Controllable Connection of Fe2Se3 Double Chains and Fe(dien)2 Complexes for Organic–Inorganic Hybrid Ferrimagnet with a Large Coercivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Monoclinic (Fe2Se3)2[Fe(dien)2]0.9 Nanorods
2.3. Characterization
3. Results
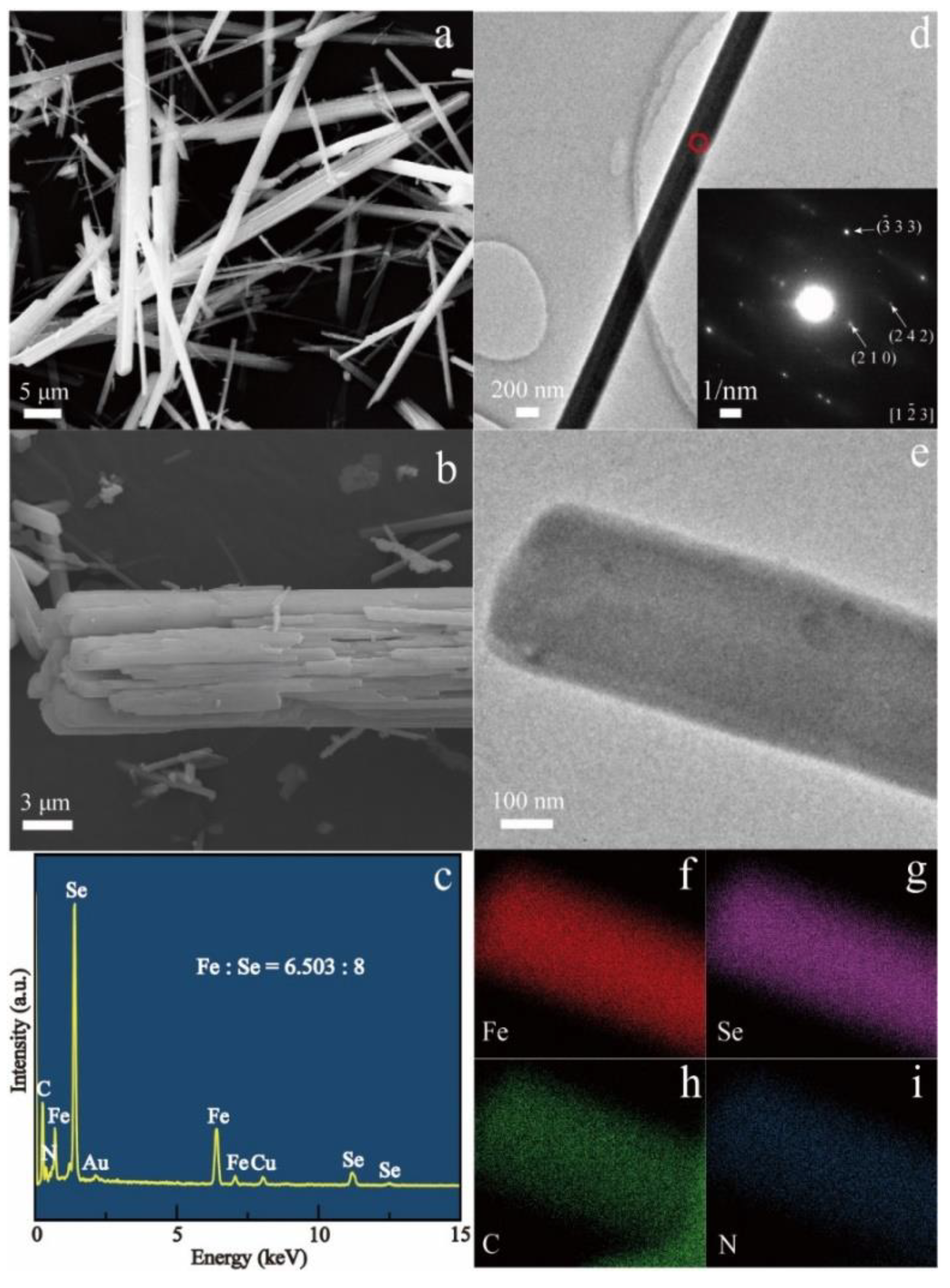
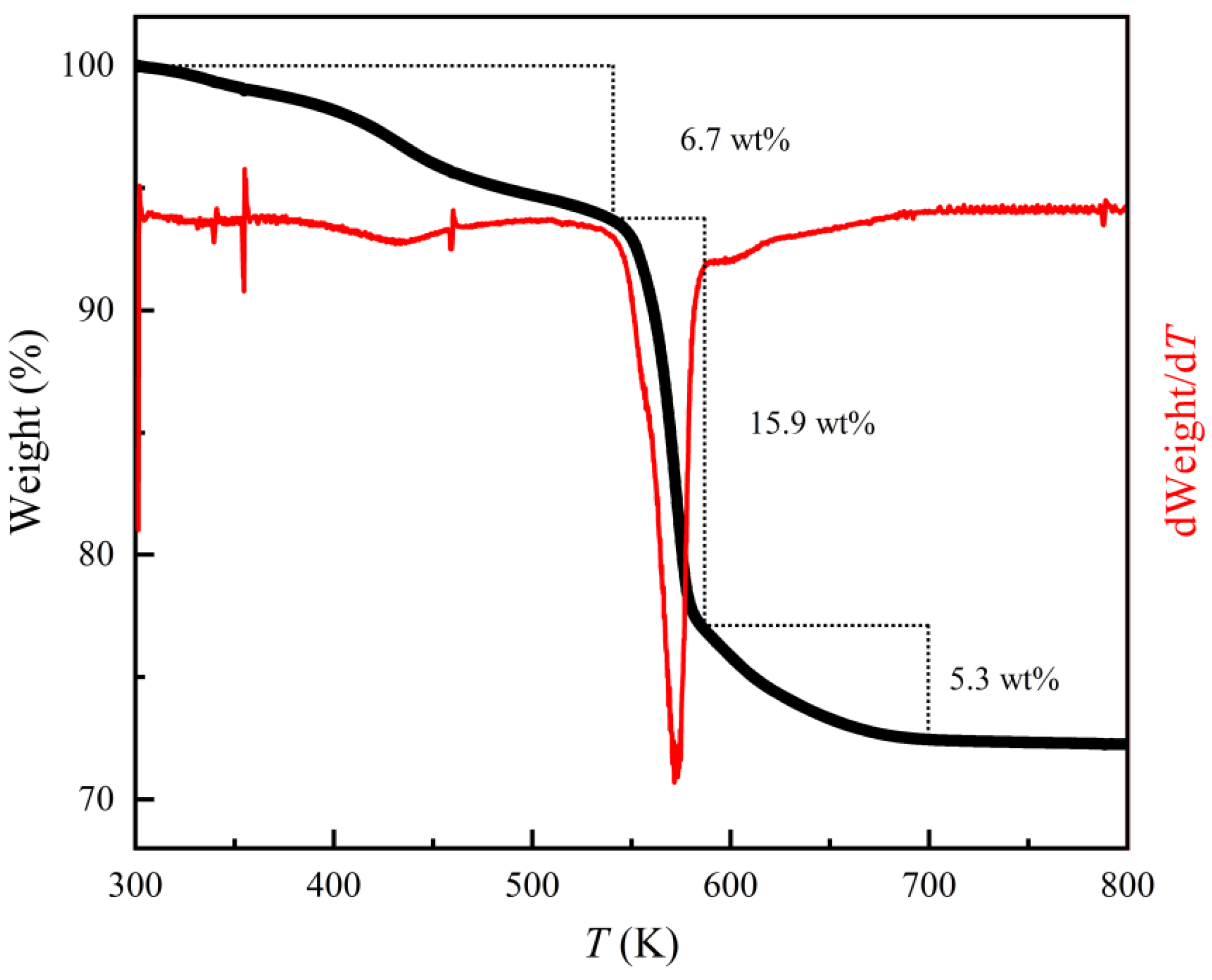
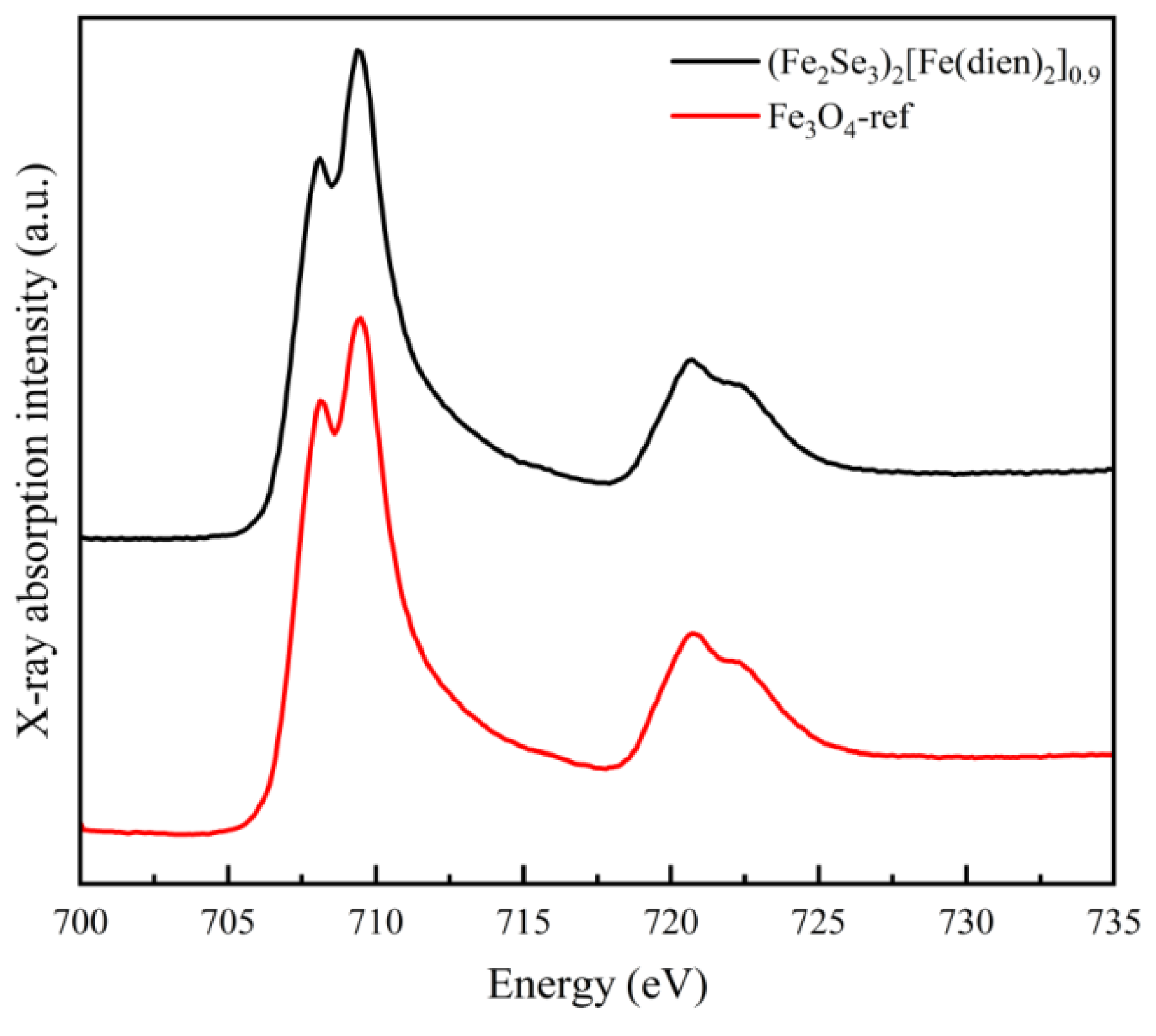
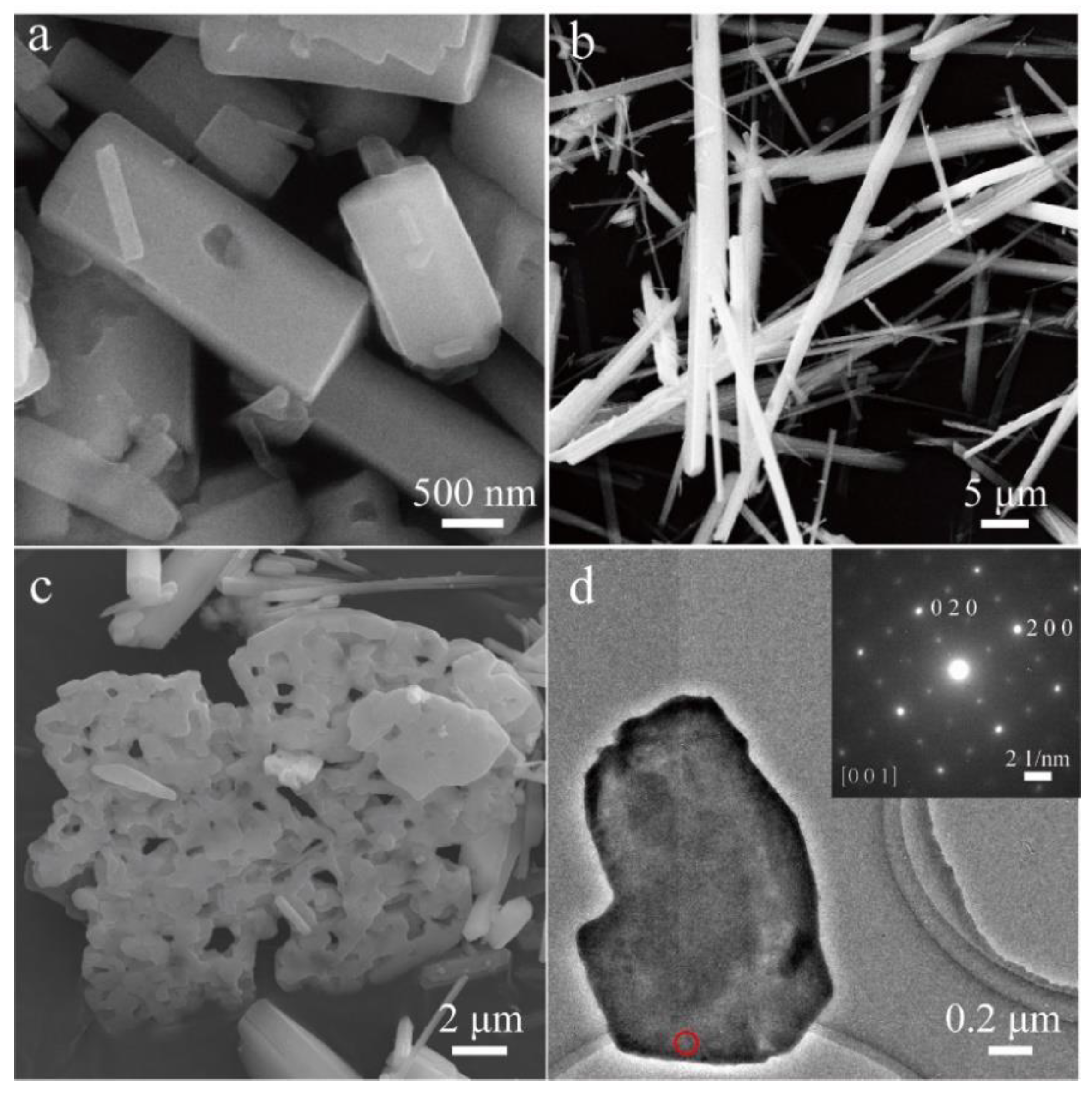
3.1. Structural Properties
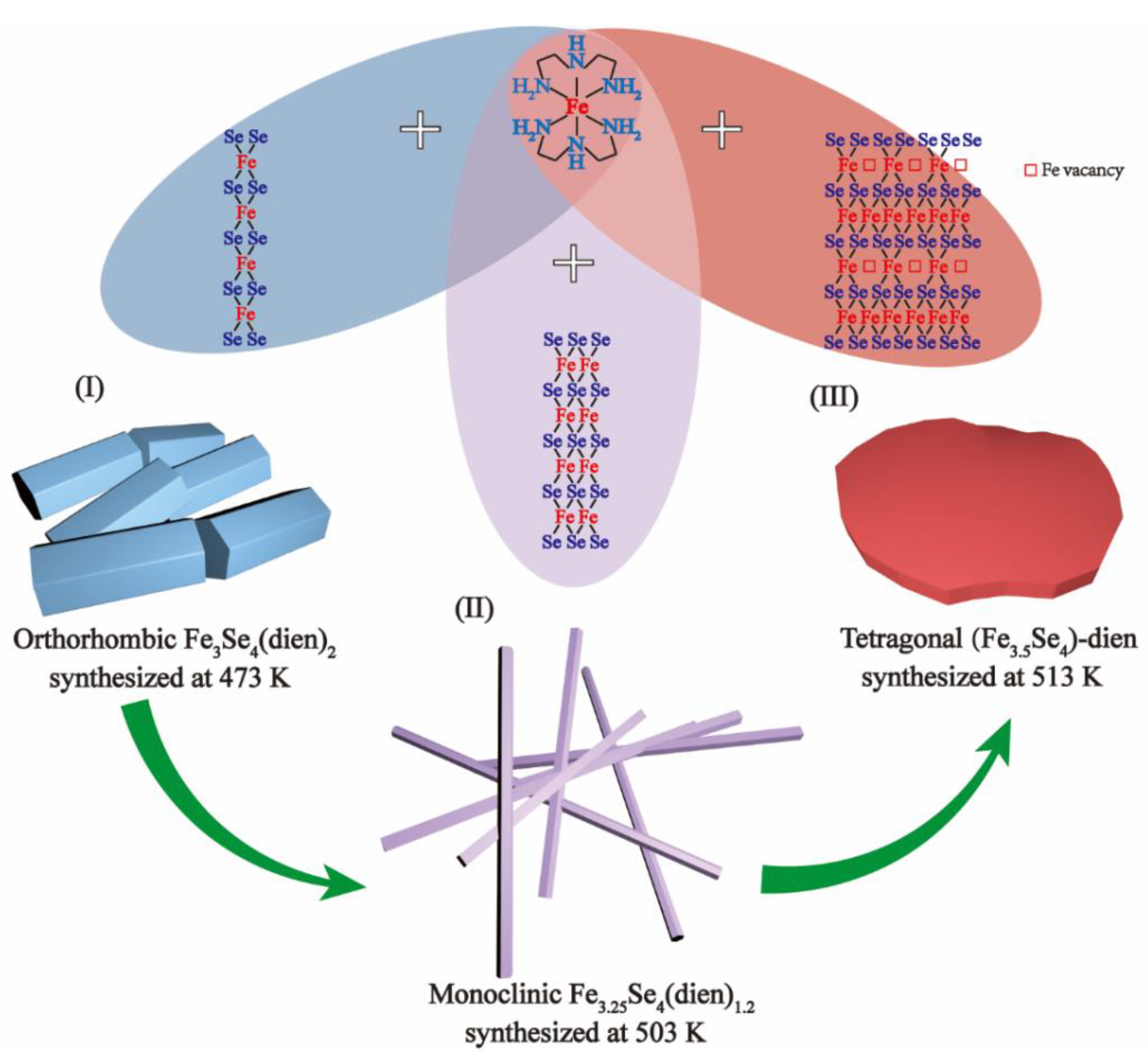
3.2. Growth Mechanism
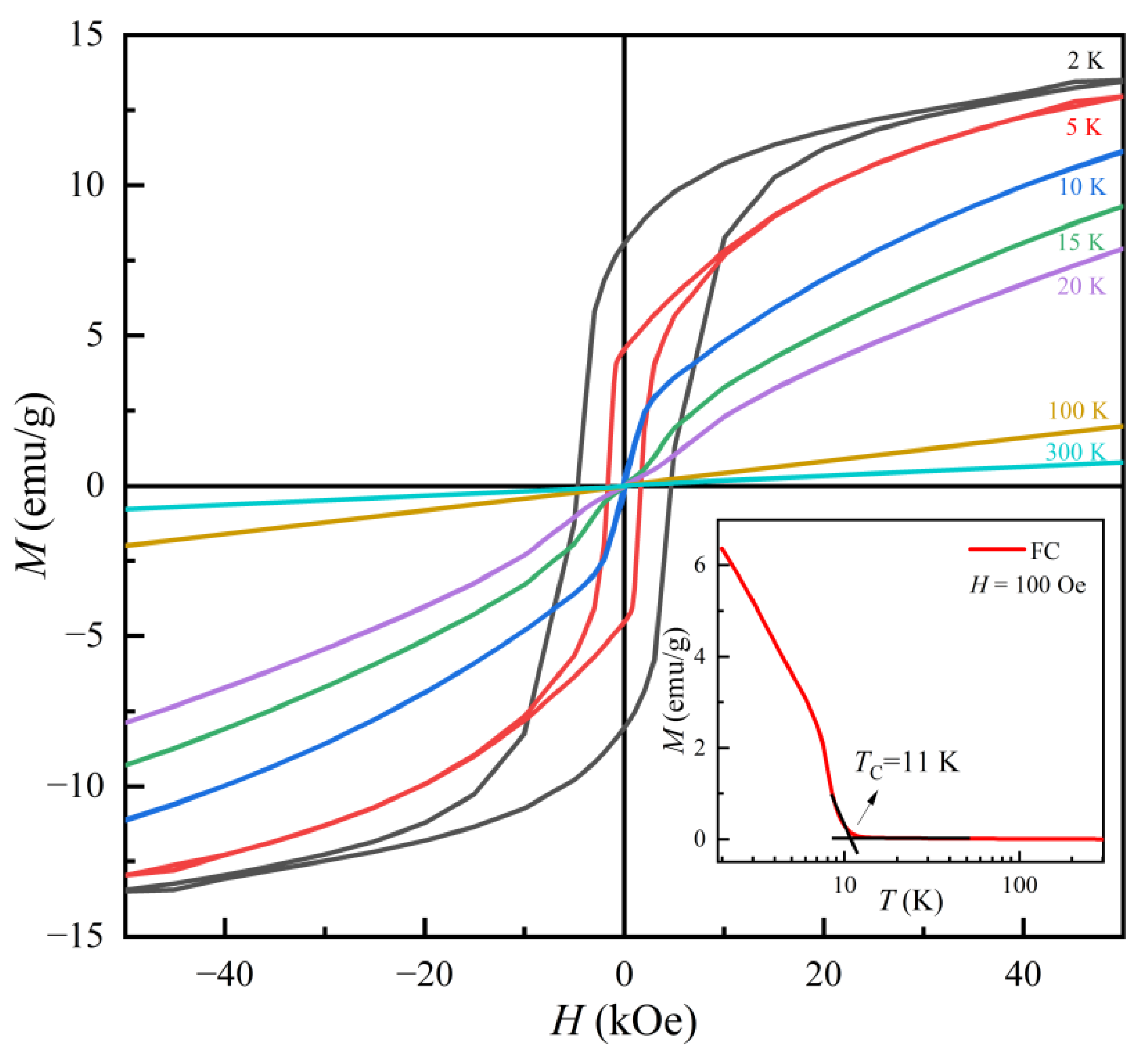
3.3. Magnetic Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, M.M.; Fan, Y.C.; Qin, W. Progress of organic magnetic materials. Sci. China Phys. Mech. 2019, 62, 977501. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar]
- Welbes, L.L.; Borovik, A.S. Confinement of metal complexes within porous hosts: Development of functional materials for gas binding and catalysis. Acc. Chem. Res. 2005, 38, 765–774. [Google Scholar] [CrossRef]
- Ansari, F.; Ding, Y.C.; Berglund, L.A.; Dauskardt, R.H. Toward sustainable multifunctional coatings containing nanocellulose in a hybrid glass matrix. ACS Nano 2018, 12, 5495–5503. [Google Scholar] [CrossRef]
- Li, W.; Xia, F.; Qu, J.; Li, P.; Chen, D.H.; Chen, Z.; Yu, Y.; Lu, Y.; Caruso, R.A.; Song, W.G. Versatile inorganic-organic hybrid WOx-ethylenediamine nanowires: Synthesis, mechanism and application in heavy metal ion adsorption and catalysis. Nano Res. 2014, 7, 903–916. [Google Scholar] [CrossRef]
- Jin, S.F.; Fan, X.; Wu, X.Z.; Sun, R.J.; Wu, H.; Huang, Q.Z.; Shi, C.L.; Xi, X.K.; Li, Z.L.; Chen, X.L. High-TC superconducting phases in organic molecular intercalated iron selenides: Synthesis and crystal structures. Chem. Commun. 2017, 53, 9729–9732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Fu, H.R.; Hu, Z.; Fu, Q.M.; Tao, H.; Weng, J.; Xiong, L.W.; Cheng, Z.X. Magnetic hybrid organic-inorganic perovskite (CH3NH3)2XCl4 (X = Mn, Cu, Co) crystals. CrystEngComm 2021, 23, 5208–5213. [Google Scholar] [CrossRef]
- Asensio, Y.; Marras, S.; Spirito, D.; Gobbi, M.; Ipatov, M.; Casanova, F.; Mateo-Alonso, A.; Hueso, L.E.; Martin-Garcia, B. Magnetic properties of layered hybrid organic-inorganic metal-halide perovskites: Transition metal, organic cation and perovskite phase effects. Adv. Funct. Mater. 2022, 32, 2207988. [Google Scholar] [CrossRef]
- Kim, K.Y.; Park, G.; Cho, J.; Kim, J.; Kim, J.S.; Jung, J.; Park, K.; You, C.Y.; Oh, I.H. Intrinsic magnetic order of chemically exfoliated 2D ruddlesden-popper organic-inorganic halide perovskite ultrathin films. Small 2020, 16, 2005445. [Google Scholar] [CrossRef]
- Zhang, S.L.; Li, X.C.; Gu, S.Y.; Guo, S.; Liu, Z.Y.; Zeng, S.Y.; Li, S.S. Crystal structures and magnetic properties of one-dimensional compounds constructed from Mn2(salen)2 building blocks and organic selenite acid ligands. N. J. Chem. 2021, 45, 21599–21605. [Google Scholar] [CrossRef]
- Sibille, R.; Mazet, T.; Diop, L.V.B.; Francois, M. Crystal structure and magnetic properties of the layered hybrid organic-inorganic compounds M2(OH)2(C14H8O4) (M = Mn, Fe). Acta Crystallogr. Sect. B 2021, 77, 801–807. [Google Scholar] [CrossRef]
- Gao, M.R.; Yao, W.T.; Yao, H.B.; Yu, S.H. Synthesis of unique ultrathin lamellar mesostructured CoSe2-amine (protonated) nanobelts in a binary solution. J. Am. Chem. Soc. 2009, 131, 7486–7487. [Google Scholar] [CrossRef]
- Greenfield, J.T.; Pak, C.; Kamali, S.; Lee, K.; Kovnir, K. Control over connectivity and magnetism of tetrahedral FeSe2 chains through coordination Fe-amine complexes. Chem. Commun. 2015, 51, 5355–5358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Xue, W.; Zheng, S.L.; Tong, M.L.; Chen, X.M. Néel temperature enhancement by increasing the in-plane magnetic correlation in layered inorganic-organic hybrid materials. Adv. Mater. 2008, 20, 1534–1538. [Google Scholar] [CrossRef]
- De Luis, R.F.; Urtiaga, M.K.; Mesa, J.L.; Vidal, K.; Lezama, L.; Rojo, T.; Arriortua, M.I. Short-range and long-range magnetic ordering, in third generation brannerite type inorganic–organic-vanadates: [{Mn(Bpy)}(VO3)2] approximate to (H2O)1.16 and [{Mn(Bpy)0.5}(VO3)2] approximate to (H2O)0.62. Chem. Mater. 2010, 22, 5543–5553. [Google Scholar] [CrossRef]
- Kurmoo, M.; Kumagai, H.; Green, M.A.; Lovett, B.W.; Blundell, S.J.; Ardavan, A.; Singleton, J. Two modifications of layered cobaltous terephthalate: Crystal structures and magnetic properties. J. Solid State Chem. 2001, 159, 343–351. [Google Scholar] [CrossRef]
- Pak, C.; Kamali, S.; Pham, J.; Lee, K.; Greenfield, J.T.; Kovnir, K. Chemical excision of tetrahedral FeSe2 chains from the superconductor FeSe: Synthesis, crystal structure, and magnetism of Fe3Se4(en)2. J. Am. Chem. Soc. 2013, 135, 19111–19114. [Google Scholar] [CrossRef] [Green Version]
- Zang, Z.-A.; Yao, H.-B.; Zhou, Y.-X.; Yao, W.-T.; Yu, S.-H. Synthesis and magnetic properties of new [Fe18S25](TETAH)14 (TETAH = protonated triethylenetetramine) nanoribbons: An efficient precursor to Fe7S8 nanowires and porous Fe2O3 nanorods. Chem. Mater. 2008, 20, 4749–4755. [Google Scholar] [CrossRef]
- Kuang, Q.; Men, X.; Shang, X.; Yang, B.; Zhou, Y.; Zhang, B.; Li, Z.; Li, D.; Zhang, Z. Magnetism of tetragonal β-Fe3Se4 nanoplates controllably synthesized by thermal decomposition of (β-Fe2Se3)4[Fe(tepa)] hybrid. Magnetism 2022, 2, 31–44. [Google Scholar] [CrossRef]
- Pan, D.S.; Li, Y.; Han, Z.; Li, B.; Wang, C.W.; Yang, T.; Li, D.; Choi, C.K.; Zhang, Z.D. Organic-inorganic hybrid (β-Fe3Se4)4[Fe(teta)1.5] (teta = triethylenetetramine) nanoplates: Solution synthesis and magnetic properties. Chem. Mater. 2018, 30, 8975–8982. [Google Scholar] [CrossRef]
- Kaiba, A.; Al Otaibi, F.; Geesi, M.H.; Riadi, Y.; Aljohani, T.A.; Guionneau, P. A new organic-inorganic hybrid compound (NH3(CH2)C6H4CO2H)[SnCl6]: Synthesis, crystal structure, vibrational, optical, magnetic properties and theoretical study. J. Mol. Struct. 2021, 1234, 130129. [Google Scholar] [CrossRef]
- Pan, D.S.; Kuang, Q.F.; Tong, P.; Tong, W.; Fan, L.B.; Zhao, J.; Li, D.; Choi, C.J.; Zhang, Z.D. Self-assembly of 1D FeSe2 chains and Fe(dien)2 complexes for ferrimagnetic inorganic-organic hybrid cuboids. J. Magn. Magn. Mater. 2022, 542, 168585. [Google Scholar] [CrossRef]
- Chen, T.K.; Chang, C.C.; Chang, H.H.; Fang, A.H.; Wang, C.H.; Chao, W.H.; Tseng, C.M.; Lee, Y.C.; Wu, Y.R.; Wen, M.H.; et al. Fe-vacancy order and superconductivity in tetragonal β-Fe1-xSe. Proc. Natl. Acad. Sci. USA 2014, 111, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezze, D.; Pereira, J.M.; Asensio, Y.; Ipatov, M.; Calavalle, F.; Casanova, F.; Bittner, A.M.; Ormaza, M.; Martin-Garcia, B.; Hueso, L.E.; et al. Tuning the magnetic properties of NiPS3 through organic-ion intercalation. Nanoscale 2022, 14, 1165–1173. [Google Scholar] [CrossRef]
- Nambu, Y.; Ohgushi, K.; Suzuki, S.; Du, F.; Avdeev, M.; Uwatoko, Y.; Munakata, K.; Fukazawa, H.; Chi, S.; Ueda, Y.; et al. Block magnetism coupled with local distortion in the iron-based spin-ladder compound BaFe2Se3. Phys. Rev. B 2012, 85, 064413. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Ryu, H.; Frenkel, A.I.; Petrovic, C. Anisotropy in BaFe2Se3 single crystals with double chains of FeSe tetrahedra. Phys. Rev. B 2011, 84, 214511. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Ohgushi, K.; Nambu, Y.; Kawakami, T.; Avdeev, M.; Hirata, Y.; Watanabe, Y.; Sato, T.J.; Ueda, Y. Stripelike magnetism in a mixed-valence insulating state of the Fe-based ladder compound CsFe2Se3. Phys. Rev. B 2012, 85, 214436. [Google Scholar] [CrossRef]
- Krzton-Maziopa, A.; Pomjakushina, E.; Pomjakushin, V.; Sheptyakov, D.; Chernyshov, D.; Svitlyk, V.; Conder, K. The synthesis, and crystal and magnetic structure of the iron selenide BaFe2Se3 with possible superconductivity at Tc = 11 K. J. Phys. Condens. Matter. 2011, 23, 402201. [Google Scholar] [CrossRef] [Green Version]
- Da Li, Q.K.; Men, X.; Zhang, B.; Shang, X.; Yang, B.; Yang, T.; Li, Z.; Zhang, Z. High-temperature superconductivity (TC = 42 K) of Fe1.2[Fe(tepa)]0.8(β-Fe3Se4)4. Research Square 2022. Preprint (Version 1). [Google Scholar] [CrossRef]
- Hua, A.; Zhou, W.Y.; Li, Y.; Li, S.Z.; Cheng, R.F.; Yang, J.X.; Luan, J.; Wang, X.H.; Jiang, C.H.; Li, D.; et al. A novel strategy for synthesizing the large size Co9S8@C nanosheets as anode for lithium-ion batteries with superior performance. J. Alloys Compd. 2022, 895, 162668. [Google Scholar] [CrossRef]
- Pan, D.S.; Xiao, B.; Wang, Q.; Wang, H. Chemical conversion synthesis of magnetic Fe1-xCox alloy nanosheets with controlled composition. Chem. Commun. 2021, 57, 2309–2312. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.T.; Yu, S.H.; Huang, X.Y.; Jiang, J.; Zhao, L.Q.; Pan, L.; Li, J. Nanocrystals of an inorganic-organic hybrid semiconductor: Formation of uniform nanobelts of [ZnSe](diethylenetriamine)0.5 in a ternary solution. Adv. Mater. 2005, 17, 2799–2802. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, C.; Zhang, T.K.; Feng, M.; Chang, L.; Yao, W.T.; Yu, S.H. Mn-substituted [Zn1−xMnxSe](DETA)0.5 (x = 0–0.3) inorganic-organic hybrid nanobelts: Synthesis, electron paramagnetic resonance spectroscopy, and their temperature- and pressure-dependent optical properties. Chem. Mater. 2009, 21, 5485–5490. [Google Scholar] [CrossRef]
- Li, Y.; Kuang, Q.F.; Men, X.L.; Wang, S.G.; Li, D.; Choi, C.J.; Zhang, Z.D. Anisotropic growth and magnetic properties of α″-Fe16N2@C nanocones. Nanomaterials 2021, 11, 890. [Google Scholar] [CrossRef]
- Aliev, N.G.; Guseinov, N.G.; Kerimov, I.G.; Sadykhov, R.Z.; Guseinov, D.A. Magnetic-properties of Fe3Se4. Inorg. Mater. 1977, 13, 519–521. [Google Scholar]
- Kamimura, T.; Kamigaki, K.; Hirone, T.; Sato, K. On Magnetocrystalline Anisotropy of Iron Selenide Fe7Se8. J. Phys. Soc. Jpn. 1967, 22, 1235–1240. [Google Scholar] [CrossRef]
- Long, G.; Zhang, H.W.; Li, D.; Sabirianov, R.; Zhang, Z.D.; Zeng, H. Magnetic anisotropy and coercivity of Fe3Se4 nanostructures. Appl. Phys. Lett. 2011, 99, 202103. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.; Men, X.; Kuang, Q.; Li, S.; Li, D.; Zhang, Z. Controllable Connection of Fe2Se3 Double Chains and Fe(dien)2 Complexes for Organic–Inorganic Hybrid Ferrimagnet with a Large Coercivity. Nanomaterials 2023, 13, 487. https://doi.org/10.3390/nano13030487
Shang X, Men X, Kuang Q, Li S, Li D, Zhang Z. Controllable Connection of Fe2Se3 Double Chains and Fe(dien)2 Complexes for Organic–Inorganic Hybrid Ferrimagnet with a Large Coercivity. Nanomaterials. 2023; 13(3):487. https://doi.org/10.3390/nano13030487
Chicago/Turabian StyleShang, Xiaolei, Xiaoling Men, Qifeng Kuang, Shaojie Li, Da Li, and Zhidong Zhang. 2023. "Controllable Connection of Fe2Se3 Double Chains and Fe(dien)2 Complexes for Organic–Inorganic Hybrid Ferrimagnet with a Large Coercivity" Nanomaterials 13, no. 3: 487. https://doi.org/10.3390/nano13030487






