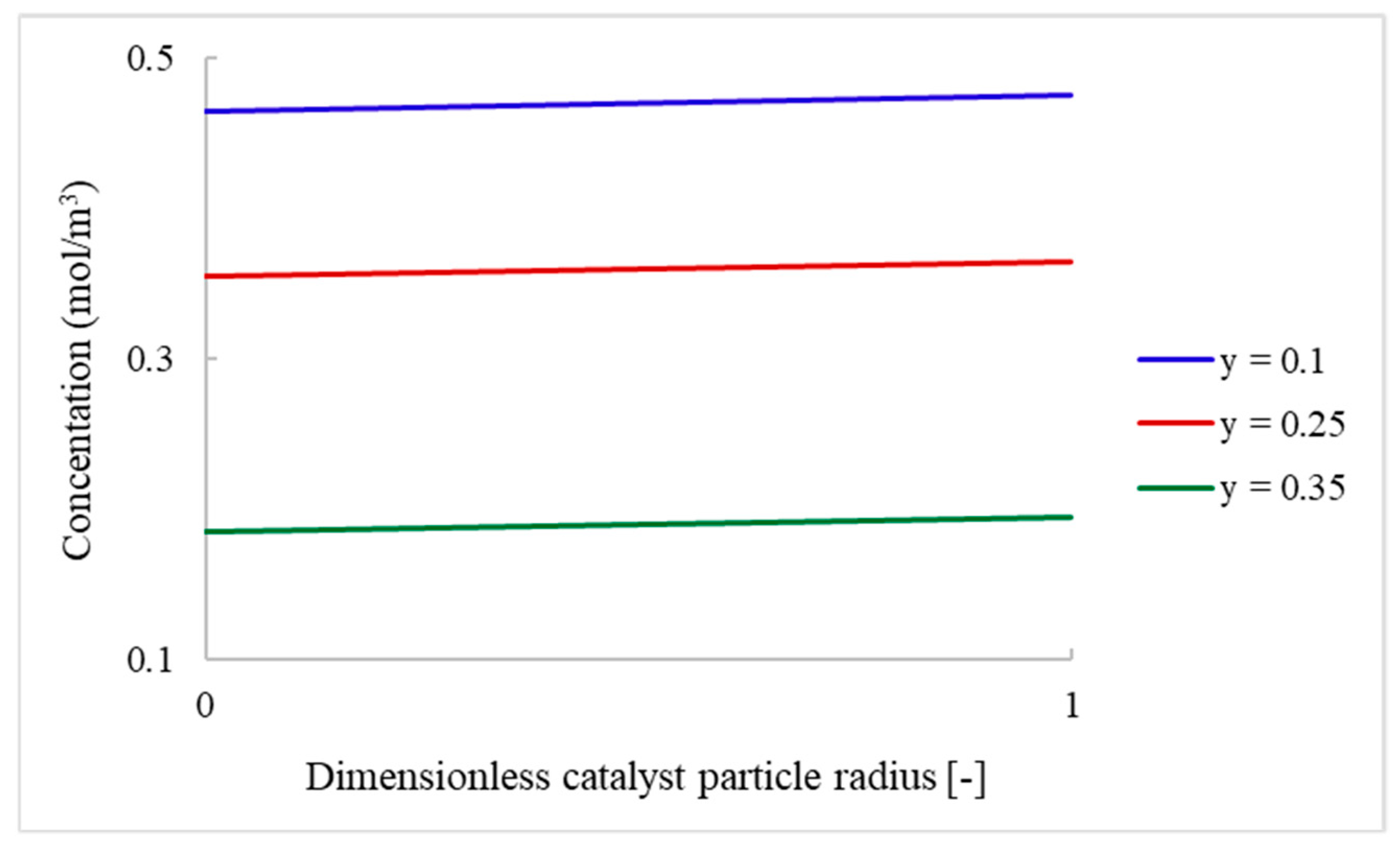
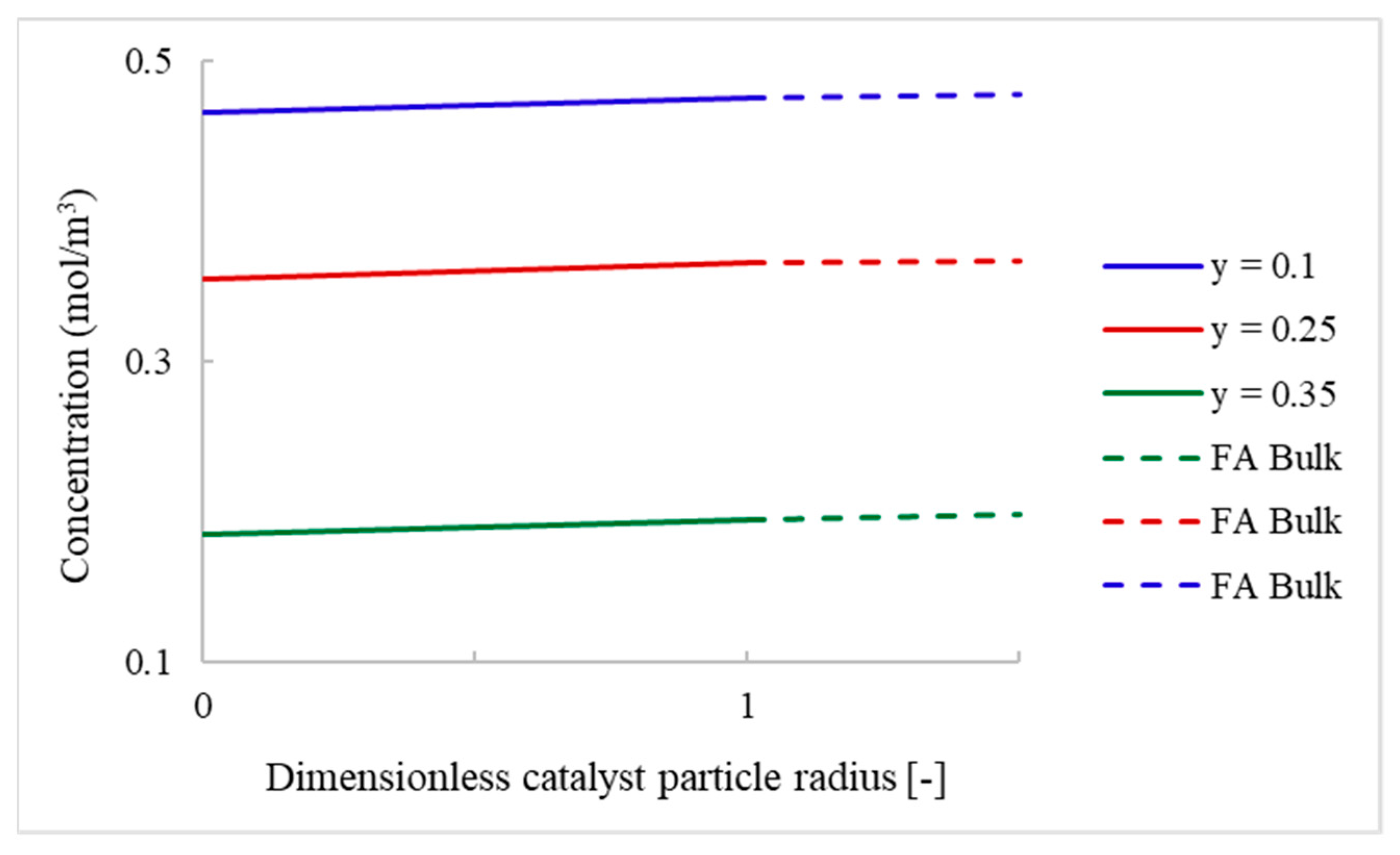
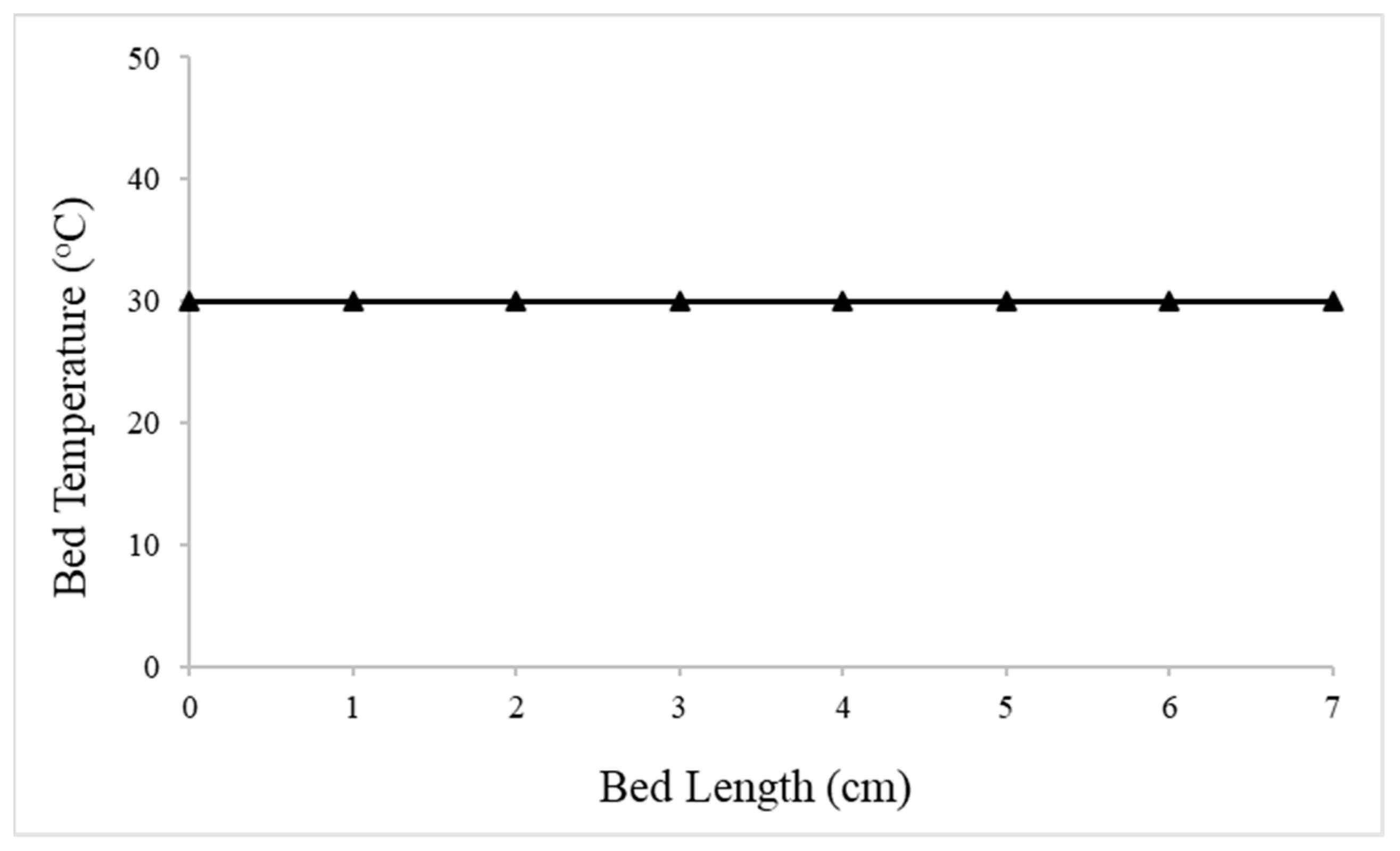
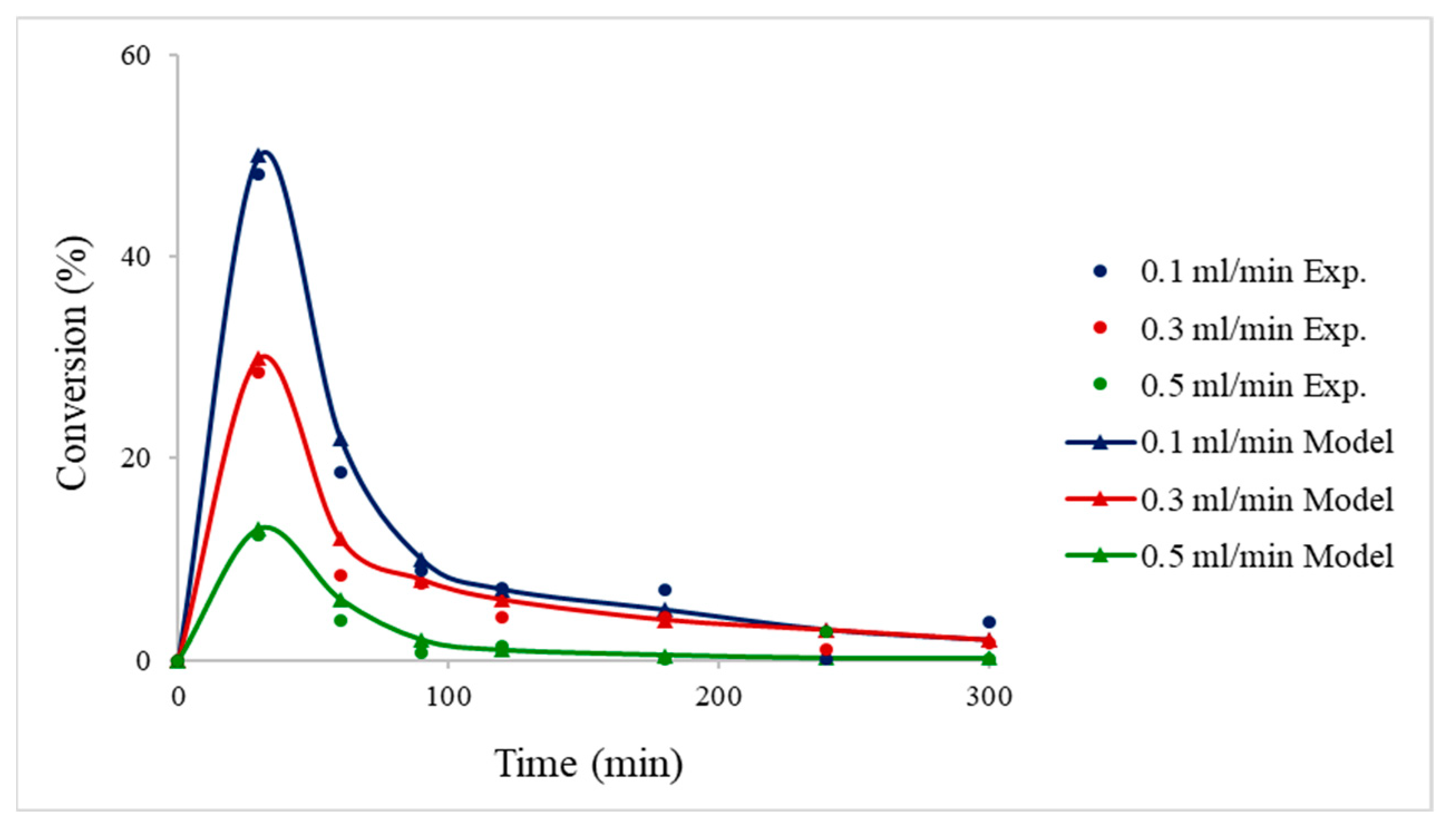
4.2.2. Packed-Bed Flow Reactor Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.; Gelman, D.; Vaccaro, L. Formic acid, a biomass-derived source of energy and hydrogen for biomass upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Carrales-Alvarado, D.H.; Dongil, A.B.; Fernández-Morales, J.M.; Fernández-García, M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Selective hydrogen production from formic acid decomposition over Mo carbides supported on carbon materials. Catal. Sci. Technol. 2020, 10, 6790–6799. [Google Scholar] [CrossRef]
- Al-Nayili, A.; Majdi, H.S.; Albayati, T.M.; Cata Saady, N.M. Formic Acid Dehydrogenation Using Noble-Metal Nanoheterogeneous Catalysts: Towards Sustainable Hydrogen-Based Energy. Catalysts 2022, 12, 324. [Google Scholar] [CrossRef]
- Joó, F. Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 2008, 1, 805–808. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Gao, L.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Recent progress in hydrogen production from formic acid decomposition. Int. J. Hydrogen Energy 2018, 43, 7055–7071. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R.H. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Arbab, M.S.; Shah, J.; Rehman, W.U. Green hydrogen storage and delivery: Utilizing highly active homogeneous and heterogeneous catalysts for formic acid dehydrogenation. Int. J. Hydrogen Energy 2022, 47, 11694–11724. [Google Scholar] [CrossRef]
- Sundramoorthy, A.K.; Paul, R.; Jiao, Y.; Li, Y.; Navlani-García, M.; Mori, K.; Salinas-Torres, D.; Kuwahara, Y.; Yamashita, H. New Approaches toward the Hydrogen Production from Formic Acid Dehydrogenation over Pd-Based Heterogeneous Catalysts. Front. Mater. 2019, 6, 4. [Google Scholar] [CrossRef]
- Mihet, M.; Dan, M.; Barbu-Tudoran, L.; Lazar, M.D.; Blanita, G. Controllable H2 Generation by Formic Acid Decomposition on a Novel Pd/Templated Carbon Catalyst. Hydrogen 2020, 1, 22–37. [Google Scholar] [CrossRef]
- Hafeez, S.; Sanchez, F.; Al-Salem, M.; Villa, A.; Manos, G.; Dimitratos, N.; Constantinou, A.; Centeno, A. Decomposition of Additive-Free Formic Acid Using a Pd/C Catalyst in Flow: Experimental and CFD Modelling Studies. Catalysts 2021, 11, 341. [Google Scholar] [CrossRef]
- Golub, F.S.; Beloshapkin, S.; Gusel’Nikov, A.V.; Bolotov, V.A.; Parmon, V.N.; Bulushev, D.A. Boosting Hydrogen Production from Formic Acid over Pd Catalysts by Deposition of N-Containing Precursors on the Carbon Support. Energies 2019, 12, 3885. [Google Scholar] [CrossRef]
- Kosider, A.; Blaumeiser, D.; Schötz, S.; Preuster, P.; Bösmann, A.; Wasserscheid, P.; Libuda, J.; Bauer, T. Enhancing the feasibility of Pd/C-catalyzed formic acid decomposition for hydrogen generation–catalyst pretreatment, deactivation, and regeneration. Catal. Sci. Technol. 2021, 11, 4259–4271. [Google Scholar] [CrossRef]
- Santos, J.L.; Megías-Sayago, C.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A. Functionalized biochars as supports for Pd/C catalysts for efficient hydrogen production from formic acid. Appl. Catal. B Environ. 2021, 282, 119615. [Google Scholar] [CrossRef]
- Xing, Z.; Guo, Z.; Chen, X.; Zhang, P.; Yang, W. Optimizing the activity of Pd based catalysts towards room-temperature formic acid decomposition by Au alloying. Catal. Sci. Technol. 2019, 9, 588–592. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Z.; Yan, L.; Zhang, Z.; Zhang, Y.; Yin, Y. Pd nanoparticles immobilized on aniline-functionalized MXene as an effective catalyst for hydrogen production from formic acid. Int. J. Hydrogen Energy 2021, 46, 33098–33106. [Google Scholar] [CrossRef]
- Sneka-Płatek, O.; Kaźmierczak, K.; Jędrzejczyk, M.; Sautet, P.; Keller, N.; Michel, C.; Ruppert, A.M. Understanding the influence of the composition of the AgPd catalysts on the selective formic acid decomposition and subsequent levulinic acid hydrogenation. Int. J. Hydrogen Energy 2020, 45, 17339–17353. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, B.W.J.; Wang, N.; He, Q.; Chang, A.; Yang, C.M.; Asakura, H.; Tanaka, T.; Hülsey, M.J.; Wang, C.H.; et al. Zeolite-Encaged Pd–Mn Nanocatalysts for CO2 Hydrogenation and Formic Acid Dehydrogenation. Angew. Chem.—Int. Ed. 2020, 59, 20183–20191. [Google Scholar] [CrossRef]
- Santos, J.L.; León, C.; Monnier, G.; Ivanova, S.; Centeno, M.Á.; Odriozola, J.A. Bimetallic PdAu catalysts for formic acid dehydrogenation. Int. J. Hydrogen Energy 2020, 45, 23056–23068. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, H.; Zheng, J.; Duan, S.; Huang, Y.; Cui, Y.; Wang, R. Pd–Zn nanocrystals for highly efficient formic acid oxidation. Catal. Sci. Technol. 2018, 8, 4757–4765. [Google Scholar] [CrossRef]
- Fathirad, F.; Mostafavi, A.; Afzali, D. Bimetallic PdeMo nanoalloys supported on Vulcan XC-72R carbon as anode catalysts for direct alcohol fuel cell. Int. J. Hydrogen Energy 2016, 42, 3215–3221. [Google Scholar] [CrossRef]
- Hafeez, S.; Barlocco, I.; Al-Salem, M.; Villa, A.; Chen, X.; Delgado, J.J.; Manos, G.; Dimitratos, N.; Constantinou, A. Experimental and process modelling investigation of the hydrogen generation from formic acid decomposition using a pd/zn catalyst. Appl. Sci. 2021, 11, 8462. [Google Scholar] [CrossRef]
- Wu, J.; Zuo, J.; Liu, K.; Lin, J.; Liu, Z. Highly Active/Selective Synergistic Catalysis of Bimetallic Pd/Co Catalyst Anchored on Air-Mediated Nanocarbons for H 2 Production by Formic Acid Dehydrogenation. Catal. Lett. 2023, 153, 2517–2526. [Google Scholar] [CrossRef]
- Barlocco, I.; Capelli, S.; Zanella, E.; Chen, X.; Delgado, J.J.; Roldan, A.; Dimitratos, N.; Villa, A. Synthesis of palladium-rhodium bimetallic nanoparticles for formic acid dehydrogenation. J. Energy Chem. 2021, 52, 301–309. [Google Scholar] [CrossRef]
- Hafeez, S.; Harkou, E.; Spanou, A.; Al-Salem, S.M.; Villa, A.; Dimitratos, N.; Manos, G.; Constantinou, A. Review on recent progress and reactor set-ups for hydrogen production from formic acid decomposition. Mater. Today Chem. 2022, 26, 101120. [Google Scholar] [CrossRef]
- Winkler, T.; Baccot, F.; Eränen, K.; Wärnå, J.; Hilpmann, G.; Lange, R.; Peurla, M.; Simakova, I.; Grénman, H.; Murzin, Y.; et al. Catalytic decomposition of formic acid in a fixed bed reactor-an experimental and modelling study. Catal. Today 2022, 387, 920–5861. [Google Scholar] [CrossRef]
- Hafeez, S.; Al-Salem, M.; Bansode, A.; Villa, A.; Dimitratos, N.; Manos, G.; Constantinou, A. Computational Investigation of Microreactor Configurations for Hydrogen Production from Formic Acid Decomposition Using a Pd/C Catalyst. Ind. Eng. Chem. Res. 2022, 614, 1655–1665. [Google Scholar] [CrossRef]
- Caiti, M.; Padovan, D.; Hammond, C. Continuous Production of Hydrogen from Formic Acid Decomposition over Heterogeneous Nanoparticle Catalysts: From Batch to Continuous Flow. ACS Catal. 2019, 9, 9188–9198. [Google Scholar] [CrossRef]
- Phuan, Y.W.; Lau, E.A.L.; Ismail, H.M.; Lee, B.K.; Chong, M.N. Computational Fluid Dynamics Modelling of Photoelectrocatalytic Reactors for the Degradation of Formic Acid. Appl. Mech. Mater. 2016, 835, 386–393. [Google Scholar] [CrossRef]
- Rostami, P.; Sharifi, M.; Aminshahidy, B.; Fahimpour, J. The effect of nanoparticles on wettability alteration for enhanced oil recovery: Micromodel experimental studies and CFD simulation. Pet. Sci. 2019, 16, 859–873. [Google Scholar] [CrossRef]
- Maslan, N.H.; Rosli, M.I.; Masdar, M.S. Three-dimensional CFD modeling of a direct formic acid fuel cell. Int. J. Hydrogen Energy 2019, 44, 30627–30635. [Google Scholar] [CrossRef]
- Jafari, A.; Hasani, M.; Hosseini, M.; Gharibshahi, R. Application of CFD technique to simulate enhanced oil recovery processes: Current status and future opportunities. Pet. Sci. 2020, 17, 434–456. [Google Scholar] [CrossRef]
- Partopour, B.; Dixon, A.G. Effect of particle shape on methanol partial oxidation in a fixed bed using CFD reactor modeling. AIChE J. 2020, 66, e16904. [Google Scholar] [CrossRef]
- Jałowiecka, M.; Bojarska, Z.; Małolepszy, A.; Makowski, Ł. Mass transport enhancement in direct formic acid fuel cell with a novel channel design. Chem. Eng. J. 2023, 451, 138474. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen from formic acid decomposition over Pd and Au catalysts. Catal. Today 2010, 154, 7–12. [Google Scholar] [CrossRef]
- Ting, S.-W.; Hu, C.; Pulleri, J.K.; Chan, K.-Y. Heterogeneous Catalytic Generation of Hydrogen from Formic Acid under Pressurized Aqueous Conditions. Ind. Eng. Chem. Res. 2012, 51, 4861–4867. [Google Scholar] [CrossRef]
- Sanchez, F.; Motta, D.; Roldan, A.; Hammond, C.; Villa, A.; Dimitratos, N. Hydrogen Generation from Additive-Free Formic Acid Decomposition Under Mild Conditions by Pd/C: Experimental and DFT Studies. Top. Catal. 2018, 61, 254–266. [Google Scholar] [CrossRef]
- Reddy, K.A.; Doraiswamy, L.K. Physico-Chemical Constants of Binary Systems in Concentrated Solutions. Ind. Eng. Chem. Fundam. 1966, 6, 77–79. [Google Scholar] [CrossRef]
- Fogler, H. Chapter 10: Catalysis and Catalytic Reactors. In Elements of Chemical Reaction Engineering, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2016; pp. 454–456. [Google Scholar]
- Cheng, Y.; Lu, S.; Xu, W.; Wen, H.; Wang, J. Fabrication of superhydrophobic Au–Zn alloy surface on a zinc substrate for roll-down, self-cleaning and anti-corrosion properties. J. Mater. Chem. A 2015, 3, 16774–16784. [Google Scholar] [CrossRef]
- Harkou, E.; Adamou, P.; Georgiou, K.; Hafeez, S.; Al-Salem, S.M.; Villa, A.; Manos, G.; Dimitratos, N.; Constantinou, A. Computational Studies on Microreactors for the Decomposition of Formic Acid for Hydrogen Production Using Heterogeneous Catalysts. Molecules 2023, 28, 5399. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, S.; Harkou, E.; Adamou, P.; Barlocco, I.; Zanella, E.; Manos, G.; Al-Salem, S.M.; Chen, X.; Delgado, J.J.; Dimitratos, N.; et al. Formic Acid Decomposition Using Palladium-Zinc Preformed Colloidal Nanoparticles Supported on Carbon Nanofibre in Batch and Continuous Flow Reactors: Experimental and Computational Fluid Dynamics Modelling Studies. Nanomaterials 2023, 13, 2993. https://doi.org/10.3390/nano13232993
Hafeez S, Harkou E, Adamou P, Barlocco I, Zanella E, Manos G, Al-Salem SM, Chen X, Delgado JJ, Dimitratos N, et al. Formic Acid Decomposition Using Palladium-Zinc Preformed Colloidal Nanoparticles Supported on Carbon Nanofibre in Batch and Continuous Flow Reactors: Experimental and Computational Fluid Dynamics Modelling Studies. Nanomaterials. 2023; 13(23):2993. https://doi.org/10.3390/nano13232993
Chicago/Turabian StyleHafeez, Sanaa, Eleana Harkou, Panayiota Adamou, Ilaria Barlocco, Elisa Zanella, George Manos, Sultan M. Al-Salem, Xiaowei Chen, Juan Josè Delgado, Nikolaos Dimitratos, and et al. 2023. "Formic Acid Decomposition Using Palladium-Zinc Preformed Colloidal Nanoparticles Supported on Carbon Nanofibre in Batch and Continuous Flow Reactors: Experimental and Computational Fluid Dynamics Modelling Studies" Nanomaterials 13, no. 23: 2993. https://doi.org/10.3390/nano13232993




