Polyethylene Glycol Functionalized Silicon Nanowire Field-Effect Transistor Biosensor for Glucose Detection
Abstract
1. Introduction
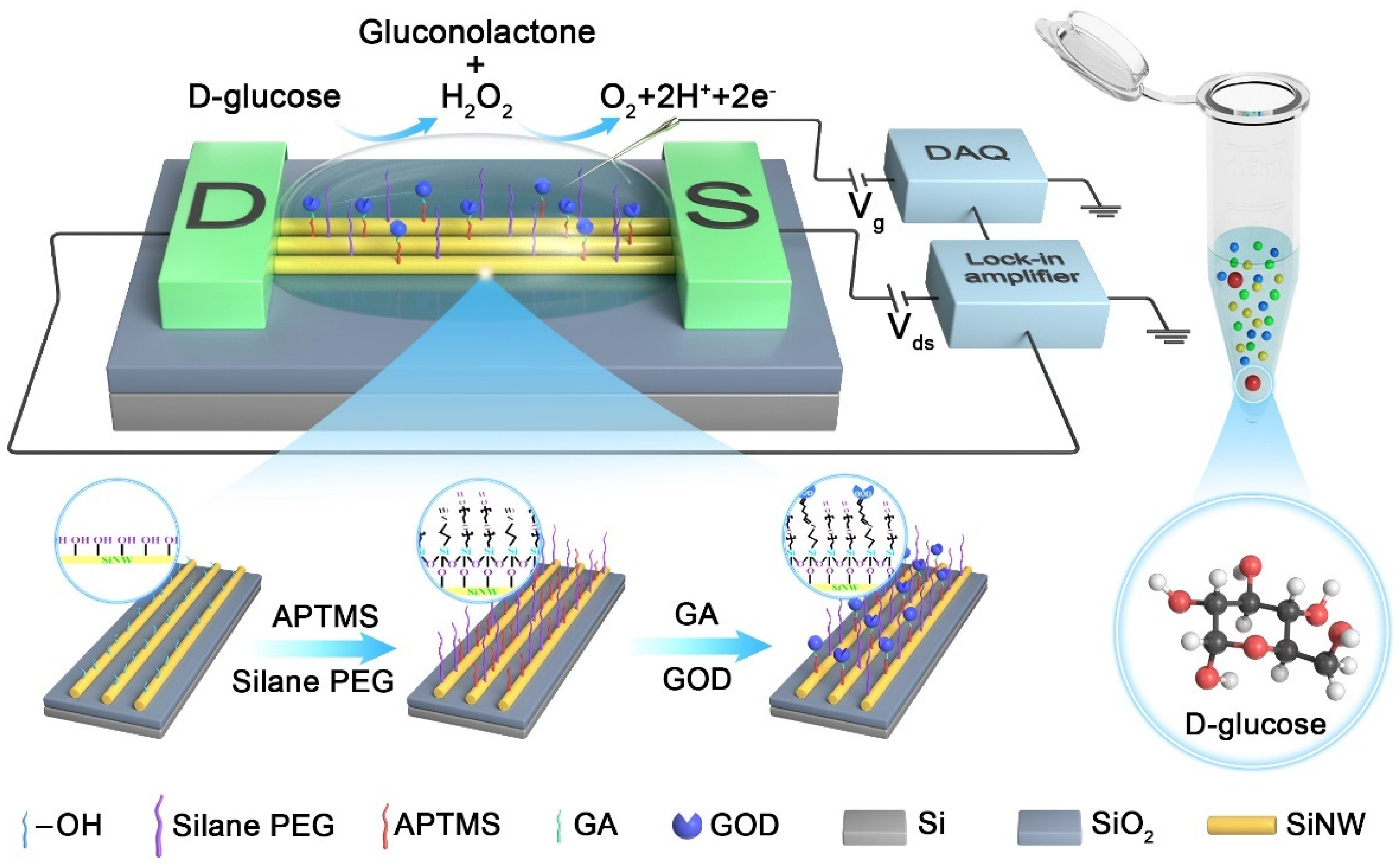
2. Materials and Methods
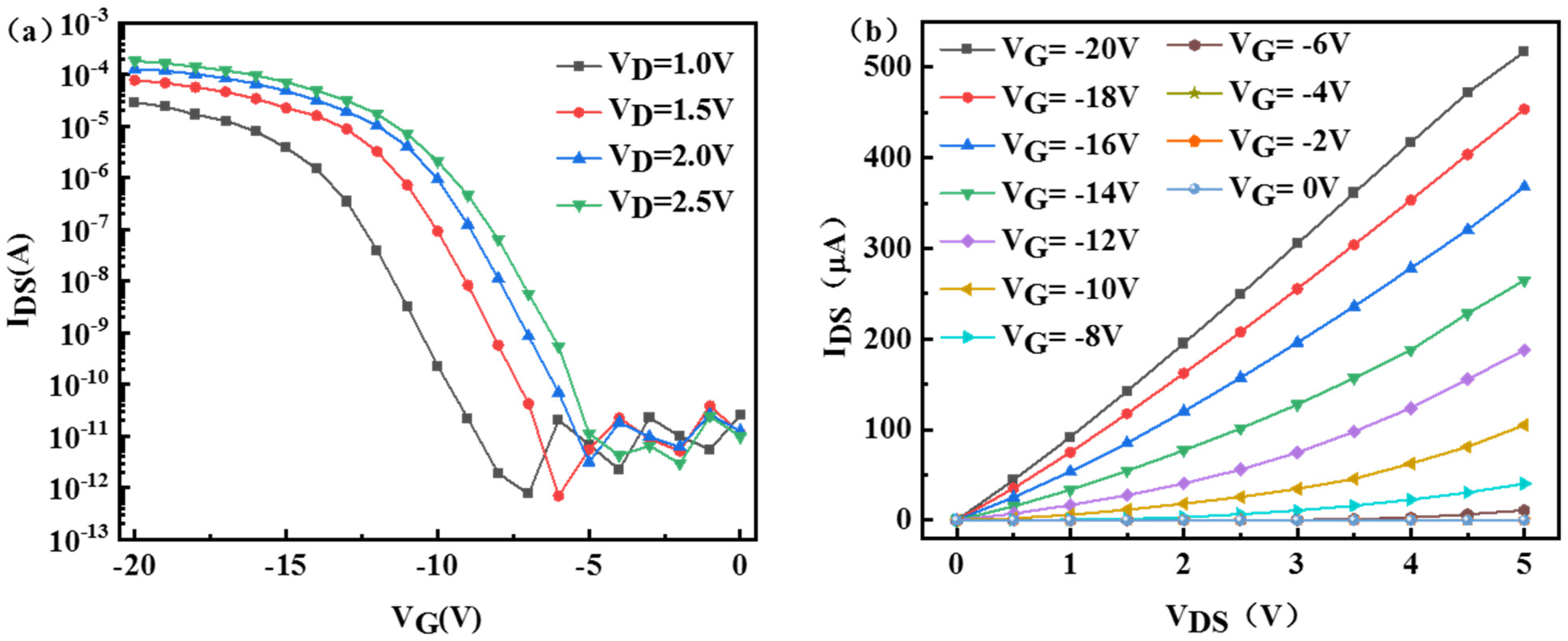
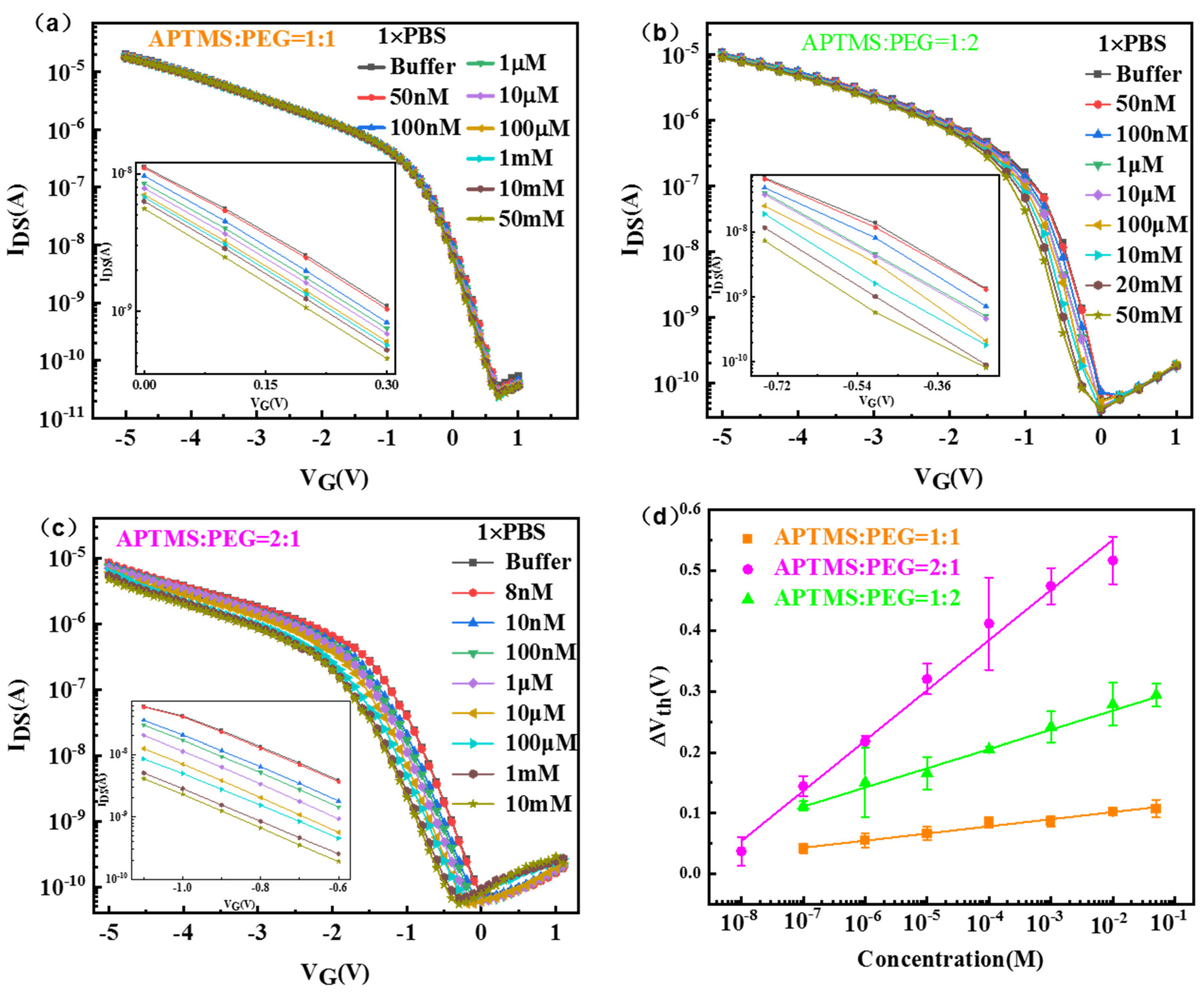
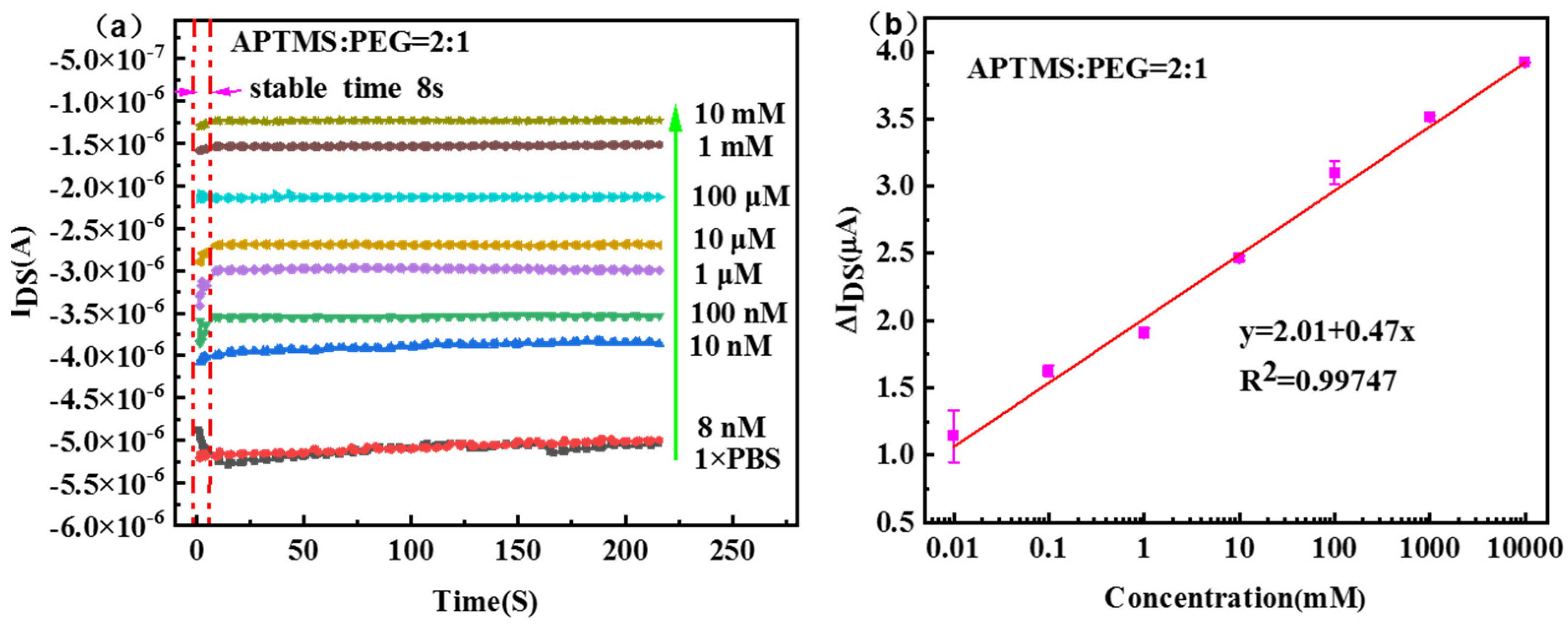
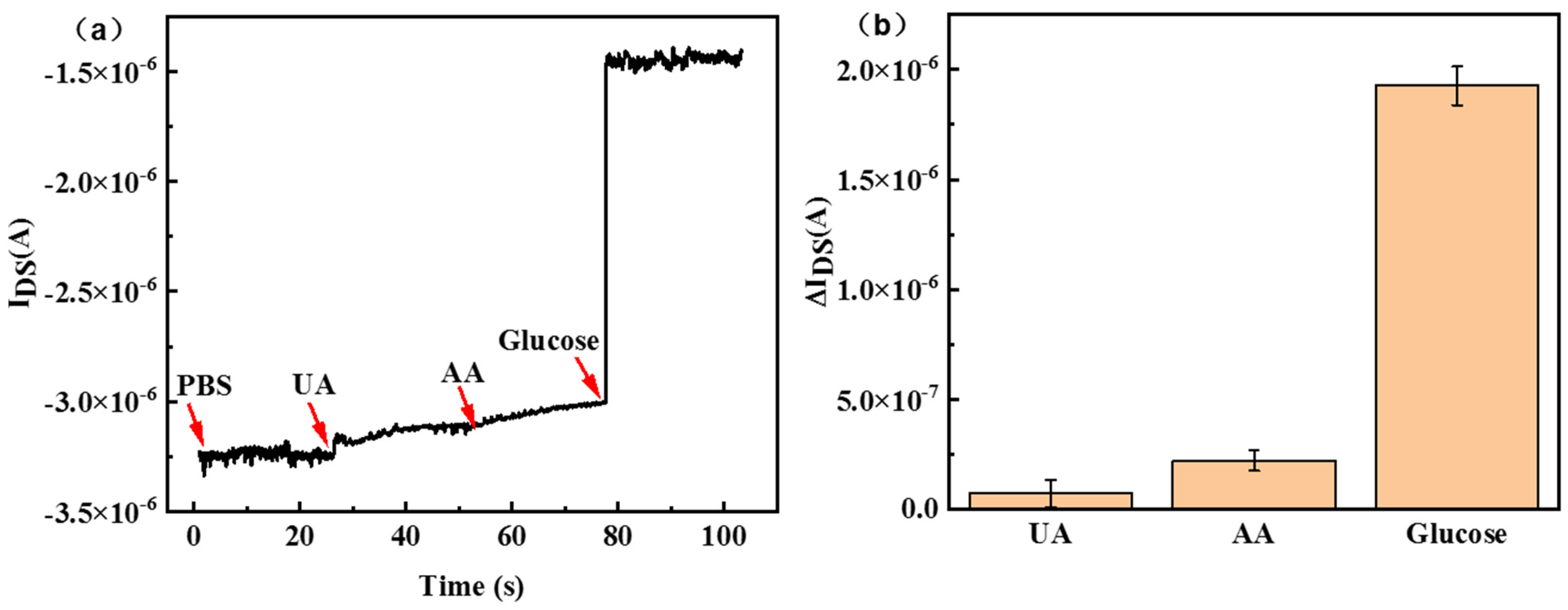
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Coster, S.; Gulliford, M.C.; Seed, P.T.; Powrie, J.K.; Swaminathan, R. Monitoring blood glucose control in diabetes mellitus: A systematic review. Health Technol. Asses. 2000, 4, 1. [Google Scholar] [CrossRef]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xia, J.; Jiang, X.; Yang, M.; Liu, S. Cellulose-based strips designed based on a sensitive enzyme colorimetric assay for the low concentration of glucose detection. Anal Chem. 2019, 91, 15461–15468. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Chen, L.; Hu, X.; Wang, F.; Yang, Y. Silver nanoprism enhanced colorimetry for precise detection of dissolved oxygen. Micromachines 2020, 11, 383. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; Fahmy, M.T.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef]
- Wasfi, A.; Awwad, F.; Gelovani, J.G.; Qamhieh, N.; Ayesh, A.I. COVID-19 Detection via Silicon Nanowire Field-Effect Transistor: Setup and Modeling of Its Function. Nanomaterials 2022, 12, 2638. [Google Scholar] [CrossRef]
- Chen, L.; Li, G.; Yang, A.; Wu, J.; Yan, F.; Ju, H. A DNA-functionalized graphene field-effect transistor for quantitation of vascular endothelial growth factor. Sens. Actuators B Chem. 2022, 351, 130964. [Google Scholar] [CrossRef]
- Gao, Z.; Xia, H.; Zauberman, J.; Tomaiuolo, M.; Ping, J.; Zhang, Q.; Johnson, A.T.C.; Ducos, P.; Ye, H.C.; Wang, S.; et al. Detection of sub-fM DNA with target recycling and self-assembly amplification on graphene field-effect biosensors. Nano Lett. 2018, 18, 3509–3515. [Google Scholar] [CrossRef]
- Shen, H.; Di, C.A.; Zhu, D. Organic transistor for bioelectronic applications. Sci. China Chem. 2017, 60, 437–449. [Google Scholar] [CrossRef]
- Zou, L.R.; Sang, D.D.; Yao, Y.; Wang, X.T.; Zheng, Y.Y.; Wang, N.Z.; Wang, C.; Wang, Q.L. Research progress of optoelectronic devices based on two-dimensional MoS2 materials. Rare Met. 2022, 42, 17–38. [Google Scholar] [CrossRef]
- Shen, M.Y.; Li, B.R.; Li, Y.K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosens. Bioelectron. 2014, 60, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rydzek, G.; Toulemon, D.; Garofalo, A.; Leuvrey, C.; Dayen, J.F.; Felder-Flesch, D.; Schaaf, P.; Jierry, L.; Begin-Colin, S.; Pichon, B. Selective Nanotrench Filling by One-Pot Electroclick Self-Constructed Nanoparticle Films. Small 2015, 11, 4638–4642. [Google Scholar] [CrossRef]
- Li, M.; Ishihara, S.; Ji, Q.; Ma, Y.; Hill, J.P.; Ariga, K. Electrochemical coupling layer-by-layer (ECC-LbL) assembly in patterning mode. Chem. Lett. 2012, 41, 383–385. [Google Scholar] [CrossRef]
- Ah, C.S.; Park, C.W.; Yang, J.H.; Lee, J.S.; Kim, W.J.; Chung, K.H.; Choi, Y.H.; Baek, I.B.; Kim, J.; Sung, G.Y. Detection of uncharged or feebly charged small molecules by field-effect transistor biosensors. Biosens. Bioelectron. 2012, 33, 233–240. [Google Scholar] [CrossRef]
- Ahn, C.; Kulkarni, A.; Kim, H.U.; Shin, C.; Xu, Y.; Jung, S.; Kim, H.; Lee, M.; Kim, T. Light-sensitive silicon nanowire array field effect transistor for glucose detection. Nano 2014, 9, 1450099. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Gibney, K.A.; Erramilli, S.; Mohanty, P. Silicon-based nanochannel glucose sensor. Appl. Phys. Lett. 2008, 92, 013903. [Google Scholar] [CrossRef]
- Tang, S.; Yan, J.; Zhang, J.; Wei, S.; Zhang, Q.; Li, J.; Fang, M.; Zhang, S.; Xiong, E.; Wang, Y. Fabrication of Low Cost and Low Temperature Poly-Silicon Nanowire Sensor Arrays for Monolithic Three-Dimensional Integrated Circuits Applications. Nanomaterials 2020, 10, 2488. [Google Scholar] [CrossRef]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.M.; Lieber, C.M. General strategy for biodetection in high ionic strength solutions using transistor-based nanoelectronic sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef]
- Cao, T.; Wang, A.; Liang, X.; Tang, H.; Auner, G.W.; Salley, S.O.; Ng, K.S. Investigation of spacer length effect on immobilized Escherichia coli pili-antibody molecular recognition by AFM. Biotechnol. Bioeng. 2007, 98, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.I.; Li, B.R.; Chen, Y.T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Kharitonov, A.B.; Zayats, M.; Lichtenstein, A.; Katz, E.; Willner, I. Enzyme monolayer-functionalized field-effect transistors for biosensor applications. Sens. Actuators B Chem. 2000, 70, 222–231. [Google Scholar] [CrossRef]
- Fathollahzadeh, M.; Hosseini, M.; Haghighi, B.; Kolahdouz, M.; Fathipour, M. Fabrication of a liquid-gated enzyme field effect device for sensitive glucose detection. Anal. Chim. Acta 2016, 924, 99–105. [Google Scholar] [CrossRef]
- Otsuka, I.; Yaoita, M.; Nagashima, S.; Higano, M. Molecular dimensions of dried glucose oxidase on a Au (111) surface studied by dynamic mode scanning force microscopy. Electrochim. Acta 2005, 50, 4861–4867. [Google Scholar] [CrossRef]
- Lee, H. Molecular dynamics studies of PEGylated single-walled carbon nanotubes: The effect of PEG size and grafting density. J. Phys. Chem. C 2013, 117, 26334–26341. [Google Scholar] [CrossRef]
- Inaba, D.; Yamaguchi, A. Integration of enzyme-encapsulated mesoporous silica between nanohole array electrode and hydrogel film for flow-type electrochemical biosensor. Anal. Sci. 2022, 39, 153–161. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, B.; Chen, S.; Zhu, Q.; Tu, T.; Wu, K.; Cao, Q.; Fang, L.; Xiao Liang, X.; Ye, X. One-step modification of nano-polyaniline/glucose oxidase on double-side printed flexible electrode for continuous glucose monitoring: Characterization, cytotoxicity evaluation and in vivo experiment. Biosens. Bioelectron. 2020, 165, 112408. [Google Scholar] [CrossRef]
- Cao, X.; Ye, Y.; Li, Y.; Xu, X.; Yu, J.; Liu, S. Self-assembled glucose oxidase/graphene/gold ternary nanocomposites for direct electrochemistry and electrocatalysis. J. Electroanal. Chem. 2013, 697, 10–14. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Park, J.H.; Hahn, Y.B. A comprehensive biosensor integrated with a ZnO nanorod FET array for selective detection of glucose. Chem. Commun. 2015, 51, 11968–11971. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Lee, M.S.; Kim, K.; Ji, S.; Kim, Y.T.; Park, J.; Na, K.; Bae, K.H.; Kim, H.K.; et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat Commun. 2017, 8, 14997. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Li, J.; Chu, X.; Xu, M.; Jin, F.; Wang, X.; Ma, L.; Fang, X.; Wei, Z.P.; Wang, X. High sensitivity glucose detection at extremely low concentrations using a MoS2-based field-effect transistor. Rsc Adv. 2018, 8, 7942–7948. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Herman, G.S. Transparent In-Ga-Zn-O field effect glucose sensors fabricated directly on highly curved substrates. Sens. Actuators B Chem. 2018, 268, 123–128. [Google Scholar] [CrossRef]
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021, 171, 112736. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Marmisollé, W.A.; Knoll, W.; Azzaroni, O. Highly sensitive urine glucose detection with graphene field-effect transistors functionalized with electropolymerized nanofilms. Sens. Diagn. 2022, 1, 139–148. [Google Scholar] [CrossRef]
- Shircliff, R.A.; Martin, I.T.; Pankow, J.W.; Fennell, J.; Stradins, P.; Ghirardi, M.L.; Cowley, S.W.; Branz, H.M. High-Resolution X-Ray Photoelectron Spectroscopy of Mixed Silane Monolayers for DNA Attachment. ACS Appl. Mater. Interfaces. 2011, 3, 3285–3292. [Google Scholar] [CrossRef]
- Shircliff, R.A.; Stradins, P.; Moutinho, H.; Fennell, J.; Ghirardi, M.L.; Cowley, S.W.; Branz, H.M.; Martin, I.T. Angle-Resolved XPS Analysis and Characterization of Monolayer and Multilayer Silane Films for DNA Coupling to Silica. Langmuir. 2013, 29, 4057–4067. [Google Scholar] [CrossRef]
- Meir, R.; Zverzhinetsky, M.; Harpak, N.; Borberg, E.; Burstein, L.; Zeiri, O.; Krivitsky, V.; Patolsky, F. Direct detection of uranyl in urine by dissociation from aptamer-modified nanosensor arrays. Anal. Chem. 2020, 92, 12528–12537. [Google Scholar] [CrossRef]


| Method | Detection Limit | Dynamic Range | Ref |
|---|---|---|---|
| GOx-ZnO NRs-FET | 0.07 μM | 0.07 μM~75 mM | Ahmad et al. [30] |
| Graphene-FET | 1 μM | 1 μM~10 mM | Kim et al. [31] |
| MoS2-FET | 300 nM | 300 nM~30 mM | Shan et al. [32] |
| IGZO-FET | 170 μM | 170 μM~30 mM | Du et al. [33] |
| GOD-GA-Ni/Cu-MOFs-FET | 0.51 μM | 1 μM~20 mM | Wang et al. [34] |
| PABA-graphene FET | 4.1 μM | 10 μM~1 mM | Fenoy et al. [35] |
| APTMS/PEG/SiNW-FET | 10 nM | 10 nM~10 mM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wei, Q.; Jin, Q.; Li, G.; Zhang, Q.; Xiao, H.; Li, T.; Wei, F.; Luo, Y. Polyethylene Glycol Functionalized Silicon Nanowire Field-Effect Transistor Biosensor for Glucose Detection. Nanomaterials 2023, 13, 604. https://doi.org/10.3390/nano13030604
Zhu Y, Wei Q, Jin Q, Li G, Zhang Q, Xiao H, Li T, Wei F, Luo Y. Polyethylene Glycol Functionalized Silicon Nanowire Field-Effect Transistor Biosensor for Glucose Detection. Nanomaterials. 2023; 13(3):604. https://doi.org/10.3390/nano13030604
Chicago/Turabian StyleZhu, Yan, Qianhui Wei, Qingxi Jin, Gangrong Li, Qingzhu Zhang, Han Xiao, Tengfei Li, Feng Wei, and Yingchun Luo. 2023. "Polyethylene Glycol Functionalized Silicon Nanowire Field-Effect Transistor Biosensor for Glucose Detection" Nanomaterials 13, no. 3: 604. https://doi.org/10.3390/nano13030604
APA StyleZhu, Y., Wei, Q., Jin, Q., Li, G., Zhang, Q., Xiao, H., Li, T., Wei, F., & Luo, Y. (2023). Polyethylene Glycol Functionalized Silicon Nanowire Field-Effect Transistor Biosensor for Glucose Detection. Nanomaterials, 13(3), 604. https://doi.org/10.3390/nano13030604







