Mechanistic Insights into a Novel Controllable Phase-Transition Polymer for Enhanced Oil Recovery in Mature Waterflooding Reservoirs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Rheological Characterization
2.2.2. Chemical Structure Analysis
2.2.3. Morphology Characterization
2.2.4. Propagation Behavior of the Phase-Transition Polymer in Porous Media
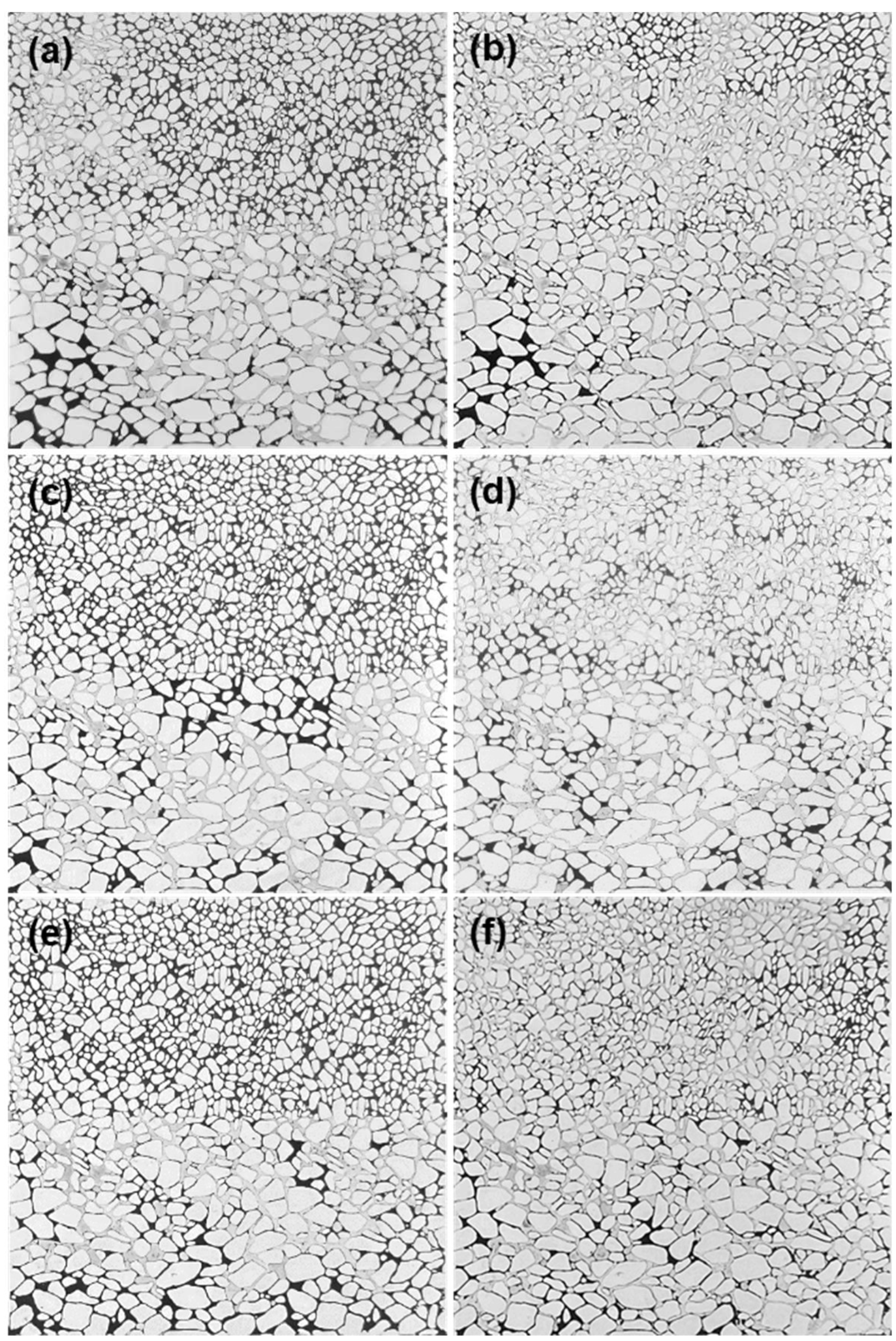
2.2.5. The 2D Glass-Etched Micromodel Flooding Experiment
2.2.6. Evaluation of Enhanced Oil Recovery Capability
3. Results and Discussion
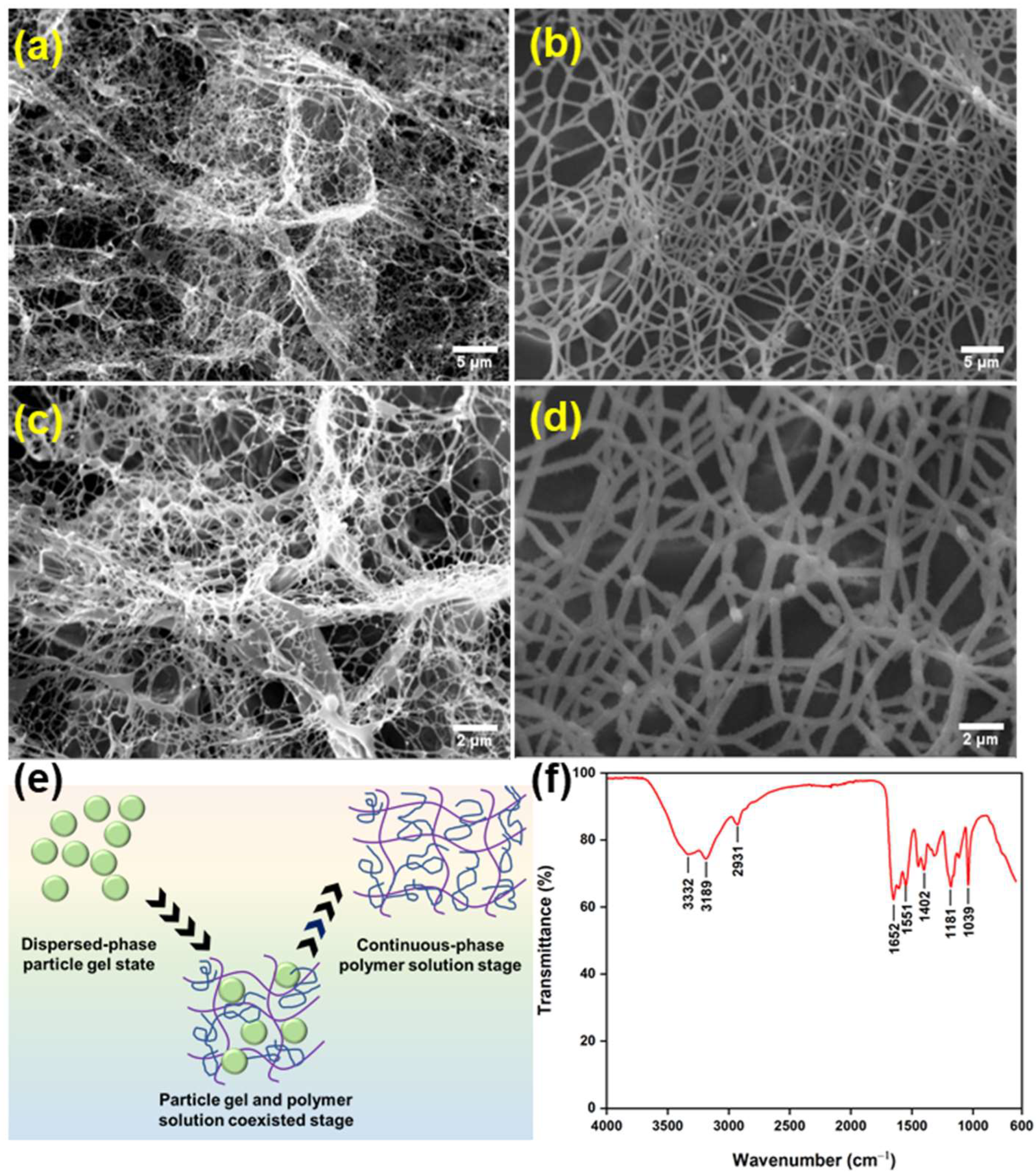
3.1. Morphology and Chemical Structure of the Phase-Transition Polymer
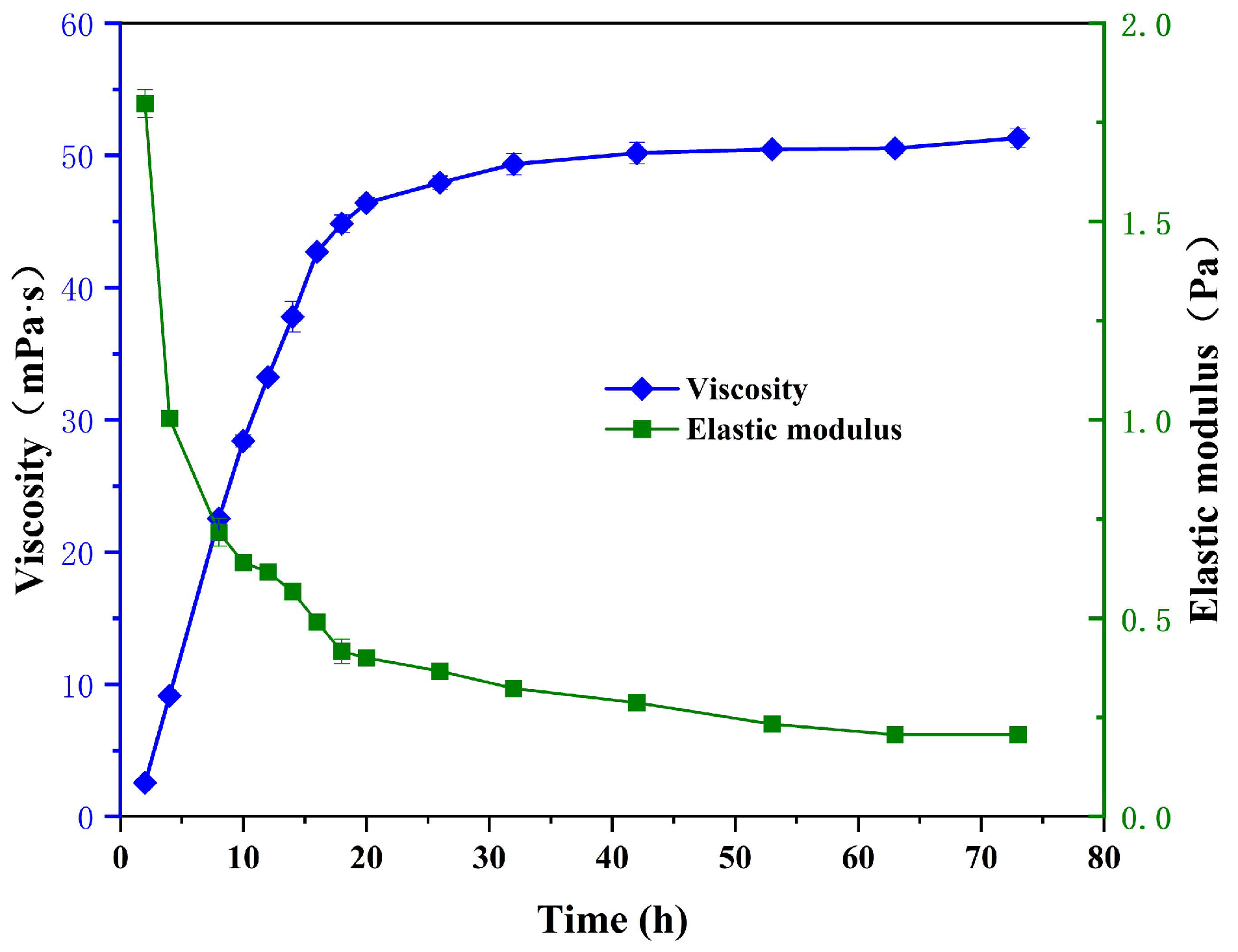
3.2. Rheological Properties of the Phase-Transition Polymer
3.3. Propagation Behavior of the Phase-Transition Polymer in Porous Media
3.4. The EOR Efficiency of the Phase-Transition Polymer
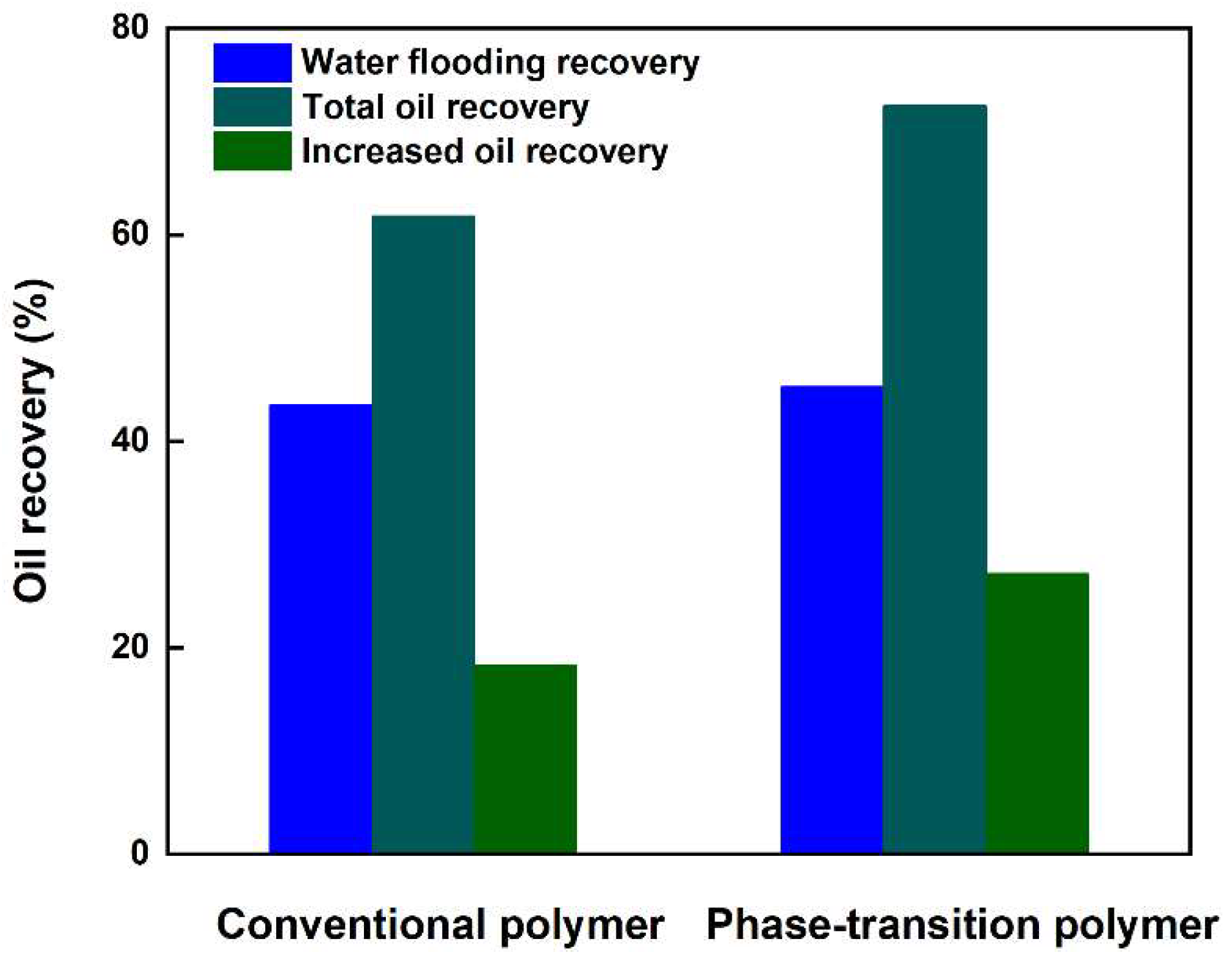
3.5. Enhanced Oil Recovery Capability of Phase-Transition Polymer over Conventional Polymer
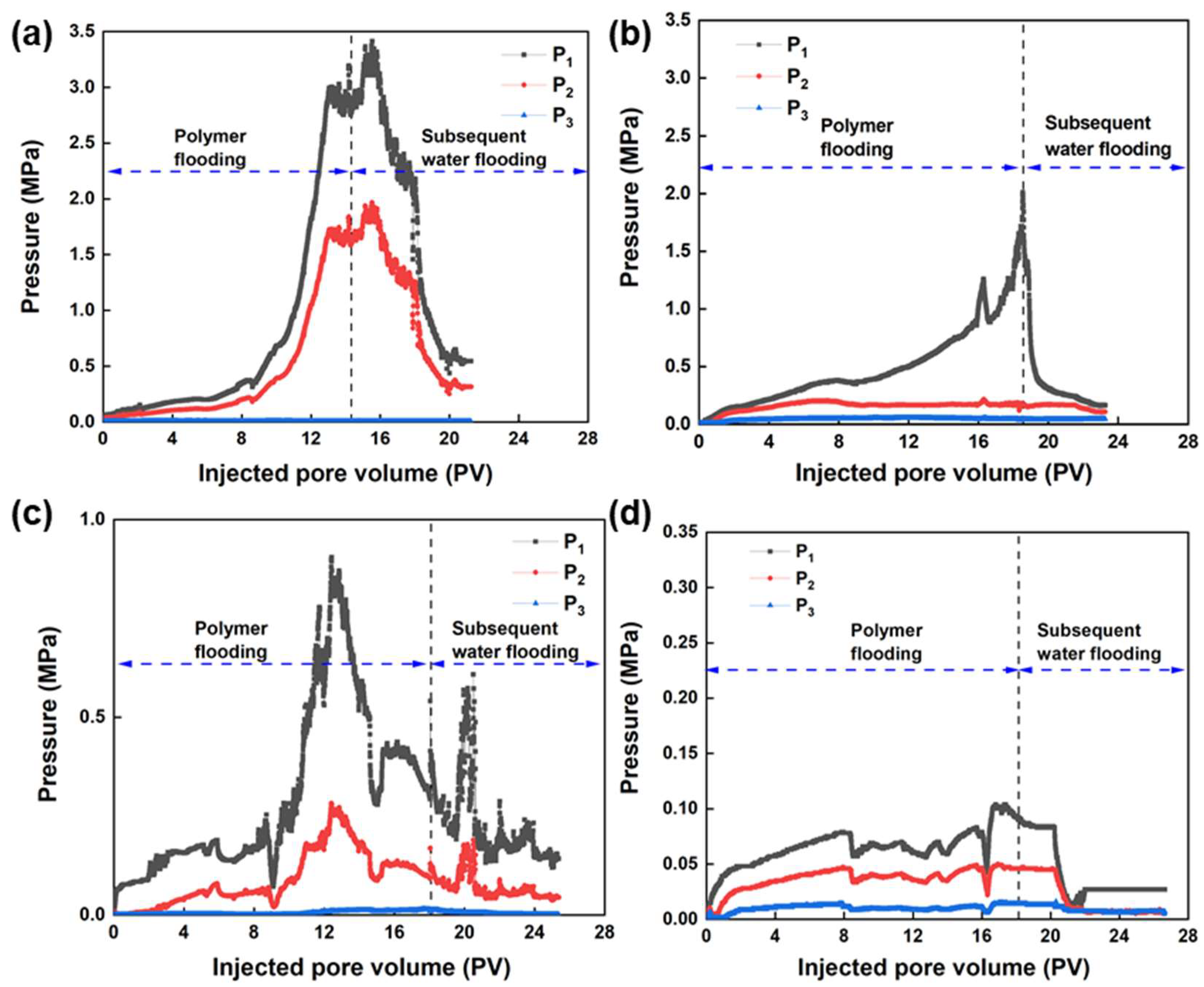
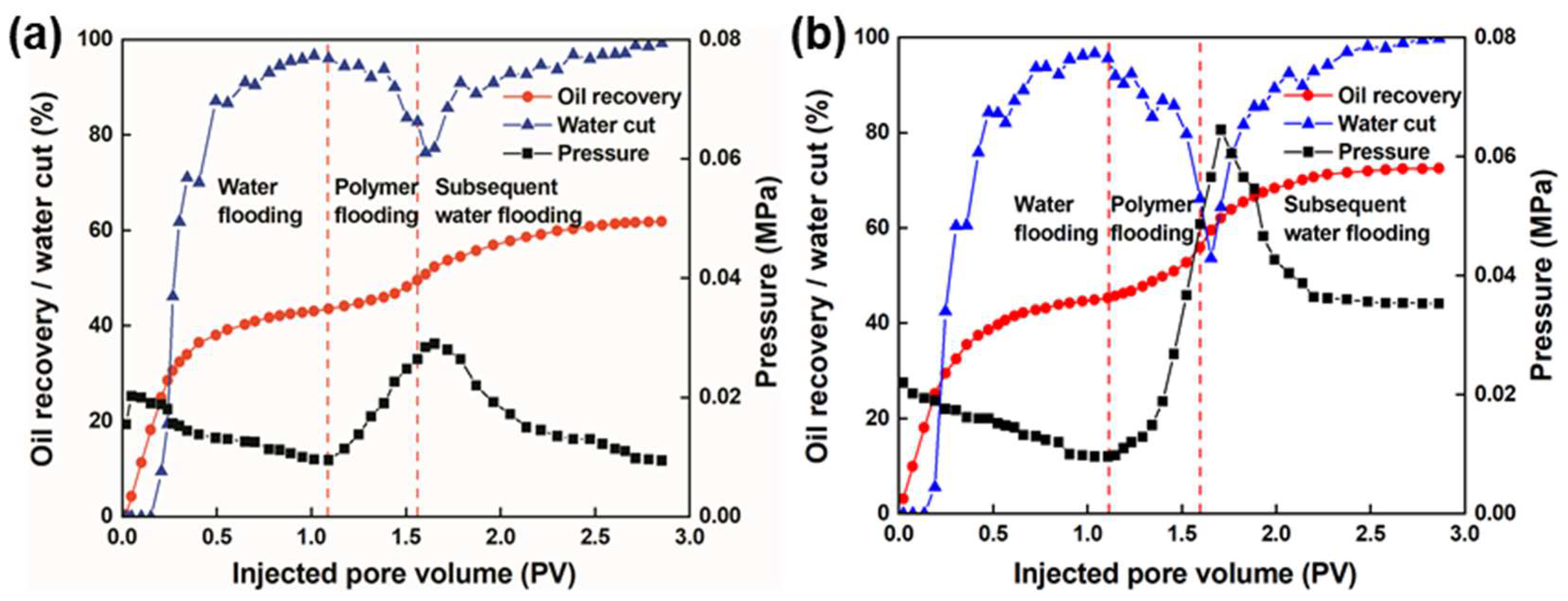
3.5.1. Analysis of the Flooding Curves
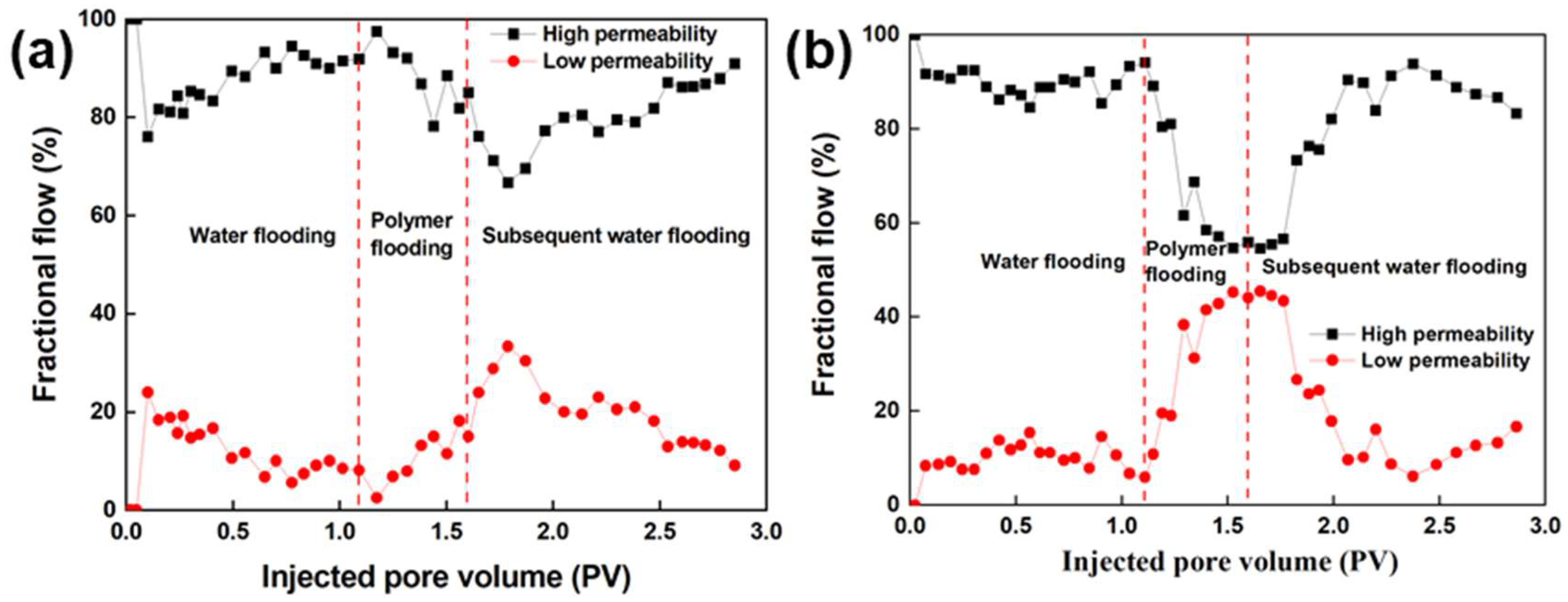
3.5.2. Analysis of Fractional Flow Curves
3.5.3. Analysis of Enhanced Oil Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on surfactant flooding: Phase behavior, retention, IFT, and field applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Yang, W.; Lu, J.; Wei, B.; Yu, H.; Liang, T. Micromodel studies of surfactant flooding for enhanced oil recovery: A review. ACS Omega 2021, 6, 6064–6069. [Google Scholar] [CrossRef]
- Guangzhi, L.; Qiang, W.; Hongzhuang, W.; Weidong, L.; Zhengmao, W. Chemical flooding development status and prospect. Acta Petrol. Sin. 2017, 38, 196–207. [Google Scholar]
- Wang, D.; Seright, R.S.; Shao, Z.; Wang, J. Key aspects of project design for polymer flooding at the Daqing oilfield. SPE Reserv. Eval. Eng. 2008, 11, 1117–1124. [Google Scholar] [CrossRef]
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of polymer-flooding technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- AlSofi, A.M.; Blunt, M.J. Polymer flooding design and optimization under economic uncertainty. J. Pet. Sci. Eng. 2014, 124, 46–59. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on polymer flooding: Rheology, adsorption, stability, and field applications of various polymer systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent advances in surfactant EOR. SPE J. 2011, 16, 889–907. [Google Scholar] [CrossRef]
- Kianinejad, A.; Ghazanfari, M.H.; Kharrat, R.; Rashtchian, D. An experimental investigation of surfactant flooding as a good candidate for enhancing oil recovery from fractured reservoirs using one-quarter five spot micromodels: The role of fracture geometrical properties. Energy Sources Part A 2013, 35, 1929–1938. [Google Scholar] [CrossRef]
- Ko, K.M.; Chon, B.H.; Jang, S.B.; Jang, H.Y. Surfactant flooding characteristics of dodecyl alkyl sulfate for enhanced oil recovery. J. Ind. Eng. Chem. 2014, 20, 228–233. [Google Scholar] [CrossRef]
- Morshedi, S.; Foroughi, S.; Beiranvand, M.S. Numerical simulation of surfactant flooding in darcy scale flow. Pet. Sci. Technol. 2014, 32, 1365–1374. [Google Scholar] [CrossRef]
- Alwated, B.; El-Amin, M.F. Enhanced oil recovery by nanoparticles flooding: From numerical modeling improvement to machine learning prediction. Adv. Geo-Energy Res. 2021, 5, 297–317. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Nano-surfactant flooding in carbonate reservoirs: A mechanistic study. Eur. Phys. J. Plus 2017, 132, 246. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Azarshin, S.; Moghadasi, J.; A Aboosadi, Z. Surface functionalization of silica nanoparticles to improve the performance of water flooding in oil wet reservoirs. Energy Explor. Exploit. 2017, 35, 685–697. [Google Scholar] [CrossRef]
- Youssif, M.I.; El-Maghraby, R.M.; Saleh, S.M.; Elgibaly, A. Silica nanofluid flooding for enhanced oil recovery in sandstone rocks. Egypt. J. Pet. 2018, 27, 105–110. [Google Scholar] [CrossRef]
- Tavakkoli, O.; Kamyab, H.; Shariati, M.; Mustafa Mohamed, A.; Junin, R. Effect of nanoparticles on the performance of polymer/surfactant flooding for enhanced oil recovery: A review. Fuel 2022, 312, 122867. [Google Scholar] [CrossRef]
- Druetta, P.; Picchioni, F. Polymer and nanoparticles flooding as a new method for Enhanced Oil Recovery. J. Pet. Sci. Eng. 2019, 177, 479–495. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Rueda, E.; Akarri, S.; Torsæter, O.; Moreno, R.B. Experimental investigation of the effect of adding nanoparticles to polymer flooding in water-wet micromodels. Nanomaterials 2020, 10, 1489. [Google Scholar] [CrossRef]
- Al-Asadi, A.; Rodil, E.; Soto, A. Nanoparticles in Chemical EOR: A Review on Flooding Tests. Nanomaterials 2022, 12, 4142. [Google Scholar] [CrossRef] [PubMed]
- Torsæter, O. Application of nanoparticles for oil recovery. Nanomaterials 2021, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Johns, R.T.; Delshad, M.; Lake, L.W.; Goudarzi, A. Oil-recovery predictions for surfactant polymer flooding. J. Pet. Sci. Eng. 2013, 112, 341–350. [Google Scholar] [CrossRef]
- Shaker Shiran, B.; Skauge, A. Enhanced oil recovery (EOR) by combined low salinity water/polymer flooding. Energy Fuels 2013, 27, 1223–1235. [Google Scholar] [CrossRef]
- Sun, C.; Guo, H.; Li, Y.; Song, K. Recent advances of surfactant-polymer (SP) flooding enhanced oil recovery field tests in China. Geofluids 2020, 2020, 8286706. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.; Zhao, F. Successful polymer flooding and surfactant-polymer flooding projects at Shengli oilfield from 1992 to 2012. J. Pet. Explor. Prod. Technol. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Zhang, J.; Zhang, A. Development and application of dilute surfactant-polymer flooding system for Shengli oilfield. J. Pet. Sci. Eng. 2009, 65, 45–50. [Google Scholar]
- Ma, Y.; Hou, J.; Zhao, F.; Song, Z. Linearly descending viscosity for alkaline-surfactant-polymer flooding mobility modification in multilayer heterogeneous reservoirs. RSC Adv. 2018, 8, 8269–8284. [Google Scholar] [CrossRef]
- Pal, N.; Saxena, N.; Mandal, A. Characterization of alkali-surfactant-polymer slugs using synthesized gemini surfactant for potential application in enhanced oil recovery. J. Pet. Sci. Eng. 2018, 168, 283–300. [Google Scholar] [CrossRef]
- Alkhatib, A.; Babaei, M. Applying the multilevel monte carlo method for heterogeneity-induced uncertainty quantification of surfactant/polymer flooding. SPE J. 2016, 21, 1192–1203. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, J.; Dong, X.; Chen, F. Formulation development and visualized investigation of temperature-resistant and salt-tolerant surfactant-polymer flooding to enhance oil recovery. J. Pet. Sci. Eng. 2019, 174, 584–598. [Google Scholar] [CrossRef]
- Al-Murayri, M.T.; Hassan, A.A.; Abdullah, M.B.; Abdulrahim, A.M.; Marlière, C.; Hocine, S.; Tabary, R.; Suzanne, G.P. Surfactant/polymer flooding: Chemical-formulation design and evaluation for Raudhatain lower Burgan Reservoir, Kuwait. SPE Reservoir Eval. Eng. 2019, 22, 923–940. [Google Scholar] [CrossRef]
- Aramideh, S.; Borgohain, R.; Naik, P.K.; Johnston, C.T.; Vlachos, P.P.; Ardekani, A.M. Multi-objective history matching of surfactant-polymer flooding. Fuel 2018, 228, 418–428. [Google Scholar] [CrossRef]
- Abdulbaki, M.; Huh, C.; Sepehrnoori, K.; Delshad, M.; Varavei, A. A critical review on use of polymer microgels for conformance control purposes. J. Pet. Sci. Eng. 2014, 122, 741–753. [Google Scholar] [CrossRef]
- He, H.; Fu, J.; Hou, B.; Yuan, F.; Guo, L.; Li, Z.; You, Q. Investigation of injection strategy of branched-preformed particle gel/polymer/surfactant for enhanced oil recovery after polymer flooding in heterogeneous reservoirs. Energies 2018, 11, 1950. [Google Scholar] [CrossRef]
- Liu, W.; He, H.; Yuan, F.; Liu, H.; Zhao, F.; Liu, H.; Luo, G. Influence of the injection scheme on the enhanced oil recovery ability of heterogeneous phase combination flooding in mature waterflooded reservoirs. ACS Omega 2022, 7, 23511–23520. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Liu, W.; Chen, Y.; Liu, H.; Liu, H.; Luo, G. Synergistic mechanism of well pattern adjustment and heterogeneous phase combined flooding on enhancing oil recovery in mature fault-block reservoirs. J. Pet. Explor. Prod. Technol. 2022, 12, 3387–3398. [Google Scholar] [CrossRef]
- Wu, D.; Zhou, K.; Hou, J.; An, Z.; Zhai, M.; Liu, W. Experimental study on combining heterogeneous phase composite flooding and streamline adjustment to improve oil recovery in heterogeneous reservoirs. J. Pet. Sci. Eng. 2020, 194, 107478. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liu, H.; Li, S.; Liu, L. Dynamic sweep experiments on a heterogeneous phase composite system based on branched-preformed particle gel in high water-cut reservoirs after polymer flooding. Gels 2023, 9, 364. [Google Scholar] [CrossRef]
- Jung, J.C.; Zhang, K.; Chon, B.H.; Choi, H.J. Rheology and polymer flooding characteristics of partially hydrolyzed polyacrylamide for enhanced heavy oil recovery. J. Appl. Polym. Sci. 2013, 127, 4833–4839. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Yu, Q.; Wen, X.; Liu, H. Optimization design of injection strategy for surfactant-polymer flooding process in heterogeneous reservoir under low oil prices. Energies 2019, 12, 3789. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-calculated IR spectrum amide I, II, and III band contributions of n-methylacetamide fine components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Jablonsky, M.J.; Mays, J.W. NMR and FT-IR studies of sulfonated styrene-based homopolymers and copolymers. Polymer 2002, 43, 5125–5132. [Google Scholar] [CrossRef]
- Yao, S.; Beginn, U.; Gress, T.; Lysetska, M.; Würthner, F. Supramolecular polymerization and gel formation of bis(merocyanine) dyes driven by dipolar aggregation. J. Am. Chem. Soc. 2004, 126, 8336–8348. [Google Scholar] [CrossRef] [PubMed]
- Katashima, T. Rheological studies on polymer networks with static and dynamic crosslinks. Polym. J. 2021, 53, 1073–1082. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, S.; Xue, X.; Zhang, J.; Xu, J.; Liu, Z. Influencing factors for effective establishment of residual resistance factor of polymer solution in porous media. J. Polym. Res. 2022, 29, 210. [Google Scholar] [CrossRef]


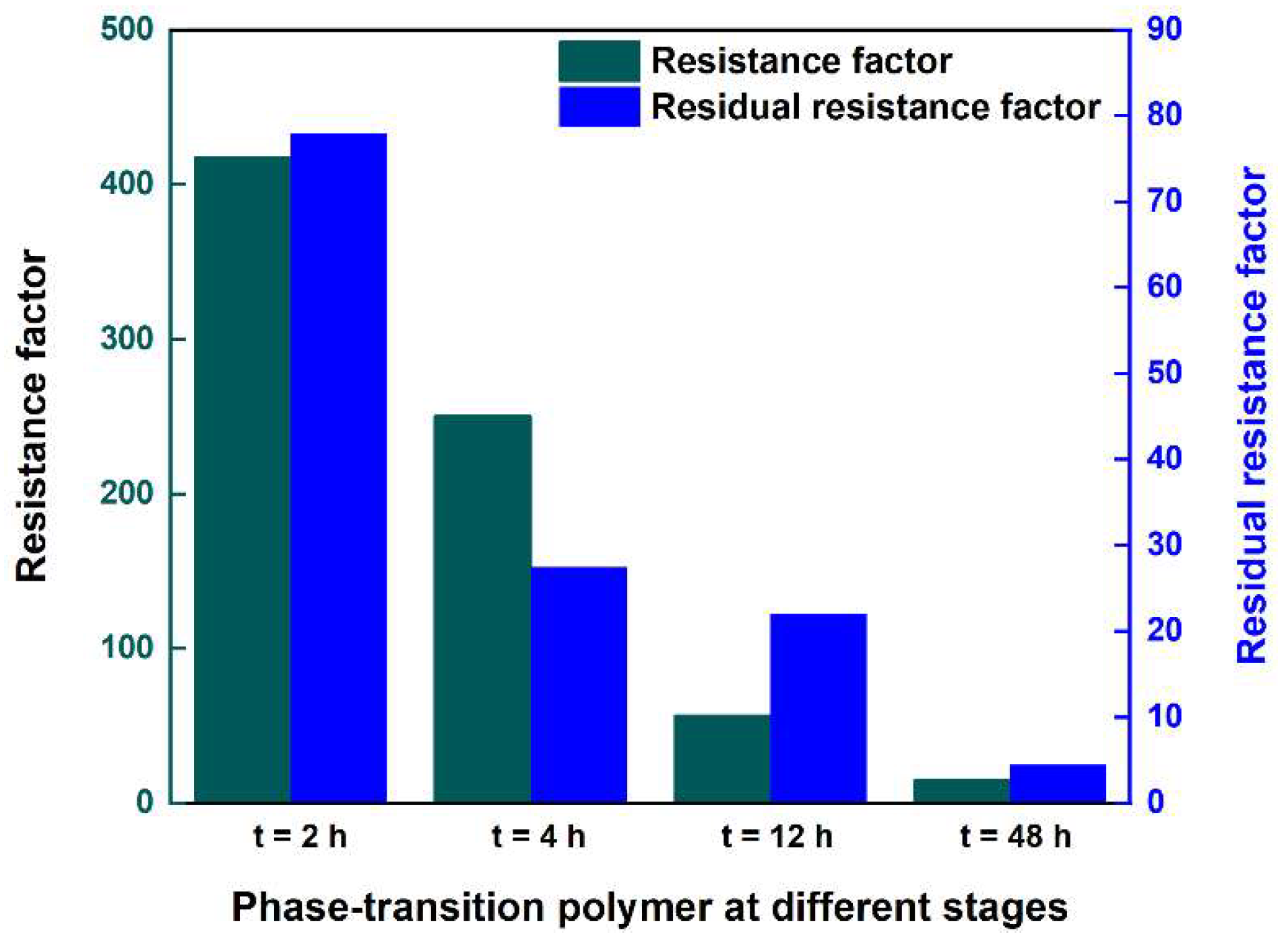
| Ageing Time/h | ∆Pwa/MPa | ∆Ppolymer/MPa | ∆Pwb/MPa | Fr | Frr |
|---|---|---|---|---|---|
| 2 | 0.007 | 2.926 | 0.546 | 418.0 | 78.0 |
| 4 | 0.006 | 1.503 | 0.165 | 250.5 | 27.5 |
| 12 | 0.0065 | 0.369 | 0.143 | 56.8 | 22.0 |
| 48 | 0.006 | 0.092 | 0.027 | 15.3 | 4.5 |
| Different Phase Stage | Waterflooding Recovery/% | Polymer Flooding Recovery/% | Increased Oil Recovery/% |
|---|---|---|---|
| Dispersed-phase particle gel | 23.6 | 46.8 | 23.2 |
| Particle gel–aqueous solution mesophase | 24.5 | 58.9 | 34.4 |
| Continuous-phase aqueous solution | 24.6 | 52.3 | 27.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Cao, X.; Ji, Y.; Zou, R. Mechanistic Insights into a Novel Controllable Phase-Transition Polymer for Enhanced Oil Recovery in Mature Waterflooding Reservoirs. Nanomaterials 2023, 13, 3101. https://doi.org/10.3390/nano13243101
Yang Y, Cao X, Ji Y, Zou R. Mechanistic Insights into a Novel Controllable Phase-Transition Polymer for Enhanced Oil Recovery in Mature Waterflooding Reservoirs. Nanomaterials. 2023; 13(24):3101. https://doi.org/10.3390/nano13243101
Chicago/Turabian StyleYang, Yong, Xiaopeng Cao, Yanfeng Ji, and Ruqiang Zou. 2023. "Mechanistic Insights into a Novel Controllable Phase-Transition Polymer for Enhanced Oil Recovery in Mature Waterflooding Reservoirs" Nanomaterials 13, no. 24: 3101. https://doi.org/10.3390/nano13243101




