A Biodegradable Polyester-Based Polymer Electrolyte for Solid-State Lithium Batteries
Abstract
:1. Introduction
2. Materials and Methods
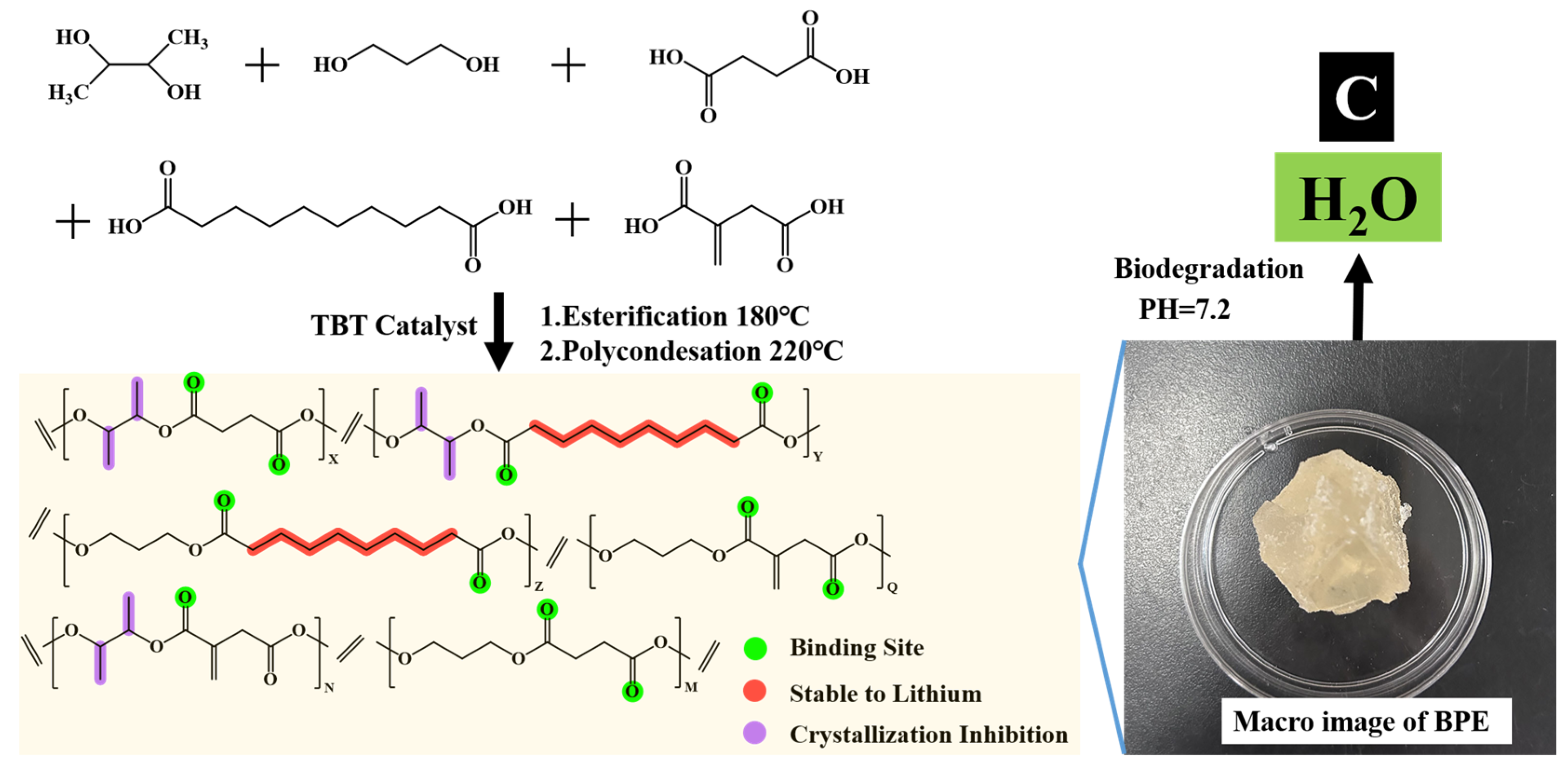
2.1. Synthesis of Biodegradable Poly (2,3-Butanediol/1,3-Propanediol/Succinic Acid/Sebacic Acid/Itaconic Acid) Ester (BPE)
2.2. Assembly of Solid-State Batteries
2.2.1. Preparation of BPSPE
2.2.2. Coin Cell
2.2.3. Pouch-Type Stacking Full Cell
2.3. Materials Characterization
2.4. Electrochemical Characterization
2.5. Analog Computation Details
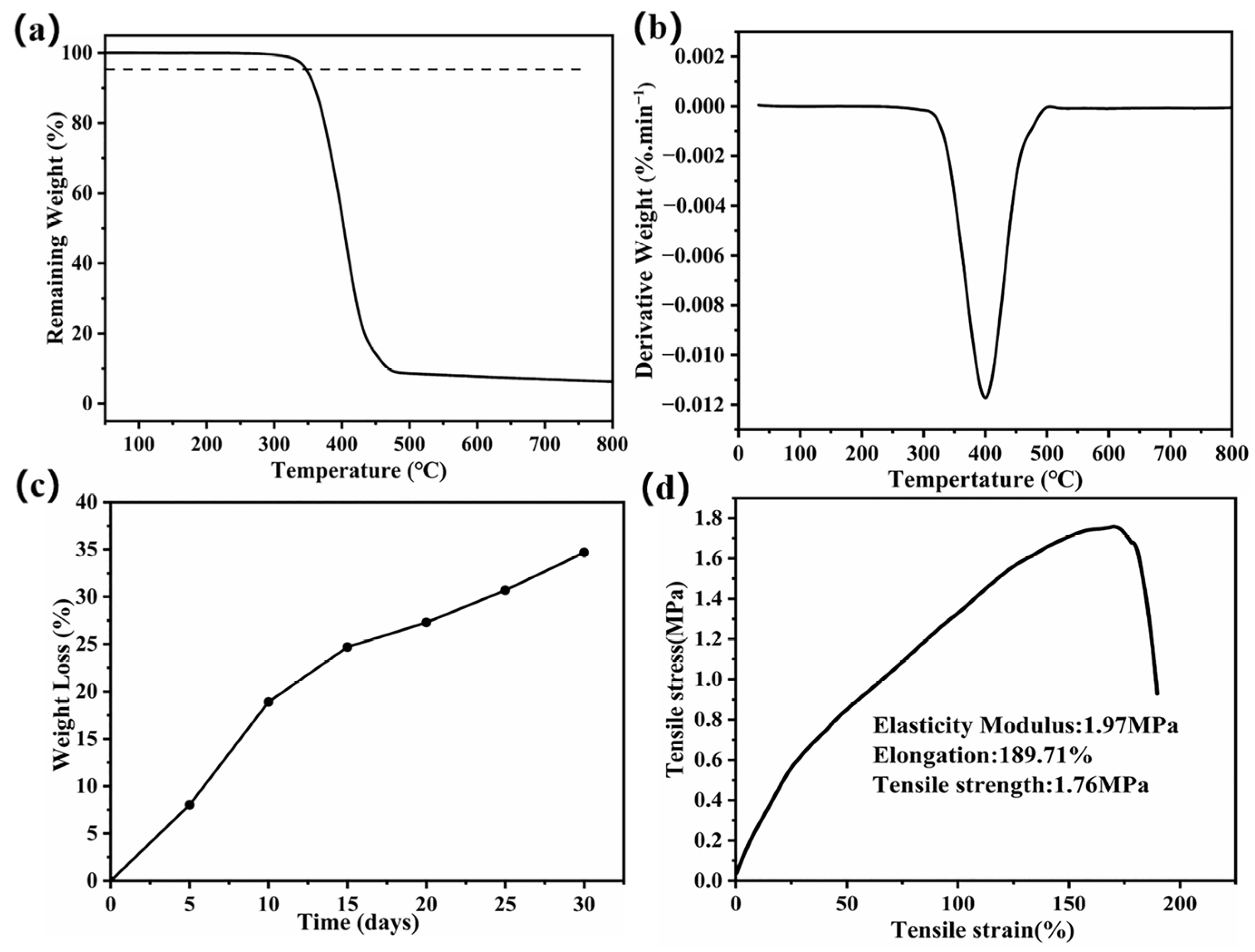
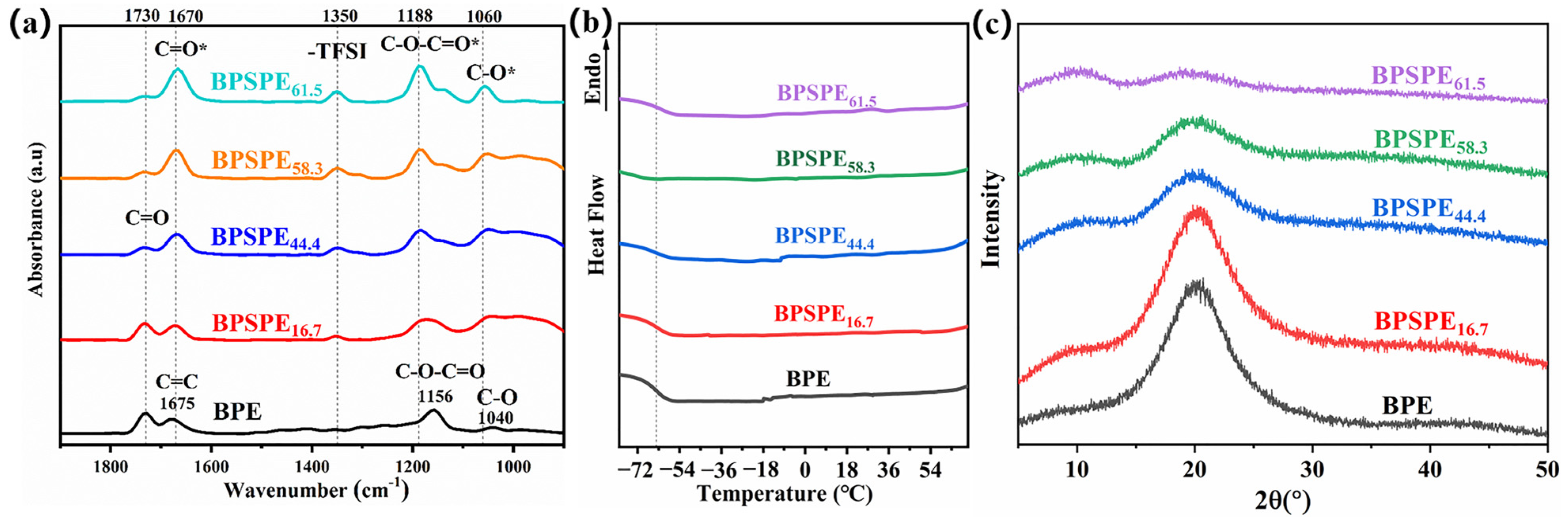
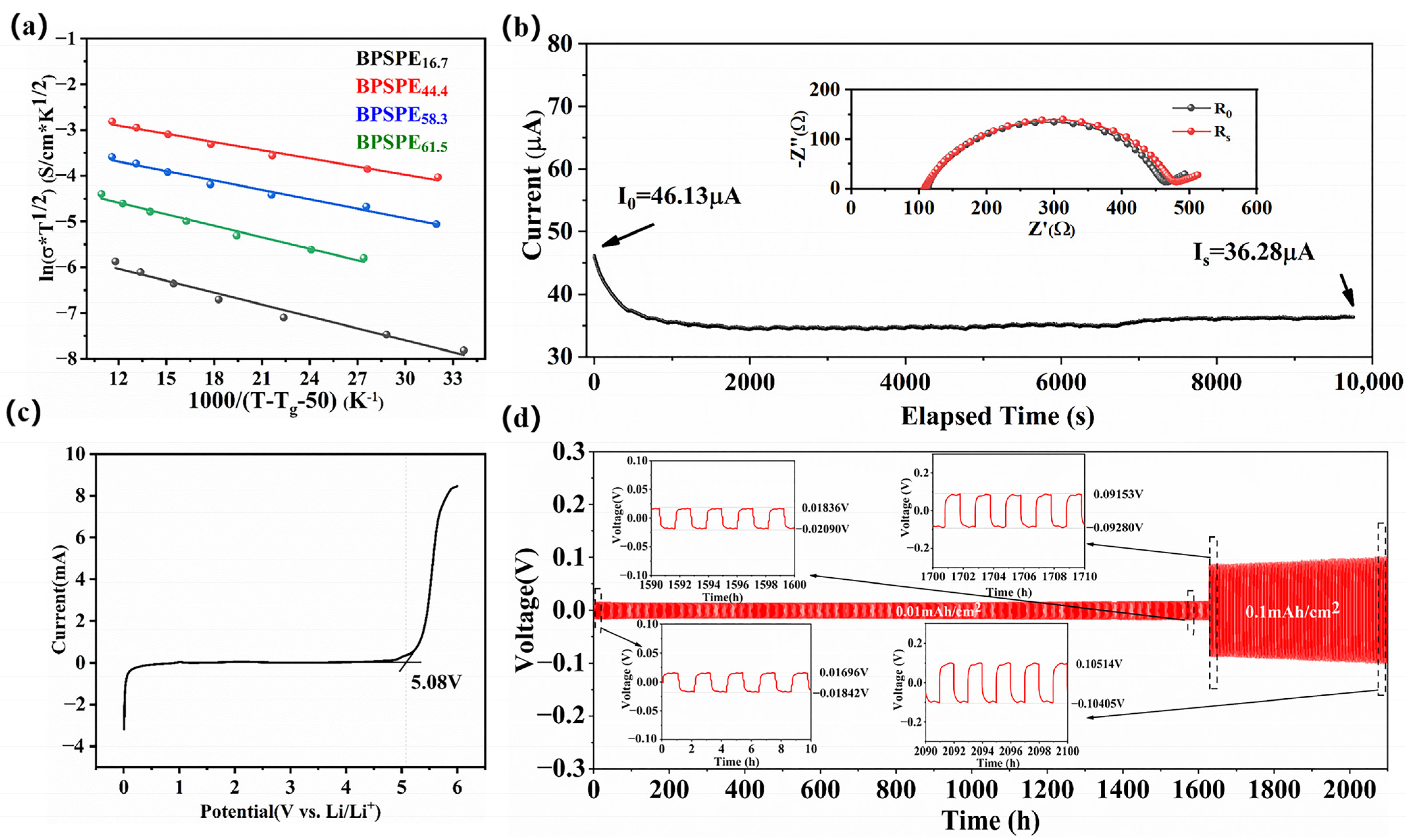
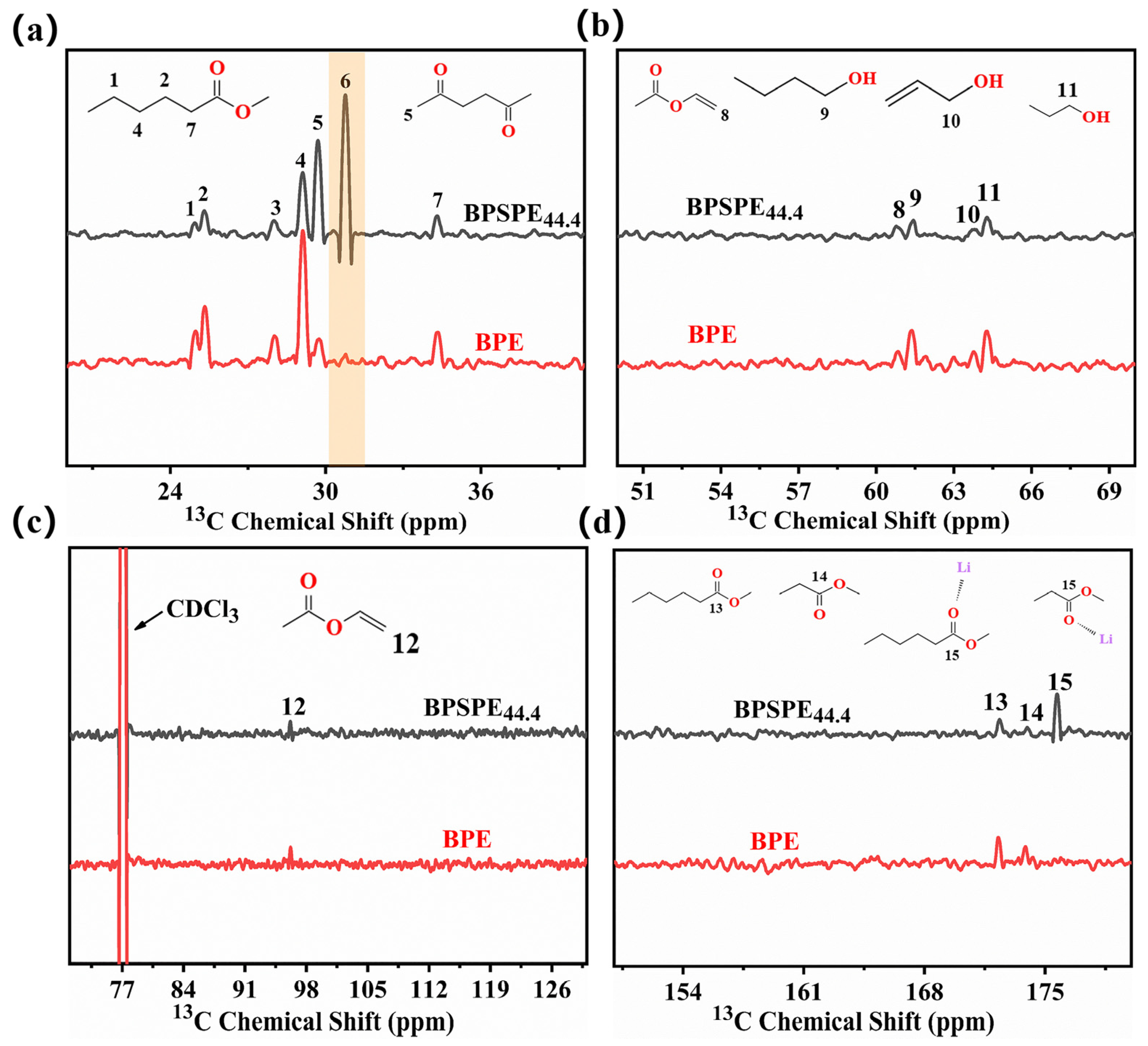
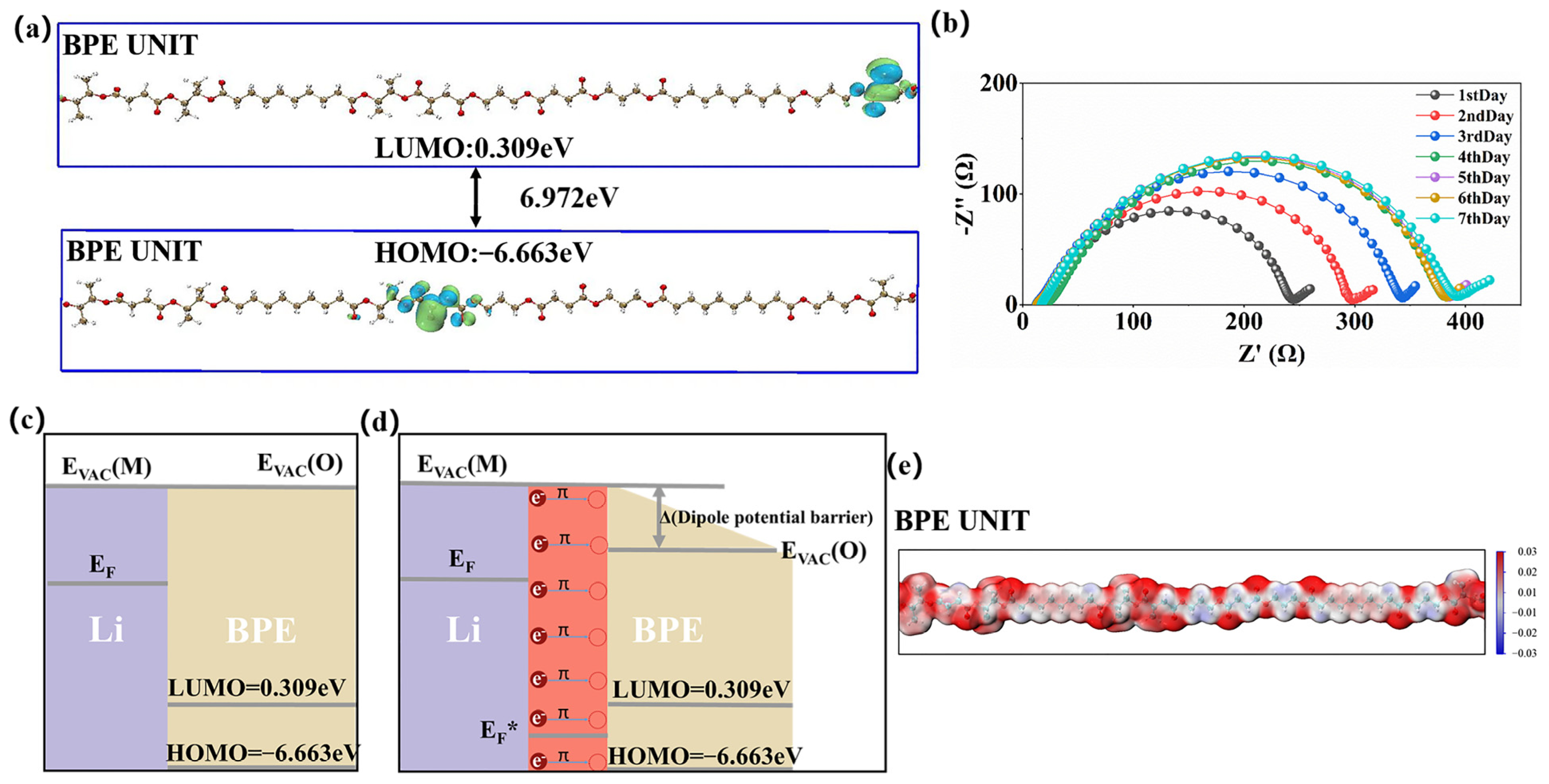
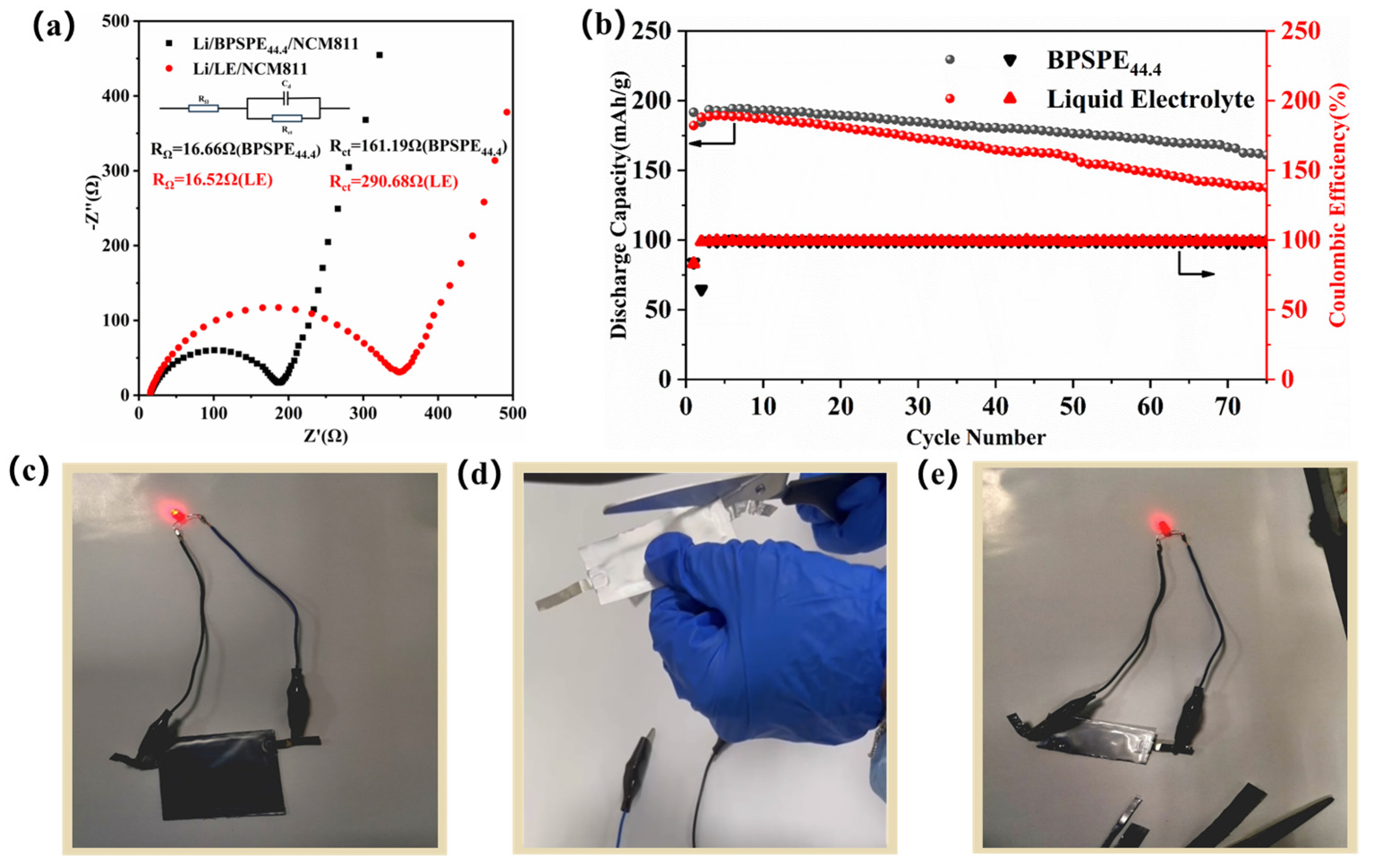
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Fu, J.L.; Zhou, X.Y.; Gui, S.W.; Wei, L.; Yang, H.; Li, H.; Guo, X. Ionic Conduction in Polymer-Based Solid Electrolytes. Adv. Sci. 2023, 10, 2201718. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Zhang, Z.; Zhao, S.; Westover, A.S. Single-Ion Conducting Polymer Electrolytes for Solid-State Lithium–Metal Batteries: Design, Performance, and Challenges. Adv. Energy Mater. 2021, 11, 2003836. [Google Scholar]
- Fan, P.; Liu, H.; Marose, Z.; Samuels, N.T.; Suib, S.L.; Sun, L.Y.; Liao, L.B. High Performance Composite Polymer Electrolytes for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101380. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, G.C.; Kim, K.G.; Zhu, Y.T.; Hood, Z.D.; Rupp, J.L.M. Processing thin but robust electrolytes for solid-state batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Liu, F.Q.; Bin, F.J.; Xue, J.X.; Wang, L.; Yang, Y.G.; Huo, H.; Zhou, J.J.; Li, L. Polymer electrolyte membrane with high ionic conductivity and enhanced interfacial stability for lithium metal Battery. ACS Appl. Mater. Interfaces 2020, 12, 22710–22720. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Guo, W.; Fu, Y.A. Advances in composite polymer electrolytes for lithium batteries and beyond. Adv. Energy Mater. 2020, 11, 2000802. [Google Scholar] [CrossRef]
- Chai, J.C.; Liu, Z.H.; Ma, J.; Wang, J.; Liu, X.C.; Liu, H.S.; Zhang, J.J.; Cui, G.L.; Chen, L.Q. In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv. Sci. 2017, 4, 1600377. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, K.; Chen, T.; Zhang, X.; Armand, M.; Chen, S.M. Recent progress in all-solid-state lithium batteries: The emerging strategies for advanced electrolytes and their interfaces. Energy Storage Mater. 2020, 31, 401–433. [Google Scholar] [CrossRef]
- Ben youcef, H.; Garcia-Calvo, O.; Lago, N.; Devaraj, S.; Armand, M. Cross-Linked Solid Polymer Electrolyte for All-Solid-State Rechargeable Lithium Batteries. Electrochim. Acta 2016, 220, 587–594. [Google Scholar] [CrossRef]
- Falco, M.; Simari, C.; Ferrara, C.; Nair, J.R.; Meligrana, G.; Bella, F.; Nicotera, I.; Mustarelli, P.; Winter, M.; Gerbaldi, C. Understanding the effect of UV-induced crosslinking on the physico-chemical properties of highly performing PEO/LiTFSI-based polymer electrolytes. Langmuir 2019, 35, 8210–8219. [Google Scholar]
- Armand, M.B.; Howells, W.S. Structure of Liquid PEO-LiTFSI Electrolyte. Phys. Rev. Lett. 2000, 84, 5536–5539. [Google Scholar]
- Porcarelli, L.; Gerbaldi, C.; Bella, F.; Nair, J.R. Super Soft All-Ethylene Oxide Polymer Electrolyte for Safe AllSolid Lithium Batteries. Sci. Rep. 2016, 6, 19892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Li, L.N.; Wu, X.C.; Wang, J.; Li, Q.K.; Pan, K.M.; He, J.L. Research Progress and Application of PEO-Based Solid State Polymer Composite Electrolytes. Front. Energy Res. 2021, 56, 276738. [Google Scholar] [CrossRef]
- Shi, L.V.; Zhang, L.X.; Yang, Y.P.; Zhang, H.P.; Yao, R.G.; Yuan, C.Q.; Chen, S.B. In Situ Nano-SiO2 Electrospun Polyethylene-Oxide-Based Nano-Fiber Composite Solid Polymer Electrolyte for High-Performance Lithium-Ion Batteries. Nanomaterials 2023, 13, 1294. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Liang, H.Y.; Wang, S.H.; Cui, C.; Zeng, C.; Zhai, T.Y.; Li, H.Q. Fabricating a PVDF skin for PEO-based SPE to stabilize the interface both at cathode and anode for Li-ion batteries. J. Energy Chem. 2022, 70, 356–362. [Google Scholar] [CrossRef]
- Noto, V.D.; Lavina, S.; Giffin, G.A.; Negro, E.; Scrosati, B. Polymer electrolytes: Present, past and future. Electrochim. Acta 2011, 47, 4–13. [Google Scholar] [CrossRef]
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Yap, Y.L.; You, A.H.; Teo, L.L. Preparation and characterization studies of PMMA–PEO-blend solid polymer electrolytes with SiO2 filler and plasticizer for lithium ion battery. Ionics 2019, 25, 3087–3098. [Google Scholar] [CrossRef]
- Zhang, B.H.; Liu, Y.L.; Pan, X.M.; Liu, J.; Doyle-Davis, K.; Sun, L.Q.; Liu, J.; Jiao, X.F.; Jie, J.; Xie, H.M.; et al. Dendrite-free lithium metal solid battery with a novel polyester based triblock copolymer solid-state electrolyte. Nano Energy 2020, 72, 104690. [Google Scholar] [CrossRef]
- Eilmes, A. A quantum-chemical study of Li+⋯C=O interactions in polyester-based polymer electrolytes. Solid State Ion. 2008, 179, 458–464. [Google Scholar] [CrossRef]
- Tominaga, Y. Ion-conductive polymer electrolytes based on poly (ethylene carbonate) and its derivatives. Polym. J. 2017, 49, 291–299. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Bai, Y.; Li, W.D.; An, M.Z.; Bai, Y.P.; Chen, G.R. Design strategies for polymer electrolytes with ether and carbonate groups for solid-state lithium metal batteries. Chem. Mater. 2020, 32, 6811–6830. [Google Scholar] [CrossRef]
- Xu, H.L.; Xie, J.B.; Liu, Z.B.; Wang, J.; Deng, Y.H. Carbonyl-coordinating polymers for high-voltage solid-state lithium batteries: Solid polymer electrolytes. MRS Energy Sustain. 2020, 7, E2. [Google Scholar] [CrossRef]
- Ding, P.P.; Lin, Z.Y.; Guo, X.Y.; Wu, L.Q.; Wang, Y.T.; Guo, H.X.; Li, L.L.; Yu, H.J. Polymer electrolytes and interfaces in solid-state lithium metal batteries. Mater. Today 2021, 51, 449–474. [Google Scholar] [CrossRef]
- Xiang, X.Y.; Shriver, D.F. Highly conductive polymer electrolytes containing rigid polymers. Chem. Mater. 1998, 10, 2307–2308. [Google Scholar]
- Chen, P.P.; Zeng, Q.H.; Li, Q.Y.; Zhao, R.H.; Li, Z.F.; Wen, X.; Wen, W.; Liu, Y.; Chen, A.Q.; Li, Z.X.; et al. A ketone-containing all-solid-state polymer electrolyte with rapid Li-ion conduction for lithium metal batteries. Chem. Eng. J. 2022, 427, 132025. [Google Scholar] [CrossRef]
- Mindemark, J.; Törmä, E.; Sun, B.; Brandell, D. Copolymers of trimethylene carbonate and ε-caprolactone as electrolytes for lithium-ion batteries. Polymer 2015, 63, 91–98. [Google Scholar] [CrossRef]
- Tang, S.; Li, J.; Wang, R.G.; Zhang, J.C.; Lu, Y.L.; Hu, G.H.; Wang, Z.; Zhang, L.Q. Current trends in bio-based elastomer materials. SusMat 2022, 2, 2–33. [Google Scholar] [CrossRef]
- Rosenwinkel, M.P.; Andersson, R.; Mindemark, J.; Schönhoff, M. Coordination effects in polymer electrolytes: Fast Li+ transport by weak ion binding. J. Phys. Chem. C 2020, 124, 23588–23596. [Google Scholar] [CrossRef]
- Tominaga, Y.; Yamazaki, K.; Nanthana, V. Effect of anions on lithium ion conduction in poly(ethylene carbonate)-based polymer electrolytes. J. Electochem. Soc. 2015, 162, A3133–A3136. [Google Scholar] [CrossRef]
- Zade, S.S.; Zamoshchik, N.; Bendikov, M. From Short Conjugated Oligomers to Conjugated Polymers. Lessons from Studies on Long Conjugated Oligomers. Acc. Chem. Res. 2011, 44, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F.W. A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Filho, S.B.D.S.; Oliveira, L.V.F.D.; Oliveira, R.D.S.; Faez, R.; Martins, V.L.; Camilo, F.F. Free-standing solid polymer electrolytes based on elastomeric material and ionic liquids for safer lithium-ion battery applications. Solid State Ion. 2022, 379, 115901. [Google Scholar] [CrossRef]
- Oprea, S. Dependence of fungal biodegradation of PEG/castor oil-based polyurethane elastomers on the hard-segment structure. Polym. Degrad. Stab. 2010, 95, 2396–2404. [Google Scholar] [CrossRef]
- Wu, I.D.; Chang, F.C. Determination of the interaction within polyester-based solid polymer electrolyte using FTIR spectroscopy. Polymer 2007, 48, 989–996. [Google Scholar] [CrossRef]
- Huang, Z.J.; Choudhury, S.; Paul, N.; Thienenkamp, J.H. Effect of polymer coating mechanics at solid-electrolyte interphase for stabilizing lithium metal anodes. Adv. Energy Mater. 2022, 12, 2103087. [Google Scholar] [CrossRef]
- Pesko, M.D.; Jung, Y.; Hasan, A.L.; Webb, M.A.; Coates, G.W.; Miller, T.F., III; Balsara, N.P. Effect of monomer structure on ionic conductivity in a systematic set of polyester electrolytes. Solid State Ion. 2016, 289, 118–124. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Dong, S.M.; Li, X.F.; Cui, G.L. Intermolecular chemistry in solid polymer electrolytes for high-energy-density lithium batteries. Adv. Mater. 2019, 31, 1902029. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Bai, Y.; Bai, Y.B.; An, M.Z.; Chen, G.R.; Li, W.D.; Li, C.; Zhou, Y.F. A rational design of solid polymer electrolyte with high salt concentration for lithium battery. J. Power Sources 2018, 407, 23–30. [Google Scholar] [CrossRef]
- Jiang, F.N.; Yang, S.J.; Liu, H.; Cheng, X.B.; Xiang, R.; Zhang, Q.; Kaskel, S.; Huang, J.Q. Mechanism understanding for stripping electrochemistry of Li metal anode. SusMat 2021, 1, 303–457. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Shen, L.; Guo, Q.Y.; He, H.; Yao, X.Y. Synergistic effects of plasticizer and 3D framework toward high-performance solid polymer electrolyte for room-temperature solid-state lithium batteries. ACS Appl. Energy Mater. 2021, 4, 4129–4137. [Google Scholar] [CrossRef]
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Tables of Spectral Data for Structure Determination of Organic Compounds, 2nd ed.; John Wilet & Sons: New York, NY, USA, 1989; pp. 69–156. [Google Scholar]
- Wang, M.Q.; Peng, Z.; Luo, W.W.; Zhang, Q.; Li, Z.D.; Zhu, Y.; Lin, H.; Cai, L.T.; Yao, X.Y.; Ouyang, C.Y.; et al. Improving the interfacial stability between lithium and solid-state electrolyte via dipole-structured lithium layer deposited on graphene oxide. Adv. Sci. 2020, 7, 2000237. [Google Scholar] [CrossRef] [PubMed]
- Caricato, M.; Trucks, G.W.; Frisch, M.J. Projected coupled cluster amplitudes from a different basis set as initial guess. J. Chem. Theory Comput. 2011, 7, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Tokgoz, S.R.; Firat, Y.E.; Safi, Z.; Peksoz, A. Electrochemical properties of Al doped polypyrrole composite polymer: Mott-Schottky approximation and density functional theory. J. Electrochem. Soc. 2019, 166, G54–G60. [Google Scholar] [CrossRef]
- Arya, R.K.; Gupta, A.K. The effect of nitrogen-rich ionic liquid [EMIMDCA] on the electronic structure of solid polymer electrolyte (PEO-LiTFSI). J. Mol. Model. 2022, 28, 363. [Google Scholar] [CrossRef]
- Khairul, W.M.; Rahamathullah, R.; Joni, J.R.; Isa, M.I.N. Density functional theory (DFT) calculations, synthesis and electronic properties of alkoxylated-chalcone additive in enhancing the performance of CMC-based solid biopolymer electrolyte. Int. J. Hydrogen Energy 2022, 47, 27866–27876. [Google Scholar] [CrossRef]
- Wang, J.B.; Okabe, J.; Komine, Y.; Notohara, H.; Urita, K.; Moriguchi, I.; Wei, M.D. The optimized interface engineering of VS2 as cathodes for high performance all-solid-state lithium-ion battery. Sci. China Technol. Sci. 2022, 65, 1859–1866. [Google Scholar] [CrossRef]
- Wang, J.B.; Huang, J.J.; Huang, S.P.; Yuki, K.; Kiroo, N.; Koki, U.; Isamu, M.; Wei, M.D. Regulating the effects of SnS shrinkage in all-solid-state lithium-ion batteries with excellent electrochemical performance. Chem. Eng. J. 2022, 429, 132424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Xue, Z.; Weng, S.; Wang, W.; Shen, H.; Xiang, Y.; Liu, L.; Peng, X. A Biodegradable Polyester-Based Polymer Electrolyte for Solid-State Lithium Batteries. Nanomaterials 2023, 13, 3027. https://doi.org/10.3390/nano13233027
Tang C, Xue Z, Weng S, Wang W, Shen H, Xiang Y, Liu L, Peng X. A Biodegradable Polyester-Based Polymer Electrolyte for Solid-State Lithium Batteries. Nanomaterials. 2023; 13(23):3027. https://doi.org/10.3390/nano13233027
Chicago/Turabian StyleTang, Chenxia, Zhiyu Xue, Shijie Weng, Wenjie Wang, Hongmei Shen, Yong Xiang, Le Liu, and Xiaoli Peng. 2023. "A Biodegradable Polyester-Based Polymer Electrolyte for Solid-State Lithium Batteries" Nanomaterials 13, no. 23: 3027. https://doi.org/10.3390/nano13233027





