Electrochemical Synthesis of Functionalized Graphene/Polyaniline Composite Using Two Electrode Configuration for Supercapacitors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Graphene Xodie and ADS-G
2.3. Preparation of ADS-G Coated Working Electrode
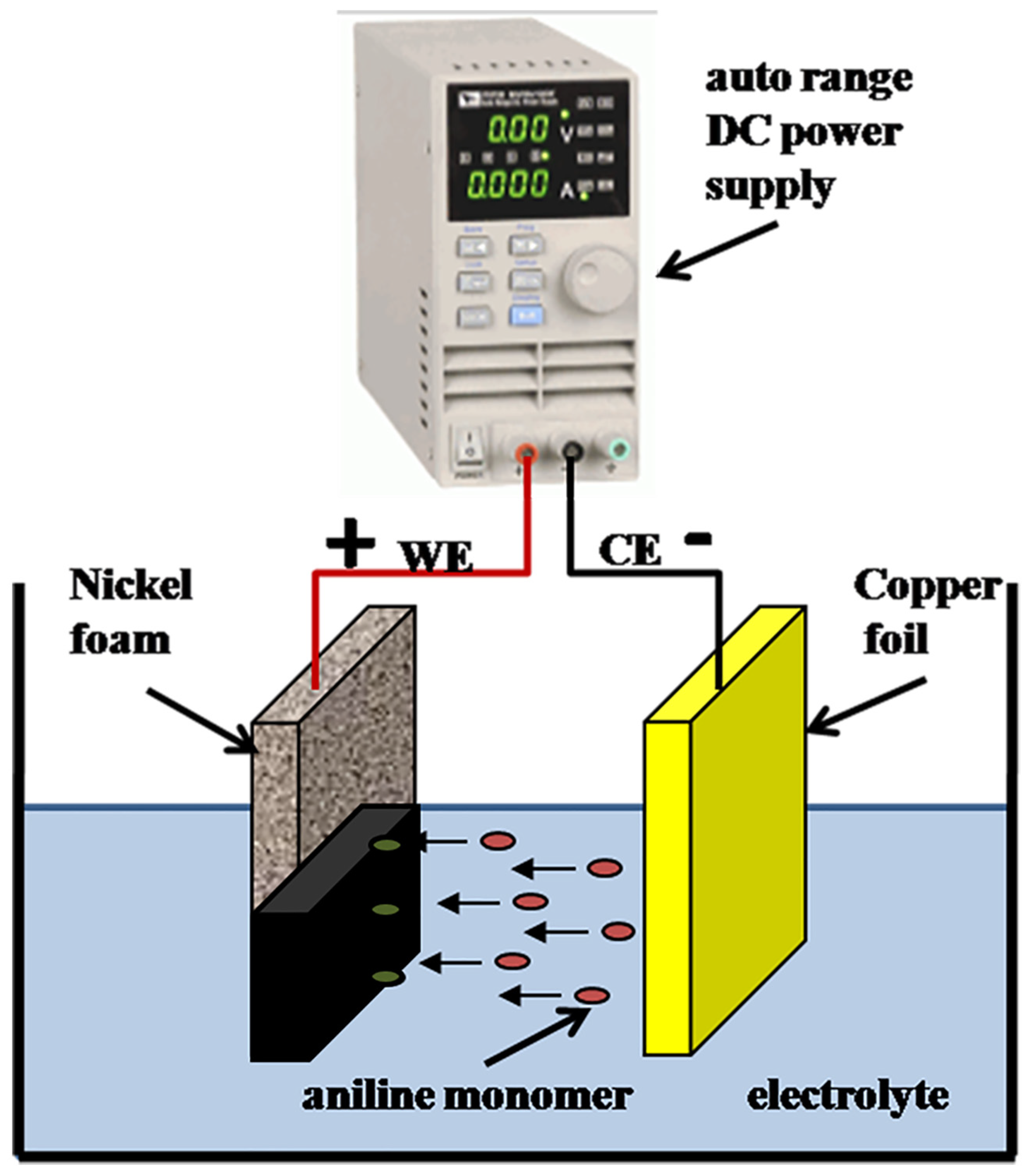
2.4. Preparation of ADS-G/PANI Composites
2.5. Characterizations
3. Results and Discussion
3.1. FT-IR Spectra Analysis
3.2. XPS
3.3. XRD
3.4. FE-SEM Analysis
3.5. TGA
3.6. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. Graphene-based materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Bunch, J.S.; Zande, A.M.V.D.; Verbridge, S.S.; Frank, L.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Electromechanical resonators from graphene sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef]
- Choi, H.J.; Jung, S.M.; Seo, J.M.; Chang, D.W.; Dai, L.M.; Baek, J.B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Faverolle, F.; Attias, A.J.; Bloch, B. Highly conducting and strongly adhering polypyrrole coating layers deposited on glass substrates by a chemical process. Chem. Mater. 1998, 10, 740–752. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Tang, Y.H.; Wu, N.; Luo, S.L.; Liu, C.B.; Wang, K.; Chen, L.Y. One-step electrodeposition to layer-by-layer graphene-conducting-polymer hybrid films. Macromol. Rapid Commun. 2012, 33, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Shao, B.; Fan, Z.J.; Qian, W.Z.; Zhang, M.L.; Wei, F. Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 2010, 48, 487–493. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Bai, H.; Xu, Y.X.; Zhao, L.; Li, C.; Shi, G.Q. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun. 2009, 13, 1667–1669. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.X.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G.Q. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Lee, R.H.; Lai, H.H.; Wang, J.J.; Jeng, R.J.; Lin, J.J. Self-doping effects on the morphology, electrochemical and conductivity properties of self-assembled polyanilines. Thin Solid Films 2008, 517, 500–505. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Li, Y.; Liu, J.; Li, Z.X. Electrochemical properties of graphene nanosheets/polyaniline nanofibers composites as electrode for supercapacitors. J. Power Sources 2011, 196, 10775–10781. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Zhao, J.P.; Ren, W.C.; Chen, Z.G.; Tan, J.; Wu, Z.S.; Gentle, l.; Lu, G.Q.; Cheng, H.M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Lee, T.M.; Yun, T.Y.; Park, B.H.; Sharma, B.; Song, H.K.; Kim, B.S. Hybrid multilayer thin film supercapacitor of graphene nanosheets with polyaniline: Importance of establishing intimate electronic contact through nanoscale blending. J. Mater. Chem. 2012, 22, 21092–21099. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J.S. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Si, Y.C.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Khanra, P.; Bose, S.; Kim, N.H.; Ku, B.C.; Moon, B.H.; Lee, J.H. Preparation of water—Dispersible graphene by facile surface modification of graphite oxide. Nanotechnology 2011, 22, 305710. [Google Scholar] [CrossRef] [PubMed]
- Li, F.H.; Bao, Y.; Chai, J.; Zhang, Q.X.; Han, D.X.; Niu, L. Synthesis and application of widely soluble graphene sheets. Langmuir 2010, 26, 12314–12320. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Qian, W.; Zhang, L.H.; Hou, Y.L. Aqueous dispersions of TCNQ-anion-stabilized graphene sheets. Chem. Commun. 2008, 48, 6576–6578. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S.; Kuila, T.; Kim, N.H.; Khanra, P.; Lee, J.H. Effects of covalent surface modifications on the electrical and electrochemical properties of graphene using sodium 4-aminoazobenzene-4′-sulfonate. Carbon 2013, 54, 310–322. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. A green approach for the reduction of graphene oxide by wild carrot root. Carbon 2012, 50, 914–921. [Google Scholar] [CrossRef]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline crosslinked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, G.S.; Yu, Z.Z.; Qi, J.S. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Yan, X.B.; Chen, J.T.; Yang, J.; Xue, Q.J.; Miele, P. Fabrication of free-standing, electrochemically active, and biocompatible graphene oxide–polyaniline and graphene–polyaniline hybrid papers. ACS. Appl. Mater. Interfaces 2010, 2, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.Q.; Wu, N.Q.; Nguyen, S.B.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Sharma, L.; Matsuoka, T.; Kimura, T.; Matsuda, H. Investigation into the surface relief grating mechanism via XPS in new azobenzene based optical material. Polym. Adv. Technol. 2002, 13, 481–486. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Liu, H.P.; Luo, W.; Liu, E.Z.; Zhao, N.Q.; Yoshino, K.; Feng, W. Covalent functionalization of graphene by azobenzene with molecular hydrogen bonds for long-term solar thermal storage. Sci. Rep. 2013, 3, 3260. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Khanra, P.; Kim, N.H.; Choi, S.K.; Yun, H.J.; Lee, J.H. One-step electrochemical synthesis of 6-amino-4-hydroxy-2-napthalene-sulfonic acid functionalized graphene for green energy storage electrode materials. Nanotechnology 2013, 24, 365706. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.Y.; He, P.Y.; Yi, L.H.; Liu, Z.L.; Yi, X. Influence of borohydride concentration on the synthesized Au/graphene nanocomposites for direct borohydride fuel cell. J. Solid State Electrochem. 2012, 16, 3929–3937. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.H.; Ruoff, R.S.; Lee, H.Y. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73–78. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Baby, T.T.; Ramaprabhu, S. Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J. Mater. Chem. 2010, 20, 8467–8469. [Google Scholar] [CrossRef]
- Pouget, J.P.; Józefowicz, M.E.; Epstein, A.J.; Tang, X.; MacDiarmid, A.G. X-ray structure of polyaniline. Macromolecules 1991, 24, 779–789. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhao, X.S. Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J. Phys. Chem. C 2012, 116, 5420–5426. [Google Scholar] [CrossRef]
- Zhu, J.H.; Chen, M.J.; Qu, H.L.; Zhang, X.; Wei, H.G.; Luo, Z.P.; Colorado, H.A.; Wei, S.Y.; Guo, Z.H. Interfacial polymerized polyaniline/graphite oxide nanocomposites toward electrochemical energy storage. Polymer 2012, 53, 5953–5964. [Google Scholar] [CrossRef]
- Haldorai, Y.; Nguyen, V.H.; Shim, J.J. Synthesis of polyaniline/Q-CdSe composite via ultrasonically assisted dynamic inverse emulsion polymerization. Colloid Polym. Sci. 2011, 289, 849–854. [Google Scholar] [CrossRef]
- Wang, H.L.; Hao, Q.L.; Yang, X.J.; Lu, L.D.; Wang, X. Effect of graphene oxide on the properties of its composite with polyaniline. ACS Appl. Mater. Interfaces 2010, 2, 821–828. [Google Scholar] [CrossRef]
- Guan, T.T.; Zhao, J.H.; Zhang, G.L.; Wang, J.L.; Zhang, D.D.; Li, K.X. Template-free synthesis of honeycomblike porous carbon rich in specific 2–5 nm mesopores from a pitch-based polymer for a high-performance supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 2116–2126. [Google Scholar] [CrossRef]
- Zhang, D.C.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.H.; Ma, Y.W. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Li, Y.N.; Xing, R.G.; Zhang, B.W.; Bulin, C. Fluoro-functionalized graphene oxide/polyaniline composite electrode material for supercapacitors. Polym. Polym. Compos. 2019, 27, 76–81. [Google Scholar] [CrossRef]










| Sample | Current Density (A g−1) | Capacitance (F g−1) | Electrolyte | Refs |
|---|---|---|---|---|
| ADS-G/PANI | 1 | 528 | 6M KOH | This work |
| PANI-doped graphene | 0.1 | 480 | 2M H2SO4 | [21] |
| Graphene-PANI | 0.4 | 475 | 1M H2SO4 | [30] |
| Honeycomblike mesoporous carbons (HPCs) | 1 | 339 | 6M KOH | [45] |
| PPy/GNS | 0.5 | 482 | 1M H2SO4 | [46] |
| Fluoro-functionalized graphene oxide (GOF)/polyaniline (PANI) | 1 | 502 | 1M H2SO4 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Li, J.; Jia, T.; Dong, B.; Han, Z.; Tian, W.; Jiang, R.; Lu, X.; Li, L. Electrochemical Synthesis of Functionalized Graphene/Polyaniline Composite Using Two Electrode Configuration for Supercapacitors. Nanomaterials 2023, 13, 3140. https://doi.org/10.3390/nano13243140
Yu D, Li J, Jia T, Dong B, Han Z, Tian W, Jiang R, Lu X, Li L. Electrochemical Synthesis of Functionalized Graphene/Polyaniline Composite Using Two Electrode Configuration for Supercapacitors. Nanomaterials. 2023; 13(24):3140. https://doi.org/10.3390/nano13243140
Chicago/Turabian StyleYu, Dongsheng, Jili Li, Tiekun Jia, Binbin Dong, Zhixiao Han, Wenjie Tian, Ruilin Jiang, Xi Lu, and Lekang Li. 2023. "Electrochemical Synthesis of Functionalized Graphene/Polyaniline Composite Using Two Electrode Configuration for Supercapacitors" Nanomaterials 13, no. 24: 3140. https://doi.org/10.3390/nano13243140
APA StyleYu, D., Li, J., Jia, T., Dong, B., Han, Z., Tian, W., Jiang, R., Lu, X., & Li, L. (2023). Electrochemical Synthesis of Functionalized Graphene/Polyaniline Composite Using Two Electrode Configuration for Supercapacitors. Nanomaterials, 13(24), 3140. https://doi.org/10.3390/nano13243140






