Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Photocatalyst Synthesis
2.2. Characterization
2.2.1. Material Characterization
2.2.2. Electrochemical Measurements
2.2.3. Photocatalytic Experiments
3. Results and Discussion
3.1. Structural and Optical Characterization of the Photocatalysts
3.2. XPS Analysis
3.3. Thermal Analysis (TGA-DTA)
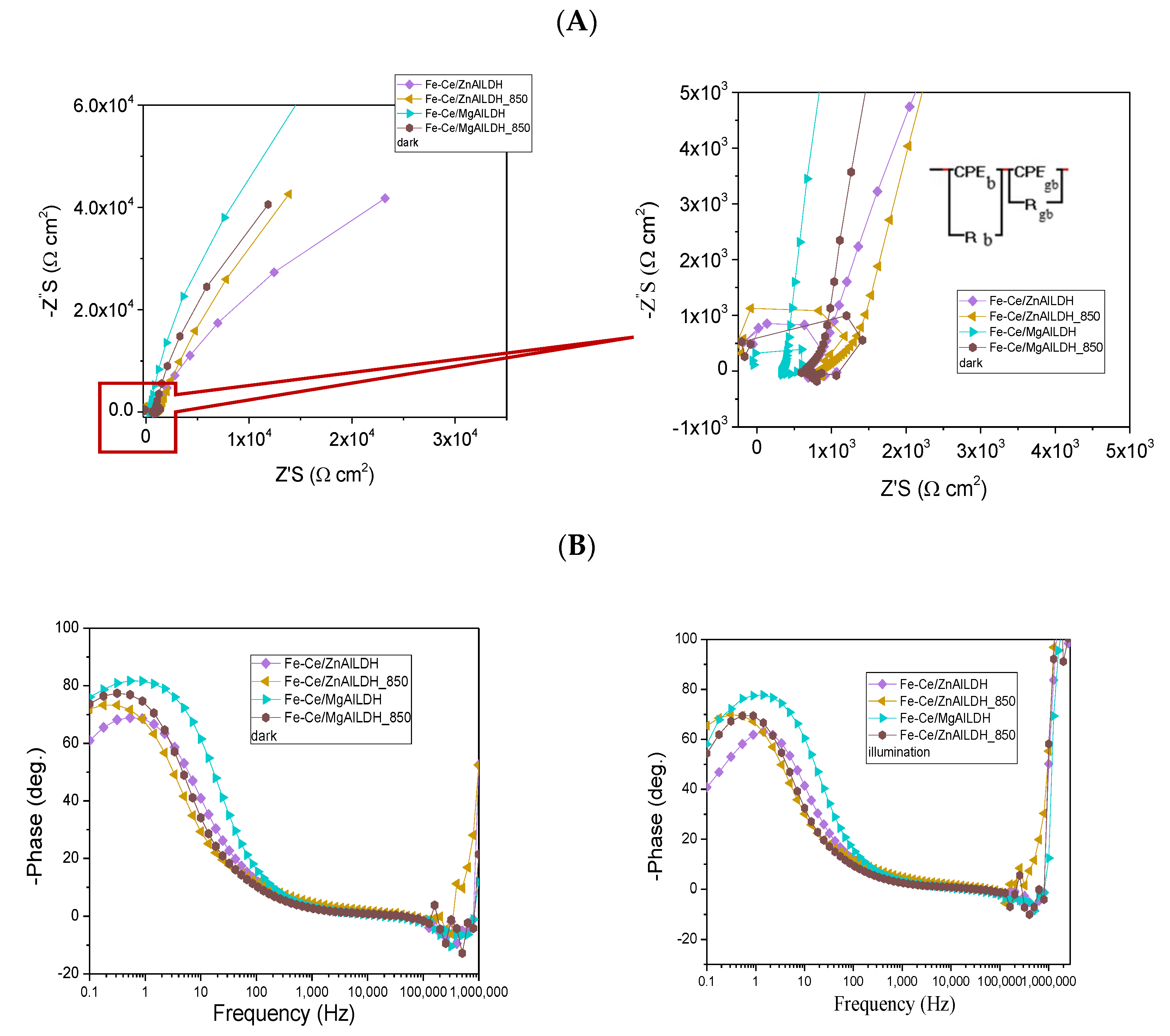
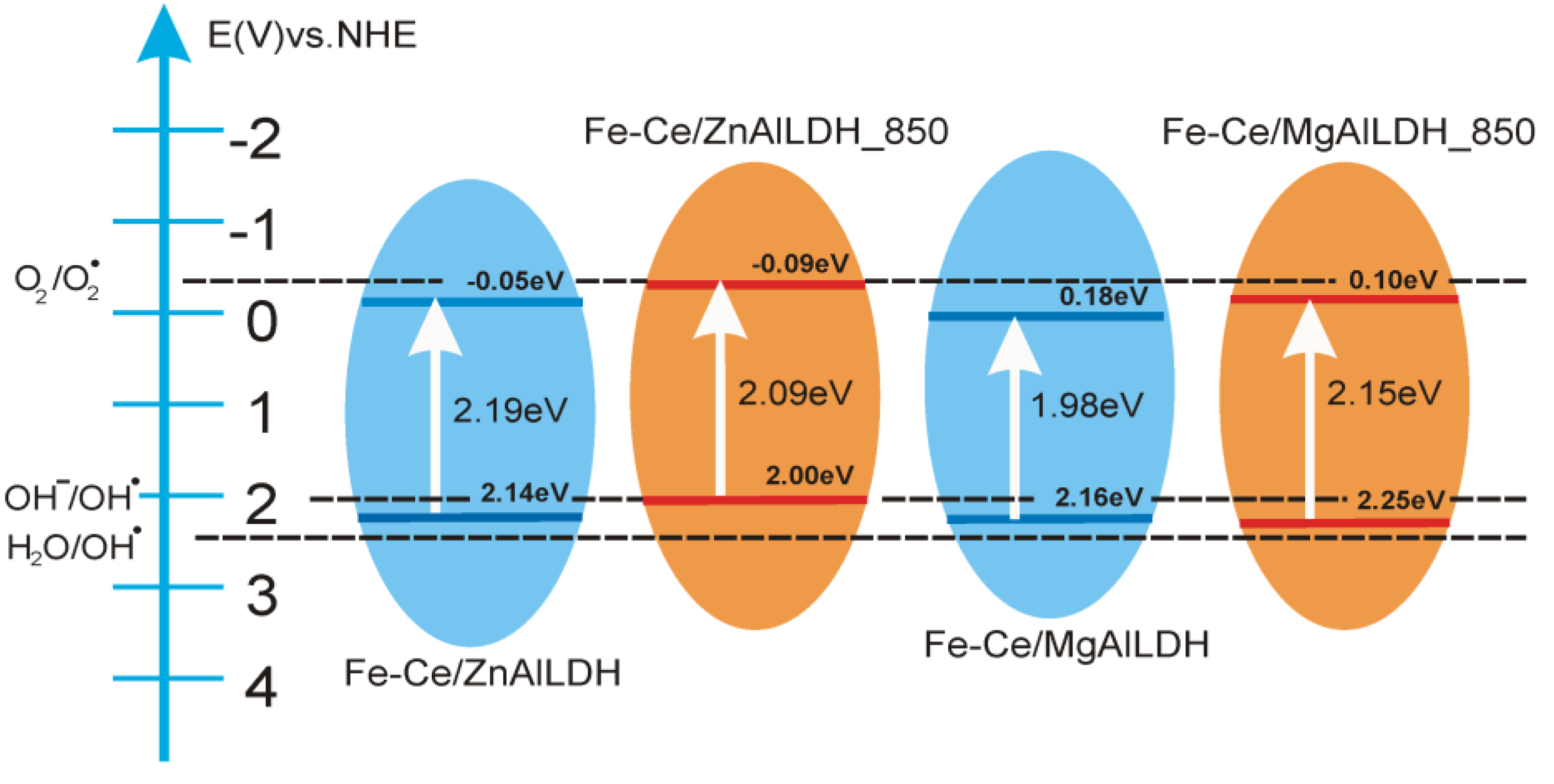
3.4. Photoelectrochemical Study
| Electrode | Voc (V) vs. Ag/AgCl | Current Density (µA/cm2) at 0.5 V vs. Ag/AgCl | Vfb (V) vs. Ag/AgCl 1 | Band Gap Eg (eV) 2 | EVB 3 | ||
|---|---|---|---|---|---|---|---|
| Dark | Illum. | Dark | Illum. | ||||
| Fe-Ce/MgAlLDH | −0.13 | −0.18 | 1.11 | 6.52 | −0.40 (0.18) | 1.98 | 2.16 |
| Fe-Ce/MgAlLDH_850 | −0.21 | −0.23 | 1.37 | 2.68 | −0.48 (0.10) | 2.15 | 2.25 |
| Fe-Ce/ZnAlLDH | −0.13 | −0.21 | 17.8 | 55.53 | −0.63 (−0.05) | 2.19 | 2.14 |
| Fe-Ce/ZnAlLDH_850 | −0.15 | −0.19 | 0.68 | 6.63 | −0.67 (−0.09) | 2.09 | 2.00 |
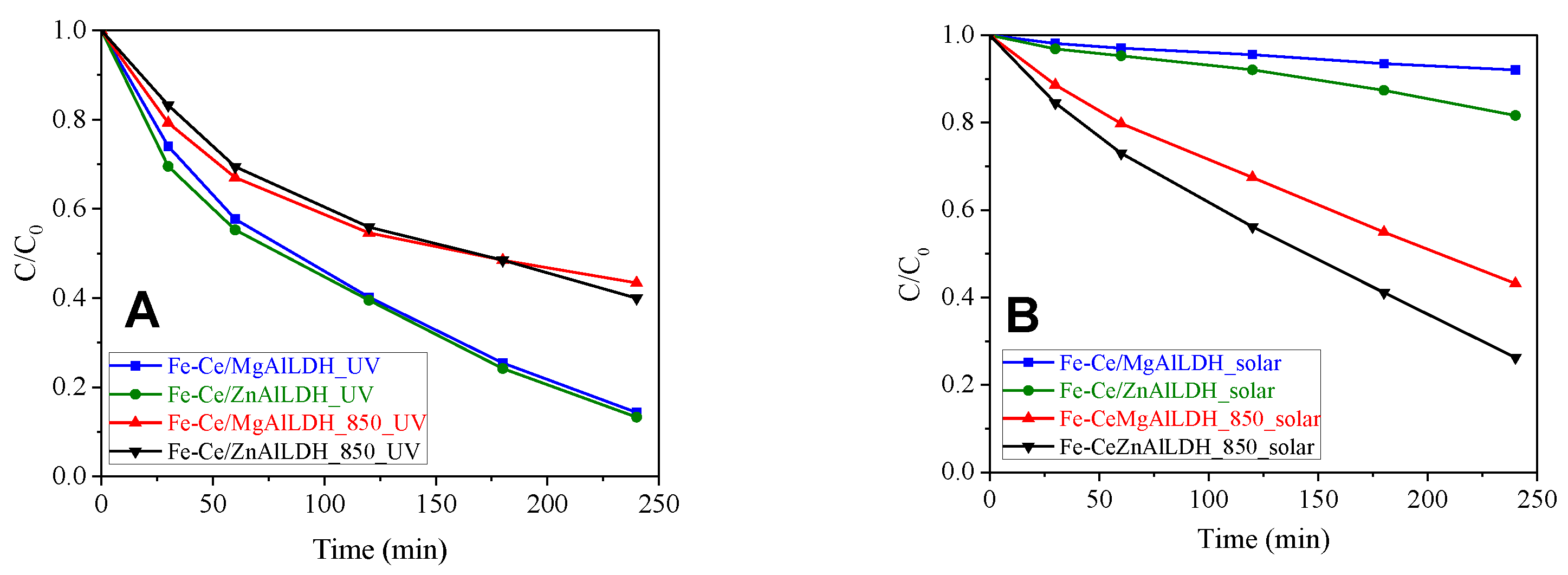
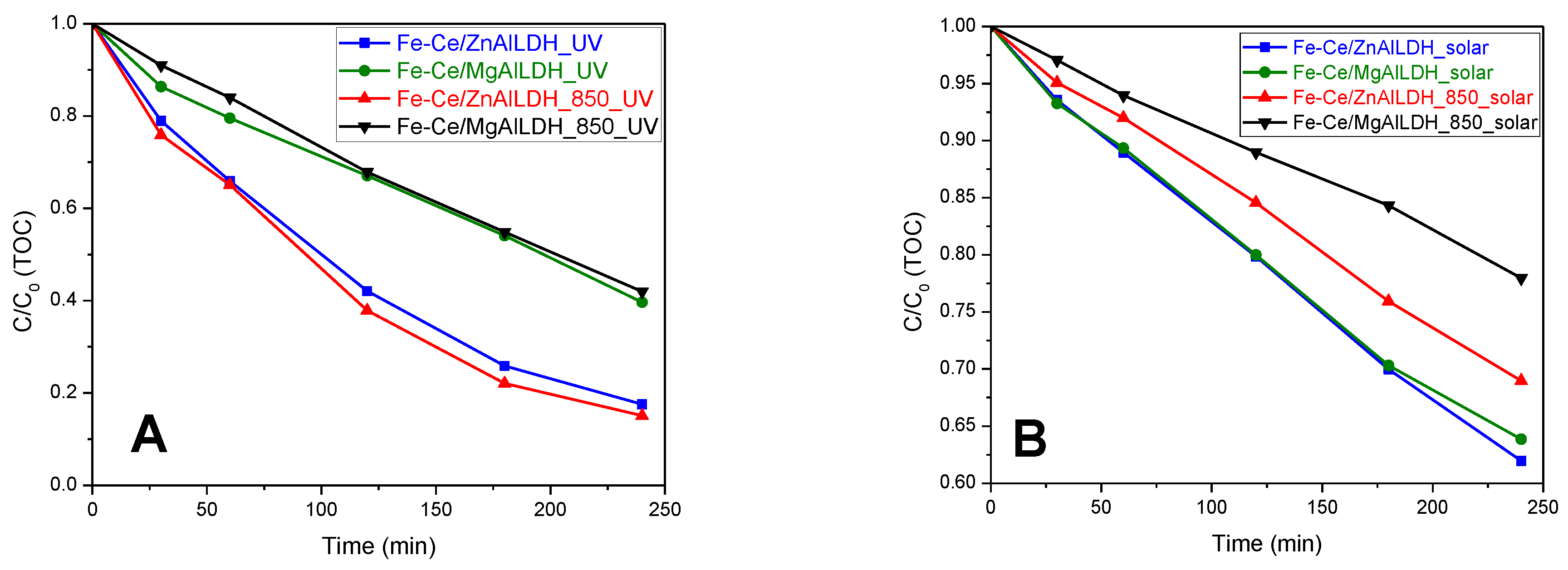
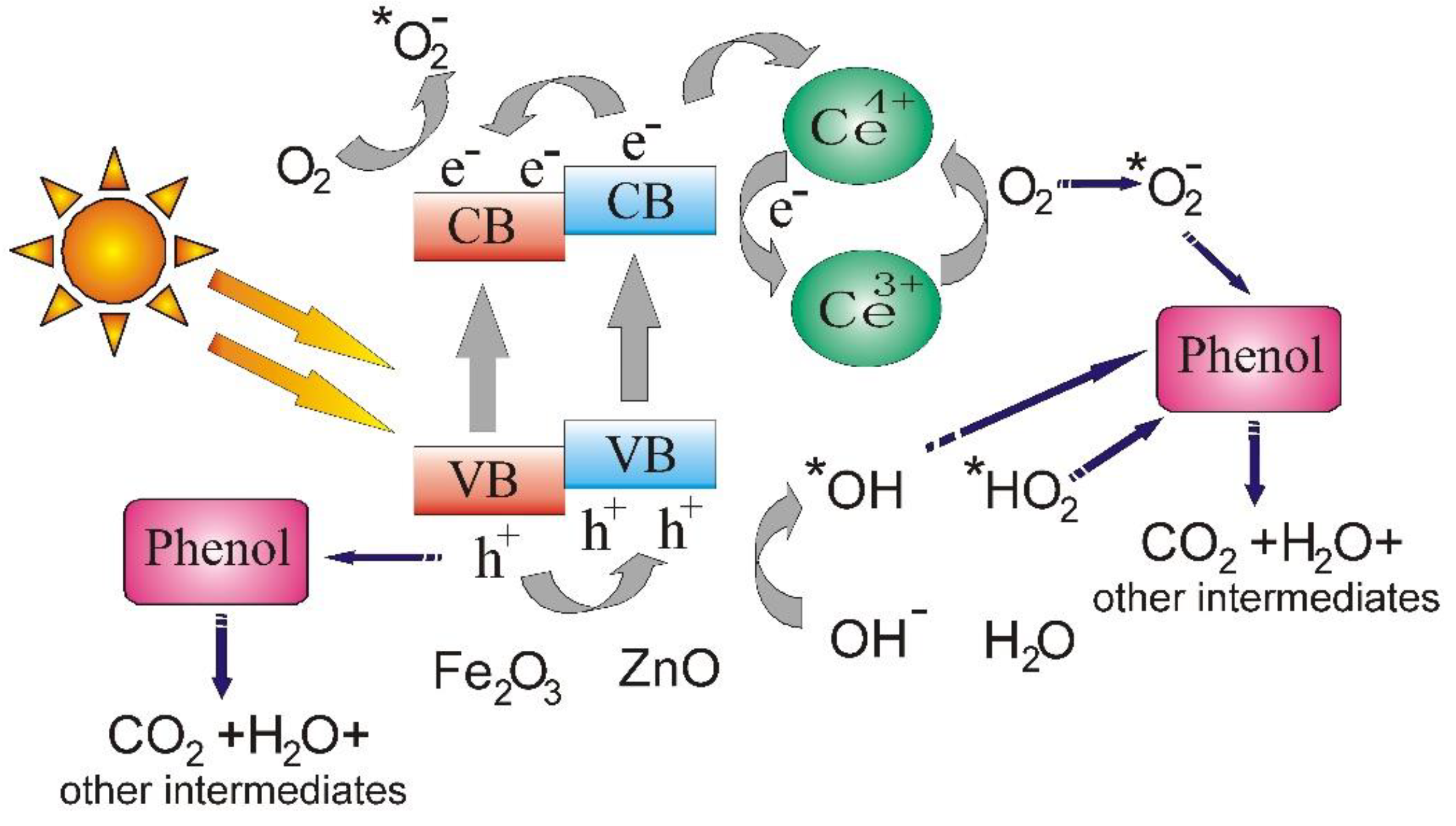
3.5. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strateg. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants. A review. Chem. Eng. J. 2020, 392, 123694. [Google Scholar] [CrossRef]
- Mukherjee, S.; Roychaudhuri, U.; Kundu, P.P. Bio-degradation of polyethylene viacomplete solubilisation by the action of pseudomonas fluorescens bio-surfactant produced by bacillus licheniformis and anionic surfactant. J. Chem. Technol. Biotechnol. 2018, 93, 1300–1311. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Norskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloys Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Liu, Q.Q.; Zulfiqar, S.; Shah, S.; Bahadur, I. An overview of semiconductors/layered double hydroxides composites: Properties, synthesis, photocatalytic ana photoelectrochemical applications. J. Mol. Liq. 2019, 289, 111–114. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P.; Li, X. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Cui, X.; Tian, L.; Xian, X.; Tang, H.; Yang, X. Solar photocatalytic water oxidation over Ag3PO4/g-C3N4 composite materials mediated by metallic Ag and graphene. Appl. Surf. Sci. 2018, 430, 108–115. [Google Scholar] [CrossRef]
- Wang, S.; Yun, J.-H.; Luo, B.; Butburee, T.; Peerakiatkhajohn, P.; Thaweesak, S.; Xiao, M.; Wang, L. Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications. J. Mater. Sci. Technol. 2017, 33, 1–22. [Google Scholar] [CrossRef]
- Forno, C.; Hibino, T.; Leroux, F.; Taviot-Gueho, C. Layered double hydroxides. Appl. Clay Sci. 2006, 1, 1021–1095. [Google Scholar]
- Kang, D.; Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. Performance and mechanism of Mg/Fe layered double hydroxidea for fluoride and arsenate removal from aqueous solution. Chem. Eng. J. 2013, 228, 731–740. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Deciphering Z-scheme charge transfer dynamics in heterostructure NiFe-LDH/NrGO/g-C3N4 nanocomposite for photocatalytic pollutant removal and water splitting reactions. Sci. Rep. 2019, 9, 2458–2481. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Swain, G.; Parida, K. Enhanced Photocatalytic Activities of RhB Degradation and H2 Evolution from in Situ Formation of the Electrostatic Heterostructure MoS2/NiFe LDH Nanocomposite through the Z-Scheme Mechanism via p-n Heterojunctions. ACS Appl. Mater. Interf. 2019, 11, 20923–20942. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Dynamics of charge-transfer behavior in a plasmon-induced quasi-type-II p-n/n-n dual heterojunction in Ag@Ag3PO4/g-C3N4/NiFe LDH nanocomposites for photocatalytic Cr(VI) reduction and phenol oxidation. ASC Omega 2018, 3, 7324–7343. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Thind, S.S.; Chen, A. Nanostructured materials for water splitting—State of the art and future needs: A mini-review. Electrochem. Commun. 2016, 63, 10–17. [Google Scholar] [CrossRef]
- Ao, Y.; Wang, D.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Enhanced photocatalytic properties of the 3D flower-like Mg-Al layered double hydroxides decorated with Ag2CO3, under visible light illumination. Mater. Res. Bull. 2016, 80, 23–29. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, X.; Tian, W.; Yan, D.; Sun, X.; Lei, X. Adsolubilization of 2,4,6-trichlorophenol from aqueous solution by surfactant intercalated ZnAl layered double hydroxides. Chem. Eng. J. 2015, 279, 597–604. [Google Scholar] [CrossRef]
- Balsamo, N.; Mendieta, S.; Oliva, M.; Eimer, G.; Crivello, M. Synthesis and Characterization of Metal Mixed Oxides from Layered Double Hydroxides. Proc. Mater. Sci. 2012, 1, 506–513. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Zhu, Z.L.; Zhang, H.; Lu, H.T.; Qiu, Y.L.; Zhu, L.Y.; Küppers, S. Enhanced photocatalytic activity of Ce-doped Zn-Al multi-metal oxide composites derived from layered double hydroxide precursors. J. Colloid Interf. Sci. 2016, 481, 144–157. [Google Scholar] [CrossRef]
- Carja, G.; Grosu, E.F.; Mureseanu, M.; Lutic, D. A family of solar light responsive photocatalysts obtained using Zn2+Me3+ (Me=AlGa) LDHs doped with Ga2O3 and In2O3 and their derived mixed oxides: A case study of phenol/4-nitrophenol decomposition. Catal. Sci. Technol. 2017, 7, 5402–5412. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xiao, H.; Liu, D.; Qin, Y.; Wua, H.; Li, H.; Dub, N.; Hou, W. Preparation and properties of mixed metal oxides based layered double hydroxide as anode materials for dye-sensitized solar cell. Chem. Eng. J. 2014, 250, 1–5. [Google Scholar] [CrossRef]
- Mantilla, A.; Tzompantzi, F.; Fernández, J.L.; Góngora, J.A.I.D.; Gómez, R. Photodegradation of phenol and cresol in aqueous medium by using Zn/Al+Fe mixed oxides obtained from layered double hydroxides materials. Catal. Today 2009, 150, 353–357. [Google Scholar] [CrossRef]
- Lee, S.-B.; Ko, E.-H.; Park, J.Y.; Oh, J.-M. Mixed metal oxides by calcination of Layered Double hydroxide: Parameters affecting specific surface area. Nanomaterials 2021, 11, 1153. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Zhang, H.; Zhu, J.; Lu, H.; Zhang, W.; Qiu, Y.; Zhu, L.; Kueppers, S. Simultaneous removal of several pharmaceuticals and arsenic on Zn-Fe mixed metal oxides: Combination of photocatalysis and adsorption. Chem. Eng. J. 2017, 328, 141–151. [Google Scholar] [CrossRef]
- Chuaicham, C.; Karthikeyan, S.; Song, J.T.; Ishihara, T.; Ohtani, B.; Sasaki, K. Importance of ZnTiO3 phase in ZnTi-mixed metal oxide photocatalysts derived from layered double hydroxide. ACS Appl. Mater. Interf. 2020, 12, 9169–9180. [Google Scholar] [CrossRef]
- Suarez-Quezada, M.; Romero-Ortiz, G.; Suarez, V.; Morales-Mendoza, G.; Lartundo- Rojas, L.; Navarro Ceron, E.; Tzompantzi, F.; Robles, S.; Gomez, R.; Mantilla, A. Photodegradation of phenol using reconstructed Ce doped Zn/Al layered double hydroxides as photocatalysts. Catal. Today 2016, 271, 213–219. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wena, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A critical review on visible-light-response CeO2-based photocatalysts with enhanced photooxidation of organic pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Furle, P.; Scheffe, J.R.; Steinfeld, A. Syngas production by simultaneous splitting of H2O and CO2 via ceria redox reactions in a high-temperature solar reactor. Energy Environ. Sci. 2012, 5, 6098–6103. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Murayama, N.; Kanzaki, S. Resistive Oxygen Gas Sensors Based on CeO2 Fine Powder Prepared Using Mist Pyrolysis. Sens. Actuat. B 2002, 87, 95–98. [Google Scholar] [CrossRef]
- Lu, X.H.; Zheng, D.Z.; Gan, J.Y.; Liu, Z.Q.; Liang, C.L.; Liu, P.; Tong, Y.X. Porous CeO2 nanowires/nanowire arrays: Electrochemical synthesis and application in water treatment. J. Mater. Chem. 2010, 20, 7118–7122. [Google Scholar] [CrossRef]
- Corma, A.; Atienzar, P.; Garcia, H.; Ching, J.Y.C. Hierarchically mesostructured doped CeO2 with potential for solar-cell use. Nat. Mater. 2004, 3, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, Y.; Zhong, Y.; Ning, J.; Hu, Y. Microwave-assisted deposition of metal sulfide/oxide nanocrystals onto a 3D hierarchical flower-like TiO2 nanostructure with improved photocatalytic activity. J. Mater. Chem. A 2013, 1, 8101–8104. [Google Scholar] [CrossRef]
- Chivu, V.; Gilea, D.; Cioatera, N.; Carja, G.; Mureseanu, M. Heterostructures of Ce-Ti/layered double hydroxides and the derived MMOs for photoenergy applications. Appl. Surf. Sci. 2020, 513, 145853. [Google Scholar] [CrossRef]
- Wu, H.; Wu, L.; Shen, S.; Liu, Y.; Cai, G.; Wang, X.; Qiu, Y.; Zhong, H.; Xing, Z.; Tang, J.; et al. Enhanced photoelectrochemical performance of an α-Fe2O3 nanorodsphotoanode with embedded nanocavities formed by helium ions implantation. Int. J. Hydrogen Energy 2020, 45, 9408–9415. [Google Scholar] [CrossRef]
- Ma, H.; Hwang, J.B.; Chae, W.S.; Chung, H.S.; Choi, S.H.; Mahadik, M.A.; Lee, H.H.; Jang, J.S. Magnetron sputtering strategy for Zr-Fe2O3 nanorod photoanode fabricated from ZrOx/β-FeOOH nanorods for photoelectrochemical water splitting. Appl. Surf. Sci. 2021, 549, 149233. [Google Scholar] [CrossRef]
- Luan, P.; Xie, M.; Liu, D.; Fu, X.; Jing, L. Effective charge separation in the rutile TiO2 nanorod-coupled α-Fe2O3 with exceptionally high visible activities. Sci. Rep. 2014, 4, 61801–61807. [Google Scholar] [CrossRef]
- Sabari Arul, N.; Mangalaraj, D.; Ramachandran, R.; Nirmala Grace, A.; In Han, J. Fabrication of CeO2/Fe2O3 composite nanospindles for enhanced visible light driven photocatalyst and supercapacitor electrode. J. Mater. Chem. A 2015, 29, 15248–15258. [Google Scholar] [CrossRef]
- Mureseanu, M.; Georgescu, I.; Bibire, L.E.; Carja, G. CuII(Sal-Ala)/MgAlLDH and CuII(Sal-Phen)/MgAlLDH as novel catalytic systems for cyclohexene oxidation by H2O2. Catal. Commun. 2014, 54, 39–44. [Google Scholar] [CrossRef]
- Mikami, G.; Grosu, F.; Kawamura, S.; Yoshida, Y.; Carja, G.; Izumi, Y. Harnessing self-supported Au nanoparticles on layered double hydroxides comprising Zn and Al for enhanced phenol decomposition under solar light. Appl. Catal. B Environ. 2016, 199, 260–271. [Google Scholar] [CrossRef]
- Machida, M.; Kawada, T.; Fujiri, H.; Hinokuma, S. The Role of CeO2 as a Gateway for Oxygen Storage over CeO2-Grafted Fe2O3 Composite Materials. J. Phys. Chem. C 2015, 119, 4932–24941. [Google Scholar] [CrossRef]
- Lahkale, R.; Sadik, R.; Elhatimi, W.; Bouragba, F.Z.; Assekouri, A.; Chouni, K.; Rhalmi, O.; Sabbar, E. Optical, electrical and dielectric properties of mixed metal oxides derived from Mg-Al Layered Double Hydroxides based solid solution series. Phys. B Phys. Cond. Matter 2022, 626, 413367. [Google Scholar] [CrossRef]
- Perkowitz, S. Optical Characterization of Semiconductors: Infrared, Raman, and Photoluminescence Spectroscopy; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar] [CrossRef]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef]
- Seftel, E.M.; Puscasu, M.; Mertens, M.; Cool, P.; Carja, G. Photo-responsive behavior of γ-Fe2O3 NPs embedded into ZnAlFe-LDH matrices and their catalytic efficiency in wastewater remediation. Catal. Today 2015, 252, 7–13. [Google Scholar] [CrossRef]
- Lei, Y.; Huo, J.; Liao, H. Fabrication and catalytic mechanism study of CeO2-Fe2O3-ZnO mixed oxides on double surfaces of polyimide substrate using ion-exchange technique. Mater. Sci. Semicond. Proc. 2018, 74, 154–164. [Google Scholar] [CrossRef]
- Mureseanu, M.; Filip, M.; Somacescu, S.; Baran, A.; Carja, G.; Parvulescu, V. Ce, Ti modified MCM-48 mesoporous photocatalysts: Effect of the synthesis route on support and metal ion properties. Appl. Surf. Sci. 2018, 444, 235–242. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Sun, G.; Gao, H.; Yu, X.; Tang, S.; Zhao, X.; Yi, Z.; Wang, Y.; Wei, Y. Facile preparation of MgAl2O4/CeO2/Mn3O4 heterojunction photocatalyst and enhanced photocatalytic activity. Mater. Today Chem. 2021, 19, 100390. [Google Scholar] [CrossRef]
- Gilea, D.; Lutic, D.; Carja, G. Heterostructures of silver and zinc based layered double hydroxides for pollutant removal under simulated solar light. Environ. Eng. Manag. 2019, 18, 1765–1772. [Google Scholar]
- Zhou, X.; Liu, J.Y.; Wang, C.; Sun, P.; Hu, X.L.; Li, X.W.; Shimanoe, K.; Yamazoe, N.; Lu, G.Y. Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens. Actuat. B Chem. 2015, 206, 577–583. [Google Scholar] [CrossRef]
- Mureseanu, M.; Chivu, V.; Osiac, M.; Ciobanu, M.; Bucur, C.; Parvulescu, V.; Cioatera, N. New photoactive mesoporous Ce-modified TiO2 for simultaneous wastewater treatment and electric power generation. Catal. Today 2021, 366, 164–176. [Google Scholar] [CrossRef]
- Kusmierek, E. A CeO2 Semiconductor as a Photocatalytic and Photoelectrocatalytic Material for the Remediation of Pollutants in Industrial Wastewater: A Review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Chaudhary, D.; Singh, S.; Vankar, V.D.; Khare, N. A ternary Ag/TiO2/CNT photoanode for efficient photoelectrochemical water splitting under visible light irradiation. Int. J. Hydrogen Energ. 2017, 42, 7826–7835. [Google Scholar] [CrossRef]
- EIS Spectrum Analyser. Available online: http://www.abc.chemistry.bsu.by/vi/analyser/ (accessed on 20 January 2023).
- Cioatera, N.; Parvulescu, V.; Rolle, A.; Vannier, R.N. Enhanced ionic conductivity of Sm, Gd-doped ceria induced by modification of powder synthesis procedure. Ceram. Int. 2012, 38, 5461–5468. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Chen, K.; Feng, X.; Hu, R.; Li, Y.; Xie, K.; Li, Y.; Gu, H. Effect of Ag nanoparticle size on the photoelectrochemical properties of Ag decorated TiO2 nanotube arrays. J. Alloys Compd. 2013, 554, 72–79. [Google Scholar] [CrossRef]
- Xia, S.; Meng, Y.; Zhou, X.; Xue, J.; Pan, G.; Ni, Z. Ti/ZnO–Fe2O3 composite: Synthesis, characterization and application as a highly efficient photoelectrocatalyst for methanol from CO2 reduction. Appl. Catal. B Environ. 2016, 187, 122–133. [Google Scholar] [CrossRef]
- Shooshtari, N.M.; Ghazi, M.M. An investigation of the photocatalytic activity of nano α-Fe2O3/ZnO on the photodegradation of cefixime trihydrate. Chem. Eng. J. 2017, 315, 527–536. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Comparison of NiFe-LDH based heterostructure material towards photocatalytic rhodamine B and phenol degradation with water splitting reactions. Mat. Today Proc. 2021, 35, 243–246. [Google Scholar] [CrossRef]
- Lestari, P.R.; Takei, T.; Yanagida, S.; Kumada, N. Facile and controllable synthesis of Zn-Al layered double hydroxide/silver hybrid by exfoliation process and its plasmonic photocatalytic activity of phenol degradation. Mater. Chem. Phys. 2020, 250, 122988. [Google Scholar] [CrossRef]
- Valente, J.S.; Tzompantzi, F.; Prince, J. Highly efficient photocatalytic elimination of phenol and chlorinated phenols by CeO2/MgAl layered double hydroxides. Appl. Catal. B Environ. 2011, 102, 276–285. [Google Scholar] [CrossRef]
- Prince, J.; Tzompantzi, F.; Mendoza-Damian, G.; Hernandez-Beltran, F.; Valente, J.S. Photocatalytic degradation of phenol by semiconducting mixed oxides derived from Zn(Ga)Al layered double hydroxides. Appl. Catal. B Environ. 2015, 163, 352–360. [Google Scholar] [CrossRef]
- Morales-Mendoza, G.; Alvarez-Lemus, M.; López, R.; Tzompantzi, F.; Adhikari, R.; Lee, S.W.; Torres-Martínez, L.M.; Gómez, R. Combination of Mn oxidation states improves the photocatalytic degradation of phenol with ZnAlLDH materials without a source of O2 in the reaction system. Catal. Today 2016, 266, 62–71. [Google Scholar] [CrossRef]
- Mendoza-Damián, G.; Tzompantzi, F.; Mantilla, A.; Pérez-Hernández, R.; Hernández-Gordillo, A. Improved photocatalytic activity of SnO2–ZnAl LDH prepared by one step Sn4+ incorporation. Appl. Clay Sci. 2016, 121–122, 127–136. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Mendoza-Damian, G.; Rico, J.L.; Mantilla, A. Enhanced photoactivity for the phenol mineralization on ZnAlLa mixed oxides prepared from calcined LDHs. Catal. Today 2014, 220–222, 56–60. [Google Scholar] [CrossRef]
- Seftel, E.M.; Puscasu, M.C.; Mertens, M.; Cool, P.; Carja, G. Fabrication of CeO2/LDHs self-assemblies with enhanced photocatalytic performance: A case study on ZnSn-LDH matrix. Appl. Catal. B Environ. 2015, 164, 251–260. [Google Scholar] [CrossRef]
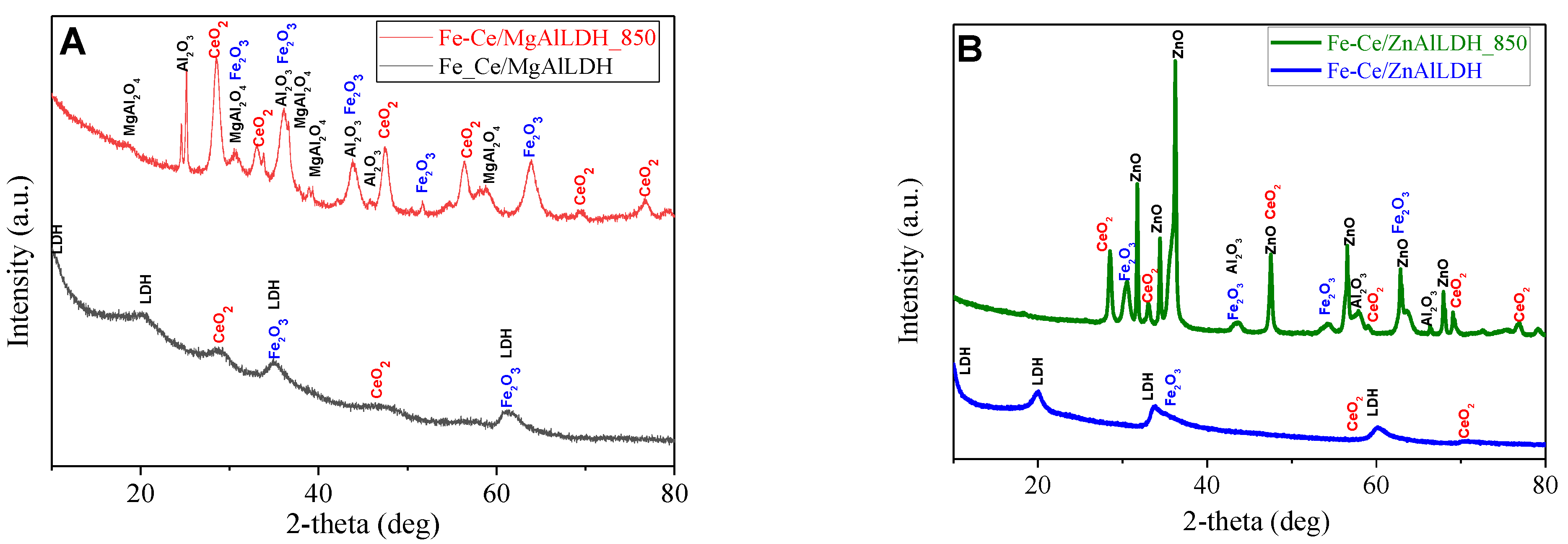

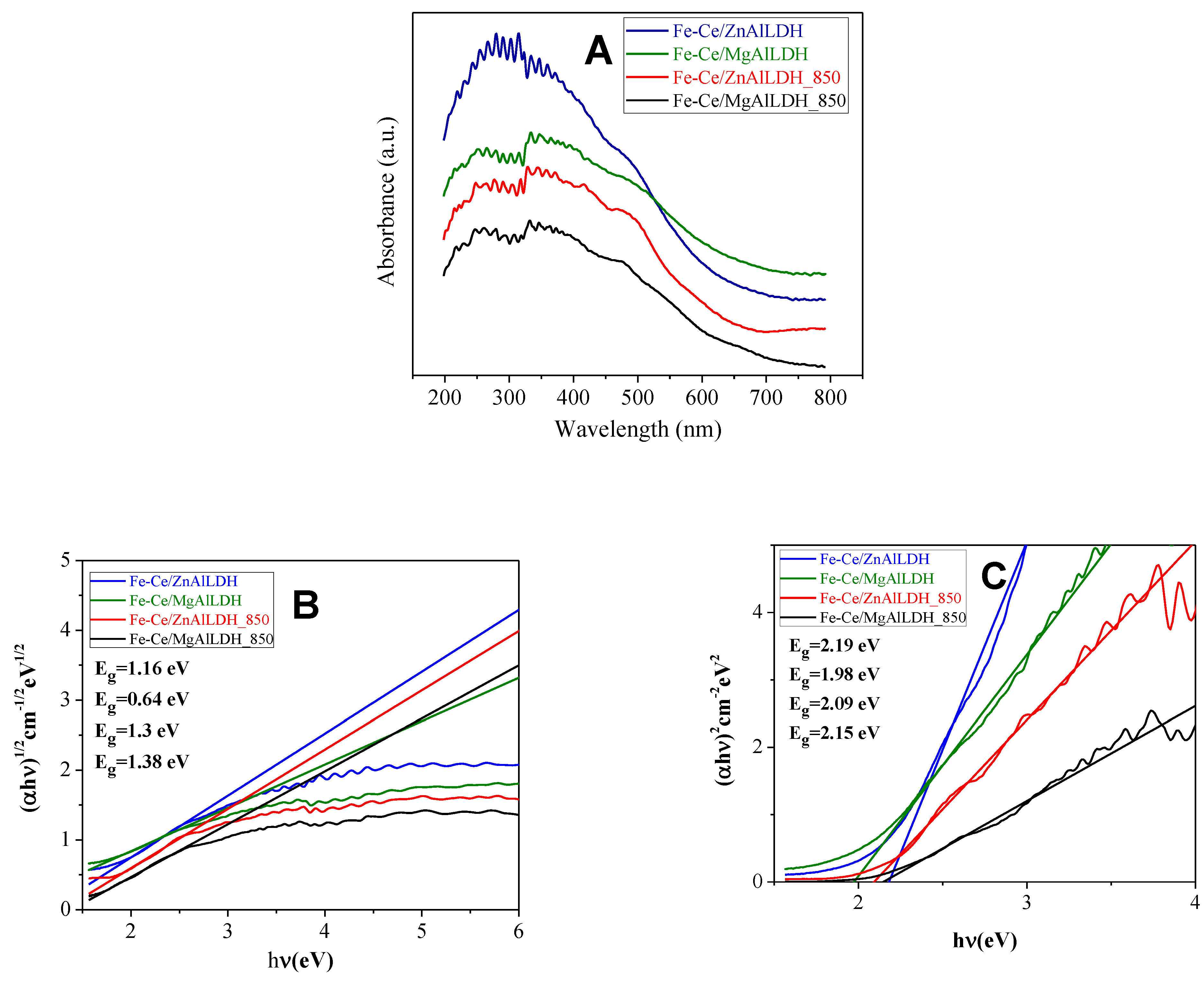
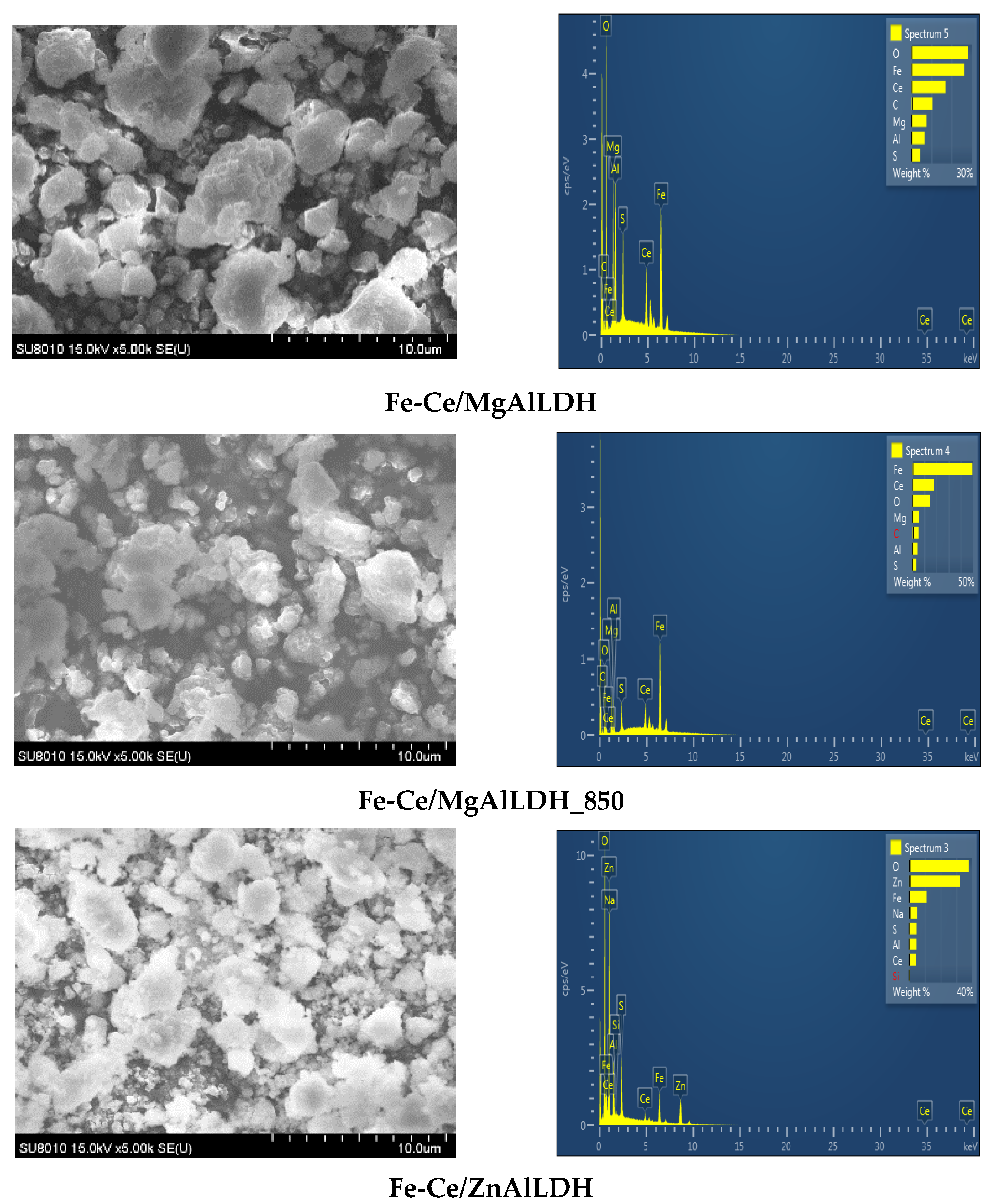
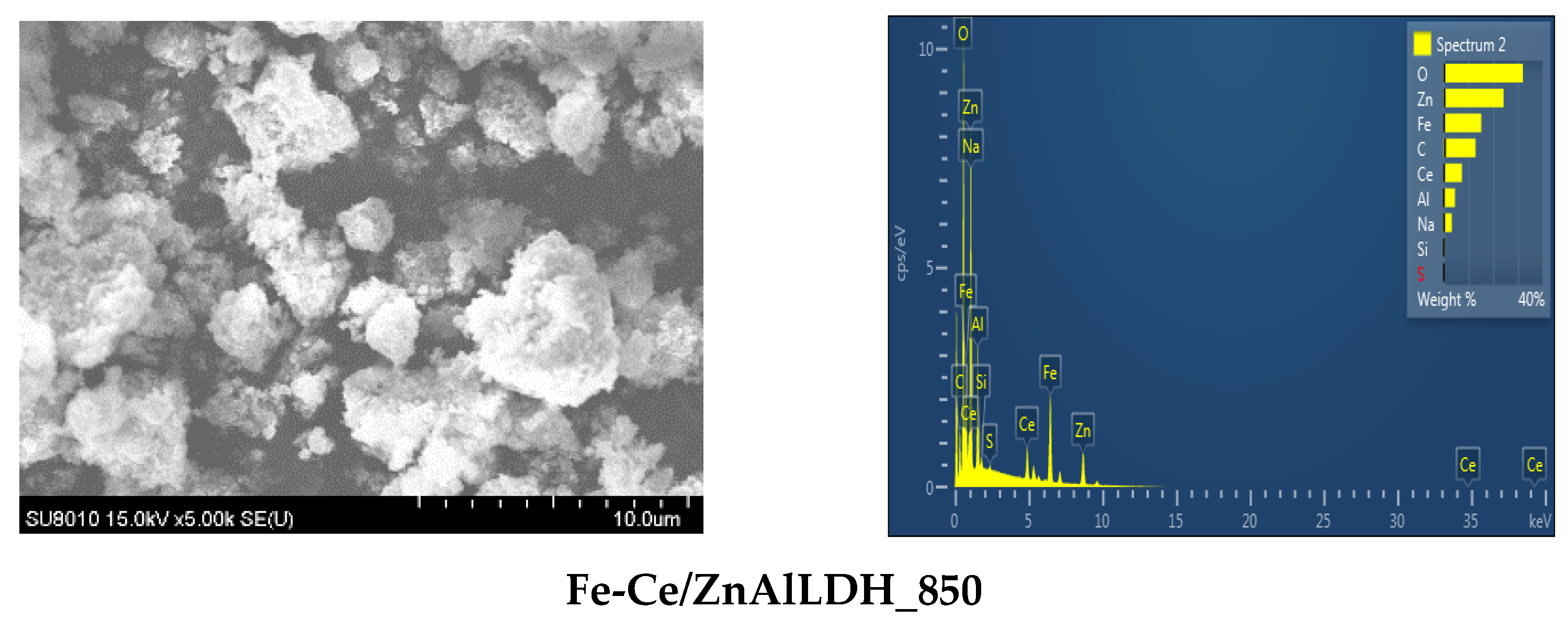
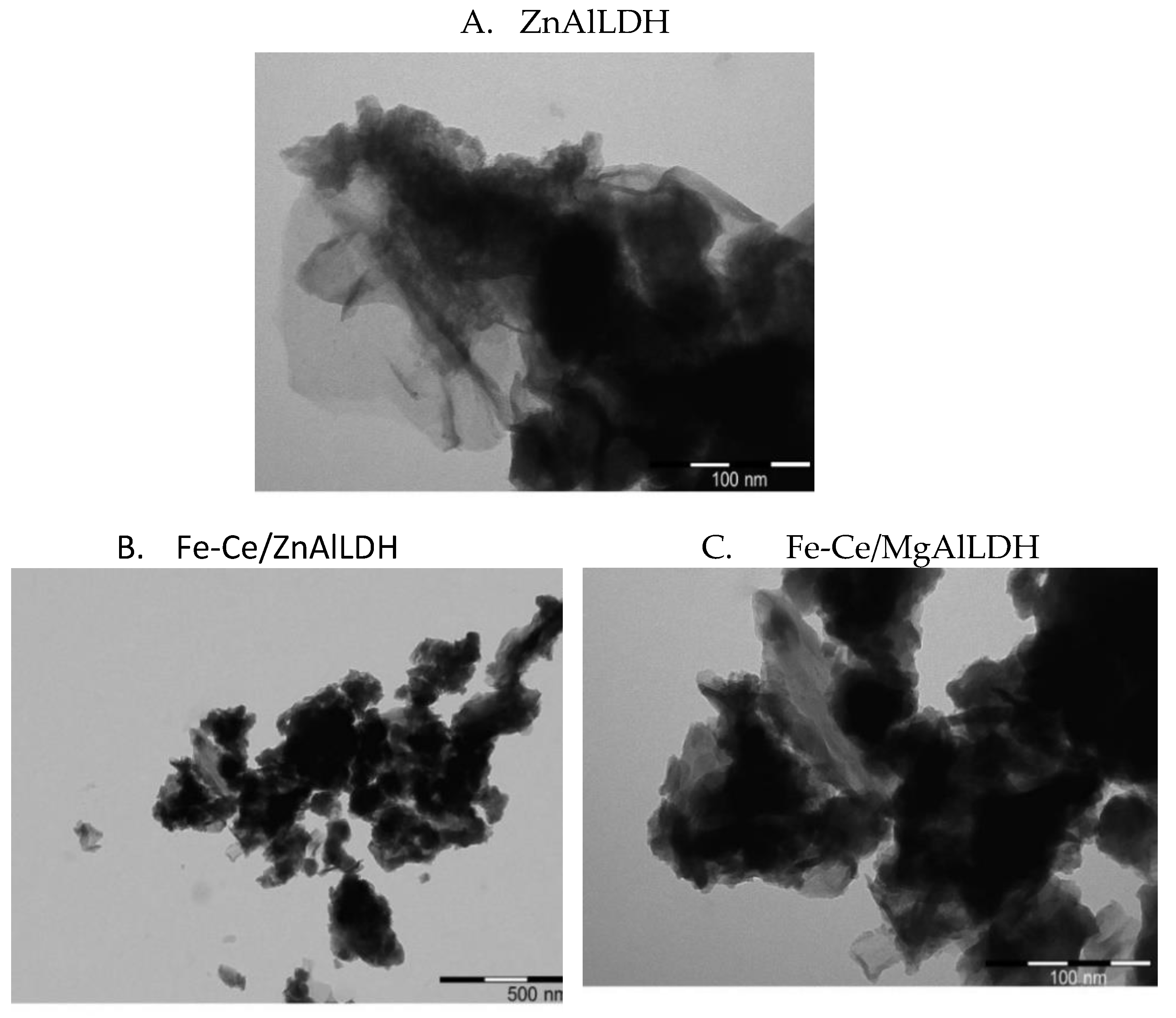

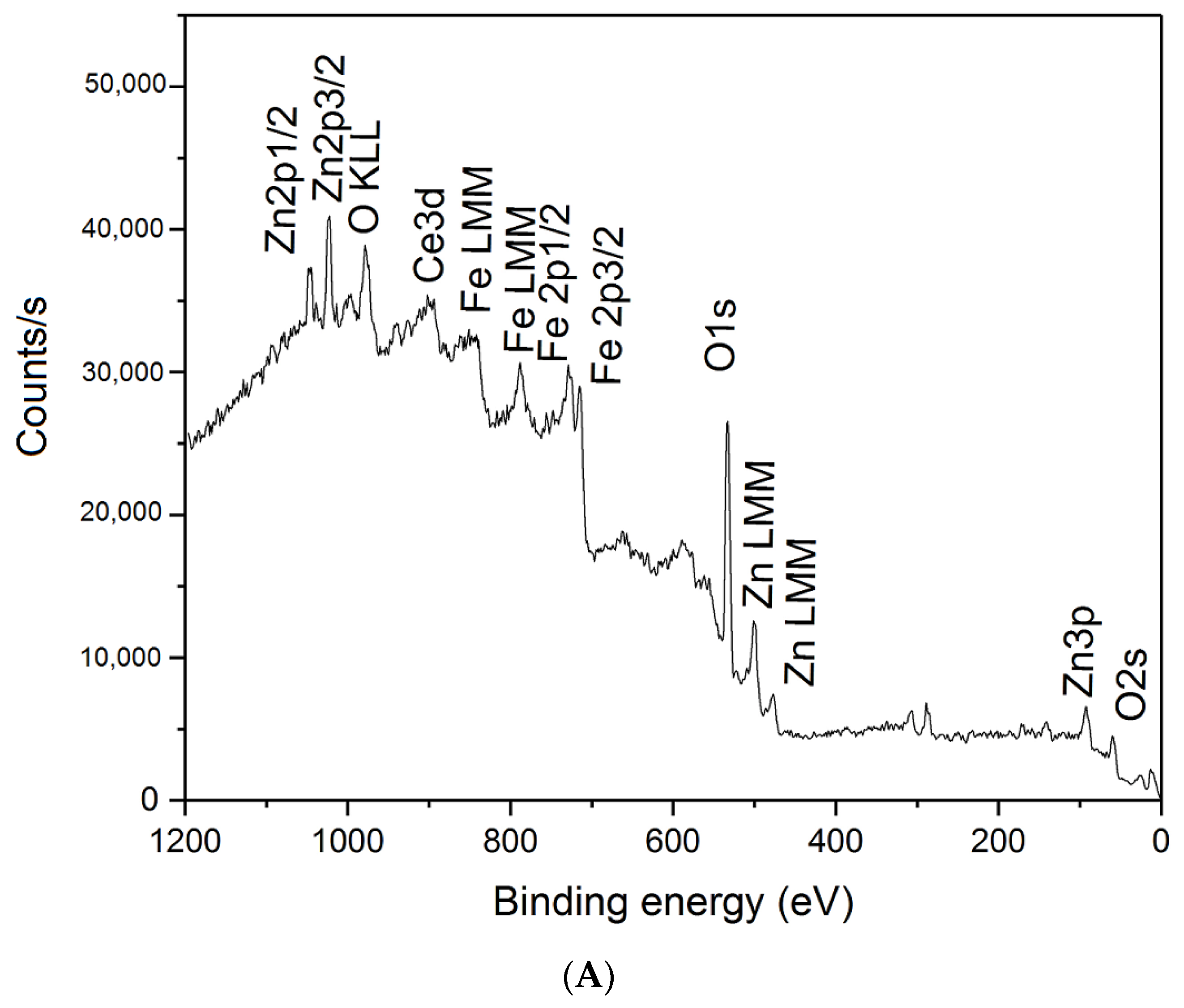
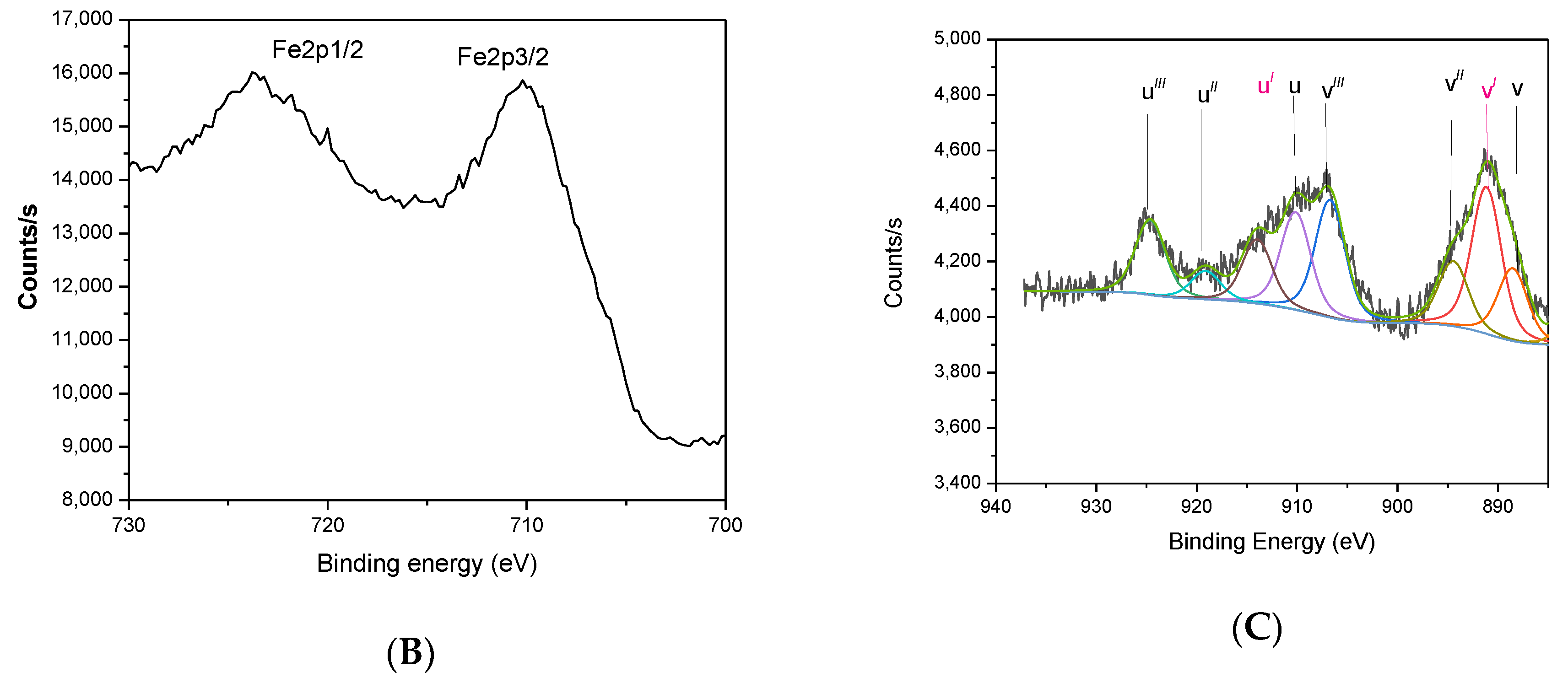
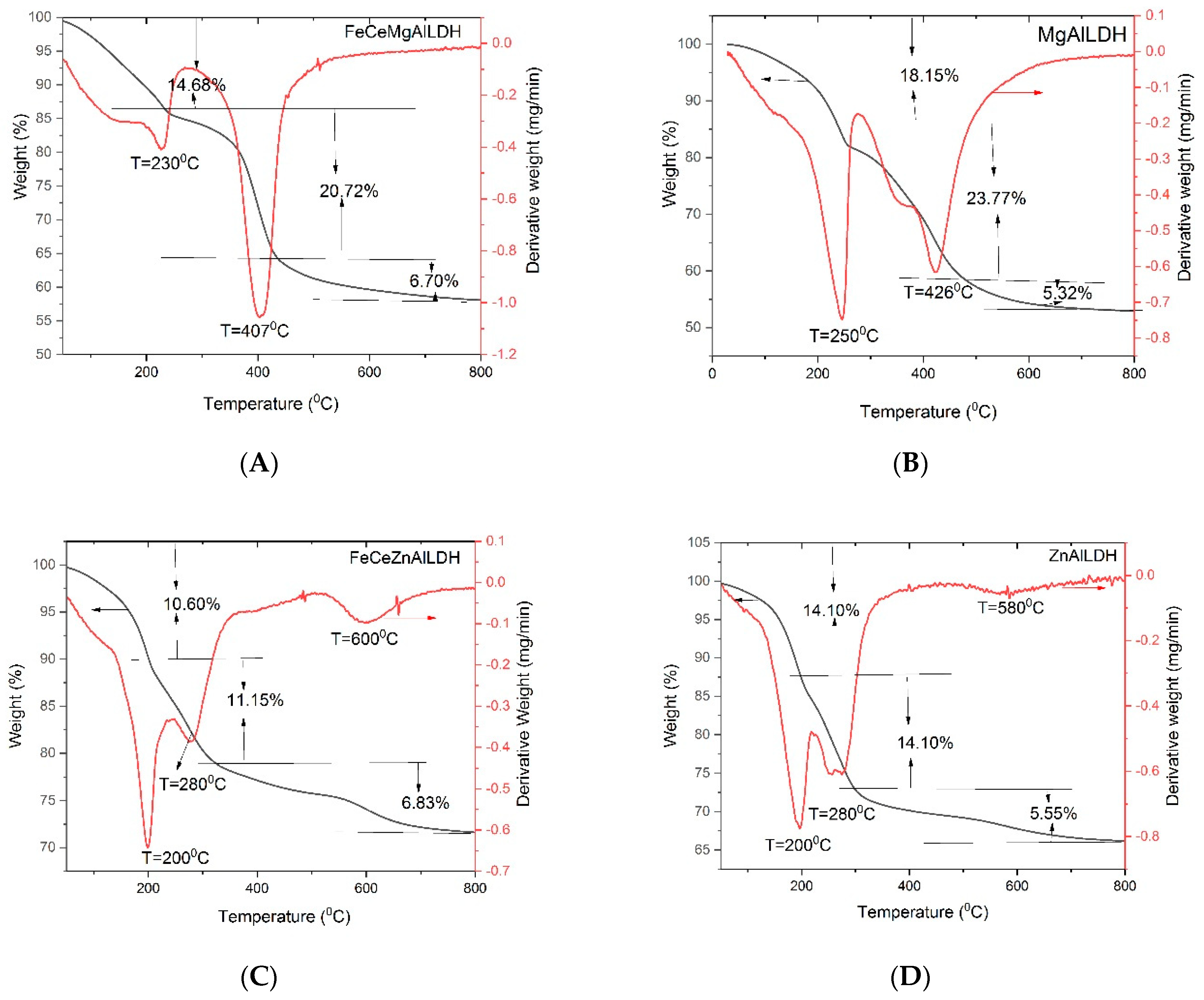
| Sample | Mg/Al Molar Ratio | Zn/Al Molar Ratio | Fe/Ce Molar Ratio | LDH Cell Parameters (nm) | |
|---|---|---|---|---|---|
| a | c | ||||
| Fe-Ce/MgAlLDH | 1.99 | - | 5.07 | 2.57 | |
| Fe-Ce/MgAlLDH_850 | 2.32 | - | 5.28 | - | - |
| Fe-Ce/ZnAlLDH | - | 2.90 | 5.42 | 0.31 | 2.54 |
| Fe-Ce/ZnAlLDH_850 | - | 3.06 | 5.33 | - | - |
| Sample | Rb, Ω | Rgb, Ω |
|---|---|---|
| Fe-CeZnAlLDH | 347 | 1.24 × 105 |
| Fe-CeZnAlLDH_850 | 633 | 7.03 × 104 |
| Fe-CeMgAlLDH | 702 | 8.09 × 104 |
| Fe-CeMgAlLDH_850 | 861 | 2.35 × 105 |
| Electrode | Mott–Schottky Slope | Photoelectron Life Time (τ) | |
|---|---|---|---|
| Dark | Illum. | ||
| Fe-Ce/MgAlLDH | 176.61 | 1.11 | 6.52 |
| Fe-Ce/MgAlLDH_850 | 193.33 | 1.37 | 2.68 |
| Fe-Ce/ZnAlLDH | 4.20 | 17.8 | 55.53 |
| Fe-Ce/ZnAlLDH_850 | 87.95 | 0.68 | 6.63 |
| Sample | UV Light Irradiation | Simulated Solar Light Irradiation | ||
|---|---|---|---|---|
| Kapp (min−1) | R2 | Kapp (min−1) | R2 | |
| Fe-Ce/MgAlLDH | 0.0077 | 0.9948 | 0.0003 | 0.8936 |
| Fe-Ce/ZnAlLDH | 0.0079 | 0.9906 | 0.0009 | 0.9457 |
| Fe-Ce/MgAlLDH_850 | 0.0033 | 0.9288 | 0.0034 | 0.9969 |
| Fe-Ce/ZnAlLDH_850 | 0.0037 | 0.9721 | 0.0054 | 0.9907 |
| Photocatalyst | Phenol Initial Conc. (mg/L) | Light Source | Time | Phenol Degrad. (%) | Ref. |
|---|---|---|---|---|---|
| NiFe-LDH | 20 mL, 20 ppm | UV λmax = 510 nm | 120 min | [59] | |
| Ag@Ag3PO4/g-C3N4/NiFeLDH | Sunlight | 2 h | 90% | [14] | |
| NiFe-LDH/N-rGO/g-C3N4 | 20 mL, 20 ppm | 125 W medium Hg lamp (λmax = 420 nm) | 120 min | 75% | [12] |
| ZnAlLDH/Ag | 20 mg/mL | 300 W Xenon lamp | 210 min | 80% | [60] |
| CeO2/MgAlLDH | 80 mg/L | UV lamp (115 V, 254 nm, 4400 μW/cm2, without filter) | 7 h | 50 | [61] |
| ZnAl and ZnGaAlLDH and MMOs | 40 mg/L 80 mg/L | UV lamp (115 V, 254 nm, 4400 μW/cm2, without filter) | 6 h | 80 60 | [62] |
| Mn-doped ZnAlLDH | 30 mg/L | UV lamp (254 nm and 2.8 W) | 6 h | 95 | [63] |
| SnO2-ZnAlLDH | 40 mg/L | UV lamp (254 nm and 4.4 mW/cm2) | 2 h | 91 | [64] |
| ZnAlCeLDH Ce (3.5%, 5.0%, and 10% mol) | 40 mg/L | UV lamp (254 nm and 4400 μW/cm2) | 4 h | 90 | [26] |
| ZnAlLa MMOs | 40 mg/L | UV-Vis lamp (254 nm, 4400 μW/cm2) | 300 min | 97 | [65] |
| CeO2/ZnSn-LDH | 50 mg/L | UV lamp | 4 h | 92 | [66] |
| Fe-Ce/ZnAlLDH Fe-Ce-MMOs | 20 mg/L 20 mg/L | UV lamp (125 W) Xenon lamp (300 W) | 4 h 4 h | 87 74 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureseanu, M.; Cioatera, N.; Carja, G. Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water. Nanomaterials 2023, 13, 981. https://doi.org/10.3390/nano13060981
Mureseanu M, Cioatera N, Carja G. Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water. Nanomaterials. 2023; 13(6):981. https://doi.org/10.3390/nano13060981
Chicago/Turabian StyleMureseanu, Mihaela, Nicoleta Cioatera, and Gabriela Carja. 2023. "Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water" Nanomaterials 13, no. 6: 981. https://doi.org/10.3390/nano13060981
APA StyleMureseanu, M., Cioatera, N., & Carja, G. (2023). Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water. Nanomaterials, 13(6), 981. https://doi.org/10.3390/nano13060981





