Recent Advances in Biomass-Based Materials for Oil Spill Cleanup
Abstract
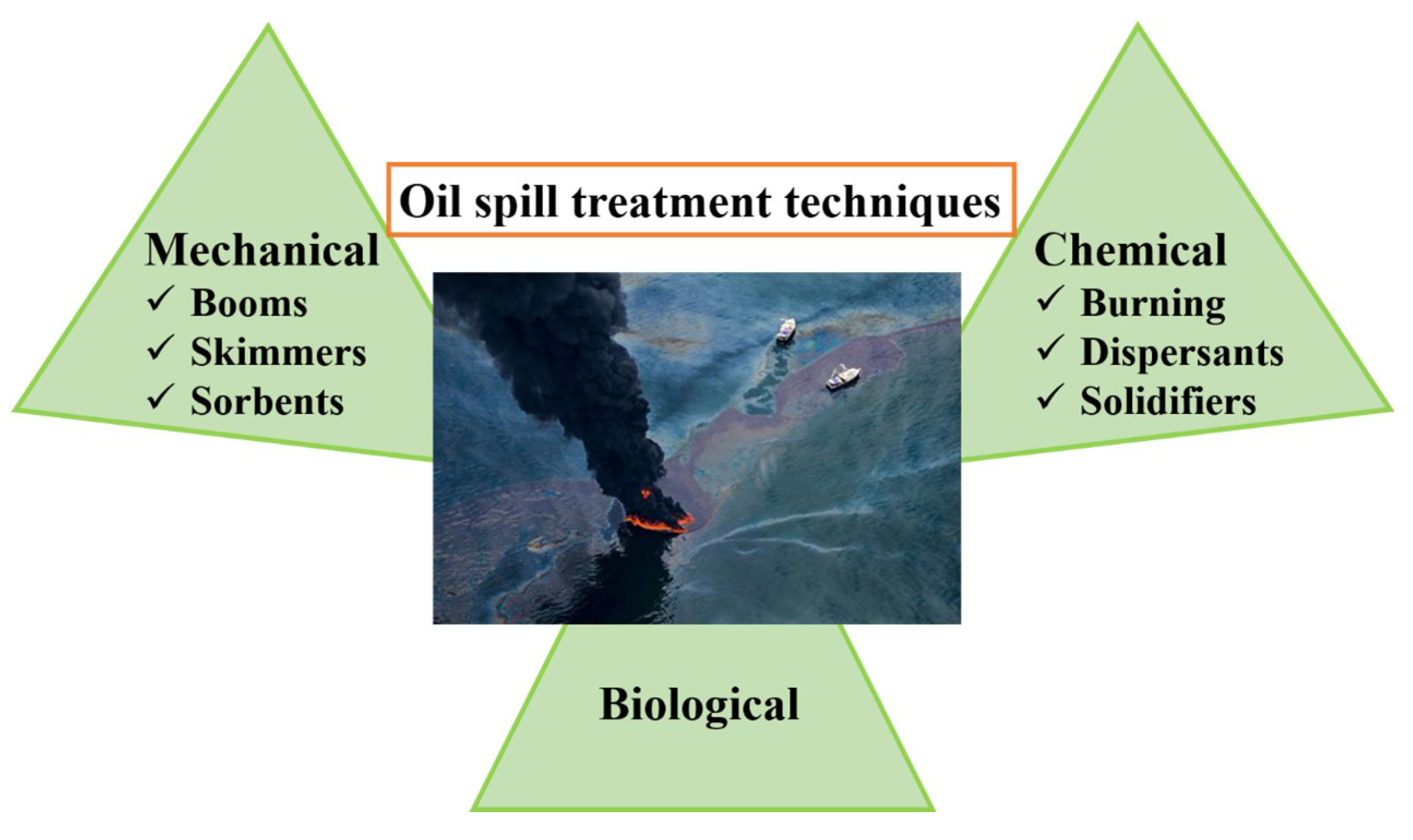
:1. Introduction
2. Basic Theories and Material Characteristics
2.1. Oil/Water Separation
2.1.1. Basic Theories
2.1.2. The Material Characteristics for the Separation of Oil and Water
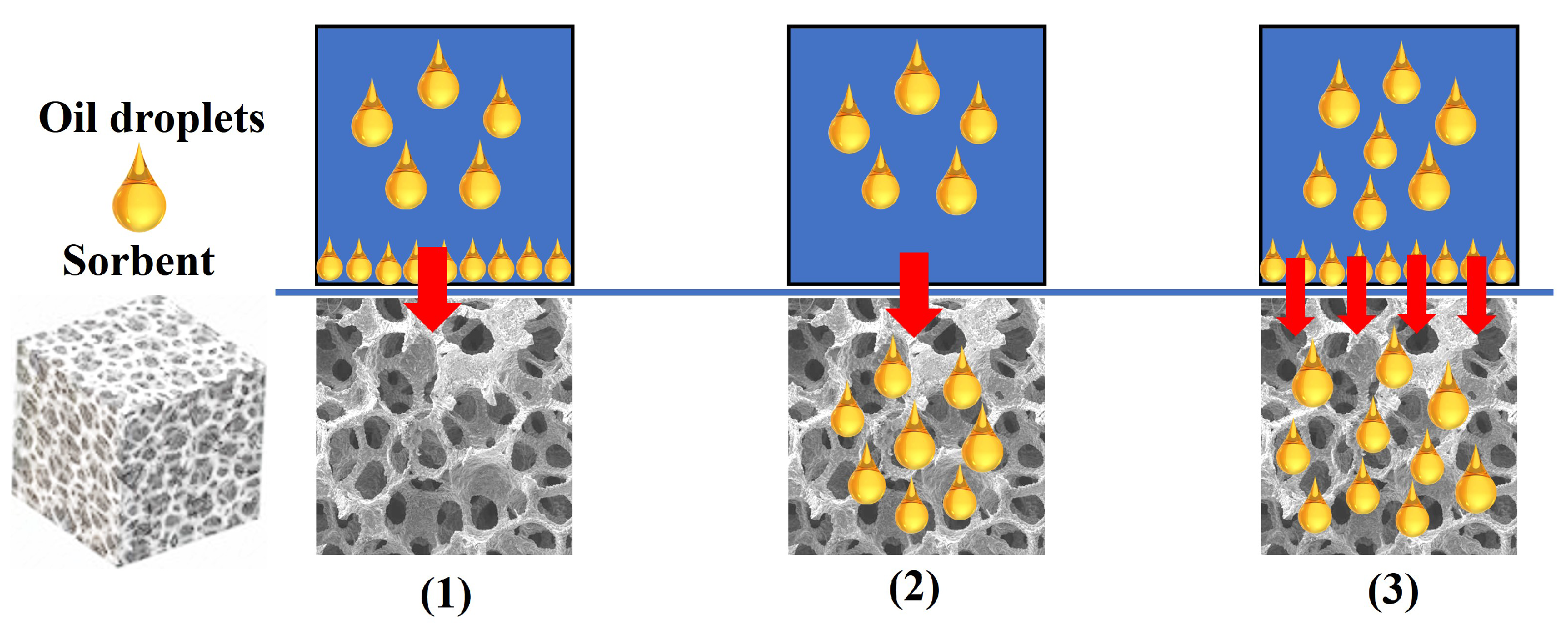
2.2. Oil Sorption
2.2.1. Basic Theories
2.2.2. The Material Characteristics for Oil Adsorption
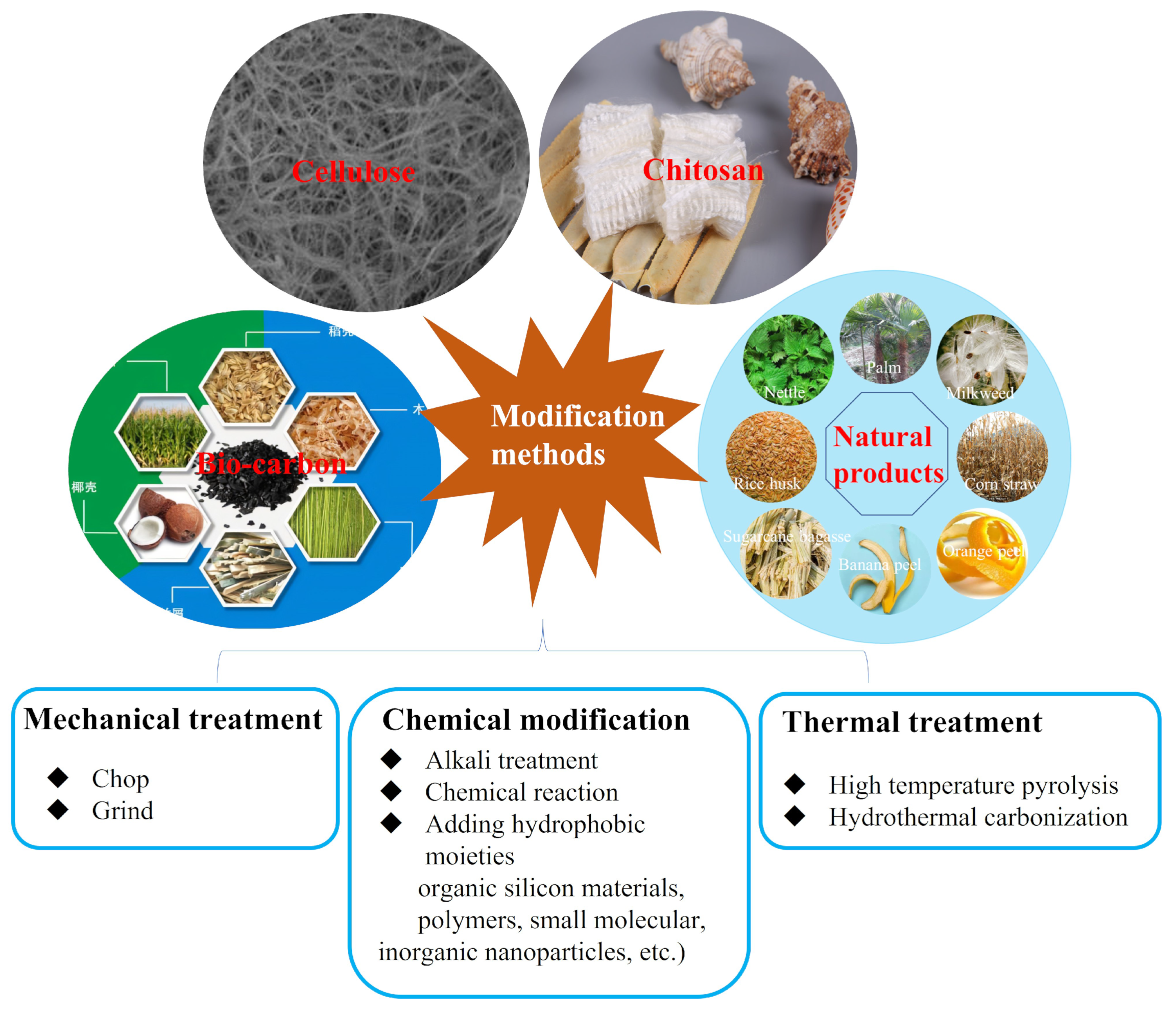
3. Modification Method for Biomass-Based Materials
3.1. Mechanical Treatment
3.2. Thermal Treatment
3.3. Chemical Modification Treatment
3.3.1. Organic Materials

3.3.2. Inorganic Materials

3.3.3. Others
3.4. Environmentally Friendly Modifications
4. Types of Biomass-Based Adsorption Materials
4.1. Cellulose
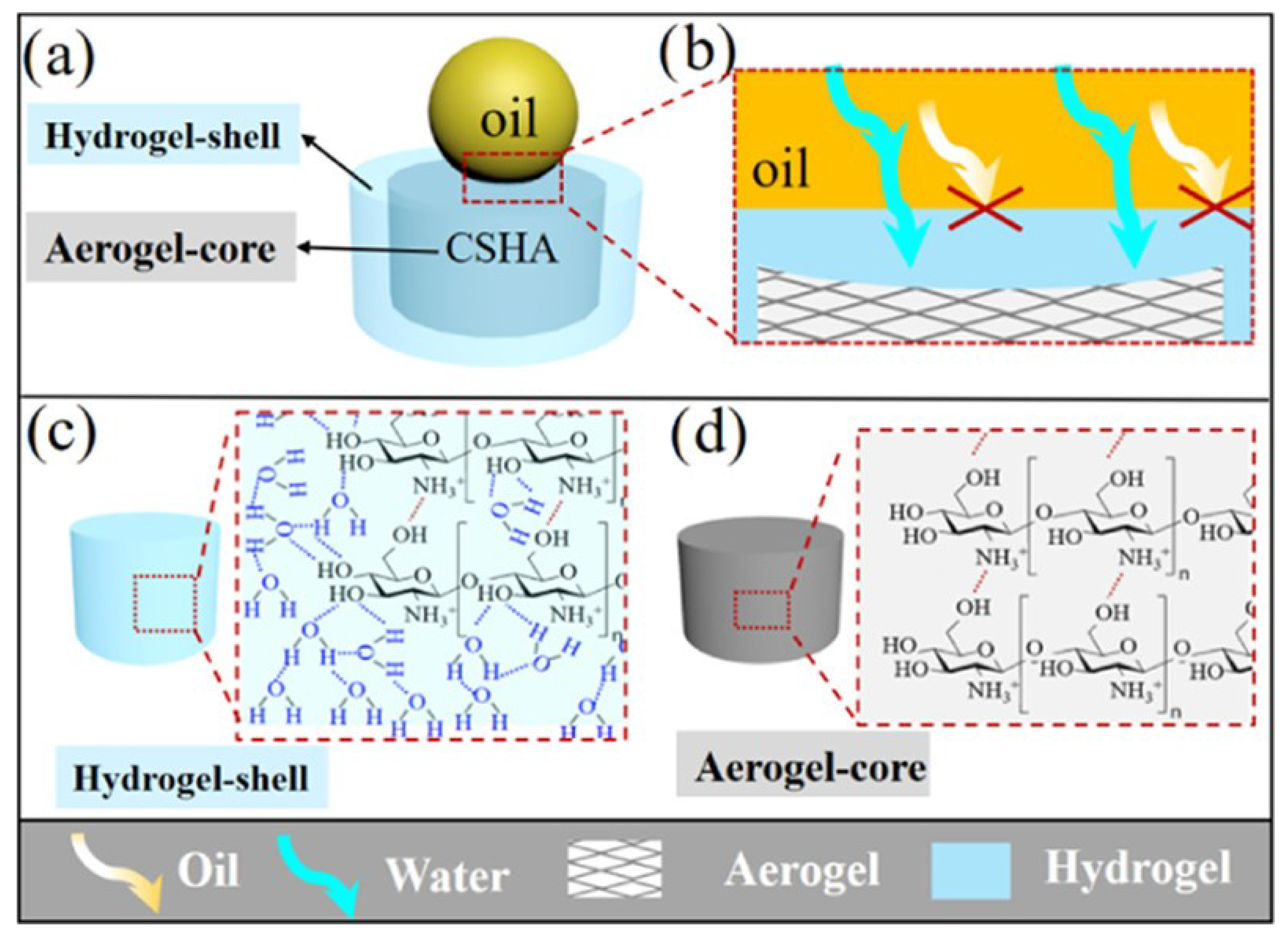
4.2. Chitosan
| Materials | Adsorption/Separation Performance | Recovery, Cycle | Refs. |
|---|---|---|---|
| Cellulose nanocrystals | 35,000 water flux >97% separation efficiency | No water flux declen after 12 >95% after 40 | [28] [127] |
| Ethyl cellulose/carboxymethyl cellulose/ | 92.76% separation efficiency | >95% after 5 | [128] |
| Cellulose pulp gelator | 25–55 times of its own weight | - | [129] |
| Nanocellulose sponge | 90–188 times of its own weight | Squeezing, 94% after 10 | [36] |
| Cellulose acetate membrane | >99% separation efficiency | >98% after 10 | [101] |
| Cellulose fibrous/expanded graphite foam | 8–24 | Squeezing, 60% after 15 | [107] |
| Cellulose nanofibrils/natural rubber latex foam | 13–42 | Squeezing, 48–93.5% after 20 | [29] |
| Cellulose microcrystalline/chitosan membrane | 98.6% separation efficiency | - | [132] |
| Cellulose nanofiber sponge | >99% separation efficiency | - | [133] |
| Pinus elliotii based aerogel | 13.73–19.55 | - | [75] |
| Cellulose nanofiber | 78.8–162.4 | Squeezing, >99% after 20 | [52] |
| Bamboo leaf based cellulose nanofiber | 160–273 times of its own weight | - | [135] |
| Eichhornia crassipes/PVA aerogel | 60.33–152.21 | Squeezing, 66.33% after 16 | [136] |
| Recycled waste cellulose nanofibrils aerogel | 65–205 | Squeezing, 80% after 30 | [137] |
| Cotton fiber aerogel | 19.8–41.5 times of its own weight | Vacuum filtration, >99% after 18 | [81] |
| Lignocellulosic fiber | >99% separation efficiency | >99% separation efficiency after 50 | [30] |
| Nanocellulose/ aerogel | 33.24–68.06 | - | [106] |
| Sugarcane bagasse/PVA aerogel | 25 times of its own weight | - | [73] |
| Cellulose nanocrystals/PVA | 21.2–32.7 times of its own weight | Squeezing, 88.5% after 10 | [87] |
| CNF/tannic acid/castor oil aerogel | 53.2–113.8 | >55% after 10 | [138] |
| Bacterial cellulose/ aerogel | 8–14 | Squeezing, 88% after 1 | [140] |
| Porous biochar/nanofibrous aerogel | 118.5–120.3 | Squeezing, 84% after 5 | [139] |
| Cellulose nanofibris | 38–68 | Heating, 92-95% after 10 | [91] |
| Cellulose/GO/silica NPs aerogel | 67.8–164.5 | Heating, >90% after 10 | [116] |
| Ethyl cellulose/ sponge | 37.8 | Heating, 87.6% after 50 | [79] |
| CNF/PVA/ aerogel | 59–136 times of its own weight | - | [37] |
| Cellulose hydrogel/Wire mesh | 98.9% separation efficiency | 98.2% separation efficiency after 60 | [142] |
| Cellulose hydrogel/Stainless mesh | >99% separation efficiency | 98.9% separation efficiency after 10 | [31] |
4.3. Bio-Carbon Based Materials
| Materials | Adsorption/Separation Performance | Recovery, Cycle | Refs. |
|---|---|---|---|
| Chitosan/sponge | 23-60 | Squeezing, 80% after 10 | [109] |
| Nanofibrillated cellulose/chitosan foam | - | - | [143] |
| Chitosan// | 98% separation efficiency | - | [84] |
| Chitosan/aerogel | 34.1–54.2 | - | [38] |
| Chitosan-acetic acid/aerogel | 31–63 times of its own weight | Squeezing, >99% after 10 | [76] |
| Chitosan-hydrogel/chitosan aerogel | 99% separation efficiency | Extraction, >99% after 20 | [145] |
| Unidirectional chitosan aerogel | 53–117 times of its own weight | Squeezing, 94.17% after 50 | [89] |
| Chitosan/r-GO/polydopamine | 12–21 times of its own weight | Extraction, >90% after 11 | [110] |
| Chitosan/nanofibrillated cellulose aerogel | >99% separation efficiency | Heating, 98% after 40 | [32] |
| Raw Materials | Aadsorption/Separation Performance | Recovery, Cycle | Refs. |
|---|---|---|---|
| Lignin | 24 times of its own weight | burning, 95% after 7 | [50] |
| Typa orientalis fibers | 42–160 | - | [39] |
| Poplars catkin microfibers | 81-161 | Heating, >99% after 10 | [146] |
| Liquidambar formosana | 2–2.90 | Extraction, >96%after 10 | [147] |
| Sisal leaves | 90-188 times of its own weight | Squeezing, >86% after 10 | [148] |
| Bamboo pulp fibers | 510–150 times of its own weight | Extraction, >89% after 5 | [149] |
| Sisal and premna microphylla | 77.7–147.3 | Extraction, >99% after 10 | [150] |
| Corn bracts | 77.67–143.63 times of its own weight | Squeezing, >90% after 10 | [152] |
| Banana peel/wastepaper | 35-115 times of its own weight | - | [153] |
| Peanut shells | 27–50 | Extraction, 94% after 9 | [154] |
| Pomelo peels | 5–36 | Extraction, 72.15–98% after 5 | [19] |
| Waste durian shell | 3-19 | Extraction, 75.31–95% after 5 | [155] |
| Starch | 36–45 | - | [156] |
| Rice straw | 29–33 times of its own weight | Heating, >99% after 5 | [65] |
| Enteromorpha | 62–140 | Heating and extraction, >99% after 10 | [120] |
| Lignin/GO | 32.5–34 | - | [117] |
| Bacterial cellulose/reduced GO | 245–598 times of its own weight | Burning, 94.6% after 10 | [157] |
| Cotton/reduced GO | 16–27 times of its own weight | Burning, 98% after 10 | [158] |
| Konjac glucomannan/reduced GO | 54-360 times of its own weight | Burning, >99% after 10 | [159] |
| CNF/PVA/GO | 57–97 | Burning, 51% after 10 | [160] |
| Anisotropic GO/PVA/CNF | 155–287 | Burning, 90% after 10 | [161] |
| Cellulose// | 60–120 | Squeezing, 99% after 10 | [115] |
| Kapok fibers/ | 28.3-58.1 times of its own weight | Heating, 85.3–90.1% after 5 | [162] |
| Starch/ZnO | 23–30 times of its own weight | Burning, 98.9% after 20 | [163] |
| Popcorn | 10–10.83 | Heating, 65.7–93.6% after 5 | [164] |
| Fe/egg yolk | 28–78 times of its own weight | Heating, >99% after 6 | [45] |
| Cotton balls | 61–113 times of its own weight | Heating, >99% after 5 | [93] |
| Bacterial cellulose | 37–87 | Heating, 71% after 5 | [165] |
4.4. Natural Products
4.5. Others
| Materials | Adsorption/Separation Performance | Recovery, Cycle | Refs. |
|---|---|---|---|
| Lignin/GO | 42–102 | Combustion, 90% after 20 | [40] |
| Lignin/melamine/PVA | 2–11 times of its own weight, >94% separtion | Extraction and distillation, 90% after 10 | [77] |
| Lignin/cotton | 36–46 times of its own weight | Extraction, >90.92% | [98] |
| Lignin/GO sheets/nanofibers | 43–103 | Squeezing, around 50% after 1 | [178] |
| Alginate-Ca/Zr | 11.2–25.9 | Extraction, 50% after 6 | [179] |
| Alginate/TiO2 NPs | 98.7–99.7% separation efficiency | Reusing, 99.5% after 60 | [180] |
| Alginate/TiO2/RGO | 99.96% separation efficiency | 69.5% after 60 and 30 min irradiation to recover | [33] |
| Alginate/nano- | 75.5–115.7% weight gain | 53.7% after 1 | [51] |
| Alginate/GO/ | 17.92–43.92 | Squeezing, 89.8% after 10 | [41] |
| Alginate/Typha orientalis fibers/GO | 98.2% separation efficiency | Squeezing, 90% after 20 | [181] |
| Alginate/tubular Kapok fibers | 29.6–62.8 | Squeezing, 81-89.8% after 10 | [182] |
| Peptide/polysaccharide Konjac glucomannan | 30–180% weight gain | - | [183] |
| Gelatin/silica | 2–27 times of its own weight | Heating, >99% after 10 | [83] |
| Gelatin//PEI | 99.72% oil rejection coefficient | - | [34] |
| Seeweed polysaccharide agar/genipin | >97% water rejection | - | [88] |
| Silk fibroin/polymethylsilsesquioxane | >2500 | Squeezing, 99% after 6 | [97] |
| Silk fibroin/sodium dodecyl sulfate | 81.2–130 | Squeezing, 89% after 10 | [42] |
| Collagen/cellulose | 20–60 times of its own weight | Burning, 95% after 7 | [124] |
| Collagen | 99.95% separation efficiency | 99.9% separtion efficiency after 10 | [69] |
| Collagen/ZIF-8 | 99.99% separation efficiency | Extraction, 78.63% after 6 | [35] |
5. Discussion, Future Perspectives, and Conclusions
5.1. Discussion
5.2. Future Perspectives
- Deeply understanding the sorption mechanisms. For materials with different surface chemistry and nanostructure, the oil uptake kinetics and mechanisms of sorption process may different, and it is also different when they under different working environmental conditions. Therefore, to efficient control and monitor the whole sorption process and achieve the durable application of sorbents, the kinetic and mechanisms of the oil sorption process under special environmental conditions, such as low temperature, strong acid, strong base, or high salinity, and so forth, still need to deeply investigate and understand.
- Developing environmental-friendly and cost-effectiveness modification technologies. It’s inevitable to do modification for the designed biomass-based materials so that to obtain superior wettability even though many researchers have done numerous efforts to prepare biomass-based sorbents via a straightforward and feasible approach. It is better to produce effective spill oil sorption materials with environmental-friendly, low-cost, and feasible modification methods without scarifies the superior properties of biomass materials.
- Designing high-performance and intelligent biomass composites. Typically, biomass-based materials are carbon-based small molecular or polymer, it is desirable to take the best advantage of different materials in various field for achieving highly efficient and intelligent oil sorbents.
- Promoting the mass and industrial production techniques. Currently, most of biomass-based sorbents have been fabricated in the laboratory and cannot be produced in large scale. The information and research relating to the mass production techniques for the industry is limited.
5.3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced sorbents for oil-spill cleanup: Recent advances and future perspectives. Adv. Mater. 2016, 28, 10459–10490. [Google Scholar] [CrossRef] [PubMed]
- EPA. Oil Spill Response Techniques. 2017. Available online: https://www.epa.gov/emergency-response/epas-response-techniques (accessed on 1 December 2022).
- Adofo, Y.K.; Nyankson, E. Dispersants as an oil spill clean-up technique in the marine environment: A review. Heliyon 2022, 8, e10153. [Google Scholar] [CrossRef] [PubMed]
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [PubMed]
- Fingas, M. The Basics of Oil Spill Cleanup, 3rd ed.; Lewis Publication: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Elkin, A.A.; Makarov, S.O.; Cunningham, C.J.; Peshkur, T.A.; Atlas, R.M.; Philp, J.C. Oil spill problems and sustainable response strategies through new technologies. Environ. Sci. Process Imp. 2015, 17, 1201–1219. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Thomas, A.G.; Matthews, A.; Lewis, D.J. Surface engineering of ceramic nanomaterials for separation of oil/water mixtures. Front. Chem. 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.V.; Pinna, A.; Carbonaro, C.M.; Malfatti, L.; Guardia, P.; Cabot, A.; Casula, M.F. Performance of oil sorbents based on reduced graphene oxide-silica composite aerogels. J. Environ. Chem. Eng. 2020, 8, 103632. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Khaskhoussi, A.; Proverbio, E.; Milone, C. Thermo-physical characterization of carbon nanotube composite foam for oil recovery applications. Nanomaterials 2020, 10, 86. [Google Scholar] [CrossRef]
- Viju, S.; Brindha, R.; Thilagavathi, G. Surface modification of nettle fibers by grafting to improve oil sorption capacity. J. Ind. Text. 2019, 50, 1314–1329. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodyńska, D.; Franus, W. Application of mineral sorbents for removal of petroleum substances: A review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef]
- Wen, G.; Guo, Z.; Liu, W. Biomimetic polymeric superhydrophobic surfaces and nanostructures: From fabrication to applications. Nanoscale 2017, 9, 3338–3366. [Google Scholar] [CrossRef]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Tayeb, A.M.; Farouq, R.; Mohamed, O.A.; Tony, M.A. Oil spill clean-up using combined sorbents: A comparative investigation and design aspects. Int. J. Environ. Anal. Chem. 2019, 100, 311–323. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Zhang, X.; Tang, Y.; Zhou, X.; Zhang, K.; Chen, Z.; Lai, Y. Rational design of materials interface at nanoscale towards intelligent oil-water separation. Nanoscale Horiz. 2018, 3, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Qing, G.; Su, B.; Jiang, L. Functional biointerface materials inspired from nature. Chem. Soc. Rev. 2011, 40, 2909–2921. [Google Scholar] [CrossRef]
- Zamparas, M.; Tzivras, D.; Dracopoulos, V.; Ioannides, T. Application of sorbents for oil spill cleanup focusing on natural-based modified materials: A review. Molecules 2020, 25, 4522. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Rasit, N.; Ooi, C.K.; Ma, N.L.; Ng, J.H.; Lam, W.H.; Chong, C.T.; Chase, H.A. Pyrolysis production of fruit peel biochar for potential use in treatment of palm oil mill effluent. J. Environ. Manag. 2018, 213, 400–408. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Wang, Y.; You, L.; Shen, X.; Li, S. An environmentally friendly carbon aerogels derived from waste pomelo peels for the removal of organic pollutants/oils. Microporous Mesoporous Mater. 2017, 241, 285–292. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Johnson, A. Nonconventional low-cost cellulose- and keratin-based biopolymeric sorbents for oil/water separation and spill cleanup: A review. Environ. Sci. Technol. 2017, 47, 964–1001. [Google Scholar] [CrossRef]
- Nakajima, A. Design of hydrophobic surfaces for liquid droplet control. NPG Asia Mater. 2011, 3, 49–56. [Google Scholar] [CrossRef]
- Kamarudin, N.H.; Harun, Z.; Othman, M.H.D.; Abdullahi, T.; Syamsul Bahri, S.; Kamarudin, N.H.; Yunos, M.Z.; Wan Salleh, W.N. Waste environmental sources of metakaolin and corn cob ash for preparation and characterisation of green ceramic hollow fibre membrane (h-MCa) for oil-water separation. Ceram. Int. 2020, 46, 1512–1525. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. J. Environ. Manag. 1944, 240, 546–551. [Google Scholar] [CrossRef]
- Fan, Q.W.; Lu, T.; Deng, Y.K.; Zhang, Y.Y.; Ma, W.J.; Xiong, R.H.; Huang, C.B. Bio-based materials with special wettability for oil-water separation. Sep. Purif. Technol. 2022, 297, 121445. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Guo, Z. Designing novel superwetting surfaces for high-efficiency oil-water separation: Design principles, opportunities, trends and challenges. J. Mater. Chem. A 2020, 8, 16831–16853. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Si, Y.; Yu, J.; Ding, B. Rational design of electrospun nanofibrous materials for oil/water emulsion separation. Mater. Chem. Front. 2021, 5, 97–128. [Google Scholar] [CrossRef]
- Wu, M.-B.; Zhang, C.; Pi, J.-K.; Liu, C.; Yang, J.; Xu, Z.-K. Cellulose nanocrystals as anti-oil nanomaterials for separating crude oil from aqueous emulsions and mixtures. J. Mater. Chem. A 2019, 7, 7033–7041. [Google Scholar] [CrossRef]
- Lorevice, M.V.; Mendonça, E.O.; Orra, N.M.; Borges, A.C.; Gouveia, R.F. Porous cellulose nanofibril-natural rubber latex composite foams for oil and organic solvent absorption. Appl. Nano Mater. 2020, 3, 10954–10965. [Google Scholar] [CrossRef]
- Kang, L.; Wang, B.; Zeng, J.; Cheng, Z.; Li, J.; Xu, J.; Gao, W.; Chen, K. Degradable dual superlyophobic lignocellulosic fibers for high-efficiency oil/water separation. Green Chem. 2020, 22, 504–512. [Google Scholar] [CrossRef]
- Xie, X.; Liu, L.; Zhang, L.; Lu, A. Strong cellulose hydrogel as underwater superoleophobic coating for efficient oil/water separation. Carbohydr. Polym. 2020, 229, 115467. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Shi, R.; Chen, L.; Fan, M. A robust salt-tolerant superoleophobic chitosan/nanofibrillated cellulose aerogel for highly efficient oil/water separation. Carbohydr. Polym. 2018, 200, 611–615. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, J.; Ghaffar, S.H.; Yu, Y.; Tian, Q.; Fan, M. Development of highly efficient, renewable and durable alginate composite aerogels for oil/water separation. Surf. Coat. Technol. 2020, 388, 125551. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem. Eng. J. 2019, 358, 1539–1551. [Google Scholar] [CrossRef]
- Xiao, H.; Cui, Y.; Wang, Y.; Li, H.; Chen, G.; Huang, X.; Shi, B. Synergistic combination of the capillary effect of collagen fibers and size-sieving merits of metal–organic frameworks for emulsion separation with high flux. Ind. Eng Chem Res. 2020, 59, 14925–14934. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Kongparakul, S.; Samart, C.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Fabrication and evaluation of nanocellulose sponge for oil/water separation. Carbohydr. Polym. 2018, 190, 184–189. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, X.; Zhou, H.; Li, J. Preparation of magnetic hydrophobic polyvinyl alcohol (PVA)—Cellulose nanofiber (CNF) aerogels as effective oil absorbents. Cellulose 2017, 25, 1217–1227. [Google Scholar] [CrossRef]
- Bidgoli, H.; Khodadadi, A.A.; Mortazavi, Y. A hydrophobic/oleophilic chitosan-based sorbent: Toward an effective oil spill remediation technology. J. Environ. Chem. Eng. 2019, 7, 103340. [Google Scholar] [CrossRef]
- Yang, J.; Xu, P.; Xia, Y.; Chen, B. Multifunctional carbon aerogels from typha orientalis for oil/water separation and simultaneous removal of oil-soluble pollutants. Cellulose 2018, 25, 5863–5875. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, Y.; Li, J.; Cheng, W.; Han, G.; Lu, T.; Huang, C.; Wu, Q.; Jiang, J. High strength and ultralight lignin-mediated fire-resistant aerogel for repeated oil/water separation. Carbon 2022, 193, 285–297. [Google Scholar] [CrossRef]
- Liu, X.; Huang, P.; Wang, J.; He, Y.; Song, P.; Wang, R. Preparation of porous biomass-based sponge with zein-alginate for oil absorption. Water Environ. J. 2022, 36, 704–712. [Google Scholar] [CrossRef]
- Vadodariya, N.; Meena, R. Protein-functionalized aerogel membranes for gravity-driven separation. ACS Sustain. Chem. Eng. 2019, 7, 4814–4820. [Google Scholar] [CrossRef]
- Lei, E.; Li, W.; Ma, C.; Liu, S. An ultra-lightweight recyclable carbon aerogel from bleached softwood kraft pulp for efficient oil and organic absorption. Mater. Chem. Phys. 2018, 214, 291–296. [Google Scholar]
- Doshi, B.; Sillanpaa, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Rezazad, H.; Vahdati-Khajeh, S.; Eftekhari-Sis, B. Egg yolk biomass derived carbon material as a highly efficient and reusable hydrophobic oil-absorbent. J. Porous Mater. 2020, 27, 1439–1446. [Google Scholar] [CrossRef]
- Chen, C.; Li, F.; Zhang, Y.; Wang, B.; Fan, Y.; Wang, X.; Sun, R. Compressive, ultralight and fire-resistant lignin-modified graphene aerogels as recyclable absorbents for oil and organic solvents. Chem. Eng. J. 2018, 350, 173–180. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Cellulose nanofibril aerogels: Synergistic improvement of hydrophobicity, strength, and thermal stability via cross-Linking with diisocyanate. ACS Appl. Mater. Interfaces 2017, 9, 2825–2834. [Google Scholar] [CrossRef]
- Dan, Y.; Popowski, Y.; Buzhor, M.; Menashe, E.; Rachmani, O.; Amir, E. Covalent surface modification of cellulose-based textiles for oil-water separation applications. Ind. Eng. Chem. Res. 2020, 59, 5456–5465. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Lu, Z.; Chen, L.; Huang, L.; Fan, M. A robust superhydrophobic TiO2 NPs coated cellulose sponge for highly efficient oil-water separation. Sci. Rep. 2017, 7, 9428. [Google Scholar] [CrossRef]
- Rao, G.-S.; Nabipour, H.; Zhang, P.; Wang, X.; Xing, W.; Song, L.; Hu, Y. Lightweight, hydrophobic and recyclable carbon foam derived from lignin-resorcinol-glyoxal resin for oil and solvent spill capture. J. Mater. Res. and Technol. 2020, 9, 4655–4664. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, X.; Li, Y.; Yin, Z.; Bao, M. Construction of a superhydrophobic sodium alginate aerogel for efficient oil absorption and emulsion separation. Langmuir 2021, 37, 882–893. [Google Scholar] [CrossRef]
- Rafieian, F.; Hosseini, M.; Jonoobi, M.; Yu, Q. Development of hydrophobic nanocellulose-based aerogel via chemical vapor deposition for oil separation for water treatment. Cellulose 2018, 25, 4695–4710. [Google Scholar] [CrossRef]
- Li, Z.; Shao, L.; Hu, W.; Zheng, T.; Lu, L.; Cao, Y.; Chen, Y. Excellent reusable chitosan/cellulose aerogel as an oil and organic solvent absorbent. Carbohydr. Polym. 2018, 191, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Aghamiri, S.F.; Moheb, A.; Vakili-Nezhaad, G.R. Oil spill cleanup from sea water by sorbent materials. Chem. Eng. Technol. 2005, 28, 1525–1528. [Google Scholar] [CrossRef]
- Ibrahim, S.; Wang, S.; Ang, H.M. Removal of emulsified oil from oily wastewater using agricultural waste barley straw. Biochem. Eng. J. 2010, 49, 78–83. [Google Scholar] [CrossRef]
- Anuzyte, E.; Vaisis, V. Natural oil sorbents modification methods for hydrophobicity improvement. Energy Procedia 2018, 147, 295–300. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; Ahmad El Saqa, F.; Abd El Razek Amer, A.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Wang, J.; Han, F.; Liang, B.; Geng, G. Hydrothermal fabrication of robustly superhydrophobic cotton fibers for efficient separation of oil/water mixtures and oil-in-water emulsions. J. Ind. Eng. Chem. 2017, 54, 174–183. [Google Scholar] [CrossRef]
- Wang, J.; Wang, A.; Wang, W. Robustly superhydrophobic/superoleophilic kapok fiber with ZnO nanoneedles coating: Highly efficient separation of oil layer in water and capture of oil droplets in oil-in-water emulsions. Ind. Crop Prod. 2017, 108, 303–311. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Kravanja, G.; Knez, Ž. Hydrothermal treatment of biomass for energy and chemicals. Energy 2016, 116, 1312–1322. [Google Scholar] [CrossRef]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Hu, S.; Yu, Z.; Fang, S. Effect of hydrothermal carbonization temperature on combustion behavior of hydrochar fuel from paper sludge. Appl. Therm. Eng. 2015, 91, 574–582. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon aerogel from winter melon for highly efficient and recyclable oils and organic solvents absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Guan, H.; Cheng, Z.; Wang, X. Highly compressible wood sponges with a spring-like lamellar structure as effective and reusable oil absorbents. ACS Nano 2018, 12, 10365–10373. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, H.; Li, H.; Yue, X.; Jiang, L.; Shi, F.; Liu, J. Ultralight biomass-based carbon aerogel with hierarchical pore structure fabricated using unidirectional freeze casting and potassium hydroxide activation. Mater. Lett. 2022, 317, 132081. [Google Scholar] [CrossRef]
- Onwuka, J.C.; Agbaji, E.B.; Ajibola, V.O.; Okibe, F.G. Treatment of crude oil-contaminated water with chemically modified natural fiber. Appl. Water Sci. 2018, 8, 86. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, J.; Shi, Y.; Pei, C. Wettability-switchable bacterial cellulose/polyhemiaminal nanofiber aerogels for continuous and effective oil/water separation. Cellulose 2018, 25, 2987–2996. [Google Scholar] [CrossRef]
- Mahmoud, M.A. Oil spill cleanup by raw flax fiber: Modification effect, sorption isotherm, kinetics and thermodynamics. Arab. J. Chem. 2020, 13, 5553–5563. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, G.; Liu, Y.; Fan, W.; Yang, K.; Li, Z. A reusable, biomass-derived, and pH-responsive collagen fiber based oil absorbent material for effective separation of oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127906. [Google Scholar] [CrossRef]
- Zanini, M.; Lavoratti, A.; Lazzari, L.K.; Galiotto, D.; Pagnocelli, M.; Baldasso, C.; Zattera, A.J. Producing aerogels from silanized cellulose nanofiber suspension. Cellulose 2016, 24, 769–779. [Google Scholar] [CrossRef]
- Do, N.H.N.; Luu, T.P.; Thai, Q.B.; Le, D.K.; Chau, N.D.Q.; Nguyen, S.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Advanced fabrication and application of pineapple aerogels from agricultural waste. Mater. Technol. 2019, 35, 807–814. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton aerogels and cotton-cellulose aerogels from environmental waste for oil spillage cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Thai, Q.B.; Nguyen, S.T.; Ho, D.K.; Tran, T.D.; Huynh, D.M.; Do, N.H.N.; Luu, T.P.; Le, P.K.; Le, D.K.; Phan-Thien, N.; et al. Cellulose-based aerogels from sugarcane bagasse for oil spill-cleaning and heat insulation applications. Carbohydr. Polym. 2020, 228, 115365. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Qian, Y.; Tan, F.; Cai, W.; Li, Y.; Cao, Y. Controllable synthesis of pomelo peel-based aerogel and its application in adsorption of oil/organic pollutants. R. Soc. Open Sci. 2019, 6, 181823. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.B.; Godinho, M.; Zattera, A.J. Oils sorption on hydrophobic nanocellulose aerogel obtained from the wood furniture industry waste. Cellulose 2018, 25, 3105–3119. [Google Scholar] [CrossRef]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile fabrication of wood-inspired aerogel from chitosan for efficient removal of oil from Water. J. Hazard. Mater. 2020, 385, 121507. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, P.; Zhang, N.; Gibril, M.E.; Kong, F.; Wang, S. A high lignin-content, ultralight, and hydrophobic aerogel for oil-water separation: Preparation and characterization. J. Porous Mater. 2021, 28, 1881–1894. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Politowicz, A.L.; Chen, E.; Huang, H.-X.; Turng, L.-S. Highly compressible ultra-light anisotropic cellulose/graphene aerogel fabricated by bidirectional freeze drying for selective oil absorption. Carbon Interfaces 2018, 132, 199–209. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Liu, L.; Yuan, W. Environmental-friendly and magnetic/silanized ethyl cellulose sponges as effective and recyclable oil-absorption materials. Carbohydr. Polym. 2017, 173, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, W. Superhydrophobic/superoleophilic and reinforced ethyl cellulose sponges for oil/water separation: Synergistic strategies of cross-linking, carbon nanotube composite, and nanosilica modification. ACS Appl. Mater. Interfaces 2017, 9, 29167–29176. [Google Scholar] [CrossRef]
- Laitinen, O.; Suopajarvi, T.; Osterberg, M.; Liimatainen, H. Hydrophobic, superabsorbing aerogels from choline chloride-based deep eutectic solvent pretreated and silylated cellulose nanofibrils for selective oil removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Zhang, L.; Zhong, Y.; Xu, H.; Mao, Z.; Wang, B.; Sui, X. Thiol-ene click reaction on cellulose sponge and its application for oil/water separation. RSC Adv. 2017, 7, 20147–20151. [Google Scholar] [CrossRef]
- Yun, L.; Zhao, J.; Kang, X.; Du, Y.; Yuan, X.; Hou, X. Preparation and properties of monolithic and hydrophobic gelatin–silica composite aerogels for oil absorption. J. Sol-Gel Sci. Technol. 2017, 83, 197–206. [Google Scholar] [CrossRef]
- Zhang, S.; Lü, T.; Qi, D.; Cao, Z.; Zhang, D.; Zhao, H. Synthesis of quaternized chitosan-coated magnetic nanoparticles for oil-water separation. Mater. Lett. 2017, 191, 128–131. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Jiang, X.; Li, J.; Huang, F. Facile synthesis of reduced graphene oxide/trimethyl chlorosilane-coated cellulose nanofibres aerogel for oil absorption. IET Nanobiotechnol. 2017, 11, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Yuan, Y.; Ren, J.; Yuan, B.; He, Q.; Xia, G.; Chen, F.; Song, R. Facile and green fabrication of cellulosed based aerogels for lampblack filtration from waste newspaper. Carbohydr. Polym. 2017, 162, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, Y.; Zeng, H.; Betti, M.; Chen, L. Highly porous, hydrophobic, and compressible cellulose nanocrystals/poly(vinyl alcohol) aerogels as recyclable absorbents for oil-water separation. ACS Sustain. Chem. Eng. 2019, 7, 11118–11128. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Xue, C.; Yu, H.; Li, Y. Facile fabrication of bubbles-enhanced flexible bioaerogels for efficient and recyclable oil adsorption. Chem. Eng. J. 2020, 402, 126240. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.L.; Cheng, J.B.; Zhang, A.N.; Wang, Y.Z.; Zhao, H.B. Fully bio-based, low fire-hazard and superelastic aerogel without hazardous cross-linkers for excellent thermal insulation and oil clean-up absorption. J. Hazard. Mater. 2021, 403, 123977. [Google Scholar] [CrossRef]
- Deuber, F.; Mousavi, S.; Federer, L.; Adlhart, C. Amphiphilic nanofiber-based aerogels for selective liquid absorption from electrospun biopolymers. Adv. Mater. Interface 2017, 4, 1700065. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Huang, H.-X.; Peng, X.-F.; Turng, L.-S. Superhydrophobic graphene/cellulose/silica aerogel with hierarchical structure as superabsorbers for high efficiency selective oil absorption and recovery. Ind. Eng. Chem. Res. 2018, 57, 1745–1755. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; Tang, H.; Zhou, J. Fabrication of hydrophobic cellulosic materials via gas-solid silylation reaction for oil/water separation. Cellulose 2019, 26, 4021–4037. [Google Scholar] [CrossRef]
- Lu, Y.; Niu, Z.; Yuan, W. Multifunctional magnetic superhydrophobic carbonaceous aerogel with micro/nano-scale hierarchical structures for environmental remediation and energy storage. Appl. Surf. Sci. 2019, 480, 851–860. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, H.; Zang, D.; Zhou, Y.; Liu, F.; Huang, X.; Chang, J.-S.; Wang, C.; Ho, S.-H. Preparation of a new superhydrophobic/superoleophilic corn straw fiber used as an oil absorbent for selective absorption of oil from water. Bioresour. Bioprocess. 2018, 5, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Chai, W.; Liu, X.; Xu, Y.; Zhou, S. Kapok fiber as a natural source for fabrication of oil absorbent. J. Chem. Technol. Biotechnol. 2017, 92, 1613–1619. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Cai, W.; Cao, Y.; Sun, Y.; Tan, F. Preparation of corn straw based spongy aerogel for spillage oil capture. Korean J. Chem. Eng. 2018, 35, 1119–1127. [Google Scholar] [CrossRef]
- Maleki, H.; Whitmore, L.; Husing, N. Novel multifunctional polymethylsilsesquioxane-silk fibroin aerogel hybrids for environmental and thermal insulation applications. J. Mater. Chem. A 2018, 6, 12598–12612. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Jiang, Y.-H.; Qin, Y.-N.; An, Q.-D.; Xiao, L.-P.; Wang, Z.-H.; Xiao, Z.-Y.; Zhai, S.-R. Cooperative construction of oil/water separator using renewable lignin and PDMS. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128790. [Google Scholar] [CrossRef]
- Wang, K.; Liu, X.; Tan, Y.; Zhang, W.; Zhang, S.; Li, J. Two-dimensional membrane and three-dimensional bulk aerogel materials via top-down wood nanotechnology for multibehavioral and reusable oil/water separation. Chem. Eng. J. 2019, 371, 769–780. [Google Scholar] [CrossRef]
- Durgadevi, N.; Swarnalatha, V. Polythiophene functionalized hydrophobic cellulose kitchen wipe sponge and cellulose fabric for effective oil-water separation. RSC Adv. 2017, 7, 34866–34874. [Google Scholar] [CrossRef]
- Ma, W.; Guo, Z.; Zhao, J.; Yu, Q.; Wang, F.; Han, J.; Pan, H.; Yao, J.; Zhang, Q.; Samal, S.K.; et al. Polyimide/cellulose acetate core/shell electrospun fibrous membranes for oil-water separation. Sep. Purif. Technol. 2017, 177, 71–85. [Google Scholar] [CrossRef]
- Shang, Q.; Cheng, J.; Bo, C.; Hu, Y.; Liu, C.; Yang, X.; Hu, L.; Zhou, Y.; Lei, W. Durable superhydrophobic cotton fabric from cardanol/POSS-based polybenzoxazine for high-efficiency oil/water separation. Cellulose 2020, 29, 6425–6440. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Chu, B.; Hsiao, B.S. Super-hydrophobic modification of porous natural polymer “luffa sponge” for oil absorption. Polymer 2017, 126, 470–476. [Google Scholar] [CrossRef]
- Li, N.; Yue, Q.; Gao, B.; Xu, X.; Su, R.; Yu, B. One-step synthesis of peanut hull/graphene aerogel for highly efficient oil-water separation. J. Clean. Prod. 2019, 207, 764–771. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, L.; Grishkewich, N.; Tam, K.C.; Yuan, J.; Mao, Z.; Sui, X. CO2-responsive cellulose nanofibers aerogels for switchable oil-water separation. ACS Appl. Mater. Interfaces 2019, 11, 9367–9373. [Google Scholar] [CrossRef] [PubMed]
- Chhajed, M.; Yadav, C.; Agrawal, A.K.; Maji, P.K. Esterified superhydrophobic nanofibrillated cellulose based aerogel for oil spill treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef] [PubMed]
- Calcagnile, P.; Caputo, I.; Cannoletta, D.; Bettini, S.; Valli, L.; Demitri, C. A bio-based composite material for water remediation from oily contaminants. Mater. Des. 2017, 134, 374–382. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S.; et al. Magnetic nanocellulose-magnetite aerogel for easy oil adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Yang, H.; Zhao, H.; Liu, Y.; Chen, R. Recyclable and biodegradable superhydrophobic and superoleophilic chitosan sponge for the effective removal of oily pollutants from water. Chem. Eng. J. 2017, 330, 423–432. [Google Scholar] [CrossRef]
- Cao, N.; Lyu, Q.; Li, J.; Wang, Y.; Yang, B.; Szunerits, S.; Boukherroub, R. Facile synthesis of fluorinated polydopamine/chitosan/ reduced graphene oxide composite aerogel for efficient oil/water separation. Chem. Eng. J. 2017, 326, 17–28. [Google Scholar] [CrossRef]
- Gao, R.; Xiao, S.; Gan, W.; Liu, Q.; Amer, H.; Rosenau, T.; Li, J.; Lu, Y. Mussel adhesive-inspired design of superhydrophobic nanofibrillated cellulose aerogels for oil/water separation. ACS Sustain. Chem. Eng. 2018, 6, 9047–9055. [Google Scholar] [CrossRef]
- de Souza, G.; Kramer, R.K.; Carvalho, A.J.F. Urethane modified hydrophobic compact wood pulp paper for oil spill cleanup: A preliminary study. J. Renew. Mater. 2020, 8, 1257–1268. [Google Scholar] [CrossRef]
- Xu, X.; Dong, F.; Yang, X.; Liu, H.; Guo, L.; Qian, Y.; Wang, A.; Wang, S.; Luo, J. Preparation and characterization of cellulose grafted with epoxidized soybean oil aerogels for oil-absorbing materials. J. Agric. Food Chem. 2019, 67, 637–643. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.; Ling, J.; Li, N.; Wang, D.; Yue, F.; Xu, S. Cellulose acetate-based SiO2/TiO2 hybrid microsphere composite aerogel films for water-in-oil emulsion separation. Appl. Surf. Sci. 2018, 435, 609–616. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Recyclable biomass carbon@SiO2@MnO2 aerogel with hierarchical structures for fast and selective oil-water separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, flexible, and superhydrophobic functionalized cellulose aerogel for selective and versatile oil/water separation. ACS Sustain. Chem. Eng. 2019, 37, 9984–9994. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, T.; Yu, S.; Cheng, Y.; Lu, J.; Wang, H. A lignin-based carbon aerogel enhanced by graphene oxide and application in oil/water separation. Fuel 2020, 278, 118376. [Google Scholar] [CrossRef]
- Sun, F.; Liu, W.; Dong, Z.; Deng, Y. Underwater superoleophobicity cellulose nanofibril aerogel through regioselective sulfonation for oil/water separation. Chem. Eng. J. 2017, 330, 774–782. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Zhang, Q.; Zhan, X.; Chen, F. A shape recovery zwitterionic bacterial cellulose aerogel with superior performances for water remediation. Langmuir 2019, 35, 11959–11967. [Google Scholar] [CrossRef]
- Dan, H.; Ji, K.; Gao, Y.; Yin, W.; Gao, B.; Yue, Q. Fabrication of superhydrophobic Enteromorpha-derived carbon aerogels via NH4H2PO4 modification for multi-behavioral oil/water separation. Sci. Total Environ. 2022, 837, 155869. [Google Scholar] [CrossRef]
- Yang, S.; He, W.-T.; Fu, Y.; Zhang, Y.; Yuan, T.-Q.; Sun, R.-C. A bio-based coating onto the surface Populus fiber for oil spillage cleanup applications. Ind. Crop. Prod. 2017, 98, 38–45. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Cranston, E.D.; Rezende, C.A. Naturally hydrophobic foams fromlignocellulosic fibers prepared by oven drying. ACS Sustain. Chem. Eng. 2020, 8, 8267–8278. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Cao, Q.; Wang, C.; Yang, C.; Li, Y.; Zhou, J. Novel porous oil-water separation material with super-hydrophobicity and super-oleophilicity prepared from beeswax, lignin, and cotton. Sci. Total Environ. 2020, 706, 135807. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Mu, C.; Lin, W. A novel hydrophobic all-biomass aerogel reinforced by dialdehyde carboxymethyl cellulose for oil/organic solvent-water separation. Polymer 2022, 238, 124402. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Buchtova, N.; Pradille, C.; Bouvard, J.L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, S.; Lyu, S.; Fu, F. Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind. Crop. Prod. 2018, 122, 438–447. [Google Scholar] [CrossRef]
- He, X.; Liang, C.; Liu, Q.; Xu, Z. Magnetically responsive Janus nanoparticles synthesized using cellulosic materials for enhanced phase separation in oily wastewaters and water-in-crude oil emulsions. Chem. Eng. J. 2019, 378, 122045. [Google Scholar] [CrossRef]
- Prathap, A.; Sureshan, K.M. Organogelator-cellulose composite for practical and eco-friendly marine oil-spill recovery. Angew. Chem. Int. Ed. Engl. 2017, 56, 9405–9409. [Google Scholar] [CrossRef]
- Liu, H.; Geng, B.; Chen, Y.; Wang, H. Review on the aerogel-type oil sorbents derived from nanocellulose. ACS Sustain. Chem. Eng. 2016, 5, 49–66. [Google Scholar] [CrossRef]
- Keplinger, T.; Wang, X.; Burgert, I. Nanofibrillated cellulose composites and wood derived scaffolds for functional materials. J. Mater. Chem. A 2019, 7, 2981–2992. [Google Scholar] [CrossRef]
- Hardian, R.; Alammar, A.; Holtzl, T.; Szekely, G. Fabrication of sustainable organic solvent nanofiltration membranes using cellulose-chitosan biopolymer blends. J. Membr. Sci. 2022, 658, 120743. [Google Scholar] [CrossRef]
- Halim, A.; Xu, Y.; Lin, K.-H.; Kobayashi, M.; Kajiyama, M.; Enomae, T. Fabrication of cellulose nanofiber-deposited cellulose sponge as an oil-water separation membrane. Sep. Purif. Technol. 2019, 224, 322–331. [Google Scholar] [CrossRef]
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Yao, Q.; Wang, C.; Jin, C.; Wang, H.; Xiong, Y.; Li, S.; Sun, Q. Natural cellulose nanofiber extracted from cell wall of bamboo leaf and its derived multifunctional aerogel. Polym. Compos. 2021, 39, 3869–3876. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, X.; Liu, X.; Wang, C. Resource recovery of Eichhornia crassipes as oil superabsorbent. Mar. Pollut. Bull. 2017, 118, 267–274. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S. Remodeling of raw cotton fiber into flexible, squeezing-resistant macroporous cellulose aerogel with high oil retention capability for oil/water separation. Sep. Purif. Technol. 2019, 221, 303–310. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, S.; Han, F.; Li, M.; Wang, N.; Liu, L. Anisotropic cellulose nanofiber/chitosan aerogel with thermal management and oil absorption properties. Carbohydr. Polym. 2021, 264, 118033. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Shang, Q.; Han, J.; Liu, C. Enhanced oil adsorption and nano-emulsion separation of nanofibrous aerogels by coordination of pomelo peel-derived biochar. Ind. Eng. Chem. Res. 2020, 59, 8825–8835. [Google Scholar] [CrossRef]
- Shang, Q.; Cheng, J.; Hu, L.; Bo, C.; Yang, X.; Hu, Y.; Liu, C.; Zhou, Y. Bio-inspired castor oil modified cellulose aerogels for oil recovery and emulsion separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128043. [Google Scholar] [CrossRef]
- He, J.; Zhao, H.; Li, X.; Su, D.; Zhang, F.; Ji, H.; Liu, R. Superelastic and superhydrophobic bacterial cellulose/silica aerogels with hierarchical cellular structure for oil absorption and recovery. J. Hazard. Mater. 2018, 346, 199–207. [Google Scholar] [CrossRef]
- Ao, C.; Hu, R.; Zhao, J.; Zhang, X.; Li, Q.; Xia, T.; Zhang, W.; Lu, C. Reusable, salt-tolerant and superhydrophilic cellulose hydrogel-coated mesh for efficient gravity-driven oil/water separation. Chem. Eng. J. 2018, 338, 271–277. [Google Scholar] [CrossRef]
- Wang, Y.; Uetani, K.; Liu, S.; Zhang, X.; Wang, Y.; Lu, P.; Wei, T.; Fan, Z.; Shen, J.; Yu, H.; et al. Multifunctional bionanocomposite foams with a chitosan matrix reinforced by nanofibrillated cellulose. ChemNanoMat 2017, 3, 98–108. [Google Scholar] [CrossRef]
- Soares, S.F.; Rodrigues, M.I.; Trindade, T.; Daniel-da-Silva, A.L. Chitosan-silica hybrid nanosorbents for oil removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 305–313. [Google Scholar] [CrossRef]
- Zhang, E.; Li, W.; Gao, Y.; Lei, C.; Huang, H.; Yang, J.; Zhang, H.; Li, D. High-capacity reusable chitosan absorbent with a hydrogel-coated/aerogel-Core structure and superhydrophilicity under oil for water removal from oil. ACS Appl. Bio Mater. 2020, 3, 5872–5879. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, T.; Sun, H.; Zhang, J.; Wang, A. Pressure-sensitive and conductive carbon aerogels from poplars catkins for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2017, 9, 18001–18007. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, S.; Liu, G.; Yao, J. Facile and fast removal of oil through porous carbon spheres derived from the fruit of Liquidambar formosana. Chemosphere 2017, 170, 268–274. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, Y.; Zhang, T.; Qiu, F.; Yuan, D. Superhydrophobic, ultralight and flexible biomass carbon aerogels derived from sisal fibers for highly efficient oil-water separation. Cellulose 2018, 25, 3067–3078. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Zhao, J.; Li, Q.; Ao, C.; Xia, T.; Zhang, W.; Lu, C. Ultra-lightweight and highly porous carbon aerogels from bamboo pulp fibers as an effective sorbent for water treatment. Results Phys. 2017, 7, 2919–2924. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, D.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Preparation of a renewable biomass carbon aerogel reinforced with sisal for oil spillage clean-up: Inspired by green leaves to green Tofu. Food Bioprod. Process. 2019, 114, 154–162. [Google Scholar] [CrossRef]
- Cai, T.; Wang, H.; Jin, C.; Sun, Q.; Nie, Y. Fabrication of nitrogen-doped porous electrically conductive carbon aerogel from waste cabbage for supercapacitors and oil/water separation. J. Mater. Sci. Mater. Electron. 2017, 29, 4334–4344. [Google Scholar] [CrossRef]
- Jing, Z.; Ding, J.; Zhang, T.; Yang, D.; Qiu, F.; Chen, Q.; Xu, J. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation. Food Bioprod. Process. 2019, 115, 134–142. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Li, Z. Hybrid aerogels derived from banana peel and waste paper for efficient oil absorption and emulsion separation. J. Clean. Prod. 2018, 199, 411–419. [Google Scholar] [CrossRef]
- Dai, Y.; Jing, Z.; Qiu, Z.; Zhu, Y.; Qiu, F.; Pan, J.; Zhang, T.; Li, C. Multifunctional biomass carbon fiber aerogel based on resource utilization of agricultural waste-peanut shells for fast and efficient oil-water/emulsion separation. Mater. Sci. Eng. B 2022, 283, 115819. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhu, F.; You, L.; Shen, X.; Li, S. Removal of organic solvents/oils using carbon aerogels derived from waste durian shell. J. Taiwan Inst. Chem. Eng. 2017, 78, 351–358. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Du, T.; Tian, Z.; Ren, B.; Xu, Y.; Wang, S.; Zhang, L.; Liu, Z. Green and sustainable carbon aerogels from starch for supercapacitors and oil-water separation. Ceram. Int. 2021, 47, 22080–22087. [Google Scholar] [CrossRef]
- Luo, H.; Xie, J.; Wang, J.; Yao, F.; Yang, Z.; Wan, Y. Step-by-step self-assembly of 2D few-layer reduced graphene oxide into 3D architecture of bacterial cellulose for a robust, ultralight, and recyclable all-carbon absorbent. Carbon 2018, 139, 824–832. [Google Scholar] [CrossRef]
- Gu, H.; Xu, Y.; Shen, Y.; Zhu, P.; Zhao, T.; Hu, Y.; Sun, R.; Wong, C.-P. Versatile biomass carbon foams for fast oil-water separation, flexible pressure-strain sensors, and electromagnetic interference shielding. Ind. Eng. Chem. Res. 2020, 59, 20740–20748. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Zhou, L.; Ding, X.; Lin, D.; Duan, T.; Yang, G.; He, R.; Zhu, W. Bio-inspired biomass-derived carbon aerogels with superior mechanical property for oil-water separation. ACS Sustain. Chem. Eng. 2020, 8, 6458–6465. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Tan, S.; Jiang, X.; Wu, W.; Shi, J.; Chen, P. Ultralight super-hydrophobic carbon aerogels based on cellulose nanofibers/poly(vinyl alcohol)/graphene oxide (CNFs/PVA/GO) for highly effective oil-water separation. Beilstein J. Nanotechnol. 2018, 9, 508–519. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z. Ultralight, highly compressible, hydrophobic and anisotropic lamellar carbon aerogels from graphene/polyvinyl alcohol/cellulose nanofiber aerogel as oil removing absorbents. J. Hazard. Mater. 2020, 388, 121804. [Google Scholar] [CrossRef]
- Ma, S.; Xu, M.; Zhao, Z.; Pan, J.; Zhao, S.; Xue, J.; Ye, Z. Preparation of 3D superhydrophobic porous g-C3N4 nanosheets@carbonized kapok fiber composites for oil/water separation and treating organic pollutants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129298. [Google Scholar] [CrossRef]
- Priyanka, M.; Saravanakumar, M.P. Ultrahigh adsorption capacity of starch derived zinc based carbon foam for adsorption of toxic dyes and its preliminary investigation on oil-water separation. J. Clean. Prod. 2018, 197, 511–524. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, R.; Ge, W.; Xie, A.; Chang, Z.; Tian, S.; Zhou, Z.; Yan, Y. 3D macroscopic superhydrophobic magnetic porous carbon aerogel converted from biorenewable popcorn for selective oil-water separation. Mater. Des. 2018, 139, 122–131. [Google Scholar] [CrossRef]
- Ieamviteevanich, P.; Palaporn, D.; Chanlek, N.; Poo-arporn, Y.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Carbon nanofiber aerogel/magnetic core-shell nanoparticle composites as recyclable oil sorbents. ACS Appl. Nano Mater. 2020, 3, 3939–3950. [Google Scholar] [CrossRef]
- Cao, S.; Dong, T.; Xu, G.; Wang, F. Oil spill cleanup by hydrophobic natural fibers. J. Nat. Fibers 2017, 14, 727–735. [Google Scholar] [CrossRef]
- Dong, T.; Cao, S.; Xu, G. Highly efficient and recyclable depth filtrating system using structured kapok filters for oil removal and recovery from wastewater. J. Hazard. Mater. 2017, 321, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, O.; Nasr, S.M.; Thabet, W.M. Palm fibers and modified palm fibers adsorbents for different oils. Alex. Eng. J. 2017, 56, 749–755. [Google Scholar] [CrossRef]
- Panahi, S.; Moghaddam, M.K.; Moezzi, M. Assessment of milkweed floss as a natural hollow oleophilic fibrous sorbent for oil spill cleanup. J. Environ. Manage. 2020, 268, 110688. [Google Scholar] [CrossRef]
- Demirel Bayik, G.; Altin, A. Production of sorbent from paper industry solid waste for oil spill cleanup. Mar. Pollut. Bull. 2017, 125, 341–349. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Ezeofor, C.C.; Olikagu, C.S.; Odewole, O.A.; Ezeorah, C.J. Abstraction and regeneration potential of temperature-enhanced rice husk montmorillonite combo for oil spill. Environ. Sci. Pollut. Res. Int. 2018, 25, 34711–34719. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the use of banana peels for oil spill removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Mai, V.C.; Das, P.; Zhou, J.; Lim, T.T.; Duan, H. Mussel-inspired uual-superlyophobic biomass membranes for selective oil/water separation. Adv. Mater. Interface 2020, 7, 1901756. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, L.; Fu, Z.; Sun, X.; Zhou, S.; Liu, X.; Zhang, C.; Xu, W. Fabrication of polyurethane porous composite films using biomass-based Juncus effusus fibers for oil removal from water. Ind. Crop. Prod. 2020, 176, 114290. [Google Scholar] [CrossRef]
- Chen, Z.; Zhan, B.; Li, S.; Wei, D.; Zhou, W.; Liu, Y. Facile fabrication of corn stover-based aerogel for oil/water separation. Sep. Purif. Technol. 2022, 298, 121642. [Google Scholar] [CrossRef]
- Yi, L.; Xia, Y.; Tan, Z.; Fang, X.; Zhao, L.; Wu, H.; Guo, S. Design of tubelike aerogels with macropores from bamboo fungus for fast oil/water separation. J. Clean. Prod. 2020, 264, 121558. [Google Scholar] [CrossRef]
- Dong, T.; Tian, N.; Xu, B.; Huang, X.; Chi, S.; Liu, Y.; Lou, C.W.; Lin, J.H. Biomass poplar catkin fiber-based superhydrophobic aerogel with tubular-lamellar interweaved neurons-like structure. J. Hazard. Mater. 2022, 429, 128290. [Google Scholar] [CrossRef]
- Cao, M.; Hu, Y.; Cheng, W.; Huan, S.; Bai, T.; Niu, Z.; Zhao, Y.; Yue, G.; Zhao, Y.; Han, G. Lignin-based multi-scale cellular aerogels assembled from co-electrospun nanofibers for oil/water separation and energy storage. Chem. Eng. J. 2022, 436, 135233. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Yao, J. Construction of hydrophobic alginate-based foams induced by zirconium ions for oil and organic solvent cleanup. J. Colloid Interface Sci. 2019, 533, 182–189. [Google Scholar] [CrossRef]
- Dai, J.; Tian, Q.; Sun, Q.; Wei, W.; Zhuang, J.; Liu, M.; Cao, Z.; Xie, W.; Fan, M. TiO2-alginate composite aerogels as novel oil/water separation and wastewater remediation filters. Compos. Part B Eng. 2019, 160, 480–487. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Gao, K.; Li, Y.; Wang, S.; Xie, F.; Jia, X.; Song, H. Biomimetic superelastic sodium alginate-based sponges with porous sandwich-like architectures. Carbohydr. Polym. 2021, 272, 118527. [Google Scholar] [CrossRef]
- Tian, N.; Wu, S.; Han, G.; Zhang, Y.; Li, Q.; Dong, T. Biomass-derived oriented neurovascular network-like superhydrophobic aerogel as robust and recyclable oil droplets captor for versatile oil/water separation. J. Hazard. Mater. 2022, 424, 127393. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Y.; Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Self-assembled microporous peptide-polysaccharide aerogels for oil-water separation. Langmuir 2018, 34, 10732–10738. [Google Scholar] [CrossRef] [PubMed]










| Classification | Examples | Advantages | Disadvantages |
|---|---|---|---|
| Inorganic | Zeolites, silica | Availability, chemical inertness, non-flammable | Low capacity, poor selectivity, difficult recovery |
| Synthetic polymer | Polypropylene, polyurethane | Hydrophobic, moderate capacity, good reusability | Non-biodegradability, expensive, complex synthesis |
| Natural organic | Straw, peel | Abundant, inexpensive, biodegradability, eco-friendly | Low capacity, poor selectivity, poor hydrophobicity |
| Material | Definition | Characteristic | Typical Examples |
|---|---|---|---|
| Cellulose | Long chain linear polysaccharide made of glucose (-1,4), which can be extracted from plants, algae, tunicates and bacterial, etc. | Insoluble in water and general organic solvents, rich in hydroxyl groups, poor absorption capacity, rigid for natural cellulose | Lignin fiber, microfibrillated cellulose, cellulose nanocrystals, cellulose nanofibrils |
| Chitosan | Natural polysaccharide containing N-acetyl-D-glucosamine residue in its C2 position, which can be extracted from crab shells, lobsters, etc. | Contain numerous hydroxyl and amino groups, charge biopolymer, biodegradability, biocompatibility | Chitosan-based aerogel |
| Bio-carbon based materials | Carbon materials prepared by the carbonization of raw biomass materials at high temperature | Unique and various microstructure, high porosity, low apparent density, large specific surface area, high material purity, good stability | Cellulose based bio-carbon, three-dimensional nanostructured bio-carbon |
| Natural products | Products that are acquired directly from the nature without extraction or treatment | Multicomponent, versatile properties | Cotton, flax, wood fiber, corn straw, rice husk, fruit peel, luffa |
| Application | Material Characterizes | Example | Refs. |
|---|---|---|---|
| Oil/water separation | High porosity, high surface area, reusability, antifouling, high selectivity, tolerability, mechanical capacity, environmental-friendly | Cellulose nanocrystals | [28] |
| Cellulose microcrystalline | [29] | ||
| Lignocellulosic fiber | [30] | ||
| Cellulose hydrogel | [31] | ||
| NCF/chitosan aerogel | [32] | ||
| Alginate//rGO | [33] | ||
| Gelatin//PEI | [34] | ||
| Collagen/ZIF-8 | [35] | ||
| Oil sorption | high porosity, high surface area, mechanical stability, chemical stability, recyclable, environmental-friendly, low density, good buoyancy | Nanocellulose sponge | [36] |
| CNF/PVA/ aerogel | [37] | ||
| Chitosan sponge | [38] | ||
| Chitosan/rGO aerogel | [39] | ||
| Biocarbon aerogel | [40] | ||
| Lignin/GO | [41] | ||
| Alginate/GO/ | [42] | ||
| Silk fibroin | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, D.; Lei, X.; Zheng, H. Recent Advances in Biomass-Based Materials for Oil Spill Cleanup. Nanomaterials 2023, 13, 620. https://doi.org/10.3390/nano13030620
Ouyang D, Lei X, Zheng H. Recent Advances in Biomass-Based Materials for Oil Spill Cleanup. Nanomaterials. 2023; 13(3):620. https://doi.org/10.3390/nano13030620
Chicago/Turabian StyleOuyang, Dan, Xiaotian Lei, and Honglei Zheng. 2023. "Recent Advances in Biomass-Based Materials for Oil Spill Cleanup" Nanomaterials 13, no. 3: 620. https://doi.org/10.3390/nano13030620
APA StyleOuyang, D., Lei, X., & Zheng, H. (2023). Recent Advances in Biomass-Based Materials for Oil Spill Cleanup. Nanomaterials, 13(3), 620. https://doi.org/10.3390/nano13030620







