Influence of Isothermal Annealing on Microstructure, Morphology and Oxidation Behavior of AlTiSiN/TiSiN Nanocomposite Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
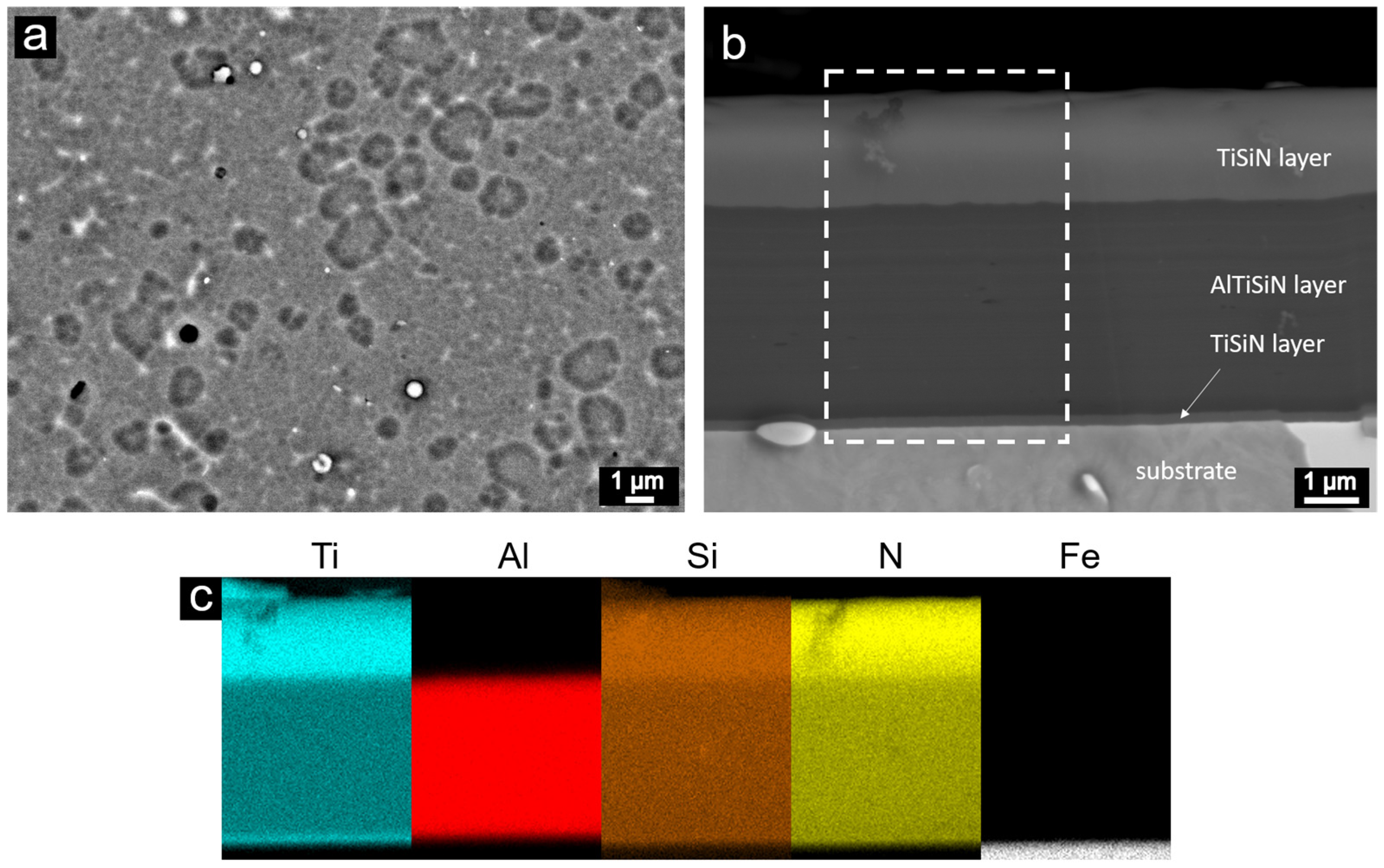
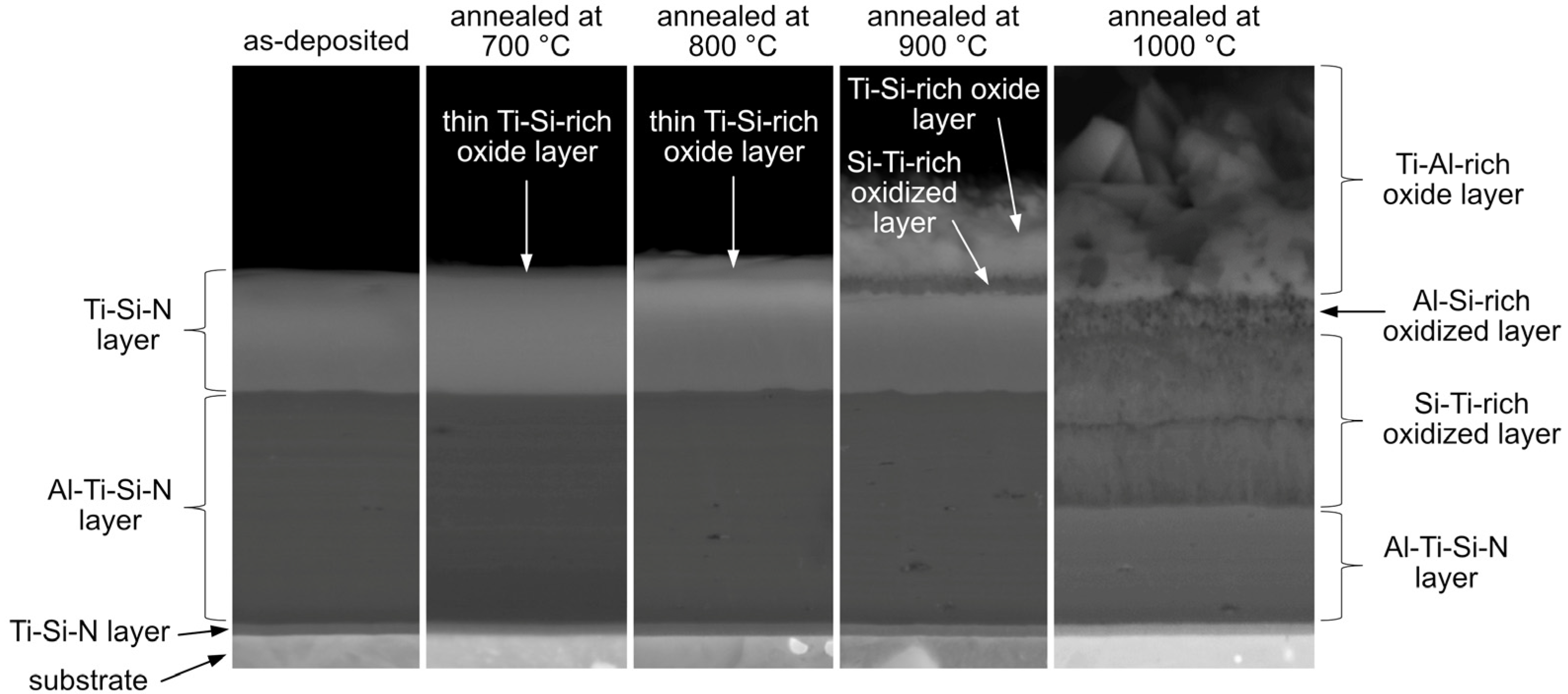
- The AlTiSiN/TiSiN nanocomposite coatings were prepared by PVD. The coatings consisted of three layers: the TiSiN adhesive layer with the thickness of about 50 nm, the middle AlTiSiN layer (∼3 μm) and the top TiSiN layer (~1.5 μm).
- The transition from the AlTiSiN layer to the TiSiN layer had a sharp boundary. At the boundary between the layers, fine grains were formed in the direction of layer growth. A detailed view of the individual layers confirmed the presence of nanocrystals in the amorphous matrix. It can be assumed that nanocrystalline particles were either TiN (in the TiSiN layer mainly) or (Ti,Al)N (in the AlTiSiN layer).
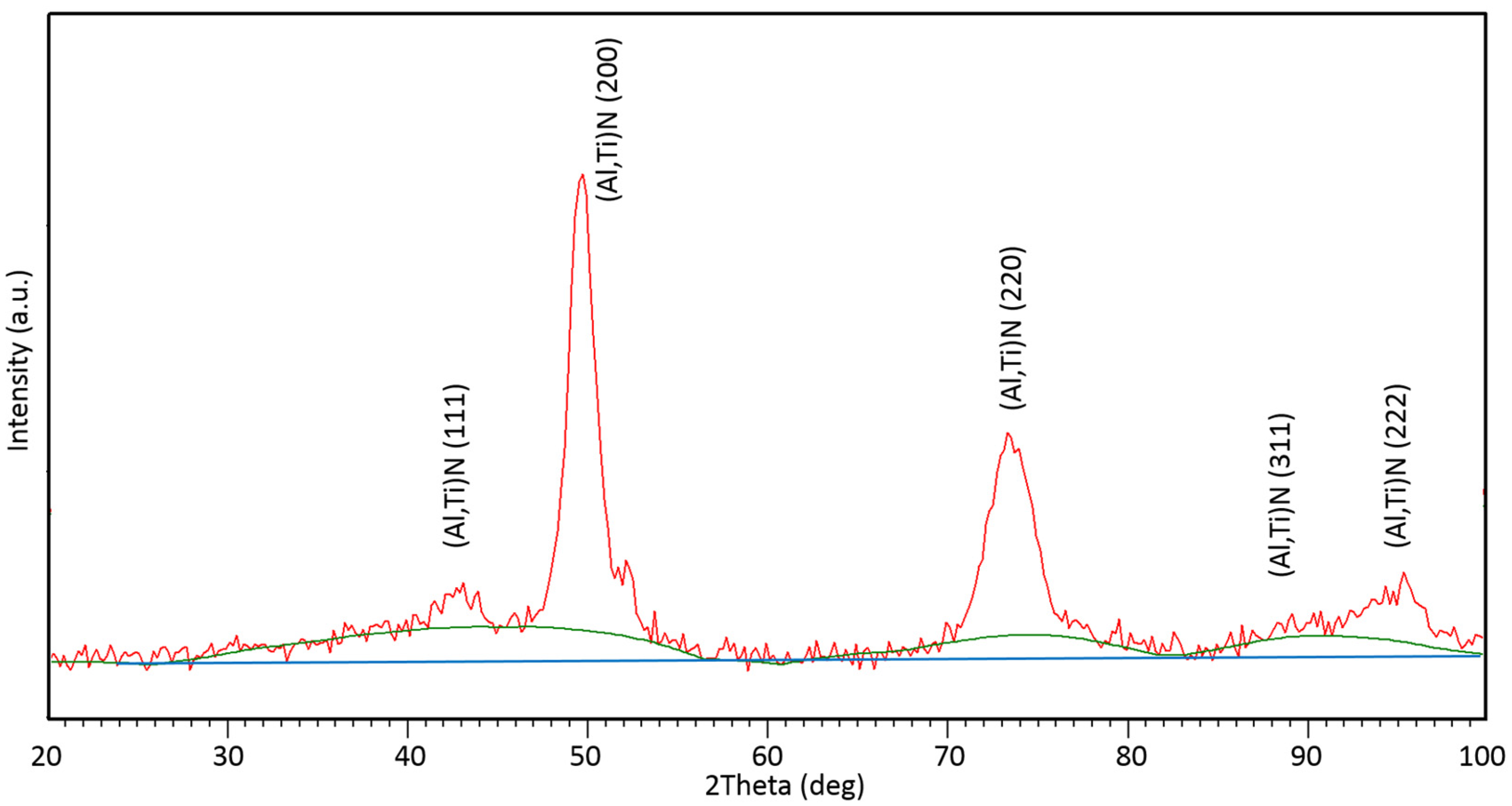
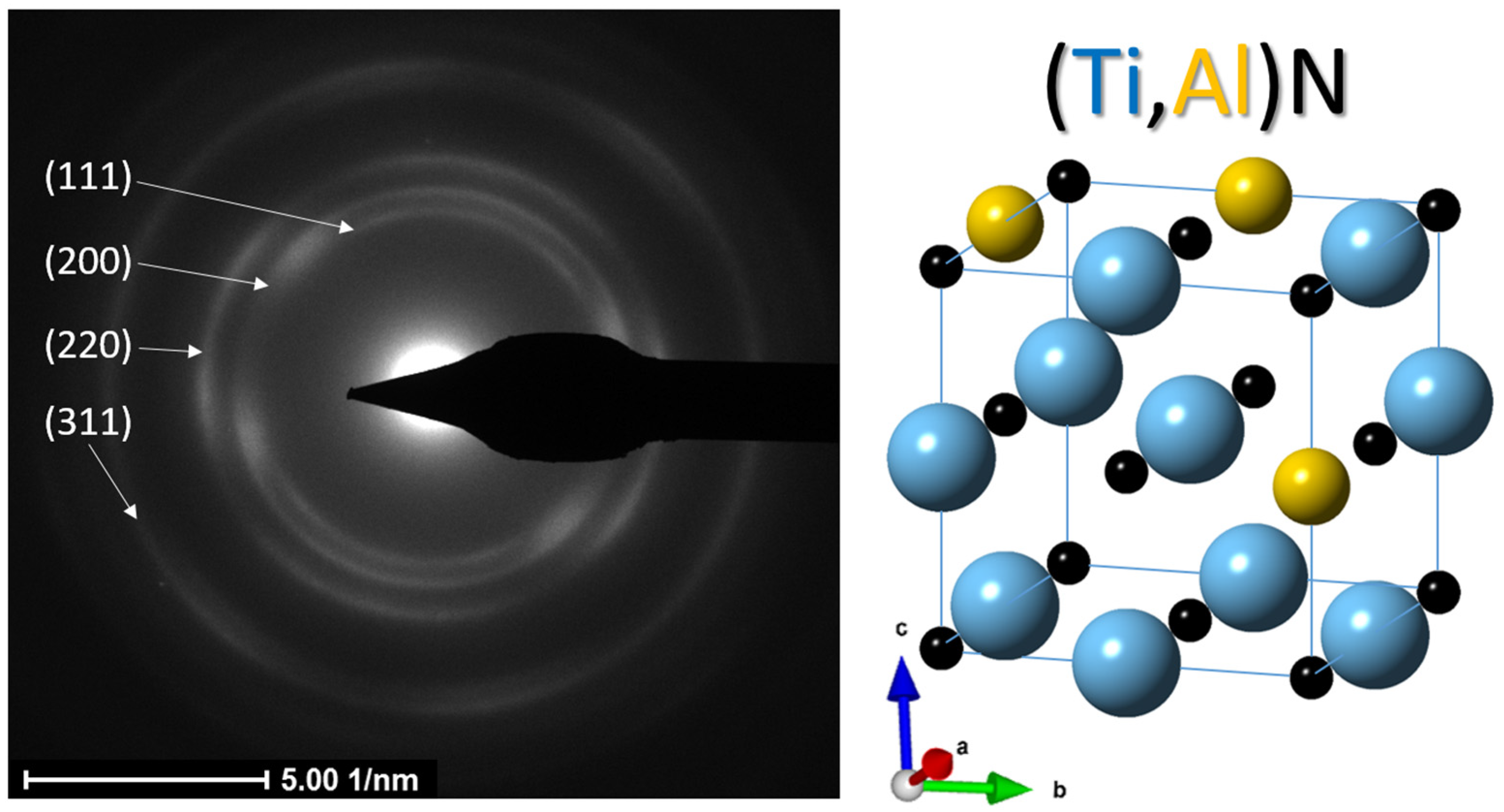
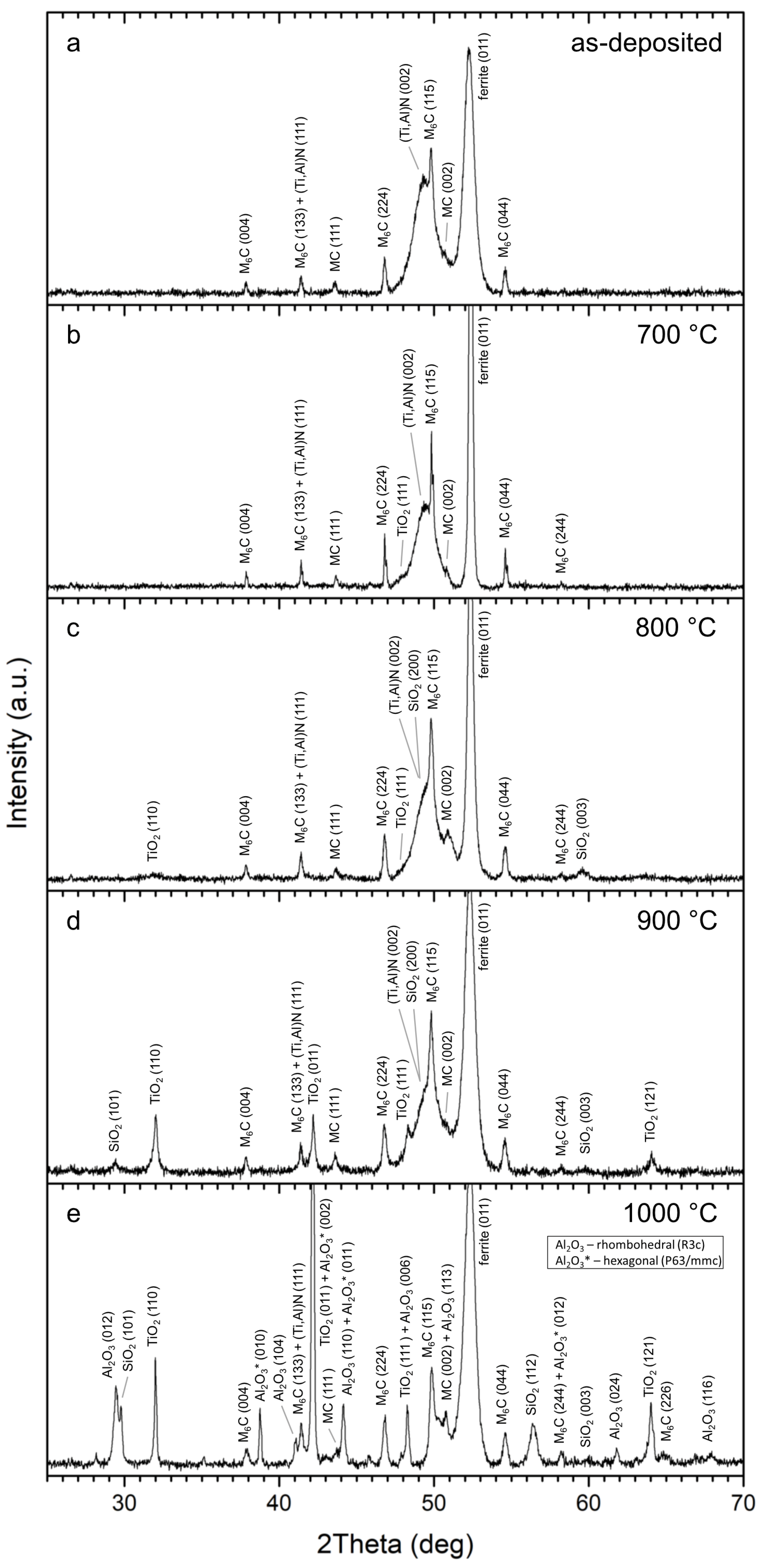
- Based on the experimental results from the TEM and XRD analyses, crystallographic data and structural parameters were obtained for the Ti0.8Al0.2N compound identified in the as-deposited coating.
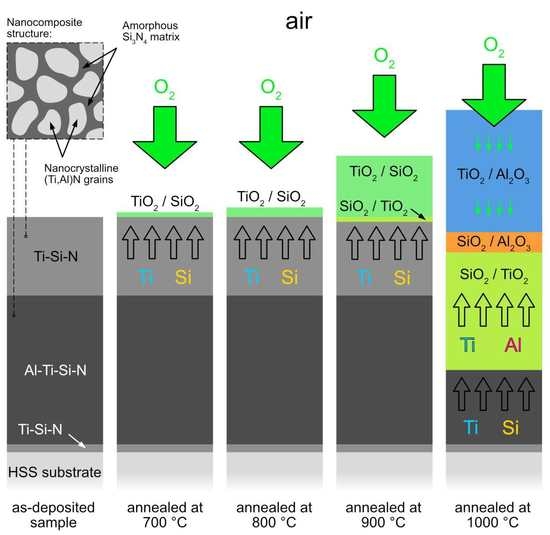
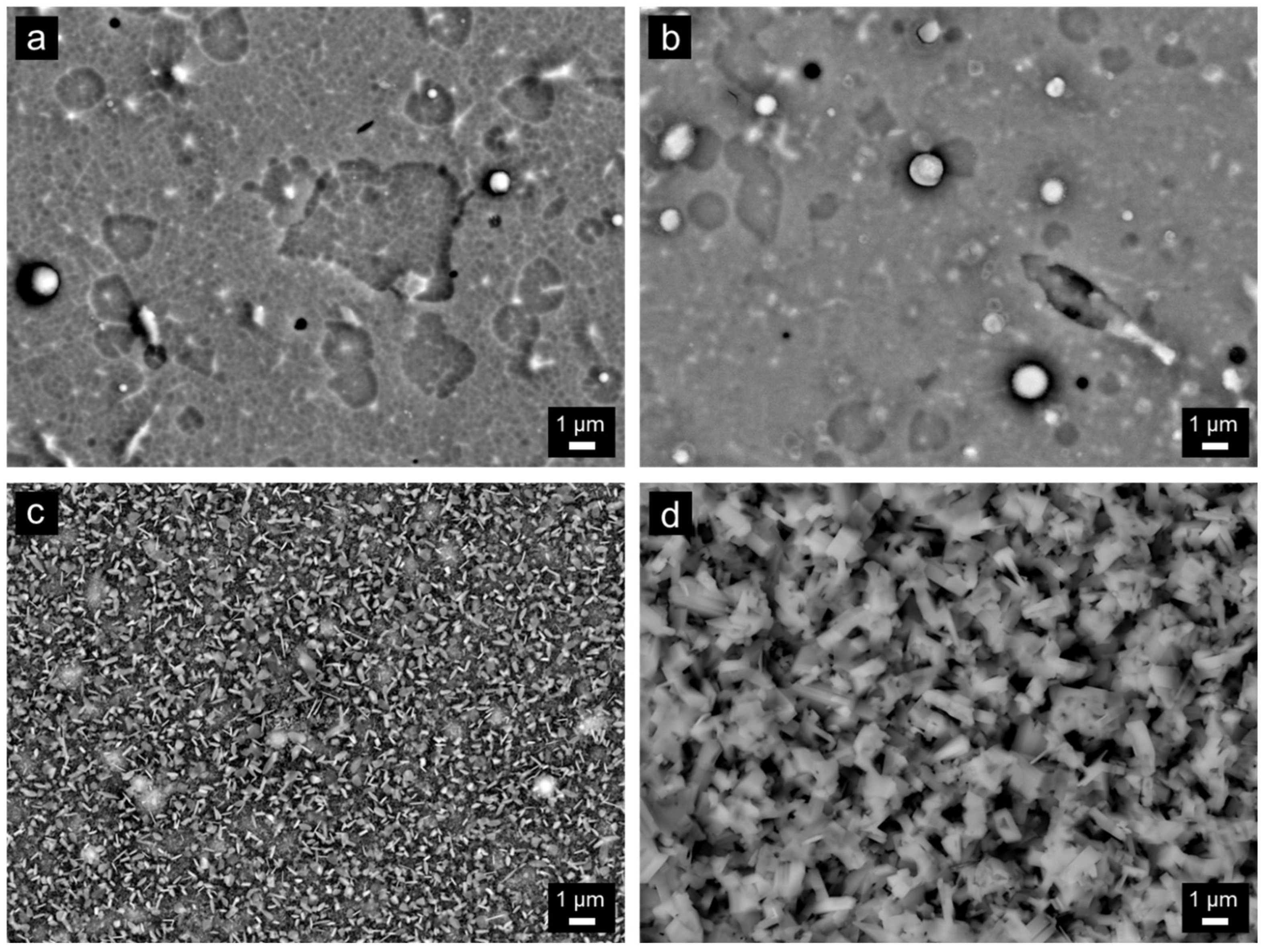
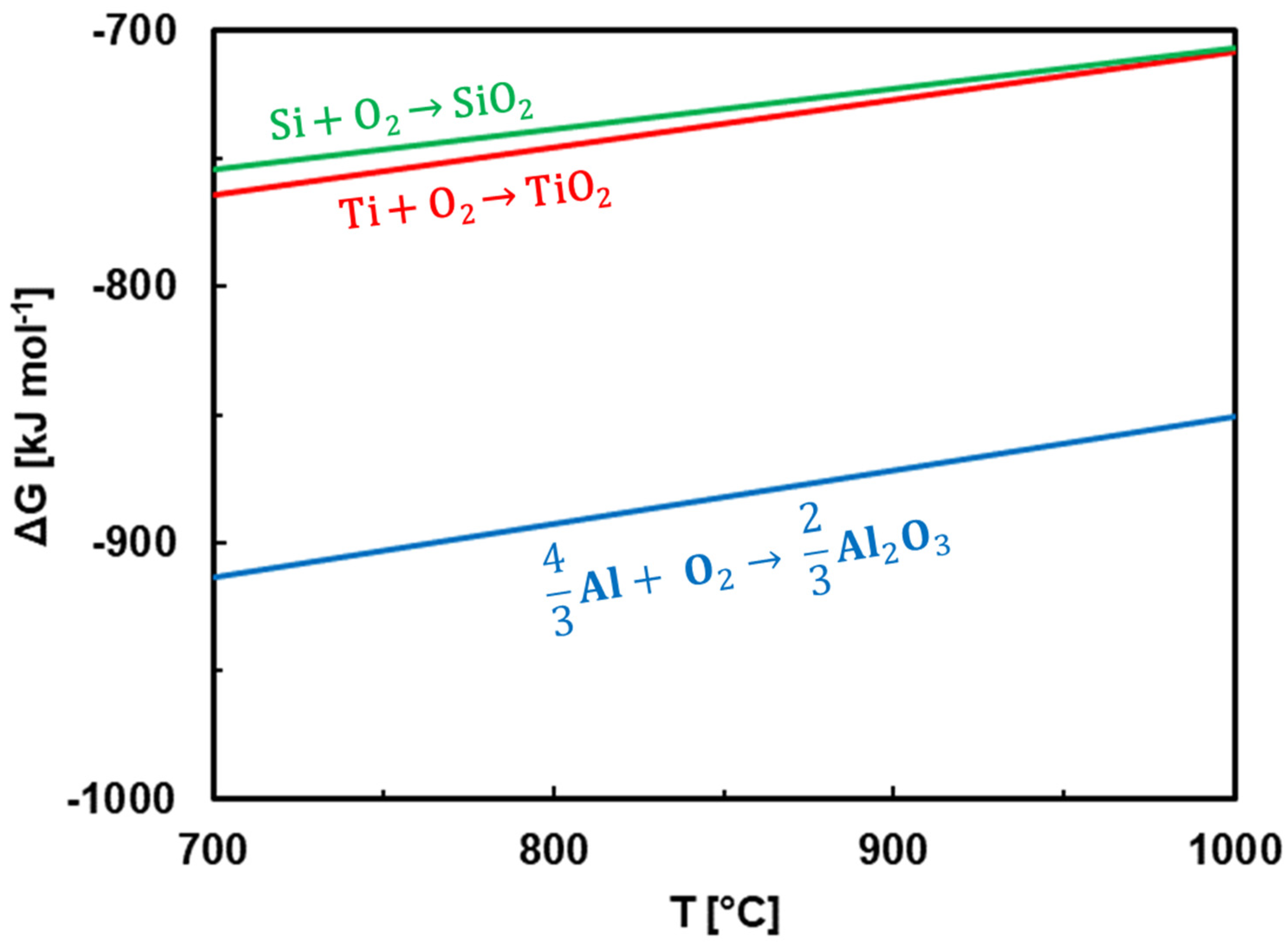
- The coatings were oxidized at 700–1000 °C in air. TiO2 and SiO2 were found at all annealing temperatures. At 1000 °C, Al2O3 was additionally identified. A significant change in surface morphology was observed in the samples annealed at 900 and 1000 °C, where the whole surface was covered with a thick oxide layer with blade-like and needle-like morphology. A network-like structure, on the contrary, was observed at the lower annealing temperature (700 °C), similar to the as-deposited sample.
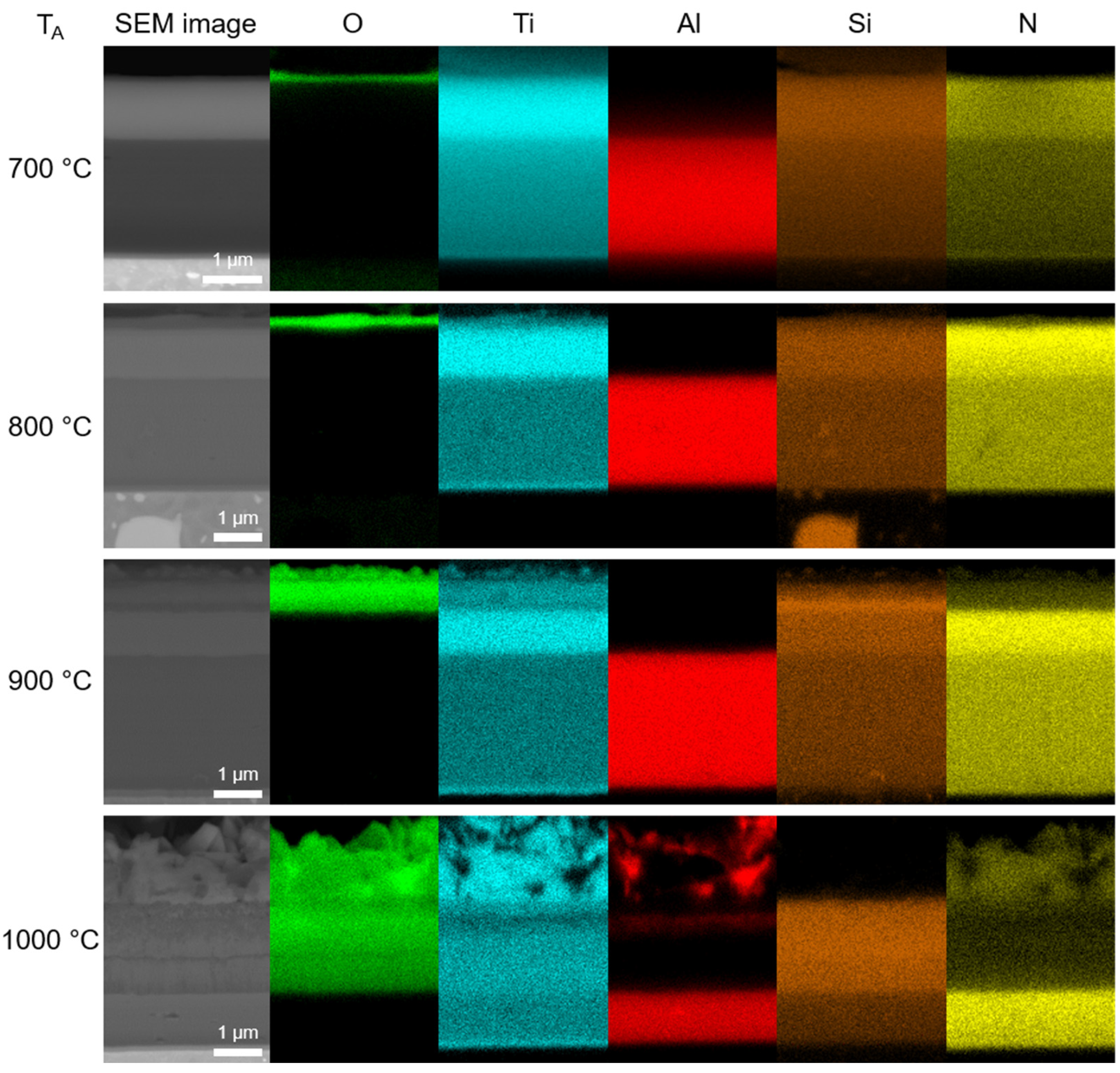
- At 700 and 800 °C, the Ti-Si-rich surface oxide layer was formed. At 900 and 1000 °C, an oxidized part of the coating was observed in addition to the surface oxide layer. It was found that Al diffused from the AlTiSiN layer to the surface of the coating, where it reacted with oxygen and formed Al2O3.
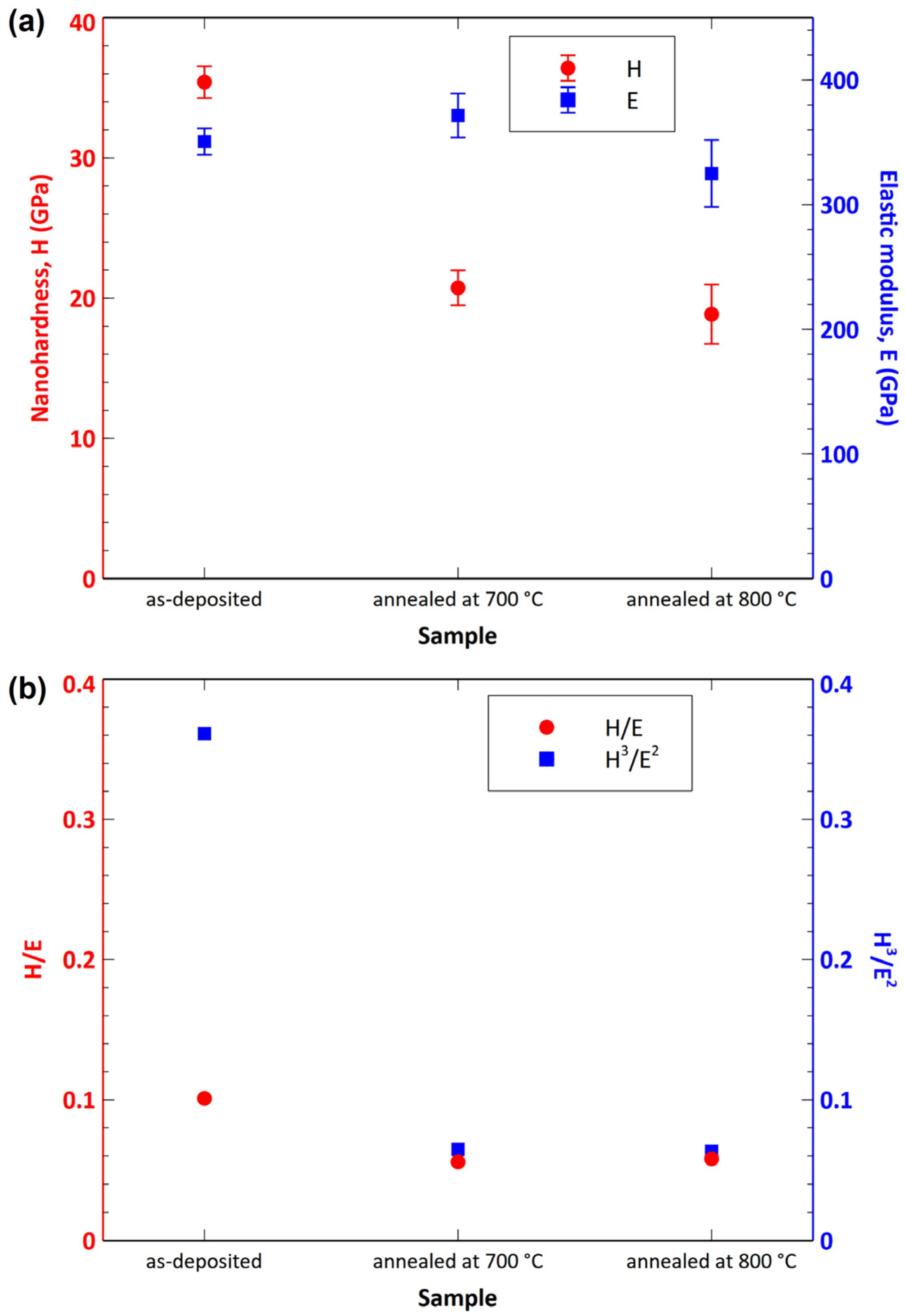
- The samples oxidized at 700 and 800 °C exhibited considerably worse mechanical properties compared to the as-deposited sample. The samples oxidized at 900 and 1000 °C were significantly degraded by oxidation. As such, it was not possible to measure their nanohardness accurately.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Çalışkan, H.; Panjan, P.; Kurbanoğlu, C. Hard Coatings on Cutting Tools and Surface Finish. In Comprehensive Materials Finishing; Elsevier: Amsterdam, The Netherlands, 2017; pp. 230–242. ISBN 978-0-12-803249-7. [Google Scholar]
- Li, C.; Wang, L.; Shang, L.; Cao, X.; Zhang, G.; Yu, Y.; Li, W.; Zhang, S.; Hu, H. Mechanical and High-Temperature Tribological Properties of CrAlN/TiSiN Multilayer Coating Deposited by PVD. Ceram. Int. 2021, 47, 29285–29294. [Google Scholar] [CrossRef]
- Strnad, G.; Buhagiar, J. Latest Developments in PVD Coatings for Tooling. Sci. Bull. Petru Maior Univ. Targu Mures 2010, 7, 32–37. [Google Scholar]
- He, C.; Zhang, J.; Song, G.; Ma, G.; Du, Z.; Wang, J.; Zhao, D. Microstructure and Mechanical Properties of Reactive Sputtered Nanocrystalline (Ti,Al)N Films. Thin Solid Films 2015, 584, 192–197. [Google Scholar] [CrossRef]
- Chauhan, K.V.; Rawal, S.K. A Review Paper on Tribological and Mechanical Properties of Ternary Nitride Based Coatings. Procedia Technol. 2014, 14, 430–437. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.X.; Du, Y.; Liu, Y. Effect of bilayer period on structure, mechanical and thermal properties of TiAlN/AlTiN multilayer coatings. Thin Solid Films 2019, 592A, 207–214. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wang, S.Q.; Wang, A.J.; Xu, H.H. Mechanical Properties and Microstructural Evolution of TiN Coatings Alloyed with Al and Si. Mater. Sci. Eng. A 2009, 502, 139–143. [Google Scholar] [CrossRef]
- Vepřek, S.; Vepřek-Heijman, M.J.G. Industrial Applications of Superhard Nanocomposite Coatings. Surf. Coat. Technol. 2008, 202, 5063–5073. [Google Scholar] [CrossRef]
- Chang, C.-L.; Chen, W.-C.; Tsai, P.-C.; Ho, W.-Y.; Wang, D.-Y. Characteristics and Performance of TiSiN/TiAlN Multilayers Coating Synthesized by Cathodic Arc Plasma Evaporation. Surf. Coat. Technol. 2007, 202, 987–992. [Google Scholar] [CrossRef]
- Quiñones-Galván, J.G.; Camps, E.; Muhl, S.; Flores, M.; Campos-González, E. Influence of Plasma Density on the Chemical Composition and Structural Properties of Pulsed Laser Deposited TiAlN Thin Films. Phys. Plasmas 2014, 21, 053509. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.d.; Wan, Q.; Cai, Y.; Chen, H.; Chen, Y.m.; Guo, J.l.; Yang, B. High-Temperature Oxidation Behaviour of AlTiSiN and AlCrSiN Coatings. Surf. Eng. 2019, 35, 652–660. [Google Scholar] [CrossRef]
- Chen, L.; Paulitsch, J.; Du, Y.; Mayrhofer, P.H. Thermal Stability and Oxidation Resistance of Ti–Al–N Coatings. Surf. Coat. Technol. 2012, 206, 2954–2960. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Cai, M.-C. Mechanical Property and Tribological Performance of AlTiSiN and AlTiBN Hard Coatings Using Ternary Alloy Targets. Surf. Coat. Technol. 2019, 374, 1120–1127. [Google Scholar] [CrossRef]
- Mei, F.; Sui, G.Z.; Gong, M.F. Residual Stress Analysis in Different Thickness TiN Coatings on High-Speed-Steel Substrates. Adv. Mater. Res. 2011, 239–242, 2331–2335. [Google Scholar] [CrossRef]
- Lyashenko, B.A.; Rutkovskii, A.V.; Soroka, E.B.; Lipinskaya, N.V. On the Reduction of Residual Stresses in Plasma-Vacuum-Deposited Coatings. Strength Mater. 2001, 33, 344–348. [Google Scholar] [CrossRef]
- Carvalho, N.J.M.; Zoestbergen, E.; Kooi, B.J.; De Hosson, J.T.M. Stress analysis and microstructure of PVD monolayer TiN and multilayer TiN/(Ti,Al)N coatings. Thin Solid Films 2003, 429, 179–189. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Single Layer and Multilayer Wear Resistant Coatings of (Ti,Al)N: A Review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Lai, H.-M. Wear Behavior and Cutting Performance of CrAlSiN and TiAlSiN Hard Coatings on Cemented Carbide Cutting Tools for Ti Alloys. Surf. Coat. Technol. 2014, 259, 152–158. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M.; Karimi, A.; Coddet, O.; Cselle, T.; Morstein, M. Characterization of Thermally Treated TiAlSiN Coatings by TEM and Nanoindentation. Surf. Coat. Technol. 2004, 188–189, 344–350. [Google Scholar] [CrossRef]
- Moritz, Y.; Saringer, C.; Tkadletz, M.; Stark, A.; Schell, N.; Letofsky-Papst, I.; Czettl, C.; Pohler, M.; Schalk, N. Oxidation Behavior of Arc Evaporated TiSiN Coatings Investigated by In-Situ Synchrotron X-Ray Diffraction and HR-STEM. Surf. Coat. Technol. 2020, 404, 126632. [Google Scholar] [CrossRef]
- Xiao, B.; Zhang, T.F.; Guo, Z.; Li, Z.; Fan, B.; Chen, G.; Xiong, Z.; Wang, Q. Mechanical, Oxidation, and Cutting Properties of AlCrN/AlTiSiN Nano-Multilayer Coatings. Surf. Coat. Technol. 2022, 433, 128094. [Google Scholar] [CrossRef]
- Diserens, M.; Patscheider, J.; Lévy, F. Mechanical Properties and Oxidation Resistance of Nanocomposite TiN–SiNx Physical-Vapor-Deposited Thin Films. Surf. Coat. Technol. 1999, 120–121, 158–165. [Google Scholar] [CrossRef]
- Vepřek, S.; Reiprich, S.; Shizhi, L. Superhard Nanocrystalline Composite Materials: The TiN/Si3N4 System. Appl. Phys. Lett. 1995, 66, 2640–2642. [Google Scholar] [CrossRef]
- Vepřek, S. Design of Novel Nanocrystalline Composite Materials by Means of Plasma CVD. Pure Appl. Chem. 1996, 68, 1023–1027. [Google Scholar] [CrossRef]
- Babincová, P.; Sahul, M.; Drobný, P.; Čaplovič, Ľ. Morphological Changes of the PVD Coatings after Isothermal Annealing. IOP Conf. Ser. Mater. Sci. Eng. 2020, 726, 012001. [Google Scholar] [CrossRef]
- Musil, J. Hard Nanocomposite Coatings: Thermal Stability, Oxidation Resistance and Toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Greczynski, G.; Bakhit, B.; Hultman, L.; Odén, M. High Si Content TiSiN Films with Superior Oxidation Resistance. Surf. Coat. Technol. 2020, 398, 126087. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Yang, S.-M. High temperature oxidation behavior of multicomponent TiAlSiN coatings. Thin Solid Films 2010, 518, S34–S37. [Google Scholar] [CrossRef]
- Chen, Q.; Sundman, B. Thermodynamic Assessment of the Ti-Al-N System. J. Phase Equilibria 1998, 19, 146–160. [Google Scholar] [CrossRef]
- Barshilia, H.C.; Deepthi, B.; Rajam, K.S. Deposition and Characterization of TiAlN/Si3N4 Superhard Nanocomposite Coatings Prepared by Reactive Direct Current Unbalanced Magnetron Sputtering. Vacuum 2006, 81, 479–488. [Google Scholar] [CrossRef]
- Qadir, A.; Pinke, P.; Dusza, J. Silicon Nitride-Based Composites with the Addition of CNTs—A Review of Recent Progress, Challenges, and Future Prospects. Materials 2020, 13, 2799. [Google Scholar] [CrossRef]
- Parlinska-Wojtan, M. Oxygen Diffusion in Columnar TiAlSiN Coatings Investigated by Electron Microscopy. Thin Solid Films 2016, 616, 437–443. [Google Scholar] [CrossRef]
- Fuentes, G.G.; Almandoz, E.; Pierrugues, R.; Martínez, R.; Rodríguez, R.J.; Caro, J.; Vilaseca, M. High Temperature Tribological Characterisation of TiAlSiN Coatings Produced by Cathodic Arc Evaporation. Surf. Coat. Technol. 2010, 205, 1368–1373. [Google Scholar] [CrossRef]
- Pei, F.; Liu, H.J.; Chen, L.; Xu, Y.X.; Du, Y. Improved Properties of TiAlN Coating by Combined Si-Addition and Multilayer Architecture. J. Alloys Compd. 2019, 790, 909–916. [Google Scholar] [CrossRef]
- Chen, M.; Cai, F.; Chen, W.; Wang, Q.; Zhang, S. Influence of Vacuum Annealing on Structures and Properties of AlTiSiN Coatings with Corrosion Resistance. Surf. Coat. Technol. 2017, 312, 25–31. [Google Scholar] [CrossRef]
- Chen, T.; Xie, Z.; Gong, F.; Luo, Z.; Yang, Z. Correlation between Microstructure Evolution and High Temperature Properties of TiAlSiN Hard Coatings with Different Si and Al Content. Appl. Surf. Sci. 2014, 314, 735–745. [Google Scholar] [CrossRef]
- Wu, J.; He, N.; Li, H.; Liu, X.; Ji, L.; Huang, X.; Chen, J. Deposition and Characterization of TiAlSiN Coatings Prepared by Hybrid PVD Coating System. Surf. Interface Anal. 2015, 47, 184–191. [Google Scholar] [CrossRef]
- Ardila-Téllez, L.C.; Sánchez-Moreno, J.M.; Moreno-Téllez, C.M. Effect of Silicon Addition on Microstructure and Mechanical Properties of Chromium and Titanium Based Coatings. Rev. Fac. Ing. 2014, 23, 9–21. [Google Scholar] [CrossRef]
- Mendez, A.; Monclus, M.A.; Santiago, J.A.; Fernandez-Martinez, I.; Rojas, T.C.; Garcia-Molleja, J.; Avella, M.; Dams, N.; Panizo-Laiz, M.; Molina-Aldareguia, J.M. Effect of Al Content on the Hardness and Thermal Stability Study of AlTiN and AlTiBN Coatings Deposited by HiPIMS. Surf. Coat. Technol. 2021, 422, 127513. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Xiao, L.; Zhang, E.; Chen, Q.; Li, W. Effects of Boron Content on Microstructure and Mechanical Properties of TiAlSiBxN Nanocomposite Films. J. Mater. Eng. Perform. 2020, 29, 2731–2736. [Google Scholar] [CrossRef]
- Chim, Y.C.; Ding, X.Z.; Zeng, X.T.; Zhang, S. Oxidation Resistance of TiN, CrN, TiAlN and CrAlN Coatings Deposited by Lateral Rotating Cathode Arc. Thin Solid Films 2009, 517, 4845–4849. [Google Scholar] [CrossRef]
- Vennemann, A.; Stock, H.-R.; Kohlscheen, J.; Rambadt, S.; Erkens, G. Oxidation Resistance of Titanium–Aluminium–Silicon Nitride Coatings. Surf. Coat. Technol. 2003, 174–175, 408–415. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzig, C. Diffusion in the Ti–Al System. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]
- Xu, W.W.; Shang, S.L.; Zhou, B.C.; Wang, Y.; Chen, L.J.; Wang, C.P.; Liu, X.J.; Liu, Z.K. A First-Principles Study of the Diffusion Coefficients of Alloying Elements in Dilute α-Ti Alloys. Phys. Chem. Chem. Phys. 2016, 18, 16870–16881. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Feng, C.; Zhu, S.; Wang, F.; Yuan, J.; Wang, P. Comparison of CrN, AlN and TiN Diffusion Barriers on the Interdiffusion and Oxidation Behaviors of Ni+CrAlYSiN Nanocomposite Coatings. Crystals 2021, 11, 1333. [Google Scholar] [CrossRef]
- Jeffes, J.H.E. Ellingham Diagrams. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 2751–2753. ISBN 978-0-08-043152-9. [Google Scholar]
- Zheng, J.; Hou, X.; Wang, X.; Meng, Y.; Zheng, X.; Zheng, L. Isothermal Oxidation Mechanism of a Newly Developed Nb–Ti–V–Cr–Al–W–Mo–Hf Alloy at 800–1200 °C. Int. J. Refract. Met. Hard Mater. 2016, 54, 322–329. [Google Scholar] [CrossRef]
- Li, J.; He, X.; Xu, B.; Tang, Z.; Fang, C.; Yang, G. Effect of Silicon on Dynamic/Static Corrosion Resistance of T91 in Lead–Bismuth Eutectic at 550 °C. Materials 2022, 15, 2862. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.H.; Lee, D.B. Oxidation of Nano-Multilayered TiAlSiN Films at 800–900 °C in Air. Oxid. Met. 2015, 84, 345–352. [Google Scholar] [CrossRef]
- Tillmann, W.; Dildrop, M. Influence of Si Content on Mechanical and Tribological Properties of TiAlSiN PVD Coatings at Elevated Temperatures. Surf. Coat. Technol. 2017, 321, 448–454. [Google Scholar] [CrossRef]
- Musil, J.; Kunc, F.; Zeman, H.; Poláková, H. Relationships between Hardness, Young’s Modulus and Elastic Recovery in Hard Nanocomposite Coatings. Surf. Coat. Technol. 2002, 154, 304–313. [Google Scholar] [CrossRef]
- Musil, J.; Vlček, J. Magnetron Sputtering of Hard Nanocomposite Coatings and Their Properties. Surf. Coat. Technol. 2001, 142–144, 557–566. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Chung, Y.-W. Commentary on Using H/E and H3/E2 as Proxies for Fracture Toughness of Hard Coatings. Thin Solid Films 2019, 688, 137265. [Google Scholar] [CrossRef]

| Chemical element | C | Mn | Si | Cr | W |
|---|---|---|---|---|---|
| Content (wt. %) | 0.87–0.95 | max 0.40 | max 0.45 | 3.80–4.50 | 5.90–6.70 |
| Chemical element | V | Mo | Co | P | S |
| Content (wt. %) | 1.70–2.10 | 4.70–5.20 | 4.50–5.00 | max 0.03 | max 0.03 |
| Crystallographic Data of (Ti,Al)N | ||||
|---|---|---|---|---|
| Pearson Symbol | Space Group | Symmetry | aTEM [nm] | |
| cF8 | 225 | 0.433 | ||
| Structural Parameters of (Ti,Al)N | ||||
| Atom | x | y | z | Site Occupancy |
| Ti | 0.5 | 0.5 | 0.5 | 0.8 |
| Al | 0.5 | 0.5 | 0.5 | 0.2 |
| N | 0 | 0 | 0 | 1 |
| Sample Condition | Chemical Composition [at. %] | ||||
|---|---|---|---|---|---|
| N | O | Al | Si | Ti | |
| as-deposited | 55.9 | - | 13.9 | 7.5 | 22.7 |
| 700 °C | 52.1 | 6.2 | 13.8 | 7.7 | 22.2 |
| 800 °C | 47.4 | 9.8 | 13.5 | 7.9 | 21.4 |
| 900 °C | 38.7 | 22.4 | 12.4 | 7.2 | 19.3 |
| 1000 °C | 7.8 | 62.4 | 8.9 | 5.9 | 15.0 |
| As-Deposited | Annealed at 700 °C | Annealed at 800 °C | |
|---|---|---|---|
| H [GPa] | 35.41 | 20.74 | 18.86 |
| E [GPa] | 350.62 | 371.49 | 324.99 |
| H/E | 0.101 | 0.056 | 0.058 |
| H3/E2 | 0.361 | 0.065 | 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šulhánek, P.; Ďuriška, L.; Palcut, M.; Babincová, P.; Sahul, M.; Čaplovič, Ľ.; Kusý, M.; Orovčík, Ľ.; Nagy, Š.; Satrapinskyy, L.; et al. Influence of Isothermal Annealing on Microstructure, Morphology and Oxidation Behavior of AlTiSiN/TiSiN Nanocomposite Coatings. Nanomaterials 2023, 13, 474. https://doi.org/10.3390/nano13030474
Šulhánek P, Ďuriška L, Palcut M, Babincová P, Sahul M, Čaplovič Ľ, Kusý M, Orovčík Ľ, Nagy Š, Satrapinskyy L, et al. Influence of Isothermal Annealing on Microstructure, Morphology and Oxidation Behavior of AlTiSiN/TiSiN Nanocomposite Coatings. Nanomaterials. 2023; 13(3):474. https://doi.org/10.3390/nano13030474
Chicago/Turabian StyleŠulhánek, Patrik, Libor Ďuriška, Marián Palcut, Paulína Babincová, Martin Sahul, Ľubomír Čaplovič, Martin Kusý, Ľubomír Orovčík, Štefan Nagy, Leonid Satrapinskyy, and et al. 2023. "Influence of Isothermal Annealing on Microstructure, Morphology and Oxidation Behavior of AlTiSiN/TiSiN Nanocomposite Coatings" Nanomaterials 13, no. 3: 474. https://doi.org/10.3390/nano13030474
APA StyleŠulhánek, P., Ďuriška, L., Palcut, M., Babincová, P., Sahul, M., Čaplovič, Ľ., Kusý, M., Orovčík, Ľ., Nagy, Š., Satrapinskyy, L., Haršáni, M., & Černičková, I. (2023). Influence of Isothermal Annealing on Microstructure, Morphology and Oxidation Behavior of AlTiSiN/TiSiN Nanocomposite Coatings. Nanomaterials, 13(3), 474. https://doi.org/10.3390/nano13030474