Rational Design of TiO2@g-C3N4/CNT Composite Separator for High Performance Lithium-Sulfur Batteries to Promote the Redox Kinetics of Polysulfide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of a TiO2@g-C3N4 Composite Separator
2.2. Preparation of the Nano-S and S/CNT Composite Cathode
2.3. Material Characterizations
2.4. Visual Adsorption Experiment of Polysulfide (Li2S6)
2.5. Assembly of Symmetric Cells
2.6. Contact Angle Test
2.7. Electrochemical Characterization
3. Results
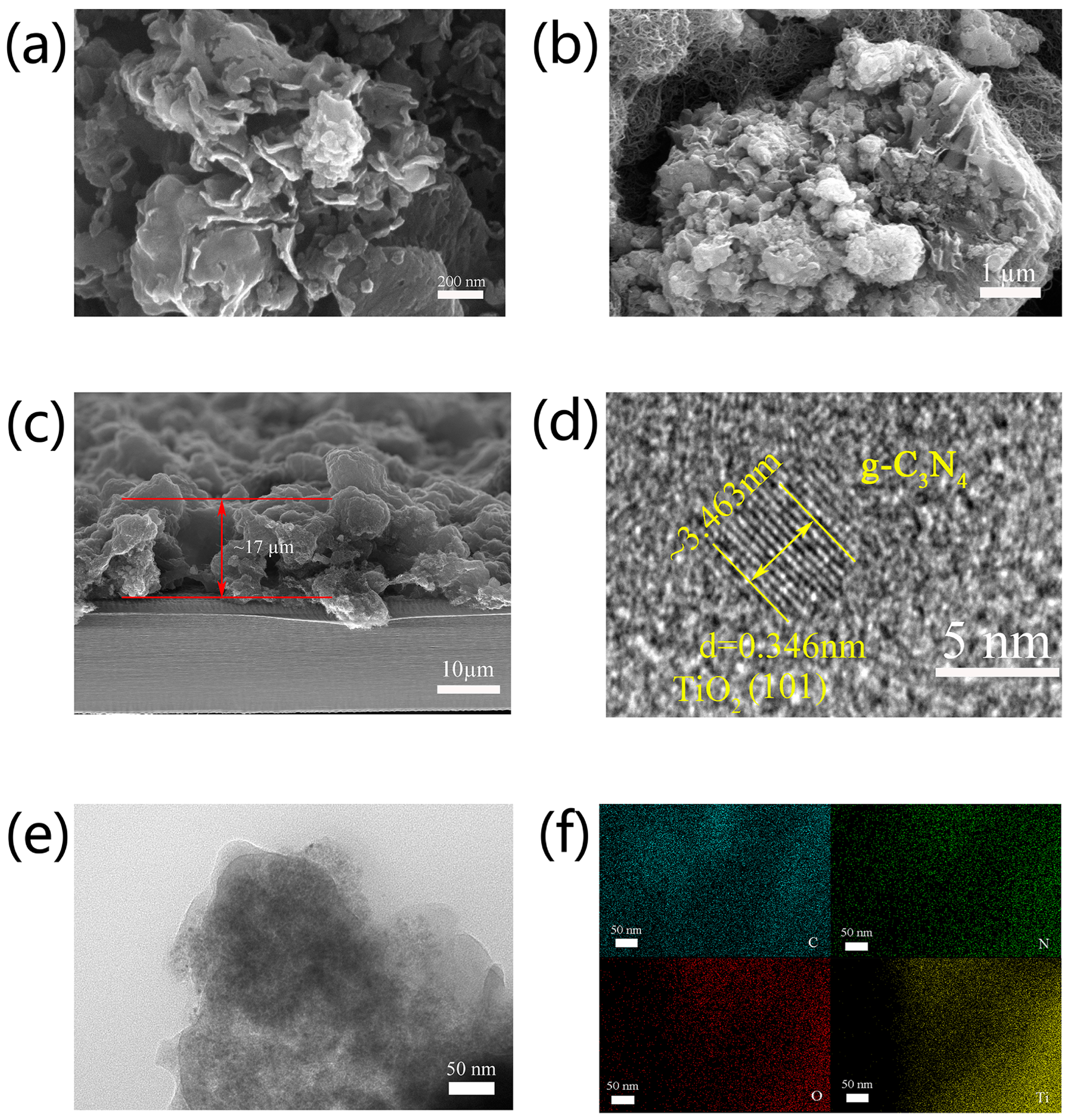
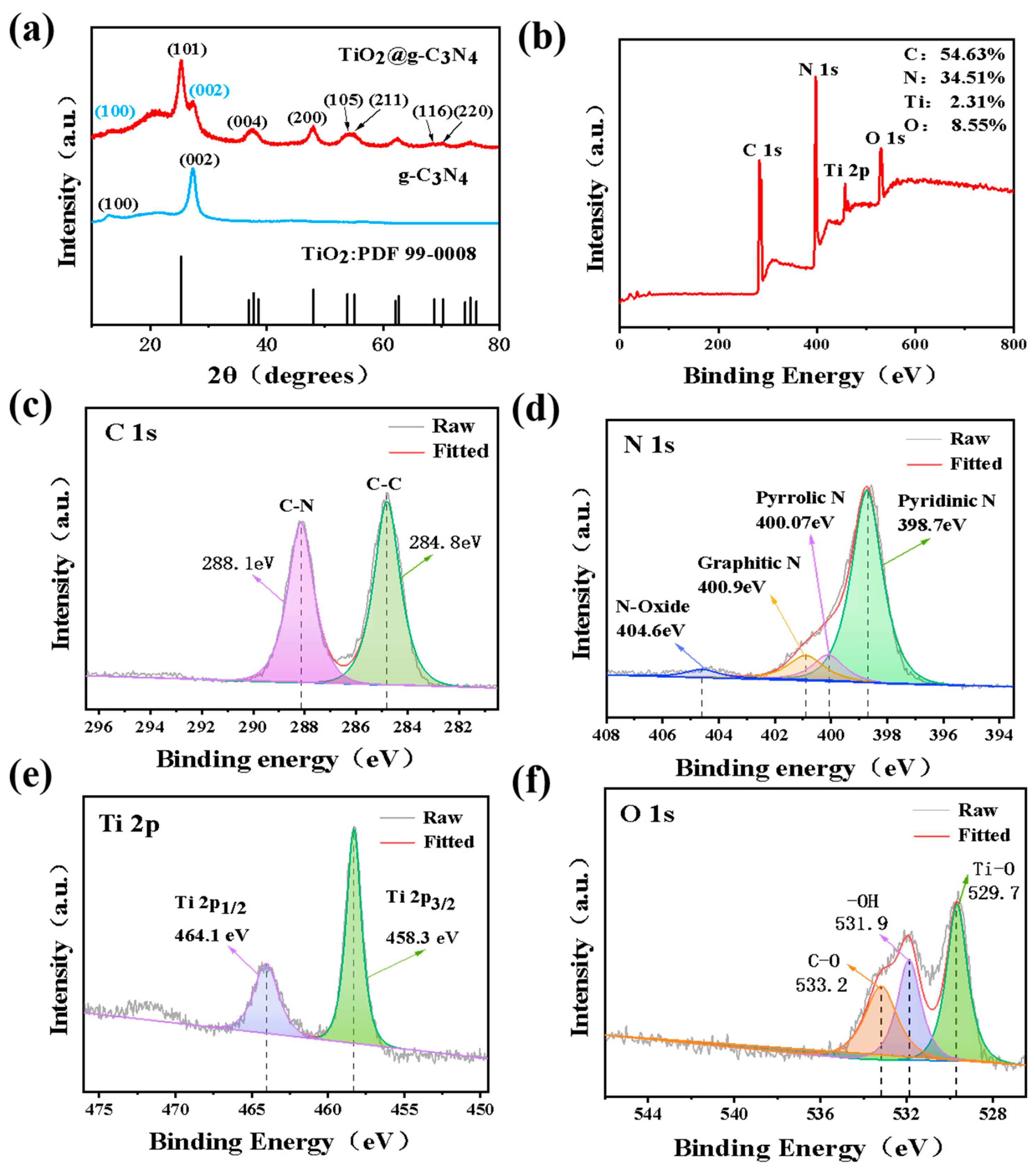
3.1. Material Characterization
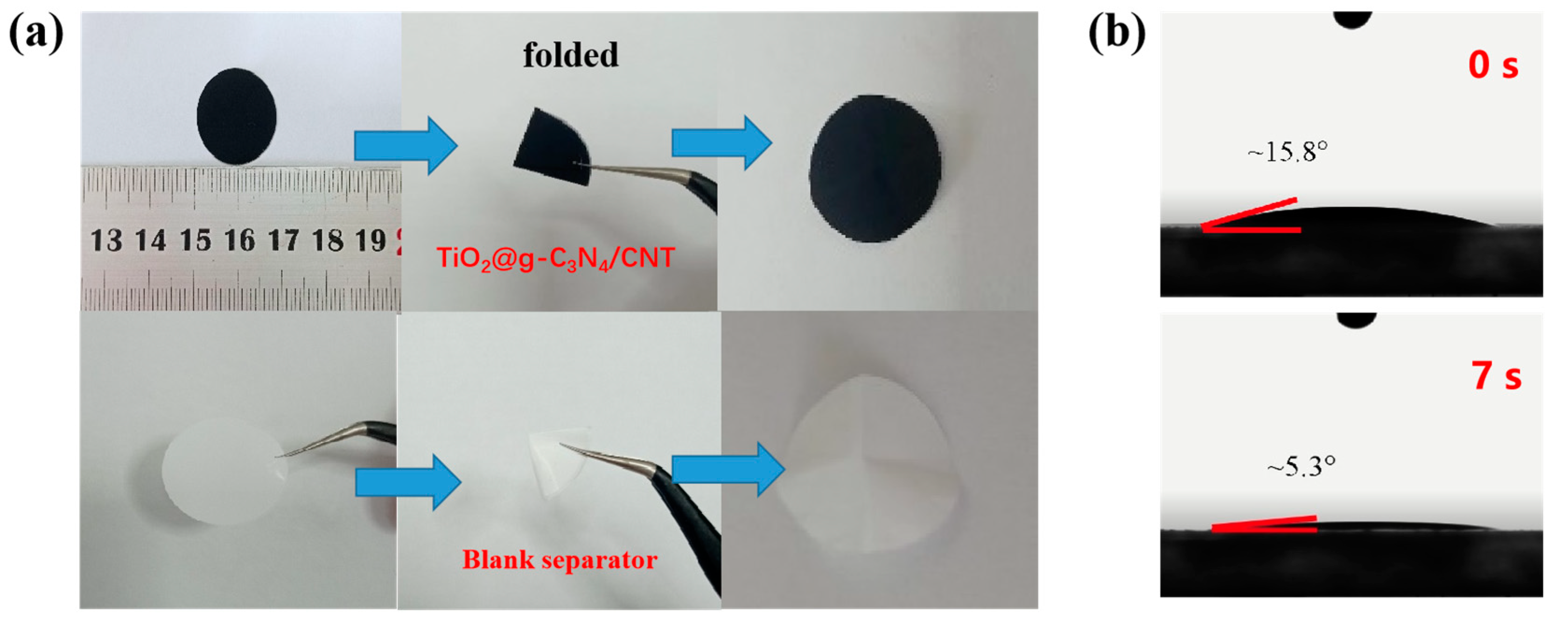
3.2. Mechanical Properties and Wettability
3.3. Adsorption Test
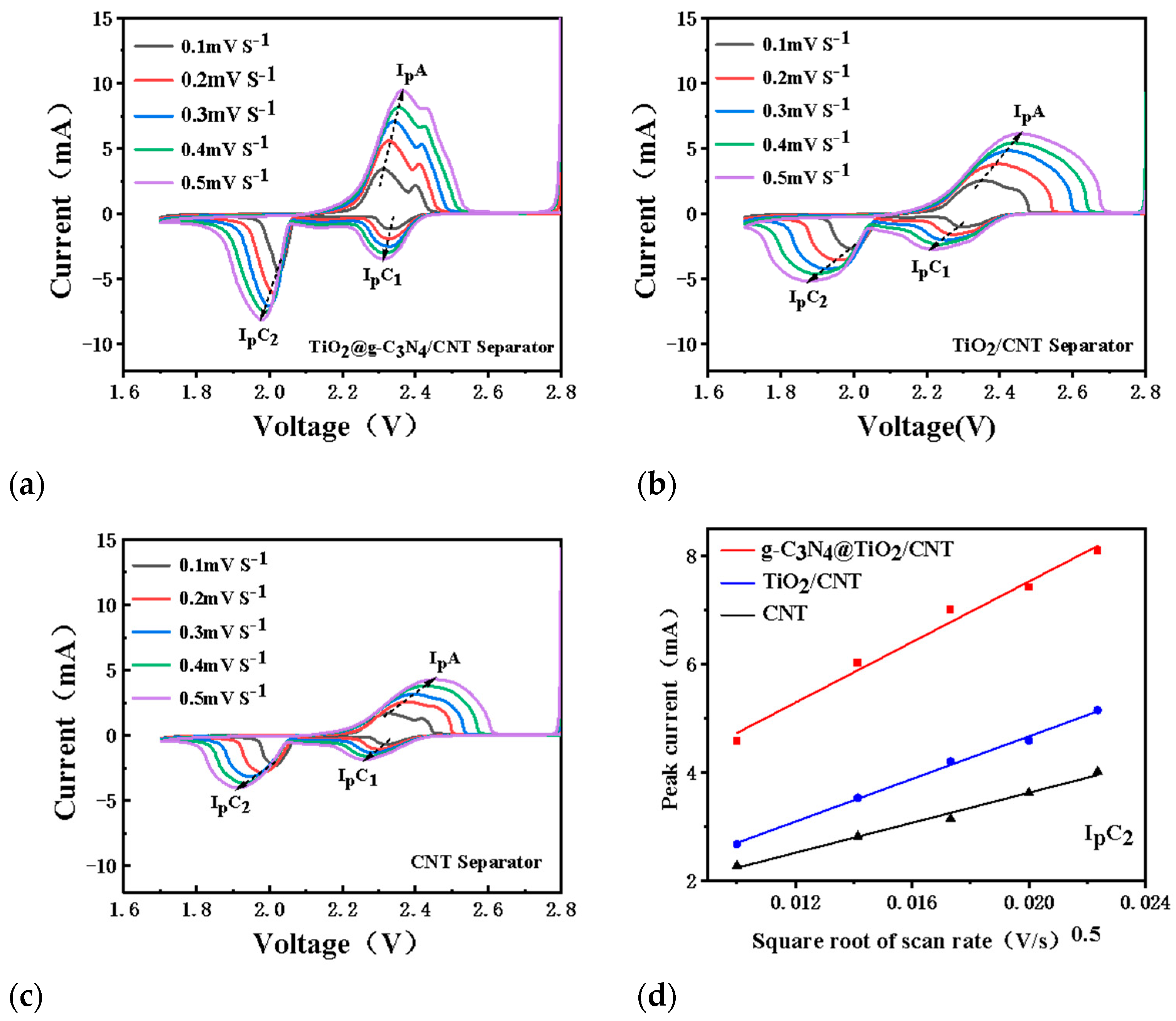
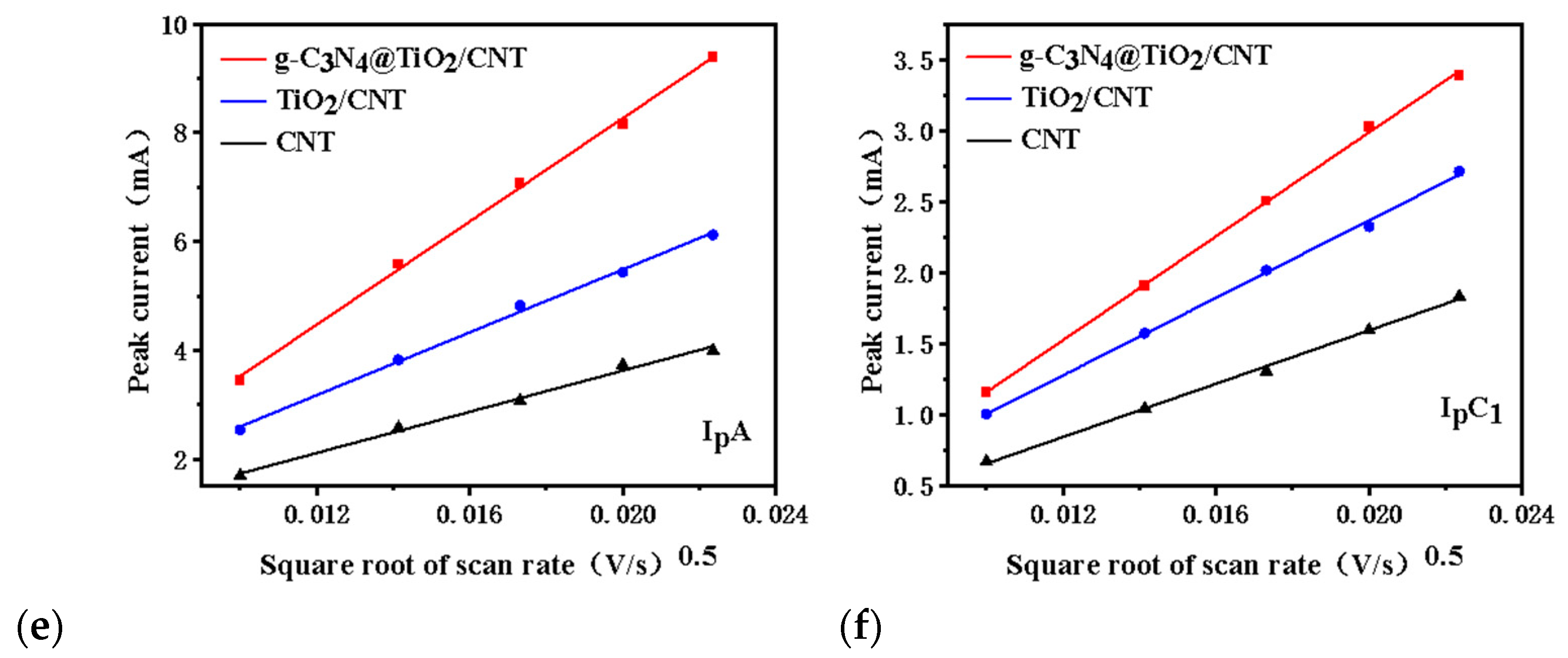
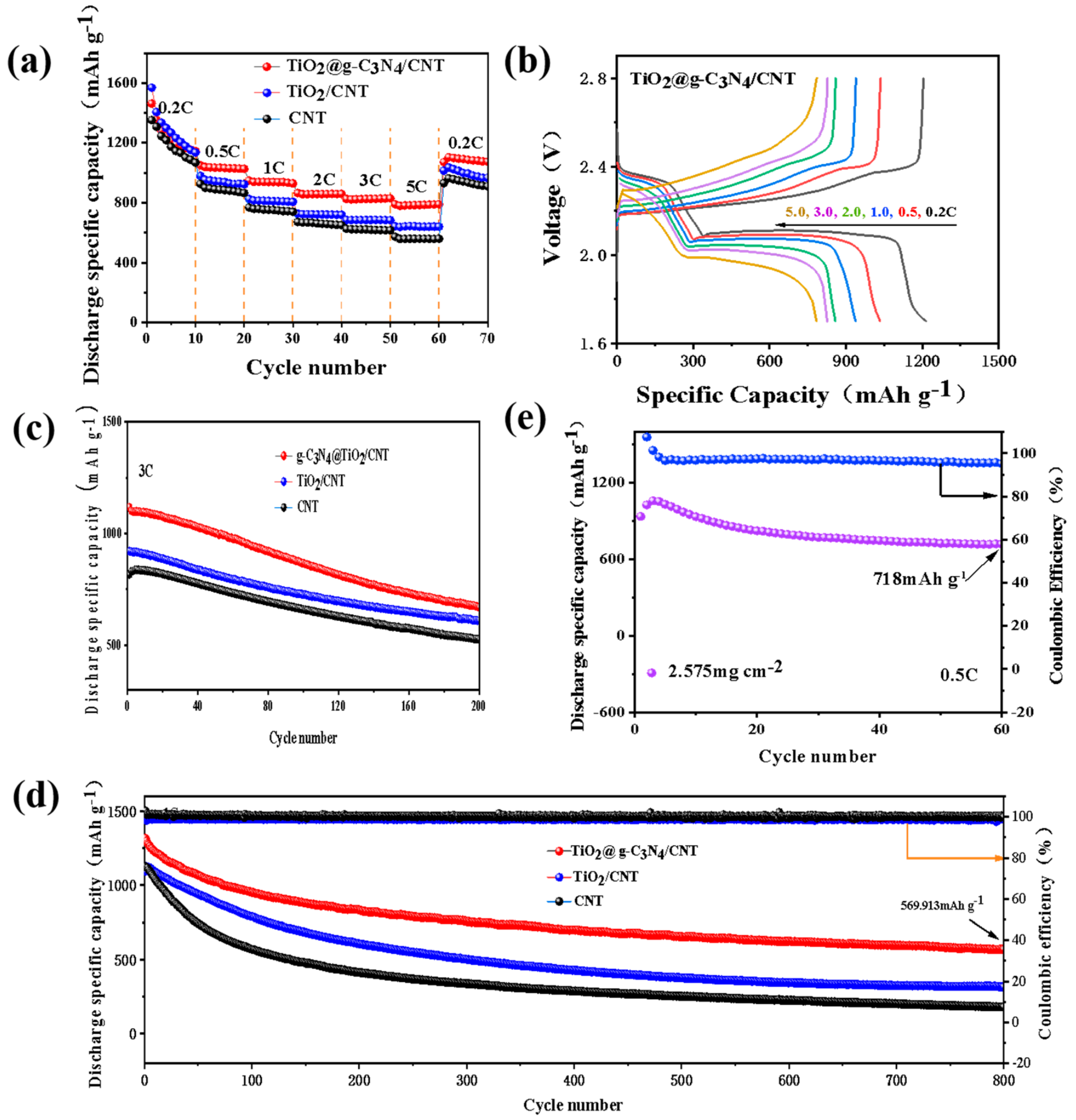
3.4. Catalytic Performance and Electrochemical Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aslam, M.K.; Jamil, S.; Hussain, S.; Xu, M. Effects of catalysis and separator functionalization on high energy lithium sulfur batteries: A complete review. Energy Environ. Mater. 2022, 6, e12420. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of Heterostructure Materials in Energy Storage: A Review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Zhao, S.; Han, K.; Zhang, W.; Pan, K.; Zhang, L. NiFe2O4/Ketjen Black Composites as Efficient Membrane Separators to Suppress the Shuttle Effect for Long-Life Lithium-Sulfur Batteries. Nanomaterials 2022, 12, 1347. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, J.; Huang, Q.; Wei, J.; Wang, C.; Ji, K.; Chen, M. Manganese-nickel bimetallic oxide electrocatalyzing redox reactions of lithium pol ysulfides in lithium–sulfur batteries. Sustain. Energy Fuels 2022, 6, 1426–1435. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Xiao, Y.; Cao, F.; Zhang, H. Recent progress of separators in lithium-sulfur batteries. Energy Storage Mater. 2021, 40, 439–460. [Google Scholar] [CrossRef]
- Sun, W.; Sun, X.; Akhtar, N.; Li, C.; Wang, W.; Wang, A.; Wang, K.; Huang, Y. Attapulgite nanorods assisted surface engineering for separator to achieve high-performance lithium-sulfur batteries. J. Energy Chem. 2020, 48, 364–374. [Google Scholar] [CrossRef]
- Wang, L.; Hua, W.; Wan, X.; Feng, Z.; Hu, Z.; Li, H.; Niu, J.; Wang, L.; Wang, A.; Liu, J.; et al. Design Rules of a Sulfur Redox Electrocatalyst for Lithium-Sulfur Batteries. Adv. Mater. 2022, 34, 2110279. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced separators for lithium-ion and lithium-sulfur batteries: A review of recent progress. ChemSusChem 2016, 9, 3023–3039. [Google Scholar] [CrossRef]
- Wei, Z.; Ren, Y.; Sokolowski, J.; Zhu, X.; Wu, G. Mechanistic understanding of the role separators playing in advanced lithium-sulfur batteries. InfoMat 2020, 2, 483–508. [Google Scholar] [CrossRef]
- Li, A.; Yuen, A.C.Y.; Wang, W.; De Cachinho Cordeiro, I.M.; Wang, C.; Chen, T.B.Y.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A review on lithium-ion battery separators towards enhanced safety performances and modelling approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef]
- Choi, J.; Kim, P.J. A roadmap of battery separator development: Past and future. Curr. Opin. Electroche 2022, 31, 100858. [Google Scholar] [CrossRef]
- Kim, H.-B.; Ngo, D.T.; Verma, R.; Singhbabu, Y.N.; Kim, D.-Y.; Le, H.T.T.; Mali, S.S.; Hong, C.-K.; Park, C.-J. Vanadium nitride and carbon nanofiber composite membrane as an interlayer for extended life cycle lithium-sulphur batteries. Ceram. Int. 2021, 47, 21476–21489. [Google Scholar] [CrossRef]
- Gao, Z.; Xue, Z.; Miao, Y.; Chen, B.; Xu, J.; Shi, H.; Tang, T.; Zhao, X. TiO2@Porous carbon nanotubes modified separator as polysulfide barrier for lithium-sulfur batteries. J. Alloys Compd. 2022, 906, 164249. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, P.; Liu, D.; Fan, Y.; Zhou, J.; Zhao, J.; Liu, H.; Guo, X.; Liu, W.; Cheng, Y. TiO2 nanotube/RGO modified separator as an effective polysulfide-barrier for high electrochemical performance Li-S batteries. J. Alloys Compd. 2022, 895, 162495. [Google Scholar] [CrossRef]
- Chen, A.; Liu, W.; Yan, J.; Liu, K. A novel separator modified by titanium dioxide nanotubes/carbon nanotubes composite for high performance lithium-sulfur batteries. Funct. Mater. Lett. 2019, 12, 1950016. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-Performance Li-S Batteries with an Ultra-lightweight MWCNT-Coated Separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cai, C.; Yu, X.; Chen, Z.; Hu, Y.; Yu, F.; Zhai, S.; Mei, T.; Yu, L.; Wang, X. Scalable 3D Honeycombed Co3O4 Modified Separators as Polysulfides Barriers for High-Performance Li-S Batteries. ACS Appl. Mater. Interfaces 2022, 14, 35894–35904. [Google Scholar] [CrossRef]
- Xia, S.; Zhou, Q.; Peng, B.; Zhang, X.; Liu, L.; Guo, F.; Fu, L.; Wang, T.; Liu, Y.; Wu, Y. Co3O4@MWCNT modified separators for Li-S batteries with improved cycling per formance. Mater. Today Energy 2022, 30, 101163. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, Y.; Dai, Y.; Gao, S.; Liao, B.; Pang, H. CoS2@montmorillonite as an efficient separator coating for high-performance lithium-sulfur batteries. Inorg. Chem. Front. 2022, 9, 3335–3347. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Zhou, Y.; Huang, Y.; Li, Y. Molybdenum carbide nanostructures for electrocatalytic polysulfide conversion in lithium-polysulfide batteries. Nanoscale Horiz. 2020, 5, 501–506. [Google Scholar] [CrossRef]
- Kim, Y.; Noh, Y.; Bae, J.; Ahn, H.; Kim, M.; Kim, W.B. N-doped carbon-embedded TiN nanowires as a multifunctional separator for Li–S batteries with enhanced rate capability and cycle stability. J. Energy Chem. 2021, 57, 10–18. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Li, M.; Liu, R.; Li, H.; Li, T.; Sun, M.; Deng, Y.; Feng, M.; Chen, Z. Reinforced polysulfide barrier by g-C3N4/CNT composite towards superior lithium-sulfur batteries. J. Energy Chem. 2021, 53, 234–240. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Hu, C.; Liu, N.; Zhao, Y. Tg-C3N4-coated functional separator as polysulfide barrier of high-performance lithium-sulfur batteries. Nanotechnology 2021, 32, 475401. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Fang, Z.; Zhao, S.; He, Y.-S.; Zhang, W.; Mu, J.; Zhang, L.; Ma, Z.-F. Constructing a catalytic reservoir using cobalt nanoparticles-MoS2@nitrogen doped carbon nanotubes on the separator to immobilize polysulfides and accelerate their conversion for lithium-sulfur batteries. Chem. Eng. J. 2022, 446, 136943. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z.; Wei, J.; Jing, Y.; Guo, W.; Xie, Z.; Qu, D.; Liu, D.; Tang, H.; Li, J. A synergistic modification of polypropylene separator toward stable lithium–sulfur battery. J. Membr. Sci. 2020, 597, 117646. [Google Scholar] [CrossRef]
- Liang, G.; Wu, J.; Qin, X.; Liu, M.; Li, Q.; He, Y.-B.; Kim, J.-K.; Li, B.; Kang, F. Ultrafine TiO2 Decorated Carbon Nanofibers as Multifunctional Interlayer for High-Performance Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2016, 8, 23105–23113. [Google Scholar] [CrossRef]
- Ma, H.; Liu, X.; Liu, N.; Zhao, Y.; Zhang, Y.; Bakenov, Z.; Wang, X. Defect-rich porous tubular graphitic carbon nitride with strong adsorption towards lithium polysulfides for high-performance lithium-sulfur batteries. J. Mater. Sci. Technol. 2022, 115, 140–147. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Z.; Zhang, B.; Zhao, J.; Liu, H.; Guo, X.; Liu, W.; Su, Z. Multi-functional TiO2 nanosheets/carbon nanotubes modified separator enhanced cycling performance for lithium-sulfur batteries. Int. J. Energy Res. 2020, 44, 3231–3240. [Google Scholar] [CrossRef]
- Shi, C.; Huang, J.; Tang, Y.; Cen, Z.; Wang, Z.; Liu, S.; Fu, R. A hierarchical porous carbon aerogel embedded with small-sized TiO2 nanoparticles for high-performance Li–S batteries. Carbon 2022, 202, 59–65. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Jiang, W.; Pan, K.; Zhang, L. Rational Design of TiO2@g-C3N4/CNT Composite Separator for High Performance Lithium-Sulfur Batteries to Promote the Redox Kinetics of Polysulfide. Nanomaterials 2023, 13, 3084. https://doi.org/10.3390/nano13243084
Dong L, Jiang W, Pan K, Zhang L. Rational Design of TiO2@g-C3N4/CNT Composite Separator for High Performance Lithium-Sulfur Batteries to Promote the Redox Kinetics of Polysulfide. Nanomaterials. 2023; 13(24):3084. https://doi.org/10.3390/nano13243084
Chicago/Turabian StyleDong, Lingling, Wen Jiang, Kefeng Pan, and Lipeng Zhang. 2023. "Rational Design of TiO2@g-C3N4/CNT Composite Separator for High Performance Lithium-Sulfur Batteries to Promote the Redox Kinetics of Polysulfide" Nanomaterials 13, no. 24: 3084. https://doi.org/10.3390/nano13243084




