Super-Branched PdCu Alloy for Efficiently Converting Carbon Dioxide to Carbon Monoxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PdCu Alloy
2.3. Electrochemical Measurements
3. Results and Discussion
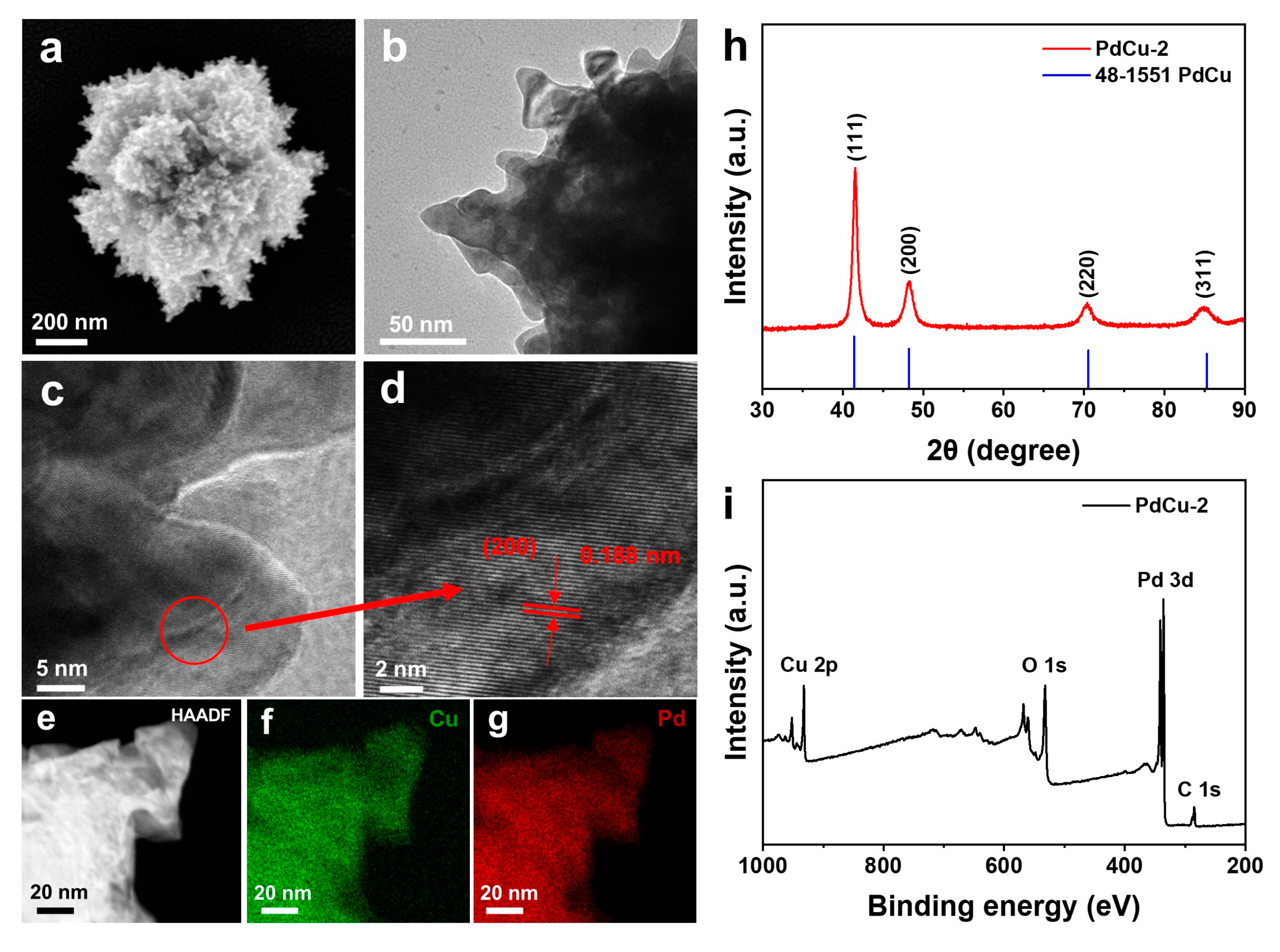
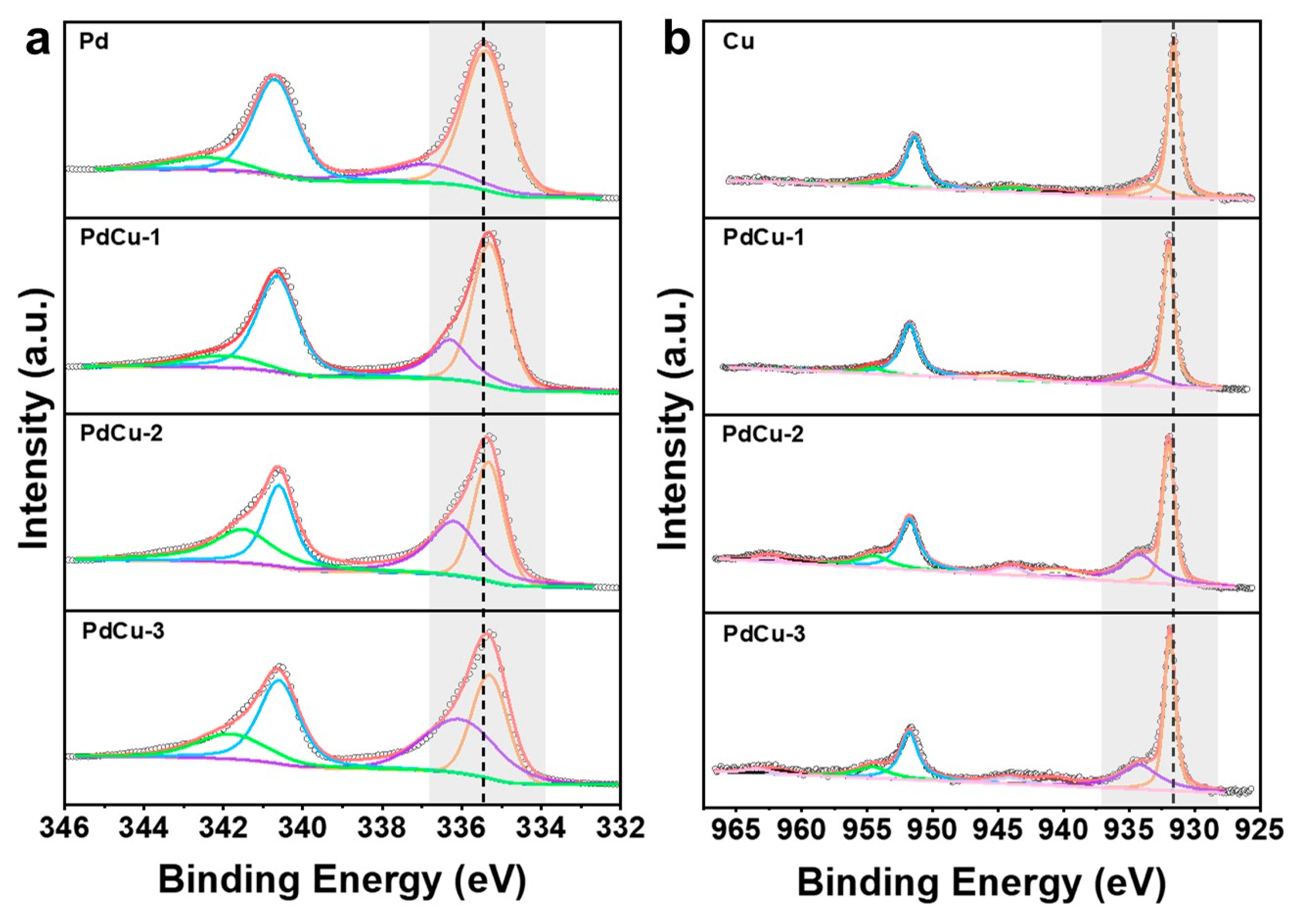
3.1. Characterizations of Catalyst
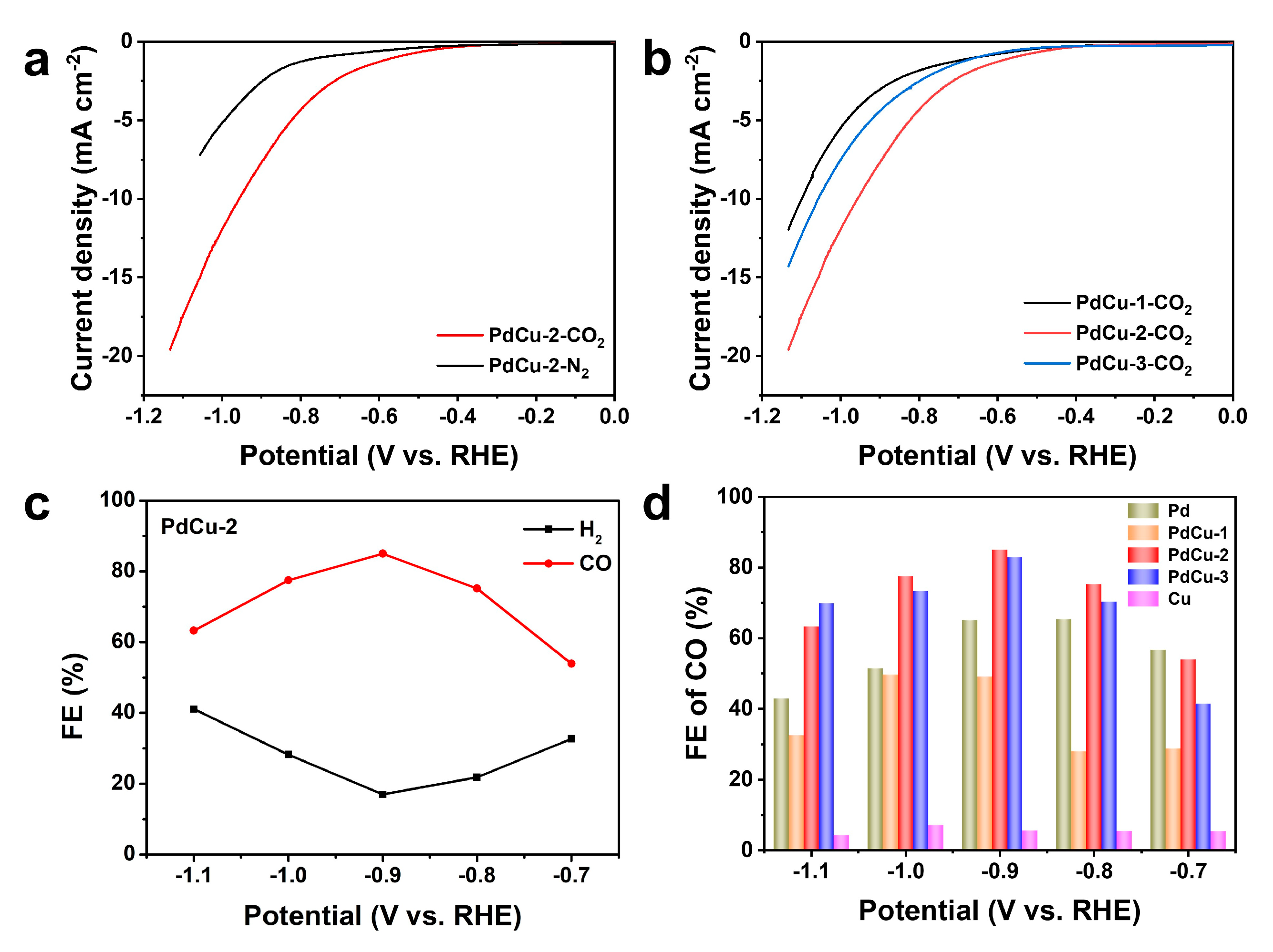
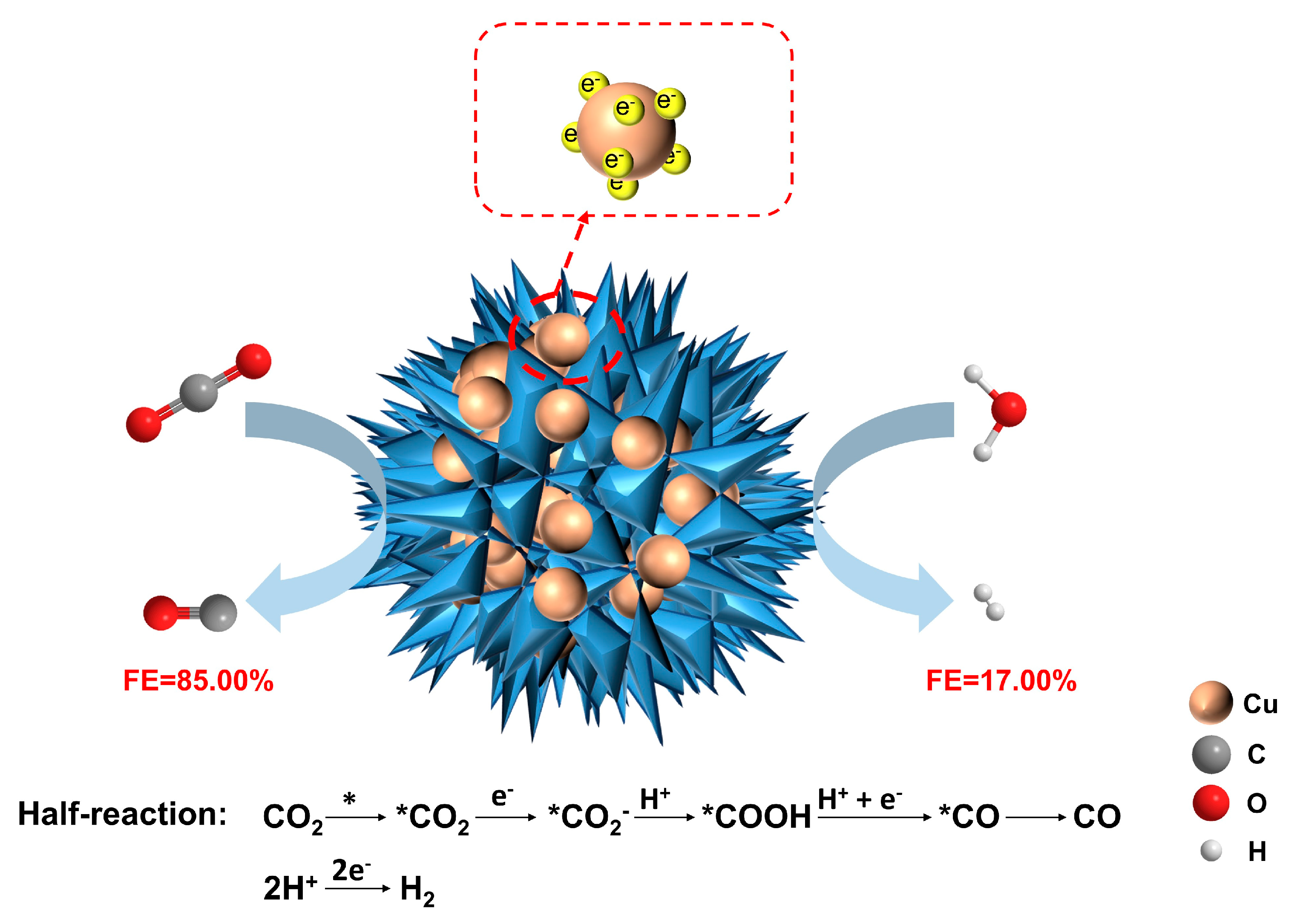
3.2. Electrocatalytic Activity of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbek, H.; DuBois, D.L.; Dupuis, M.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.; et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [Google Scholar] [CrossRef]
- Mao, J.; Iocozzia, J.; Huang, J.; Meng, K.; Lai, Y.; Lin, Z. Graphene aerogels for efficient energy storage and conversion. Energy Environ. Sci. 2018, 11, 772–799. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, W.; Chen, M.; Bu, Y. Turning the activity of Cr–Ce mixed oxide towards thermocatalytic NO oxidation and photocatalytic CO2 reduction via the formation of yolk shell structure hollow microspheres. J. Alloy Compd. 2020, 829, 154508. [Google Scholar] [CrossRef]
- Kas, R.; Hummadi, K.K.; Kortlever, R.; De Wit, P.; Milbrat, A.; Luiten-Olieman, M.W.; Benes, N.E.; Koper, M.; Mul, G. Three-dimensional porous hollow fibre copper electrodes for efficient and high-rate electrochemical carbon dioxide reduction. Nat. Commun. 2016, 7, 10748. [Google Scholar] [CrossRef]
- Ma, M.; Trześniewski, B.J.; Xie, J.; Smith, W.A. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts. Angew. Chem. Int. Edit. 2016, 128, 9900–9904. [Google Scholar] [CrossRef]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo-and electrochemical CO2 reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Zhao, Z.; Peng, X.; Liu, X.; Sun, X.; Shi, J.; Han, L.; Li, G.; Luo, J. Efficient and stable electroreduction of CO2 to CH4 on CuS nanosheet arrays. J. Mater. Chem. A 2017, 5, 20239–20243. [Google Scholar] [CrossRef]
- Cao, B.; Li, F.-Z.; Gu, J. Designing Cu-Based Tandem Catalysts for CO2 Electroreduction Based on Mass Transport of CO Intermediate. ACS Catal. 2022, 12, 9735–9752. [Google Scholar] [CrossRef]
- Choi, C.; Cheng, T.; Flores Espinosa, M.; Fei, H.; Duan, X.; Goddard, W.A., 3rd; Huang, Y. A Highly Active Star Decahedron Cu Nanocatalyst for Hydrocarbon Production at Low Overpotentials. Adv. Mater. 2019, 31, e1805405. [Google Scholar] [CrossRef]
- Christophe, J.; Doneux, T.; Buess-Herman, C. Electroreduction of carbon dioxide on copper-based electrodes: Activity of copper single crystals and copper–gold alloys. Electrocatalysis 2012, 3, 139–146. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Vasileff, A.; Qiao, S. Nanostructured 2D materials: Prospective catalysts for electrochemical CO2 reduction. Small Methods 2017, 1, 1600006. [Google Scholar] [CrossRef]
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Peterson, A.A.; Varela, A.S.; Jovanov, Z.P.; Bech, L.; Durand, W.J.; Dahl, S.; Nørskov, J.K.; Chorkendorff, I. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 2012, 14, 76–81. [Google Scholar] [CrossRef]
- Harzandi, A.M.; Tiwari, J.N.; Lee, H.S.; Jeon, H.; Cho, W.J.; Lee, G.; Baik, J.; Kwak, J.H.; Kim, K.S. Efficient CO oxidation by 50-facet Cu2O nanocrystals coated with CuO nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 2495–2499. [Google Scholar] [CrossRef]
- Raciti, D.; Livi, K.J.; Wang, C. Highly dense Cu nanowires for low-overpotential CO2 reduction. Nano Lett. 2015, 15, 6829–6835. [Google Scholar] [CrossRef]
- Shi, R.; Guo, J.; Zhang, X.; Waterhouse, G.I.; Han, Z.; Zhao, Y.; Shang, L.; Zhou, C.; Jiang, L.; Zhang, T. Efficient wettability-controlled electroreduction of CO2 to CO at Au/C interfaces. Nat. Commun. 2020, 11, 3028. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, C.; Zhu, M.; Fu, Y.; Huang, H.; Liu, Y.; Kang, Z. Highly selective and efficient electroreduction of carbon dioxide to carbon monoxide with phosphate silver-derived coral-like silver. ACS Sustain. Chem. Eng. 2019, 7, 3536–3543. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, S.; Guo, S.; Wang, H.; Sun, Y.; Yao, B.; Liu, Y.; Huang, H.; Kang, Z. Carbon quantum dot-covered porous Ag with enhanced activity for selective electroreduction of CO2 to CO. Inorg. Chem. Front. 2019, 6, 1453–1460. [Google Scholar] [CrossRef]
- He, Q.; Lee, J.H.; Liu, D.; Liu, Y.; Lin, Z.; Xie, Z.; Hwang, S.; Kattel, S.; Song, L.; Chen, J.G. Accelerating CO2 electroreduction to CO over Pd single-atom catalyst. Adv. Funct. Mater. 2020, 30, 2000407. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S.-Z. Molecular scaffolding strategy with synergistic active centers to facilitate electrocatalytic CO2 reduction to hydrocarbon/alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef]
- Aydın, R.; Doğan, H.Ö.; Köleli, F. Electrochemical reduction of carbondioxide on polypyrrole coated copper electro-catalyst under ambient and high pressure in methanol. Appl. Catal. B Environ. 2013, 140, 478–482. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.; Zhang, L.; Sun, J. Rational design strategies of Cu-based electrocatalysts for CO2 electroreduction to C2 products. J. Energy Chem. 2022, 71, 63–82. [Google Scholar] [CrossRef]
- Han, X.; Zhou, S.J.; Tan, Y.Z.; Wu, X.; Gao, F.; Liao, Z.J.; Huang, R.B.; Feng, Y.Q.; Lu, X.; Xie, S.Y. Crystal Structures of Saturn-Like C50Cl10 and Pineapple-Shaped C64Cl4: Geometric Implications of Double-and Triple-Pentagon-Fused Chlorofullerenes. Angew. Chem. Int. Edit. 2008, 47, 5340–5343. [Google Scholar] [CrossRef]
- Yang, B.; Liu, K.; Li, H.; Liu, C.; Fu, J.; Li, H.; Huang, J.E.; Ou, P.; Alkayyali, T.; Cai, C. Accelerating CO2 Electroreduction to Multicarbon Products via Synergistic Electric–Thermal Field on Copper Nanoneedles. J. Am. Chem. Soc. 2022, 144, 3039–3049. [Google Scholar] [CrossRef]
- Xiong, B.; Liu, J.; Yang, Y.; Liu, W. Tunable Pd enrichment for switching CO2 electroreduction product selectivity from HCOOH to CO. J. Environ. Chem. Eng. 2022, 10, 108056. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Jovanov, Z.P.; Hansen, H.A.; Varela, A.S.; Malacrida, P.; Peterson, A.A.; Nørskov, J.K.; Stephens, I.E.; Chorkendorff, I. Opportunities and challenges in the electrocatalysis of CO2 and CO reduction using bifunctional surfaces: A theoretical and experimental study of Au–Cd alloys. J. Catal. 2016, 343, 215–231. [Google Scholar] [CrossRef]
- Lee, C.W.; Yang, K.D.; Nam, D.H.; Jang, J.H.; Cho, N.H.; Im, S.W.; Nam, K.T. Defining a materials database for the design of copper binary alloy catalysts for electrochemical CO2 conversion. Adv. Mater. 2018, 30, 1704717. [Google Scholar] [CrossRef]
- Yin, Z.; Gao, D.; Yao, S.; Zhao, B.; Cai, F.; Lin, L.; Tang, P.; Zhai, P.; Wang, G.; Ma, D.; et al. Highly selective palladium-copper bimetallic electrocatalysts for the electrochemical reduction of CO2 to CO. Nano Energy 2016, 27, 35–43. [Google Scholar] [CrossRef]
- Chen, D.; Yao, Q.; Cui, P.; Liu, H.; Xie, J.; Yang, J. Tailoring the Selectivity of Bimetallic Copper–Palladium Nanoalloys for Electrocatalytic Reduction of CO2 to CO. ACS Appl. Energ. Mater. 2018, 1, 883–890. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Xiao, H.; Chen, W.; Zhao, M.; Jia, J. Shape-control of super-branched Pd–Cu alloys with enhanced electrocatalytic performance for ethylene glycol oxidation. Chem. Commun. 2018, 54, 13363–13366. [Google Scholar] [CrossRef]
- Mun, Y.; Lee, S.; Cho, A.; Kim, S.; Han, J.W.; Lee, J. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2019, 246, 82–88. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Z.; Chen, B.; Huang, Y.; Chen, J.; Lai, Z.; Chen, Y.; Sindoro, M.; Wang, A.L.; Cheng, H. Synthesis of ultrathin PdCu alloy nanosheets used as a highly efficient electrocatalyst for formic acid oxidation. Adv. Mater. 2017, 29, 1700769. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687–11697. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.L.; Wang, H.; Yu, Y.; Rus, E.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Facile synthesis of carbon-supported Pd–Co core–shell nanoparticles as oxygen reduction electrocatalysts and their enhanced activity and stability with monolayer Pt decoration. Chem. Mater. 2012, 24, 2274–2281. [Google Scholar] [CrossRef]
- Peterson, A.A.; Nørskov, J.K. Activity descriptors for CO2 electroreduction to methane on transition-metal catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, W.; Chen, Y.; Yin, Z.; Li, Y.; Liu, J.; Zhang, H.; Zhu, J.J.; Sun, S. Bipyridine-assisted assembly of Au nanoparticles on Cu nanowires to enhance the electrochemical reduction of CO2. Angew. Chem. Int. Edit. 2019, 131, 14238–14241. [Google Scholar] [CrossRef]
- Zou, C.; Xi, C.; Wu, D.; Mao, J.; Liu, M.; Liu, H.; Dong, C.; Du, X.W. Porous copper microspheres for selective production of multicarbon fuels via CO2 electroreduction. Small 2019, 15, 1902582. [Google Scholar] [CrossRef]
- Yan, C.; Lin, L.; Wang, G.; Bao, X. Transition metal-nitrogen sites for electrochemical carbon dioxide reduction reaction. Chin. J. Catal. 2019, 40, 23–37. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Qi, H.H.; Wu, J.; Huang, H.; Liu, Y.; Kang, Z.H. Amino Modified Carbon Dots with Electron Sink Effect Increase Interface Charge Transfer Rate of Cu-Based Electrocatalyst to Enhance the CO2 Conversion Selectivity to C2H4. Adv. Funct. Mater. 2022, 32, 2113335. [Google Scholar] [CrossRef]
- Ma, S.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R.T.; Gold, J.I.; Yamauchi, M.; Kenis, P.J. Electroreduction of Carbon Dioxide to Hydrocarbons Using Bimetallic Cu-Pd Catalysts with Different Mixing Patterns. J. Am. Chem. Soc. 2017, 139, 47–50. [Google Scholar] [CrossRef]
- Cho, B.; Lee, J.; Roh, I.P.; Lee, M.H.; Yu, T. A facile aqueous-phase synthesis method for small PdCu alloy nanocatalysts to enhance electrochemical CO2 reduction reactivity. J. Alloys Compd. 2022, 911, 164990. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Li, P.; Chang, K.; Li, C.; Wang, T.; Jiang, B.; Zhang, H.; Liu, H.; Yamauchi, Y.; et al. Mesoporous palladium–copper bimetallic electrodes for selective electrocatalytic reduction of aqueous CO2 to CO. J. Mater. Chem. A 2016, 4, 4776–4782. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, P.; Chang, X.; Dong, H.; Li, A.; Hu, C.; Huang, Z.; Zhao, Z.J.; Gong, J. Morphological and Compositional Design of Pd-Cu Bimetallic Nanocatalysts with Controllable Product Selectivity toward CO2 Electroreduction. Small 2018, 14, 1703314. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, J.; Kim, J.H.; Lee, B.J.; Lee, J.S. One-dimensional CuIn alloy nanowires as a robust and efficient electrocatalyst for selective CO2-to-CO conversion. J. Power Sources 2018, 378, 412–417. [Google Scholar] [CrossRef]
- Andrews, E.; Fang, Y.; Flake, J. Electrochemical reduction of CO2 at CuAu nanoparticles: Size and alloy effects. J. Appl. Electrochem. 2018, 48, 435–441. [Google Scholar] [CrossRef]
- Han, Z.; Choi, C.; Tao, H.; Fan, Q.; Gao, Y.; Liu, S.; Robertson, A.W.; Hong, S.; Jung, Y.; Sun, Z. Tuning the Pd-catalyzed electroreduction of CO2 to CO with reduced overpotential. Catal. Sci. Technol. 2018, 8, 3894–3900. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, K.; Zhou, Y.; Wu, J.; Li, Z.; Yan, X.; Huang, H.; Liu, Y.; Kang, Z. Super-Branched PdCu Alloy for Efficiently Converting Carbon Dioxide to Carbon Monoxide. Nanomaterials 2023, 13, 603. https://doi.org/10.3390/nano13030603
Bao K, Zhou Y, Wu J, Li Z, Yan X, Huang H, Liu Y, Kang Z. Super-Branched PdCu Alloy for Efficiently Converting Carbon Dioxide to Carbon Monoxide. Nanomaterials. 2023; 13(3):603. https://doi.org/10.3390/nano13030603
Chicago/Turabian StyleBao, Kaili, Yunjie Zhou, Jie Wu, Zenan Li, Xiong Yan, Hui Huang, Yang Liu, and Zhenhui Kang. 2023. "Super-Branched PdCu Alloy for Efficiently Converting Carbon Dioxide to Carbon Monoxide" Nanomaterials 13, no. 3: 603. https://doi.org/10.3390/nano13030603
APA StyleBao, K., Zhou, Y., Wu, J., Li, Z., Yan, X., Huang, H., Liu, Y., & Kang, Z. (2023). Super-Branched PdCu Alloy for Efficiently Converting Carbon Dioxide to Carbon Monoxide. Nanomaterials, 13(3), 603. https://doi.org/10.3390/nano13030603








