Abstract
This paper is dedicated to the discussion of applications of carbon material in electrochemistry. The paper starts with a general discussion on electrochemical doping. Then, investigations by spectroelectrochemistry are discussed. The Raman spectroscopy experiments in different electrolyte solutions are considered. This includes aqueous solutions and acetonitrile and ionic fluids. The investigation of carbon nanotubes on different substrates is considered. The optical absorption experiments in different electrolyte solutions and substrate materials are discussed. The chemical functionalization of carbon nanotubes is considered. Finally, the application of carbon materials and chemically functionalized carbon nanotubes in batteries, supercapacitors, sensors, and nanoelectronic devices is presented.
1. Introduction
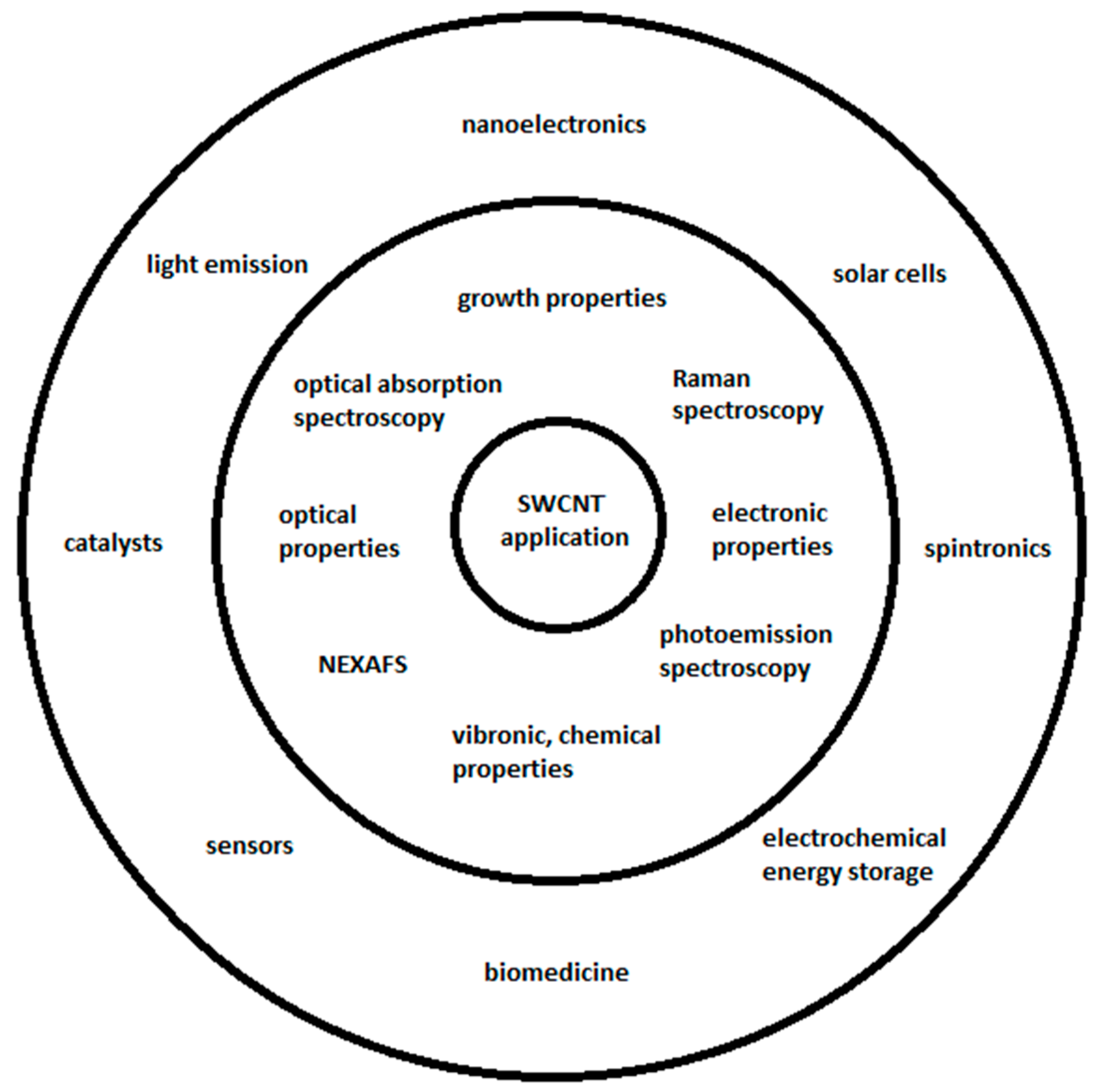
Carbon nanotubes include single-walled carbon nanotubes (SWCNTs) with unique properties [1,2] and multi-walled carbon nanotubes (MWCNTs). SWCNTs are sorted and separated to obtain uniform electronic properties [3], and the channels of SWCNTs are filled [4,5] with substances [6,7] for the investigation of kinetics and electronic properties [8,9,10,11,12,13,14,15] for applications (Figure 1). The SWCNTs are filled with inorganic compounds [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46], molecules [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76], and elementary substances [77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108]. Inorganic substances are introduced inside carbon nanotubes by the gas phase and liquid phase methods. Molecules are filled inside carbon nanotubes by the gas phase and solution methods. Elementary substances are incorporated inside carbon nanotubes by the gas, solution, and melt methods. The electronic properties of chemically modified carbon material are investigated by Raman spectroscopy, near-edge X-ray absorption fine structure spectroscopy (NEXAFS), optical absorption spectroscopy (OAS), and photoemission spectroscopy.
Figure 1.
SWCNT properties, methods, and applications.
A high current carrying capacity, long cycling stability, excellent electrical conductance, and good capability in rapid charge and discharge make SWCNTs electrodes possible, leading to a high performance [109].
The electrochemical doping allows for the alteration of the doping level [110,111,112]. The shift in the Fermi level is proportional to the applied voltage [112,113]. The experimental parameters are varied to achieve perfect conditions [112].
In 1999, the first example of electrochemical doping appeared [114]. The methods that investigated the modified electronic properties are voltamperometry [115,116,117,118,119] and spectroelectrochemistry [110,113,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142]. Voltamperometry allows for evaluating the reversibility of the p- and n-doping of SWCNTs by applied voltage using the cyclic voltamerograms [112]. Combining the spectroscopic investigations, such as Raman spectroscopy and optical absorption spectroscopy, with electrochemical charging allows for the evaluation of the modifications of the electronic properties of SWCNTs [143].
The aim of this review is to summarize the reports on the investigations of the electrochemical properties of carbon material. In Section 2, the results of the voltamperometry are discussed. In Section 3, the results of the spectroelectrochemistry with Raman spectroscopy are considered, i.e., measuring the spectra with electrochemical charging. In Section 4, the results of the spectroelectrochemistry with optical absorption spectroscopy are presented. In Section 5, investigations of chemically functionalized carbon nanotubes are highlighted. In Section 6, applications of carbon material in electrochemical devices are discussed.
2. Voltamperometry
For voltamperometry, it is important that the MWCNTs do not destroy under electrochemical charging. MWCNTs are stable up to a ~±2 eV applied potential. This allows for one to use them for voltamperometry measurements. Measurements are conducted at different scan rates. The data present the dependence of the voltage on the capacity. The insertion of the electrolyte happens. For MWCNTs, a capacity of up to ~1000 mAh/g was observed.
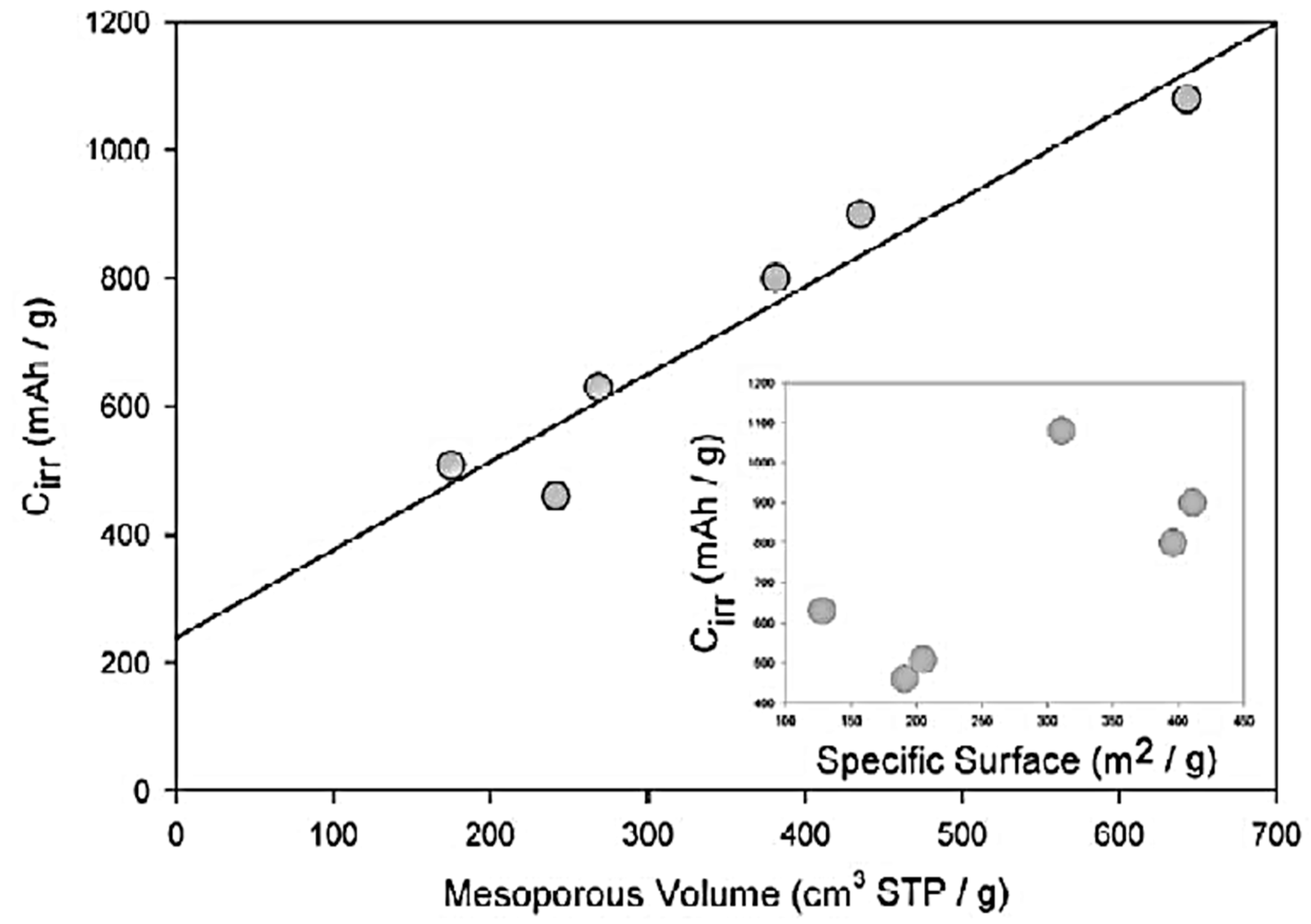
In Ref. [116], the electrochemical experiments were performed with lithium insertion into nanotubes. High values of irreversible capacity Cirr (from 460 to 1080 mAh/g) were obtained. The authors plotted the dependence of Cirr on the mesopore volume, which showed the linear behavior [116].
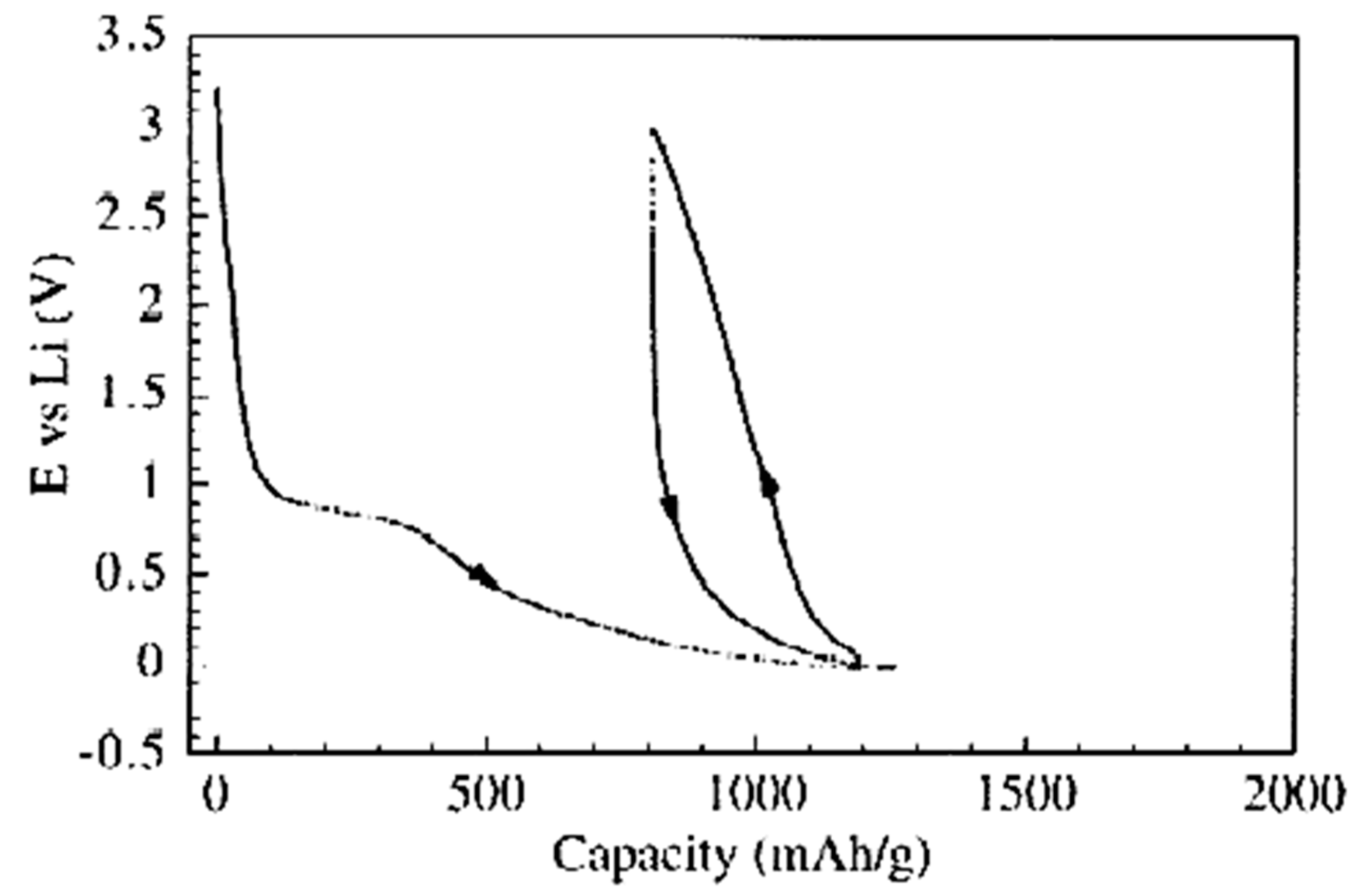
Figure 2 presents the voltamperometric data of lithium insertion and extraction in MWCNTs to test electrodes from carbon tubular form. They show the typical behavior of tubular carbon [116]. The irreversible capacity values of all forms of tubular carbon Cirr are extremely high. It is believed that one factor that makes the value of Cirr is the solid electrolyte interphase (SEI) formation.
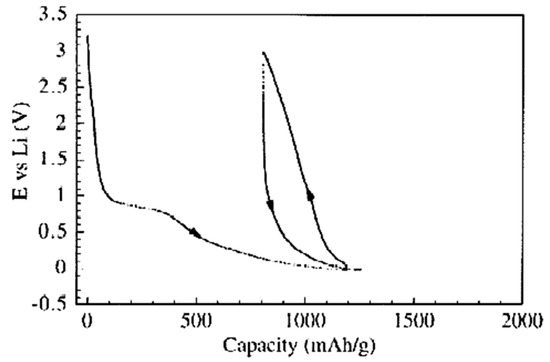
Figure 2.
Galvanostatic insertion–extraction of lithium into nanotubes. Current load of 20 mA/g. Reprinted from Frackowiak E, Beguin F Carbon 40 1775 (2002), Copyright (2002), with permission from Elsevier [116].
The authors of Ref. [116] plotted the Cirr data versus the mesopore volume. Figure 3 presents the linear dependence with the liner fitting of multi-walled carbon nanotubes, single-walled carbon nanotubes, and carbon filaments [116]. This is caused by the tubular structure of carbon forms, with an easy access of voluminous solvated lithium cations in the inner part of the electrode where it decomposes [116].
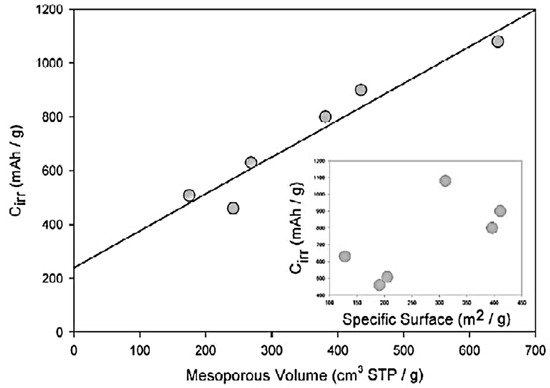
Figure 3.
Dependence of irreversible capacity Cirr versus mesopore volume and specific surface area (inset) for different types of nanotubes. Reprinted from Frackowiak E, Beguin F Carbon 40 1775 (2002), Copyright (2002), with permission from Elsevier [116].
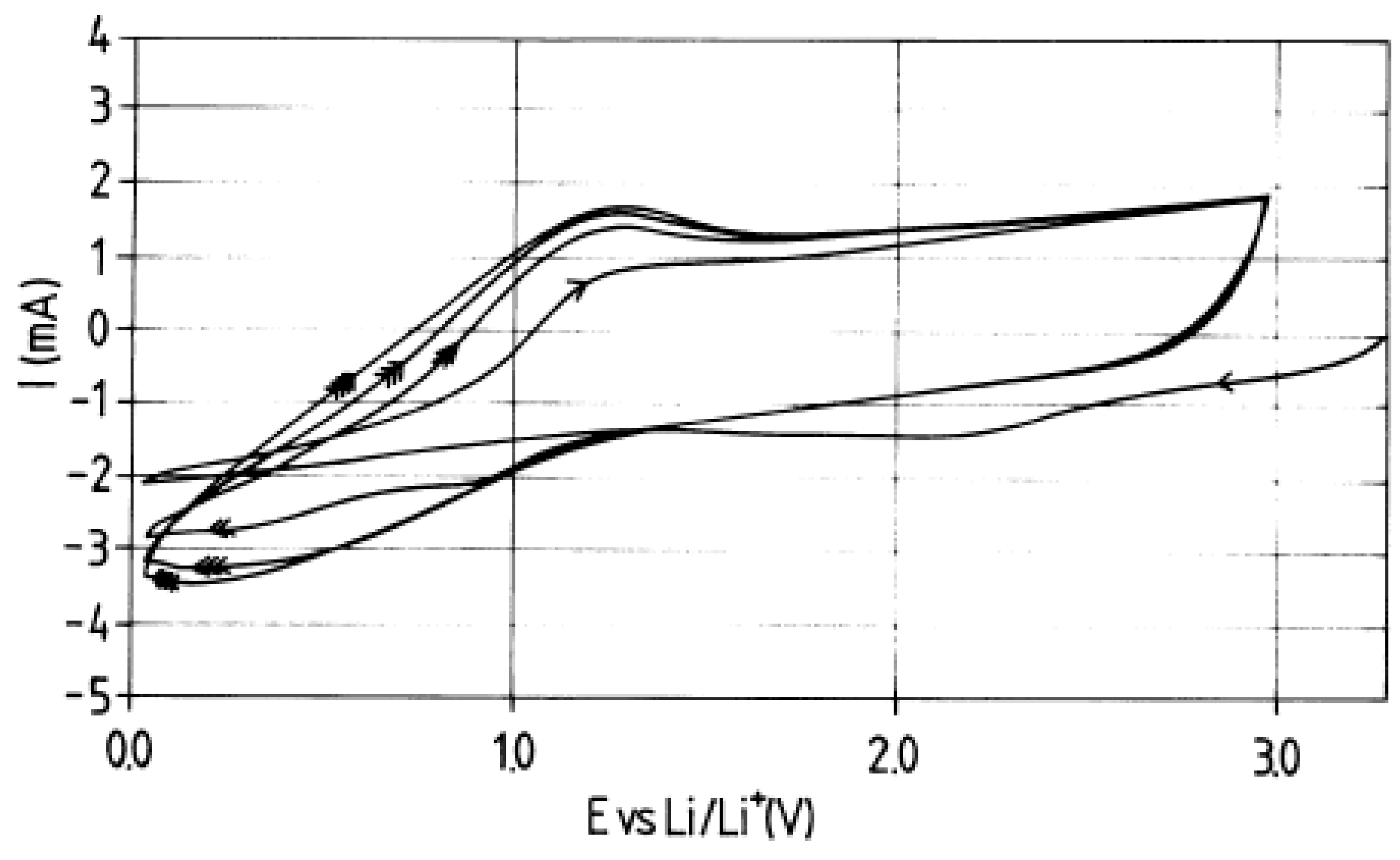
In Ref. [144], the voltamperometry data of MWCNT at different scan rates are presented (Figure 4). Until 1.5 V (open-circuit voltage (OCV)) and about 1 V, there are the reductions of chemicals, and from about 0.5 V until 0 V, there is the lithium insertion. Above 1.5 V, the region of charging of the double layer was observed, which testified to the good electrochemical properties [114].
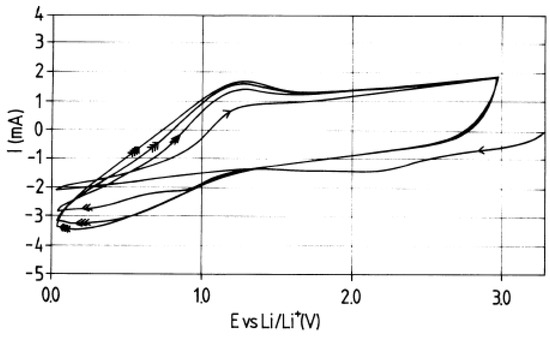
Figure 4.
Voltammetry characteristics of MWCNT. Reprinted from E. Frackowiak et al./Carbon 37 (1999) 61–69, Copyright (1999), with permission from Elsevier [144].
3. Spectroelectrochemistry with Raman Spectroscopy
The electrochemical experiments are combined with Raman spectroscopy and optical absorption spectroscopy. Yet, other methods are possible.
To present the method, in the spectroelectrochemical technique, SWCNTs serve as a working electrode. The spectrum is measured under an applied potential. The spectra obtained at different applied voltages are plotted for comparison and tracing changes.
Historically, the first experiment on the electrochemical doping of SWCNTs was performed in 1999 [142]. In this experiment, Raman spectroscopy was applied, and the electrolyte was sulfuric acid. In 2000, the experiments in aqueous [118] and aprotonic (tetrahydrofuran) [110] solutions were made. In the literature, there are also reports on experiments in aqueous [113,120,121,122,123,124,125,126,134,135,136,137,138,139,140] solutions and acetonitrile [119,127,128,130,131] and ionic fluids [129]. Thin films [118,119,121,122,128,129,130,134,135,136] and buckypaper [120,125,137,138,139,143] were analyzed.
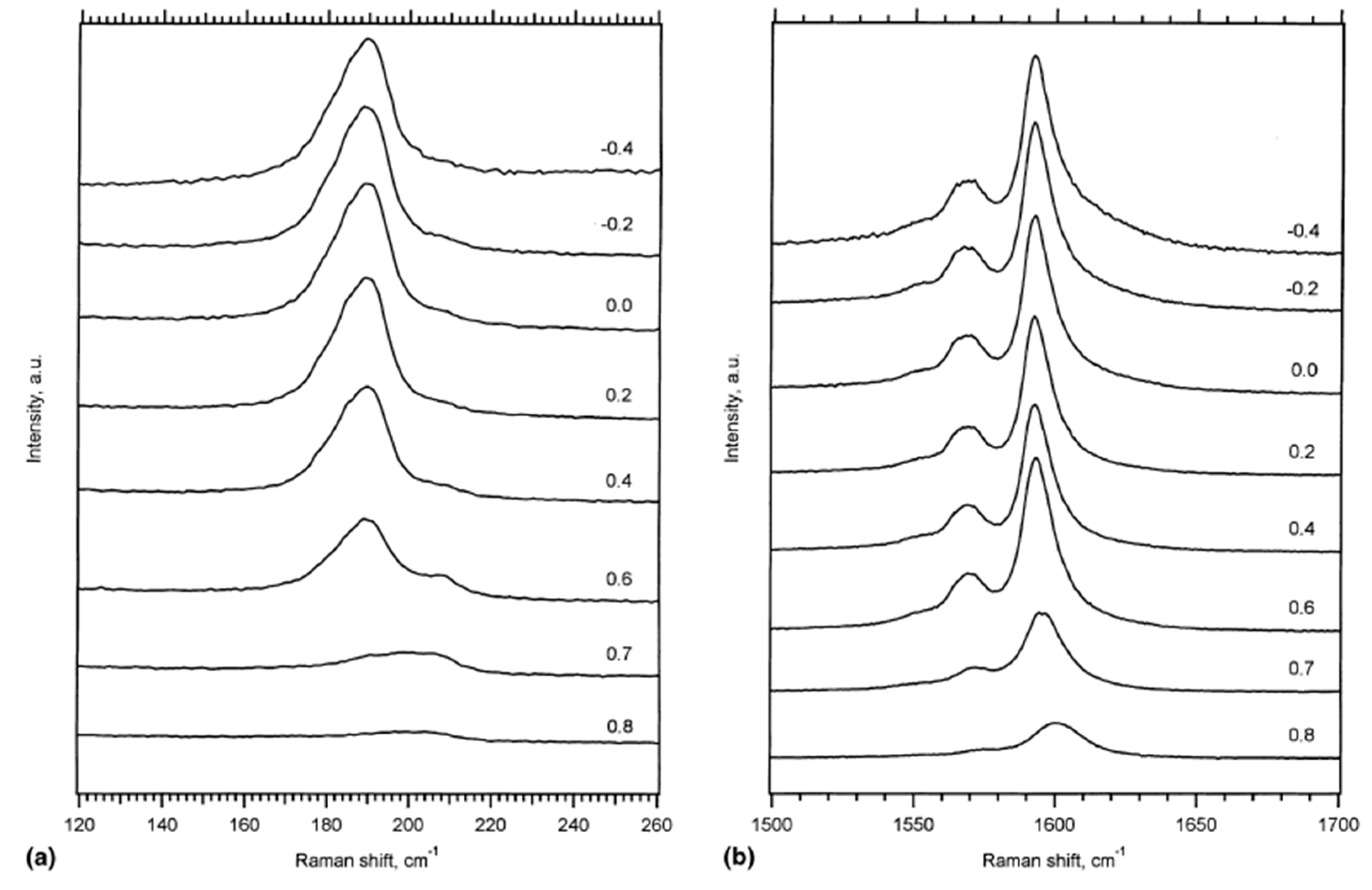
In Ref. [118], the authors studied the Raman spectra of SWCNTs upon electrochemical charging at applied potentials from −0.2 to −0.8 eV and from +0.2 to +1.4 eV. Figure 5 shows the RBM and tangential displacement modes (TDM) of the resonance Raman spectra of a buckypaper in a 1 M NaCl aqueous solution acquired at a 514 nm laser [118].
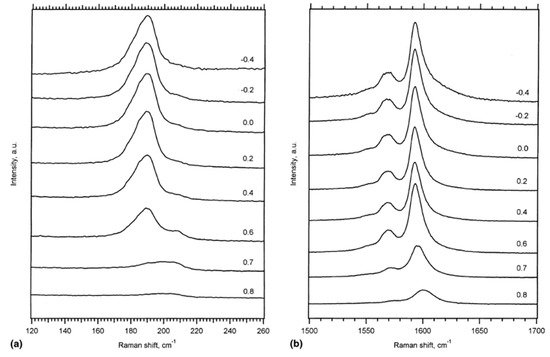
Figure 5.
The resonance Raman spectra of the RBM (a) and TDM (b) modes acquired at a laser wavelength of 514 nm under both positive and negative voltages. Reprinted from Kavan L, Rapta P, Dunsch L Chem. Phys. Lett. 328 363 (2000), Copyright (2000), with permission from Elsevier [118].
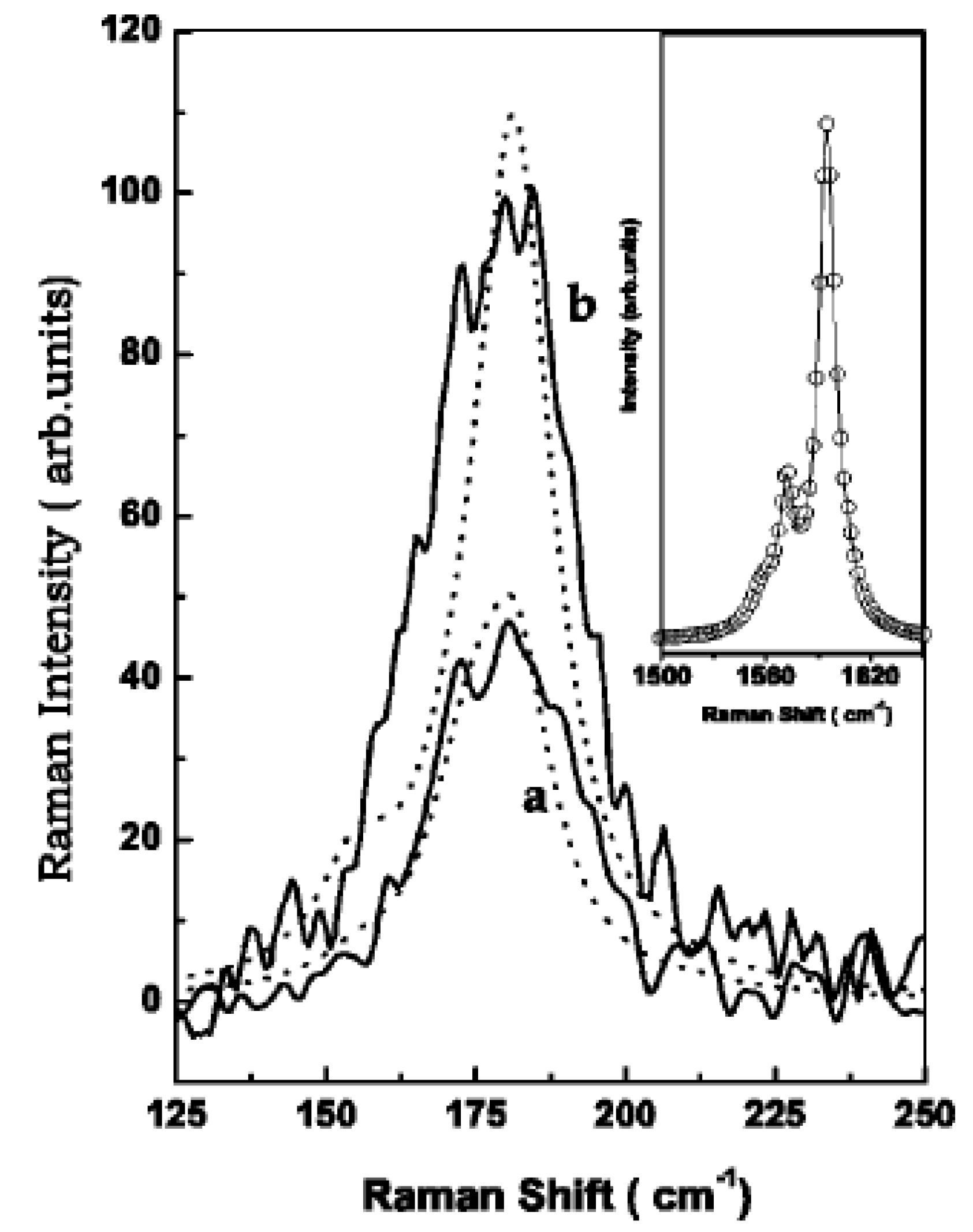
The authors of Ref. [123] investigated the RBM-band of Raman spectra. In the RBM band, there is the peak at 180 cm−1. In the G-band, there is the peak of the tangential modes (TM) at 1590 cm−1. Figure 6 shows experimental Raman spectra (solid lines) of the RBM at zero bias (Figure 6a) and −0.08 V (Figure 6b) for an applied laser energy of 2.41 eV. The dotted lines are the calculated line shapes. The inset shows the G-band at zero bias (open circles) and at −0.08 V (solid line). Thus, the intensity and position of peaks change under electrochemical charging [123].
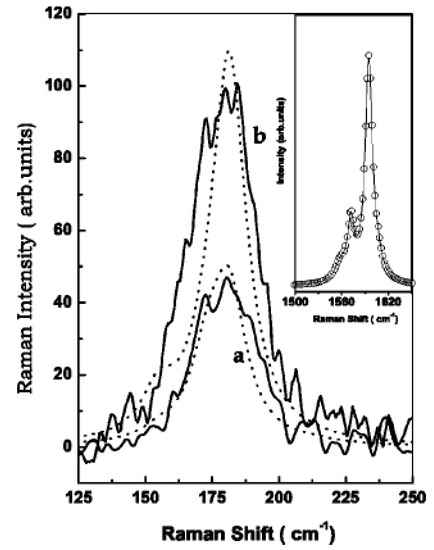
Figure 6.
Experimental Raman spectra (solid lines) of the RBM at zero bias (a) and −0.08 V (b) for a laser energy of 2.41 eV. The dotted lines are the calculated line shapes. The inset shows the G-band at zero bias (open circles) and at −0.08 V (solid line). Reprinted from Ghosh S, Sood A K, Rao C N R J. Appl. Phys. 92 1165 (2002), with the permission of AIP Publishing [123].
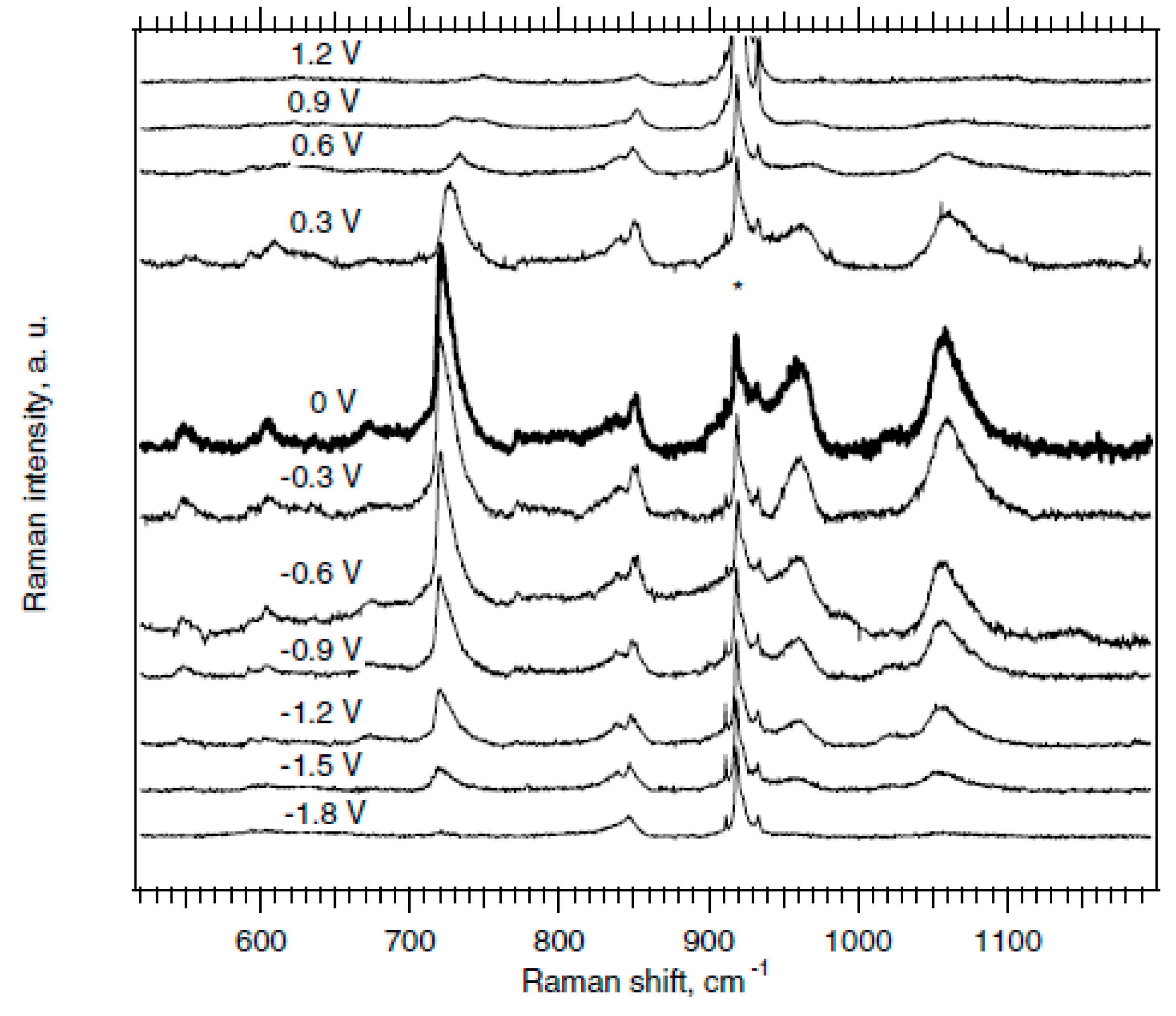
In Ref. [127], the potential dependent Raman spectra (excited at 2.18 eV) of HiPco SWCNT were obtained. There are intermediate frequency modes (IFM) in the spectrum. Figure 7 shows the dependence of IFM modes on electrochemical charging from −1.8 V to 0 V and from 0 V to 1.2 V. The dispersive IFM modes are shifted stronger than the non-dispersive IFM modes upon p-doping, whereas n-doping has the opposite effect. Thus, one laser wavelength is enough to recognize dispersive and non-dispersive features [127].
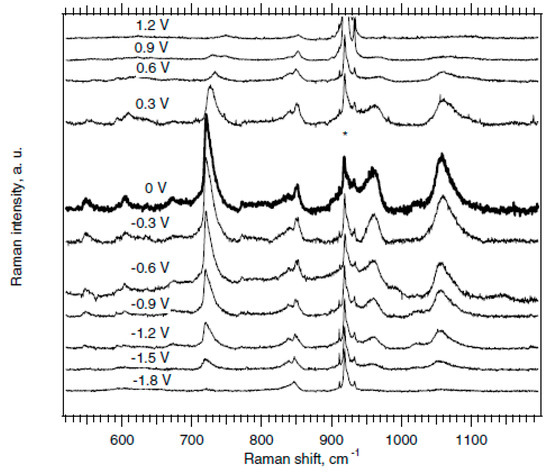
Figure 7.
Potential dependent Raman spectra (excited at 2.18 eV) of HiPco SWCNT on the Pt electrode in acetonitrile +0.2 M LiClO4. The peaks marked by * are assigned to the electrolyte solution. Reprinted with permission from Ref. [127], copyright 2006 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Thus, the spectroelectrochemistry technique is a modern, useful, and powerful method based on the Raman spectroscopy method. The substances for electrodes should be stable in electrolyte solutions, and they should not destroy under applied voltages. Raman maps are plotted using the obtained data; they are the dependence of the Raman peak position on an applied voltage. In Raman maps of carbon nanotubes, the modifications of the electronic properties are observed.
4. Spectroelectrochemistry with Optical Absorption Spectroscopy
The optical absorption spectroscopy was first combined with electrochemical charging in the year 2000 [118]. Indium-tin oxide (ITO) [118,119,124,125] and think Pt film [124,125] were used as substrates. Aqueous [118], acetonitrile [118,119,124,125], and ionic liquids [125] were used.
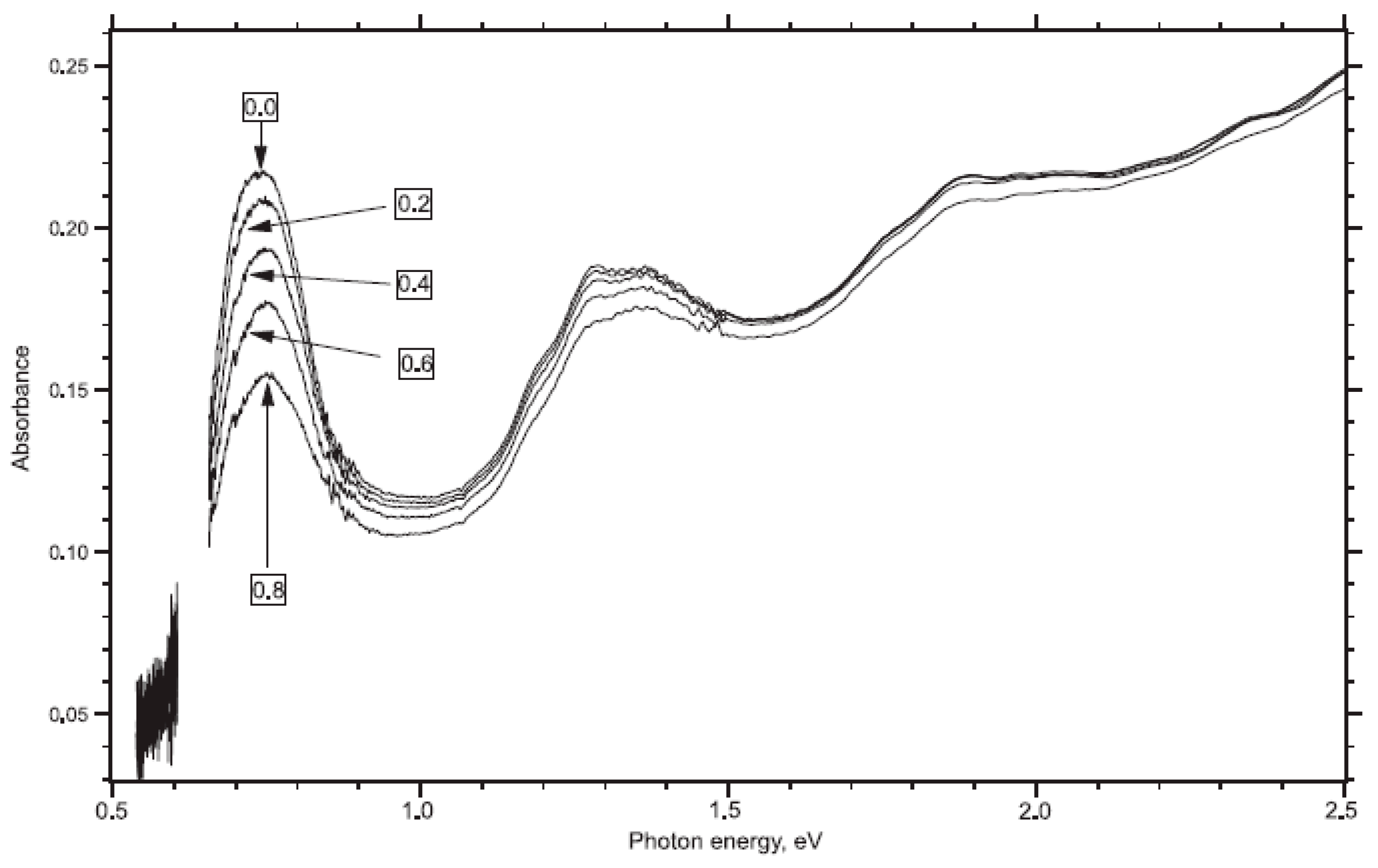
In Ref. [118], electrochemical experiments were conducted using the ITO substrate and aqueous 0.1 M KCl saturated with nitrogen as the electrolyte solution. Figure 8 shows the OAS spectra of SWCNTs measured with applied potentials from 0 V to 0.8 V [118]. The spectra contain absorption bands at 1800 nm (0.68 eV), 1000 nm (1.3 eV), and 700 nm (1.9 eV). Upon electrochemical charging, there is the suppression of the absorption bands. This was explained by a shift in the Fermi level.
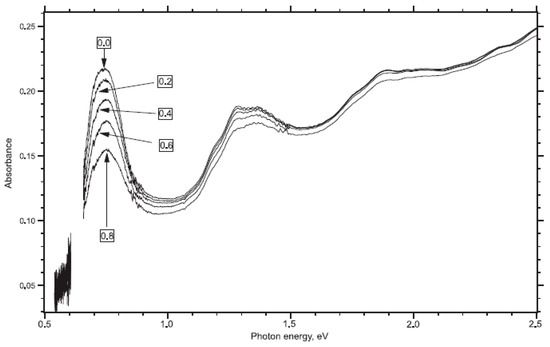
Figure 8.
Vis-NIR spectra of ITO-supported SWCNT in aqueous 0.1M KCl saturated with nitrogen. Reprinted from Kavan L, Rapta P, Dunsch L Chem. Phys. Lett. 328 363 (2000), Copyright (2000), with permission from Elsevier [118].
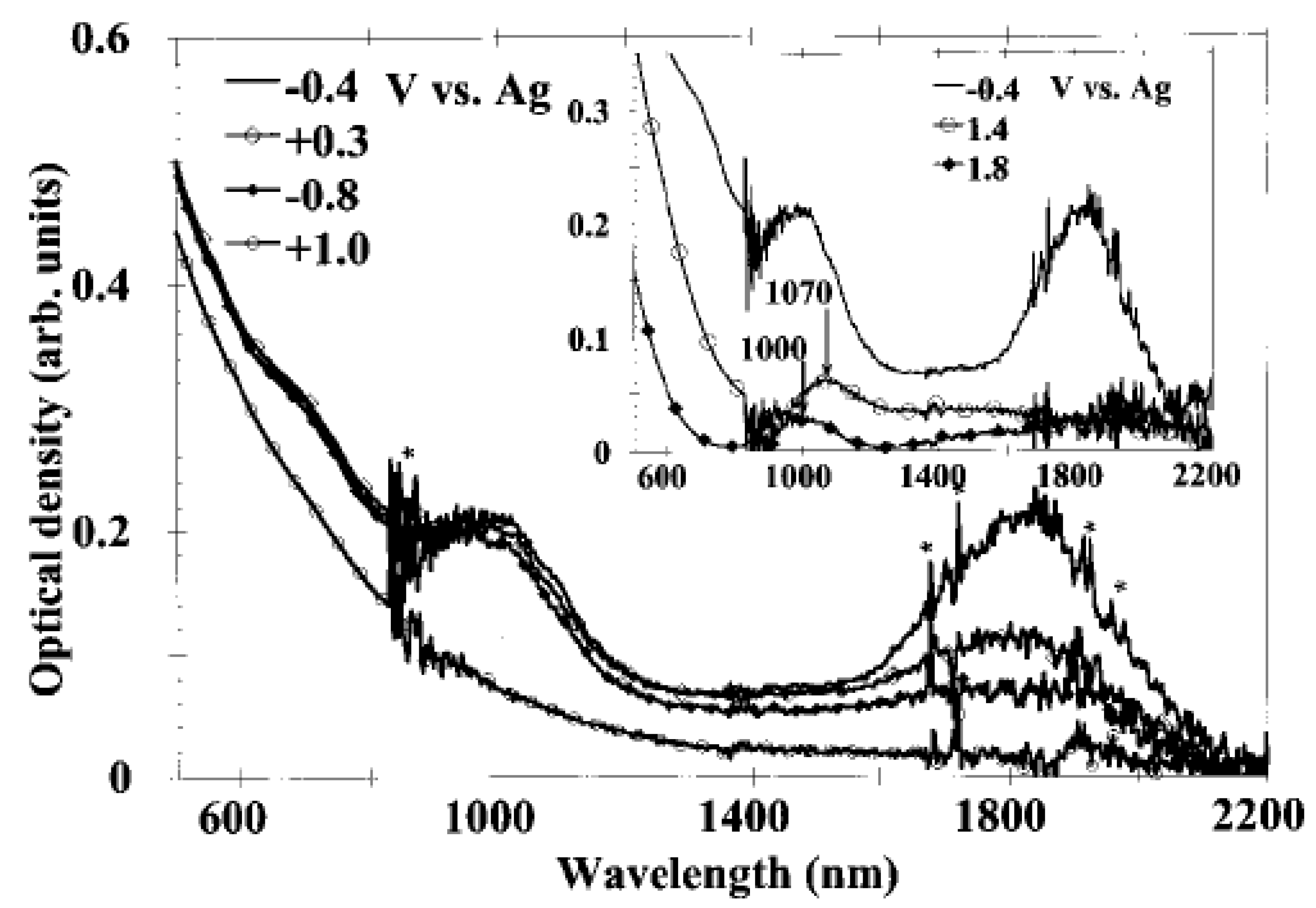
In Ref. [133], the in situ measurement of SWCNT films under electrochemical charging was performed. Figure 9 shows the OAS spectra at constant electrode potentials [133]. The potentials of −0.4 V, −0.8 V, 0.3 V, 1.0 V, 1.4 V, and 1.8 V were applied. Upon the application of the potential, the absorption bands at 1800 nm, 1000 nm, and 700 nm disappear. At high positive potentials, new broad peaks appear around 1070 nm (1.15 eV) at 1.4 V and around 1000 nm (1.24 eV) at 1.8 V. At low negative potentials, no new absorption bands, at least up to 21.4 V, were observed. At high positive potentials, the authors also observed the increased absorption background in the near-infrared field [133].
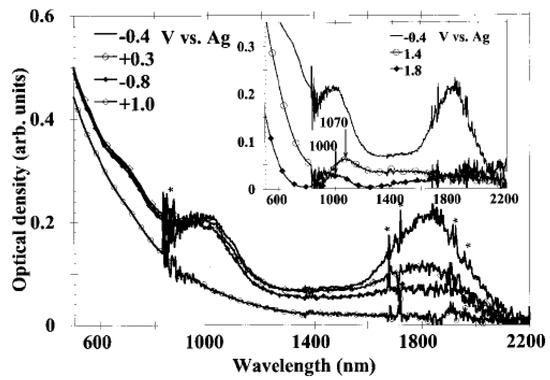
Figure 9.
In situ absorption spectra of an SWCNT film. The star indicates features coming from the solvent and also noises. Reprinted from Kazaoui S et al. Appl. Phys. Lett. 78 3433 (2001), with the permission of AIP Publishing [133].
5. Investigations of Chemically Functionalized Carbon Nanotubes
There is a large field of work on chemically functionalized carbon nanotubes. It was shown that metal halogenide-filled SWCNTs with a decreased [145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160] Fermi level, metal chalcogenide-filled SWCNTs [161,162,163], metal-filled SWCNTs [164,165,166], metal halogenide-filled SWCNTs [167], and metallocene-filled SWCNTs [168,169,170,171,172,173,174,175,176,177,178,179,180,181] with an increased Fermi level can be used for the electrochemical measurements.
5.1. Covalent Functionalization of Carbon Nanotubes
The covalent functionalization of carbon nanotubes was made with fluorination. This method is performed with different chemicals and synthesis parameters [182,183,184,185,186].
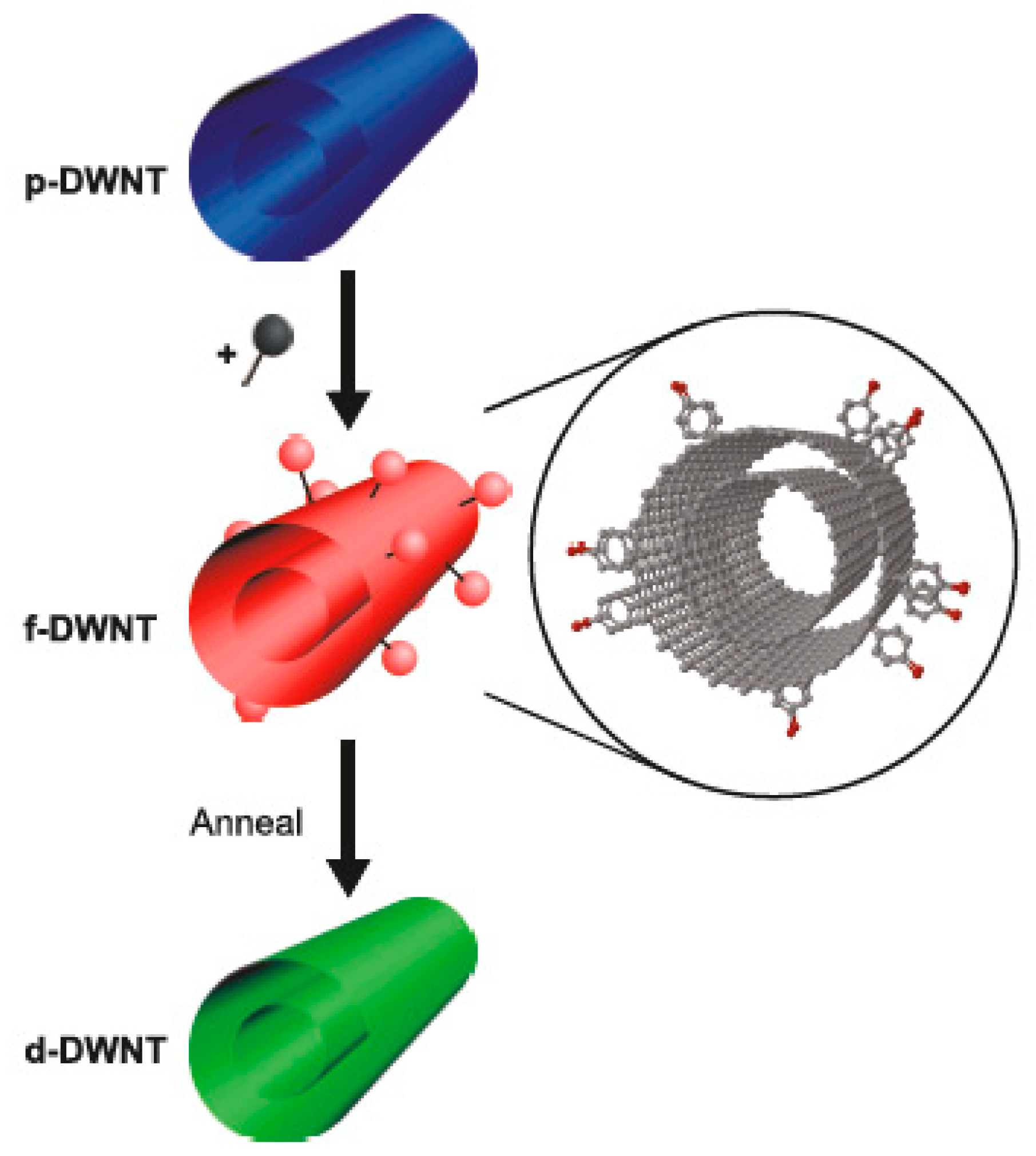
In Ref. [182], the covalent functionalization of double-walled carbon nanotubes (DWCNTs) with aryldiazonium salt was performed, and it was shown that the functionalization is reversible upon thermal treatment. The DWCNT transistors were constructed based on the functionalized DWCNTs, and the assignment of the metallicity of the inner and outer walls of DWCNTs was conducted (Figure 10).
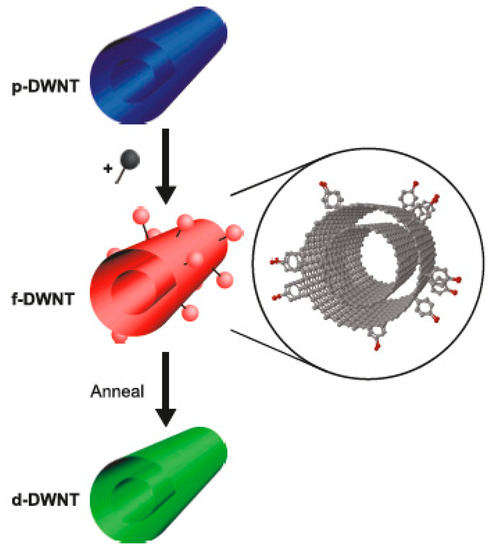
Figure 10.
The schematics of functionalization and reversible defunctionalization by the thermal treatment of DWCNTs. Reprinted with permission from Bouilly D et al. ACS Nano 2011 5 6 4927. Copyright 2011 American Chemical Society [182].
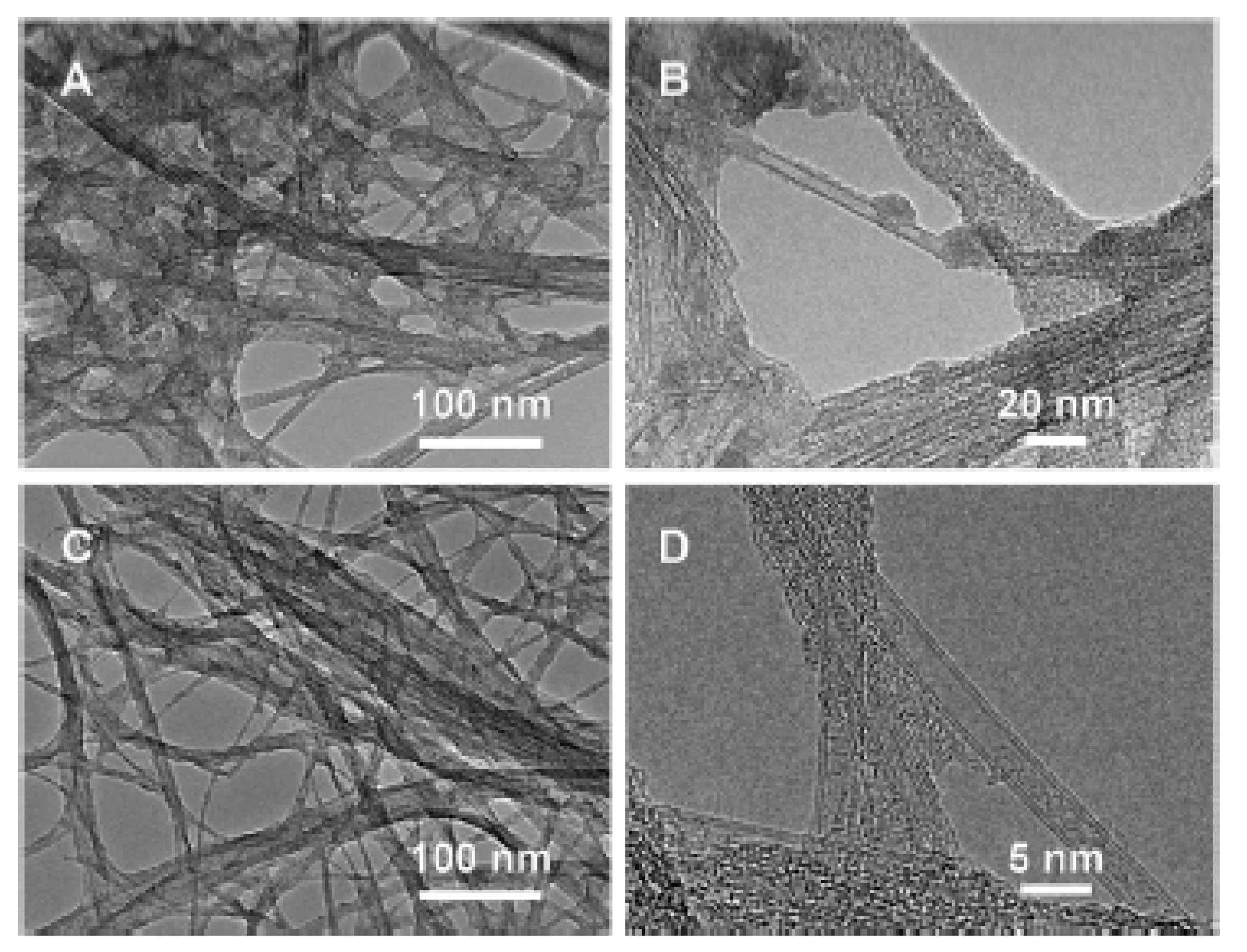
In Ref. [183], the outer walls of SWCNTs were selectively oxidized by oleum and nitric acid. Figure 11 shows the transmission electron microscopy (TEM) data of oxidized nanotubes. In Figure 11A,B low- and high-magnification images of DWCNTs treated with 5 mL of a solution for 24 h are shown. In Figure 11C,D DWCNTs treated with 10 mL of a solution for 2 h are presented [183].
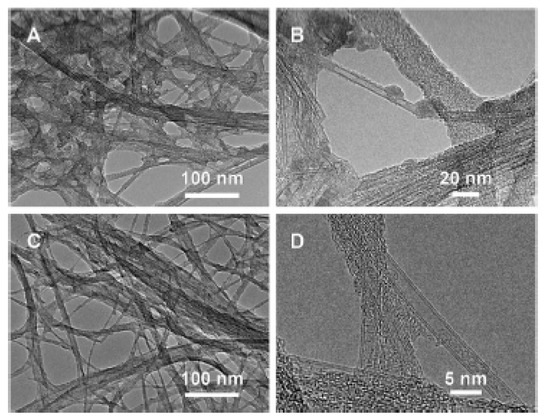
Figure 11.
The TEM data in low- and high-magnification images of DWCNTs treated with 5 mL of a solution for 24 h (A,B) and treated with 10 mL of a solution for 2 h (C,D). Reprinted with permission from Brozena A et al. JACS 2010 132 11 3932. Copyright 2010 American Chemical Society [183].
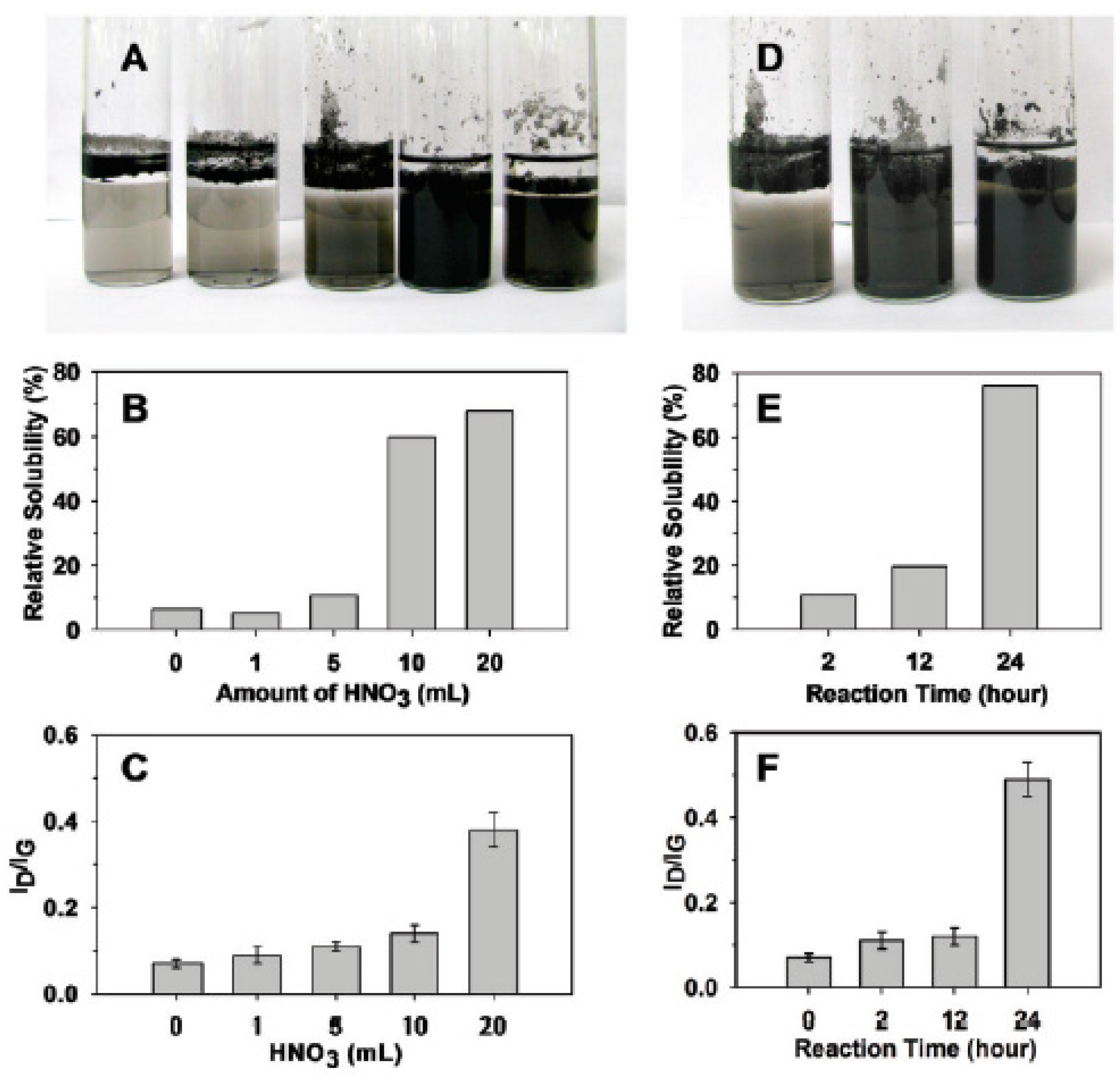
In Ref. [183], the samples were further investigated at a fixed reaction time of 2 h with nitric acid (Figure 12A–C) and an increasing reaction time using 5 mL of HNO3 (Figure 12D–F). In Figure 12B, it is seen that the relative solubility of DWCNTs is increased by increasing the amount of acid. In Figure 12C, it is visible that the nanotubes become more defective, because the ratio of the peaks in the Raman spectra of ID/IG is increased. By increasing the reaction time (Figure 12D–F), the solubility and defectiveness of nanotubes increase, too. This is caused by the appearance of more carboxylic groups on the surface of DWCNTs with oxidation. This leads to more solubility and more defects [183].
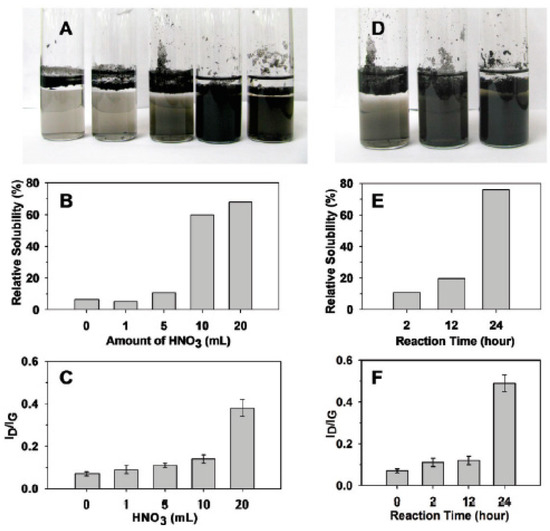
Figure 12.
The solubility and defectiveness of DWCNTs at a fixed reaction time of 2 h with nitric acid (A–C) and increasing reaction times using 5 mL of HNO3 (D–F). Reprinted with permission from Brozena A et al. JACS 2010 132 11 3932. Copyright 2010 American Chemical Society [183].
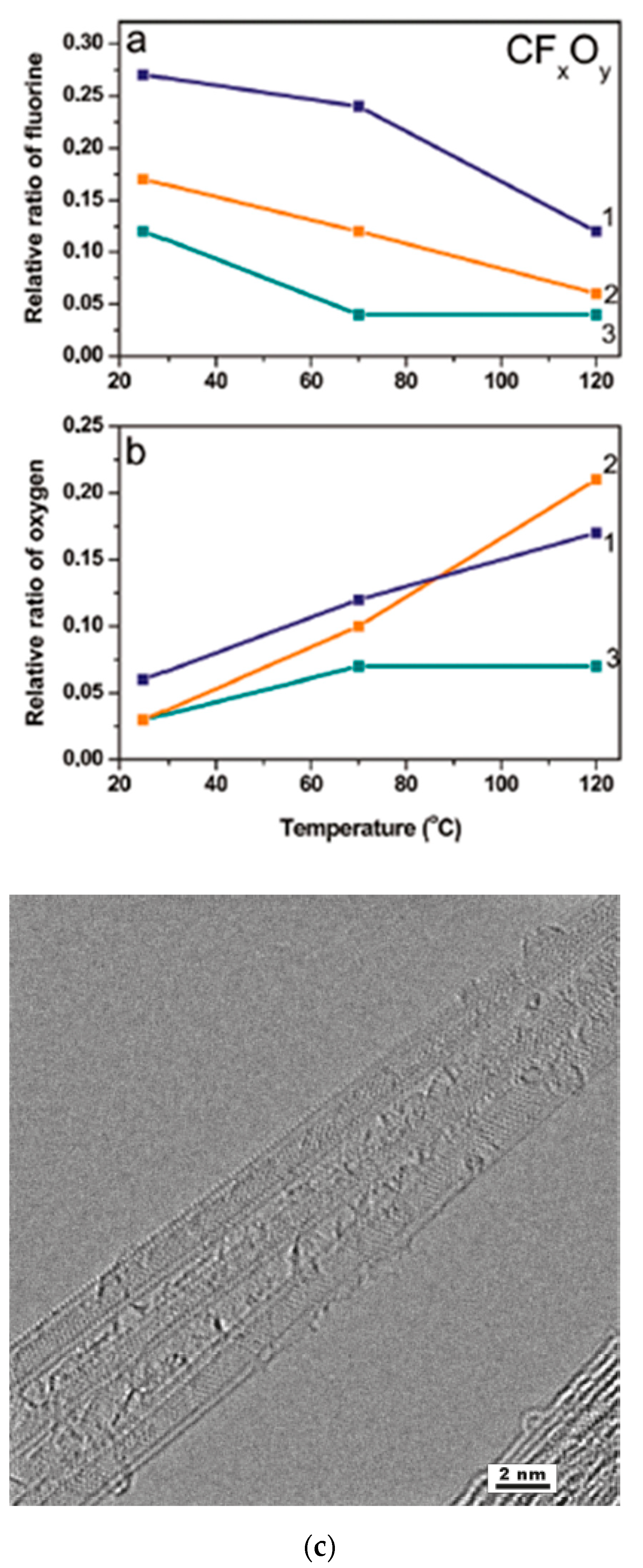
In Ref. [184], DWCNTs were fluorinated using (1) gaseous F2 at 200 °C, (2) a mixture of BrF3 and Br2 at room temperature, and (3) radio frequency CF4 plasma. Figure 13a shows the relative ratio of fluorine plotted versus the synthesis temperature. Figure 13b shows the relative ratio of oxygen plotted versus the experiment temperature. Figure 13c demonstrates the high-resolution TEM image of DWCNTs fluorinated by F2 at 200 °C [184].
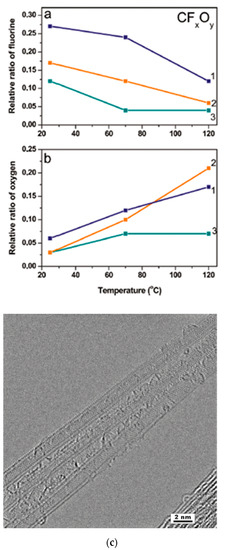
Figure 13.
Concentration of fluorine (a) and oxygen (b) in the DWCNTs fluorinated by F2 (1), BrF3 (2), and CF4 plasma (3) as a function of the annealing temperature. (c) High-resolution TEM image of DWCNTs fluorinated by F2 at 200 °C. Reprinted with permission from Bulusheva L et al. Chem Mater 2010 22 4197. Copyright 2010 American Chemical Society [184].
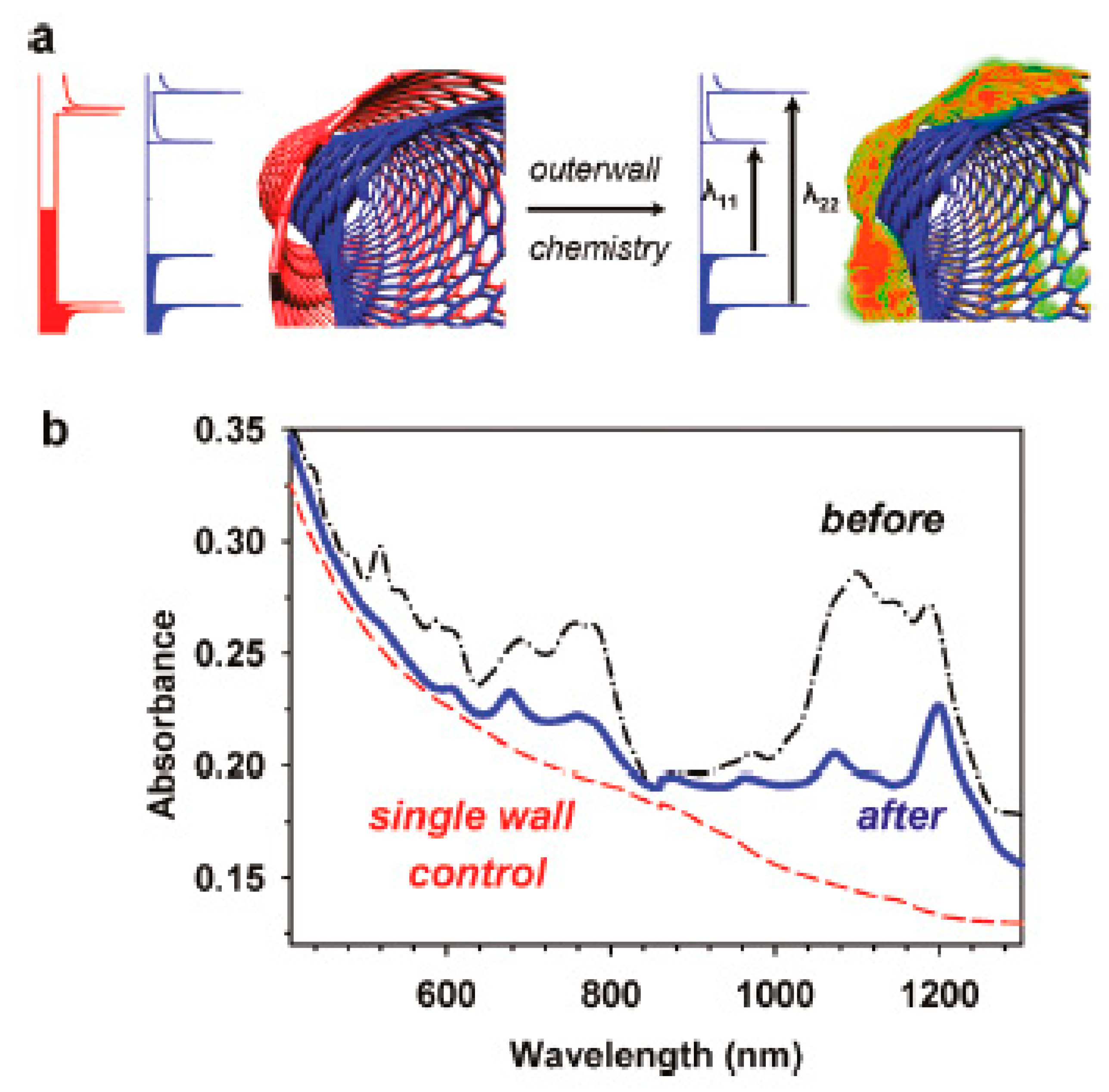
In Ref. [185], the covalent modification of DWCNTs was performed to control the sidewall chemistry. Figure 14a shows the schematics of the covalent functionalization. Figure 14b demonstrates the optical absorption spectrum of DWCNTs before and after the functionalization with diazonium salts. It is visible that the covalent functionalization leads to a charge transfer in DWCNTs [185].
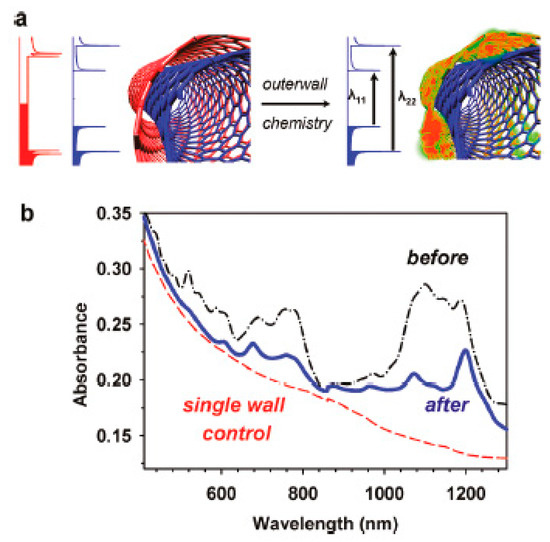
Figure 14.
(a) The schematics of the covalent functionalization of DWCNTs. (b) The optical absorption spectrum of DWCNTs before and after the functionalization with diazonium salts. Reprinted with permission from Piao Y. et al. J Phys Chem Lett 2011 2 1577. Copyright 2011 American Chemical Society [185].
5.2. Gas Sorption on Carbon Nanotubes
Gas sorption on carbon nanotubes is possible. It can be reversible depending on the experimental conditions [187,188]. This allows for using carbon nanotubes as gas sensors, biosensors, and sensors for liquids. The reversibility of adsorption is the main characteristic for the implementation in sensors.
5.3. Substitution of Carbon Atoms with Other Atoms
Many studies were dedicated to the substitution of carbon atoms of carbon nanotubes by other atoms. The electronic properties depend on the type and concentration of the doping atoms [189]. The first experiment was made in 1993 [190], and after that, many examples of doping with nitrogen [191,192,193,194,195,196,197,198,199,200,201] and boron [202,203,204,205,206,207,208,209] were made.
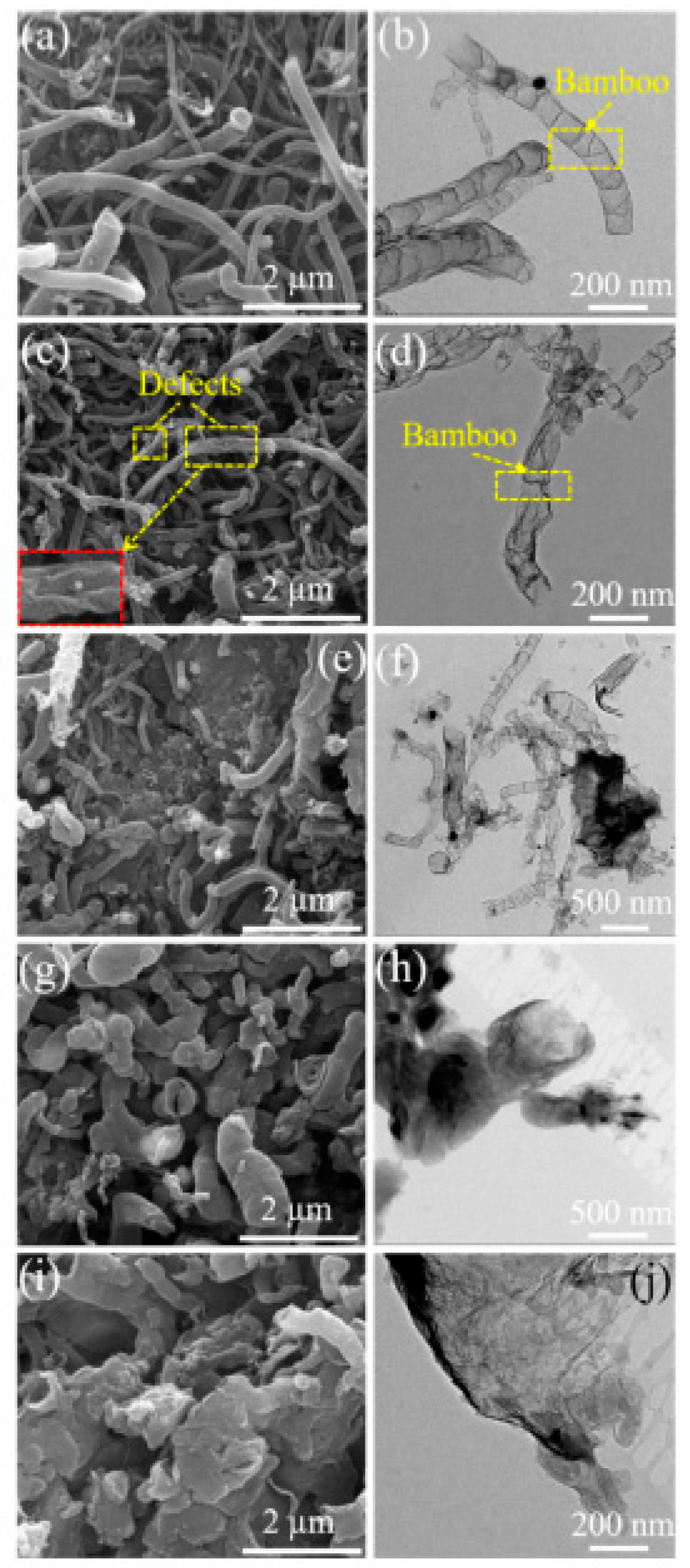
In Ref. [210], nitrogen-doped carbon nanotubes were boron-doped. Figure 15 shows the SEM and TEM images of B,N-CNTs at different addition amounts of boric acid (B,N-CNT-1, 0.05 g, B,N-CNT-2, 0.1 g, B,N-CNT-3, 0.25 g, B,N-CNT-4, 0.5 g, and B,N-CNT-5, 1 g). These images are amazing examples of the unique chemistry of carbon nanotubes.
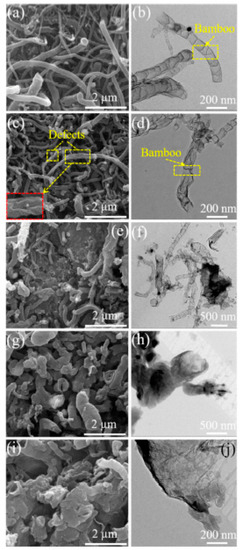
Figure 15.
The SEM and TEM images of B,N-CNTs at different addition amounts of boric acid (B,N-CNT-1, 0.05 g (a,b), B,N-CNT-2, 0.1 g (c,d), B,N-CNT-3, 0.25 g (e,f), B,N-CNT-4, 0.5 g (g,h), and B,N-CNT-5, 1 g (i,j)). Copyright 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [210].
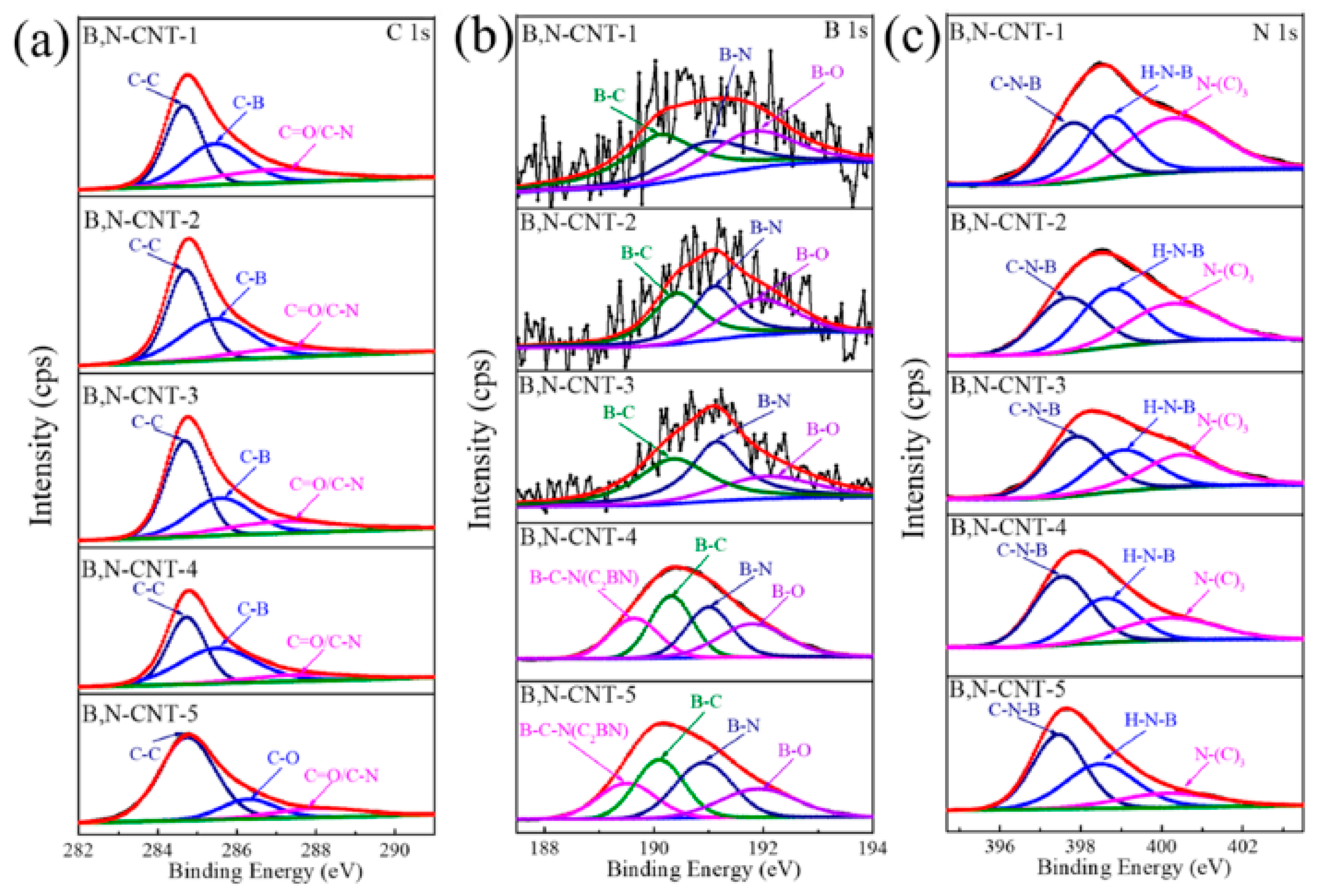
In Figure 16, the surface chemical compositions of B,N-CNTs calculations with XPS are shown. Figure 16a shows the C 1s XPS spectra of samples. Figure 16b shows the B1s XPS spectra of samples. Figure 16c shows the N 1s XPS spectra of the samples [210].
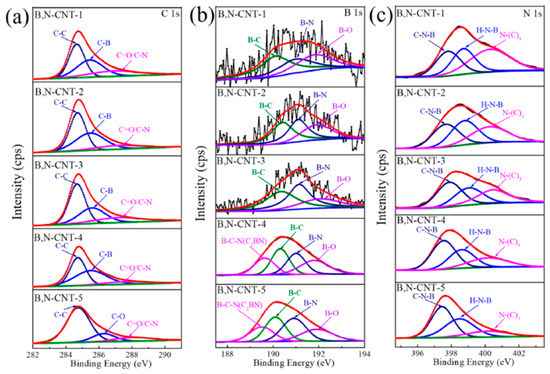
Figure 16.
The (a) C 1s XPS spectrum, (b) B 1s XPS spectrum, and (c) N 1s XPS spectrum of B,N-CNTs (B,N-CNT-1, 0.05 g, B,N-CNT-2, 0.1 g, B,N-CNT-3, 0.25 g, B,N-CNT-4, 0.5 g, and B,N-CNT-5, 1 g). Copyright 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [210].
5.4. Intercalation of Nanotube Bundles
The intercalation of carbon nanotube bundles means incorporating simple substances and chemical compounds to the space between nanotubes in the bundles. The intercalation with p- and n-dopants was demonstrated. The electronic properties of SWCNTs were investigated by Raman spectroscopy, optical absorption spectroscopy, electron energy loss spectroscopy, and X-ray photoelectron spectroscopy techniques [211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237].
5.5. Filling of Carbon Nanotubes
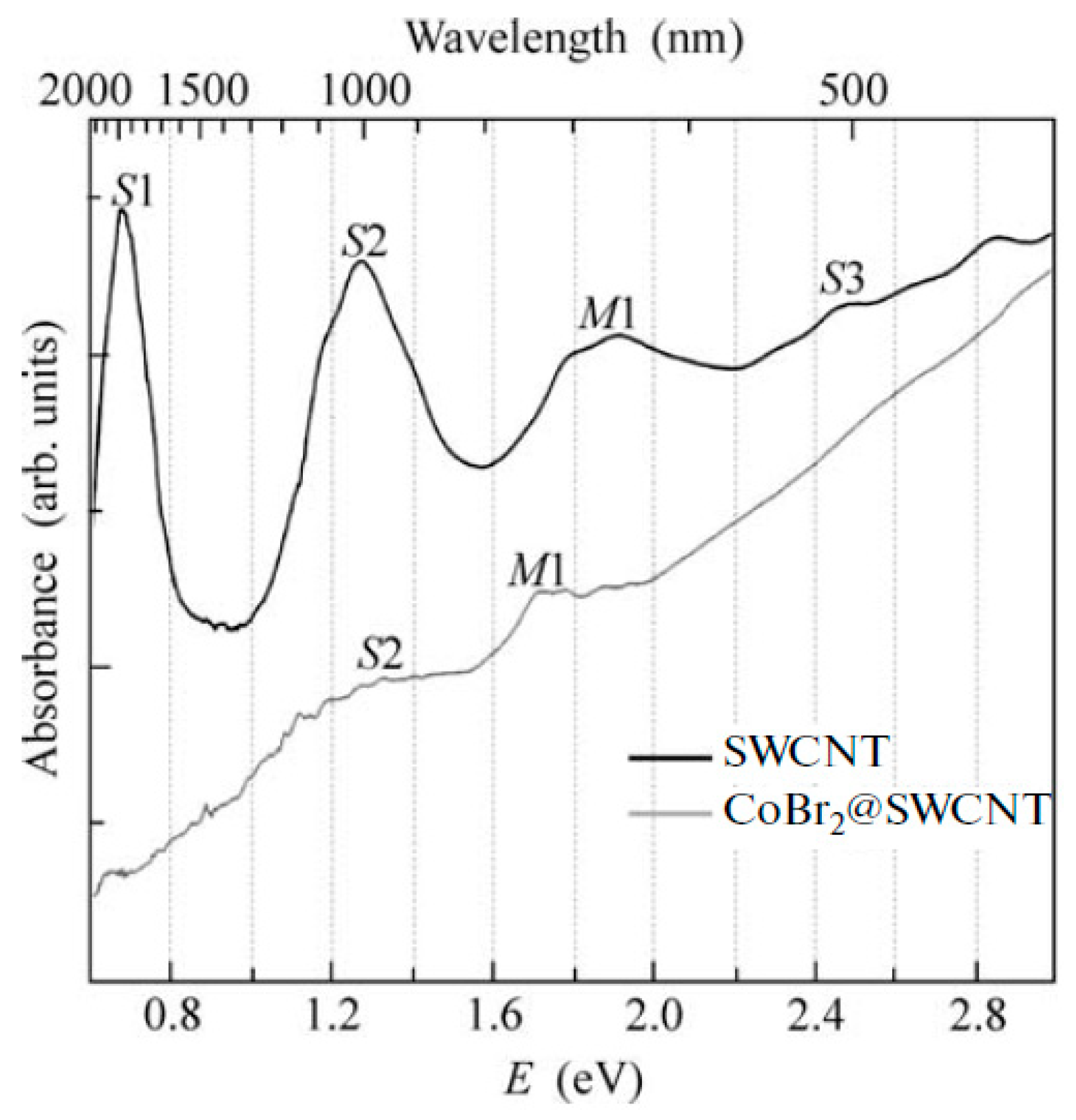
OAS spectroscopy is used to identify the charge transfer in filled SWCNTs [145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181]. Figure 17 shows the OAS spectra of pristine and cobalt bromide-filled SWCNTs [146]. The modification of spectra such as the change in the intensity of peaks, the shift of peaks, the alteration of peak profiles, and the disappearance and appearance of peaks testifies to the charge transfer in filled SWCNTs.
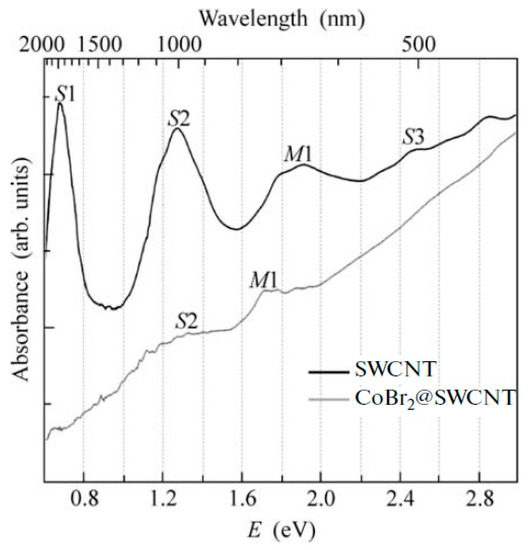
Figure 17.
The OAS spectra of pristine and cobalt bromide-filled SWCNTs. Reprinted from M.V. Kharlamova et al. Study of the electronic structure of single-walled carbon nanotubes filled with cobalt bromide, JETP Letters, V. 91, n 4, p. 196–200, 2010, Springer Nature [146].
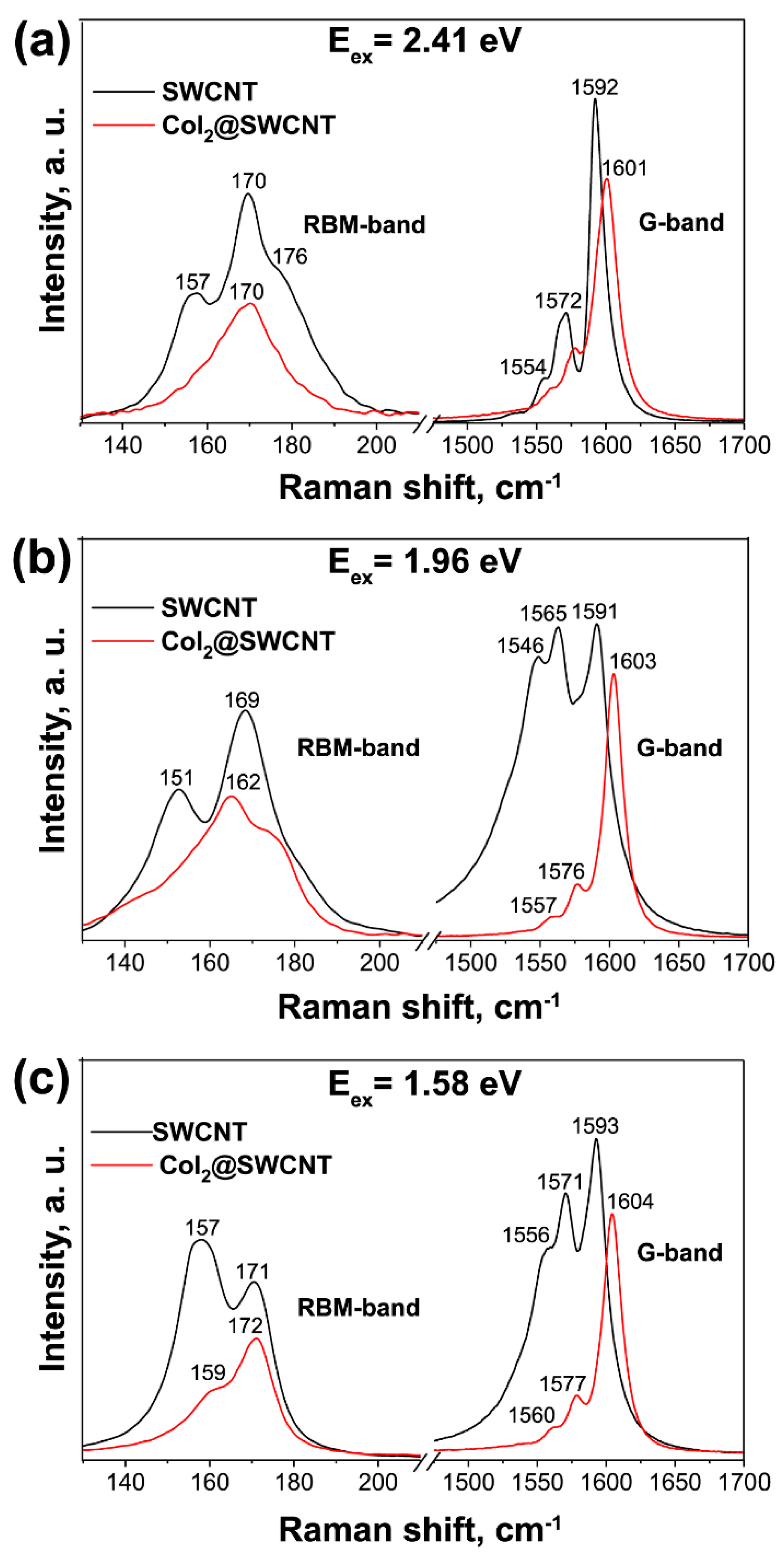
Raman spectroscopy is used to identify the charge transfer and electronic and vibronic properties of filled SWCNTs [181,182,183,184,185]. Figure 18 shows the Raman spectra of pristine and cobalt iodide-filled SWCNTs obtained at different laser wavelengths [238]. The modification of spectra such as the change in the intensity of peaks, the shift of peaks, and the alteration of peak profiles testifies to the charge transfer in filled SWCNTs.
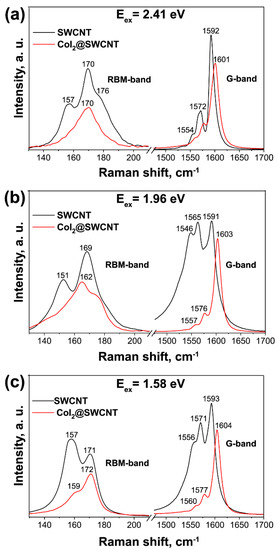
Figure 18.
The Raman spectra of pristine and cobalt iodide-filled SWCNTs obtained at laser energies of 2.41 eV (a), 1.96 eV (b), 1.58 eV (c). Copyright 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [238].
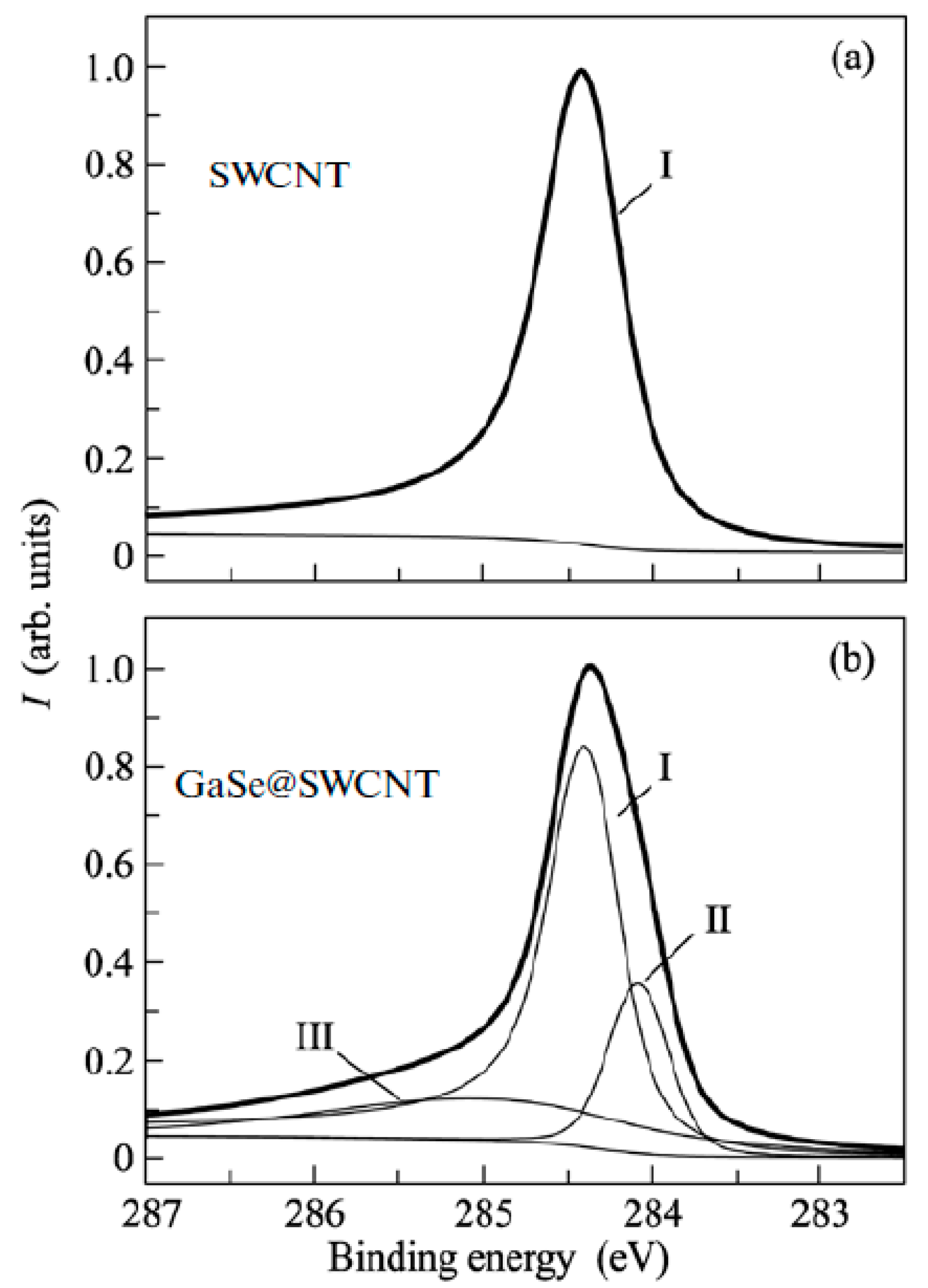
XPS is used to identify the direction and value of the Fermi level shift of the filled SWCNTs [145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181]. Figure 19 shows the XPS spectra of pristine and gallium selenide-filled SWCNTs [162]. The C 1s XPS spectra showed the shift of the peak and the change in its width. UPS was also used as a direct method of the investigation of the Fermi level shift in filled SWCNTs [145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181].
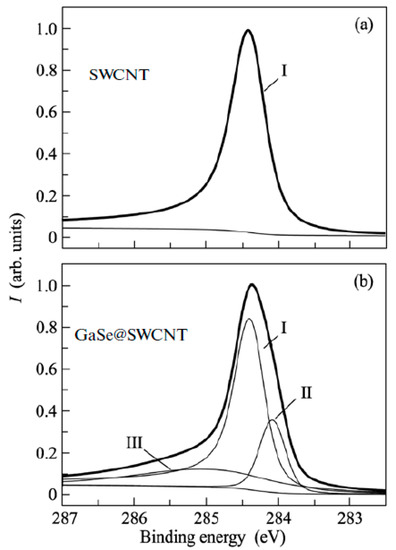
Figure 19.
The XPS spectra of pristine (a) and gallium selenide-filled SWCNTs (b). Reprinted from M.V. Kharlamova. Novel approach to tailoring the electronic properties of single-walled carbon nanotubes by the encapsulation of high-melting gallium selenide using a single-step process, JETP letters, V. 98, n 5, p. 272–277, 2013, Springer Nature [162].
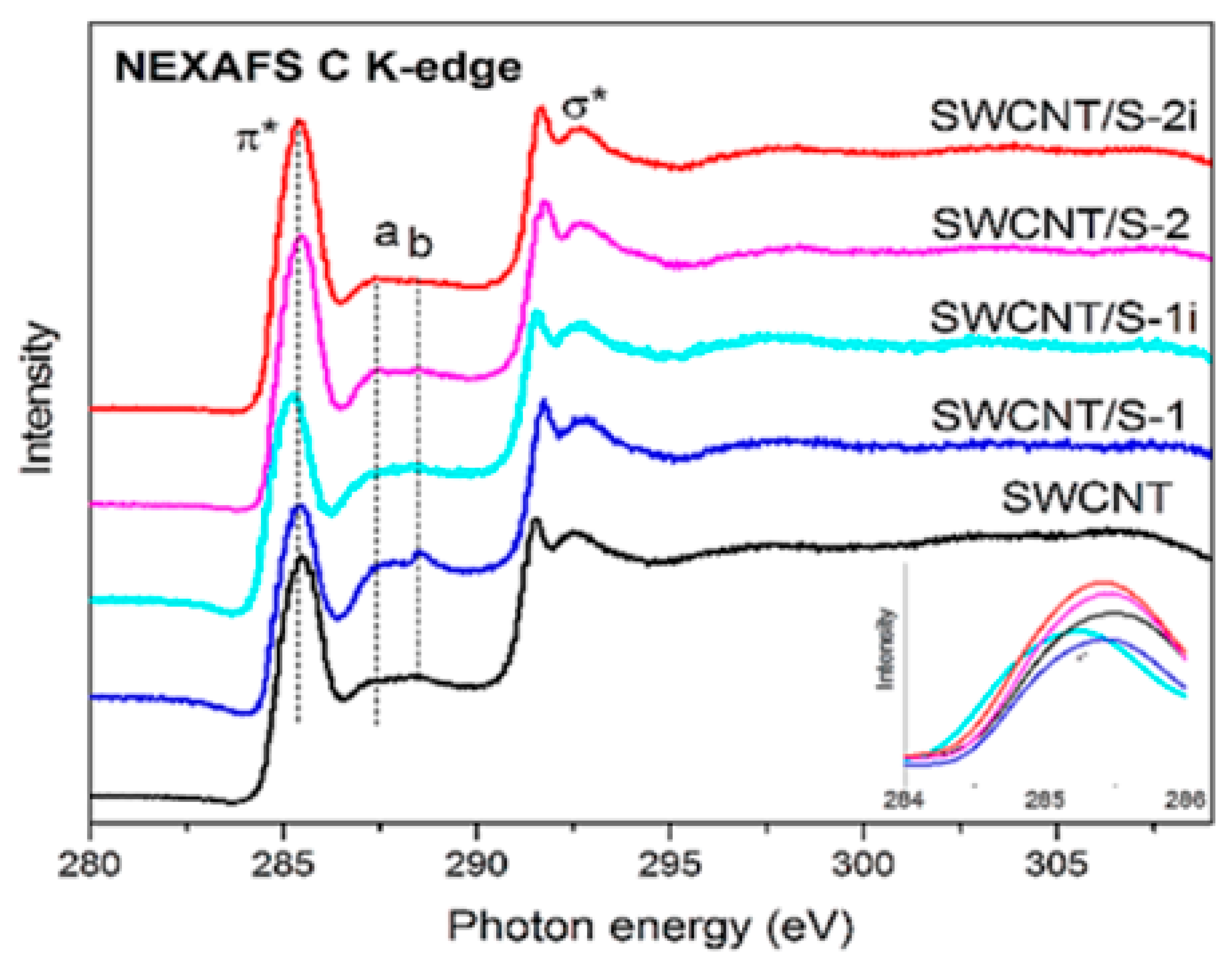
Near-edge X-ray absorption fine structure spectroscopy (NEXAFS) was applied to analyze the local interactions between encapsulated substances and SWCNTs [145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181]. Figure 20 shows the NEXAFS spectra of the pristine and sulfur-containing sample without (SWCNT/S-1) and with sonication (SWCNT/S-2) before and after light illumination (SWCNT/S-1i, SWCNT/S-2i) [239].
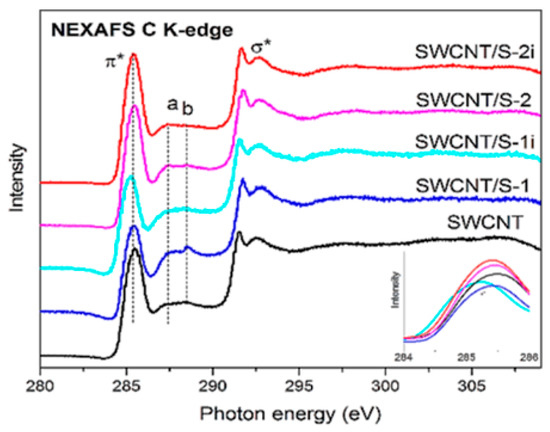
Figure 20.
The NEXAFS spectra of the pristine and sulfur-filled SWCNTs and the processed samples. Copyright 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [239].
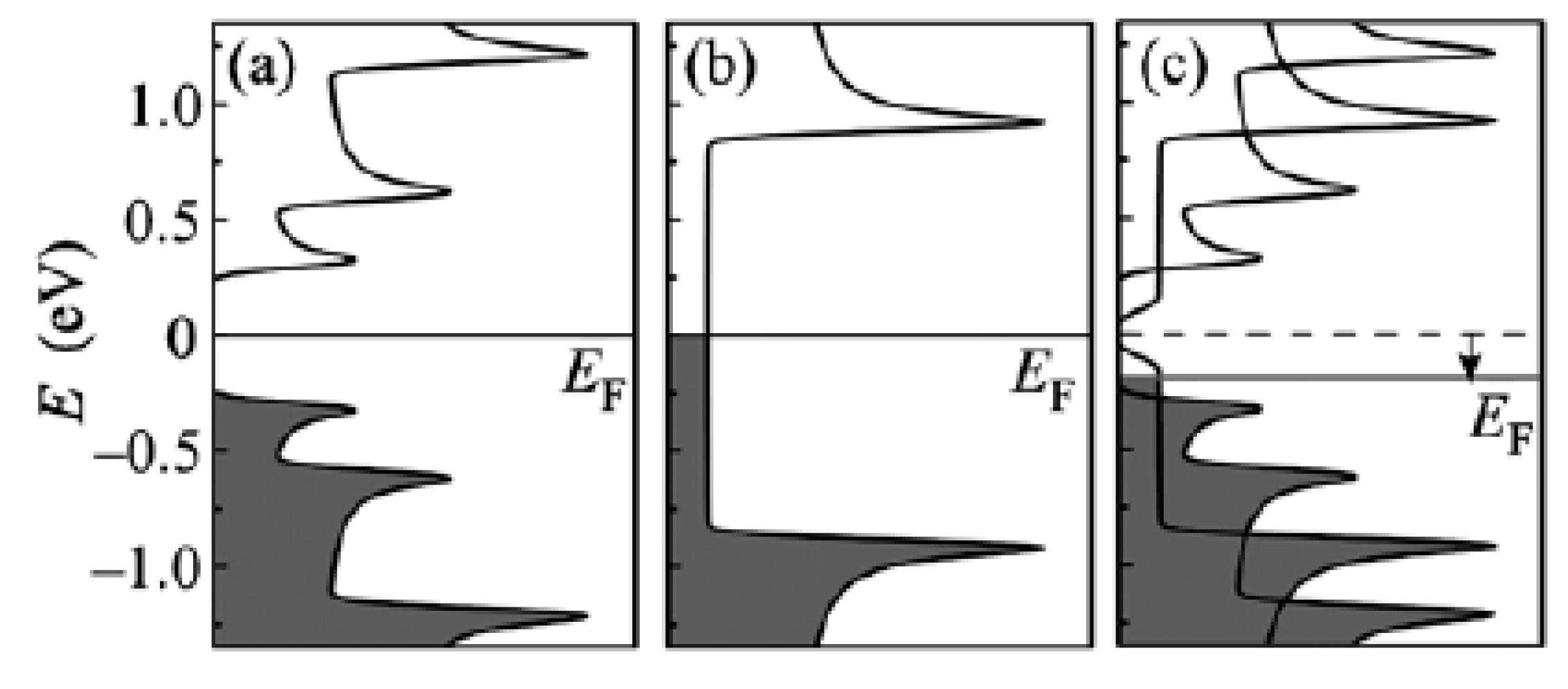
The schematics of the modification of the electronic properties of filled SWCNTs are shown in Figure 21 [145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181].
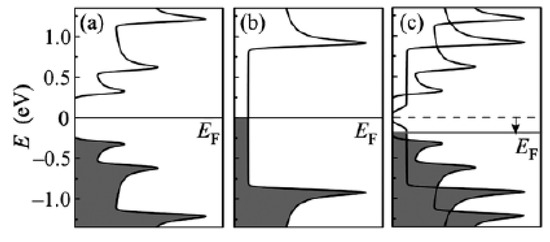
Figure 21.
The schematic of band structures of pristine semiconducting (a) and metallic (b) SWCNTS and the Fermi level (EF) shift (shown with an arrow) in gallium selenide-filled SWCNTs (c). Reprinted from M.V. Kharlamova. Novel approach to tailoring the electronic properties of single-walled carbon nanotubes by the encapsulation of high-melting gallium selenide using a single-step process, JETP letters, V. 98, n 5, p. 272–277, 2013, Springer Nature [162].
6. Applications of Carbon Material and Chemically Functionalized Carbon Material in Electrochemical Devices
The electrochemical doping of materials can find applications [240,241,242,243,244] in supercapacitors [115,116], hydrogen storage [245,246], battery construction [247,248,249], sensors [240,244], and nanoelectronic devices [113,250,251,252]. For these experiments, special electrochemical cells, electrolytes, electrode materials, and other parameters are important [141]. Recent works include electrochemical studies of carbon materials [253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281].
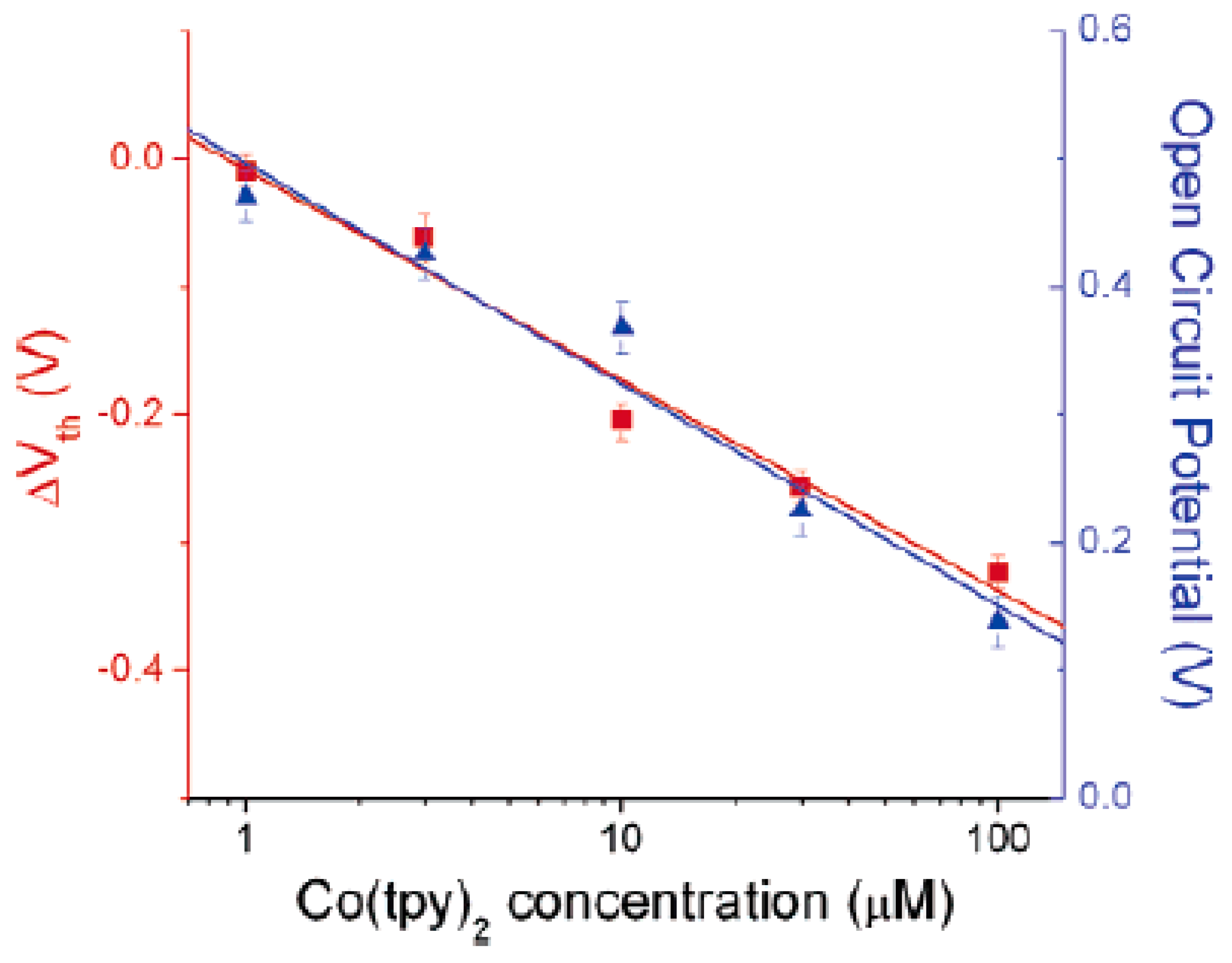
In Ref. [251], SWCNT field effect transistors (FET) can be used to reveal changes in the chemical potential. The gate-voltage dependence of the nanotube conductance was measured. This is because of the interaction of the molecules with the electrode and their redox chemistry. Figure 22 shows the threshold voltage shifts of SWCNT FET (red squares, left axis) and the open-circuit potential between the working and reference electrode (blue triangles, right axis) [251].
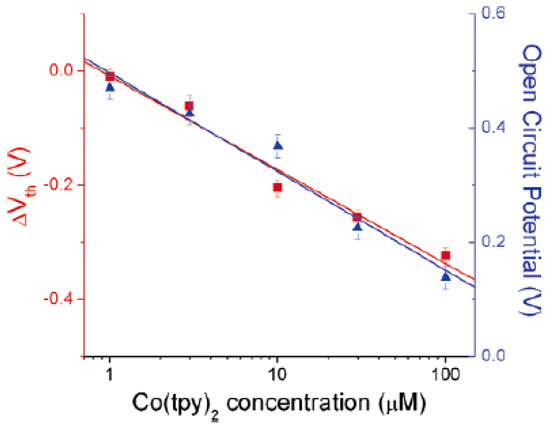
Figure 22.
Threshold voltage shifts of SWCNT FET (red squares, left axis) and open-circuit potential between the working and reference electrode (blue triangles, right axis). Reprinted with permission from Larrimore L et al. Nano Lett. 6 1329 (2006). Copyright 2006 American Chemical Society [251].
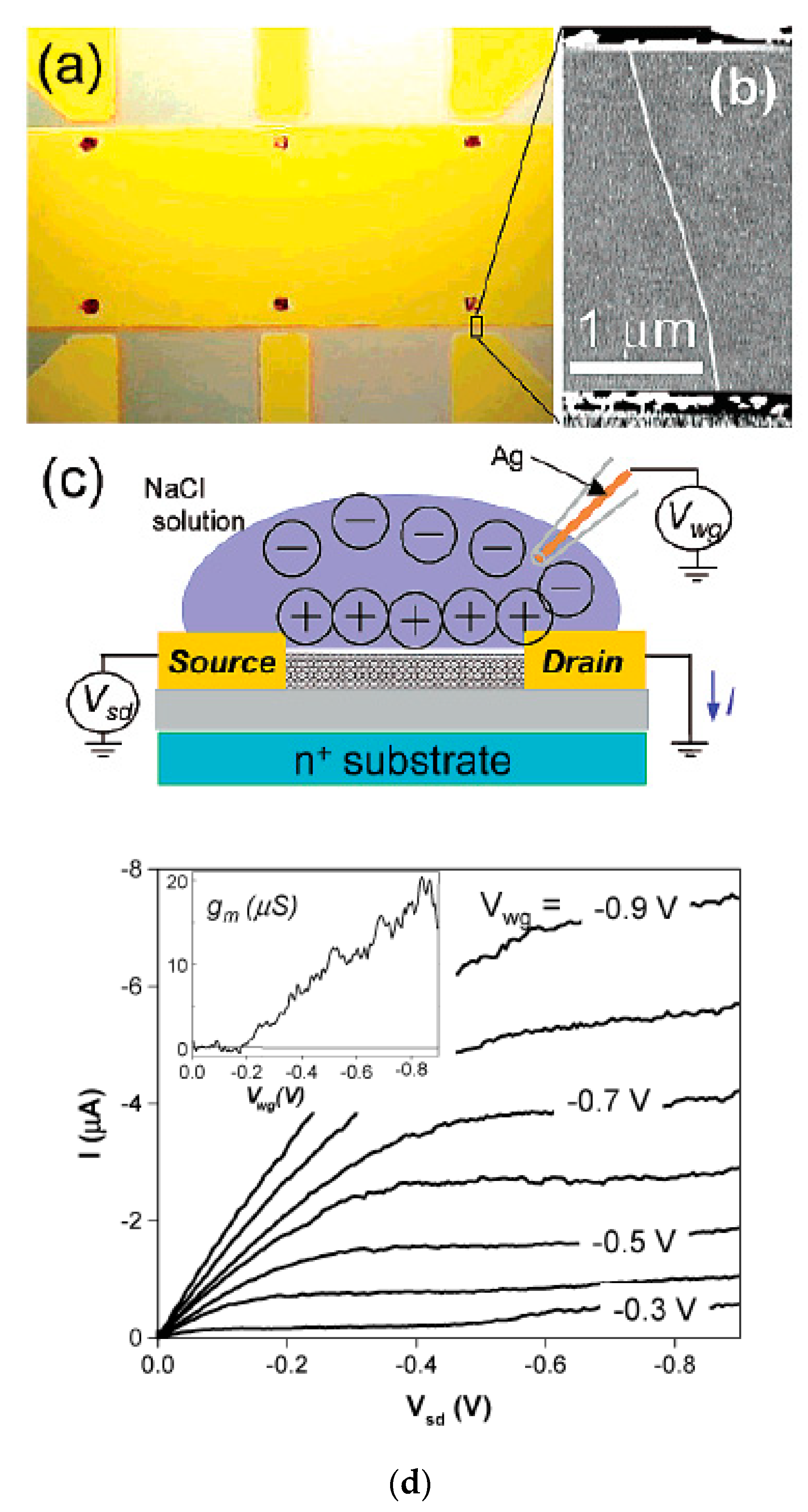
The authors of Ref. [252] made high perform FET-transistor form semiconducting SWCNTs with a diameter of 1.9 nm. Figure 23a shows an optical image of the transistor. They used six source electrodes. Figure 23b shows an atomic force microscopy (AFM) image of a tube in the FET. Figure 23c demonstrates the schematic of the electrolyte gate measurement [252]. The authors obtained very high device mobilities and transconductances. This makes semiconducting SWCNTs very useful for electronic applications and sensing (Figure 23d) [252].
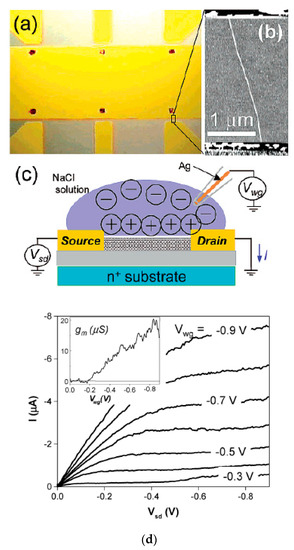
Figure 23.
(a) Optical micrograph of the device. (b) Atomic force microscopy image of a tube between two electrodes. The tube diameter is 1.9 nm. (c) Schematic of the electrolyte gate measurement. (d) Current versus source drain voltage I-Vsd characteristics of the device. The inset shows the transconductance gm = dI/dVwg taken at Vsd = −0.8 V. Reprinted with permission from Rosenblatt S et al. Nano Lett. 2 869 (2002). Copyright 2002 American Chemical Society [252].
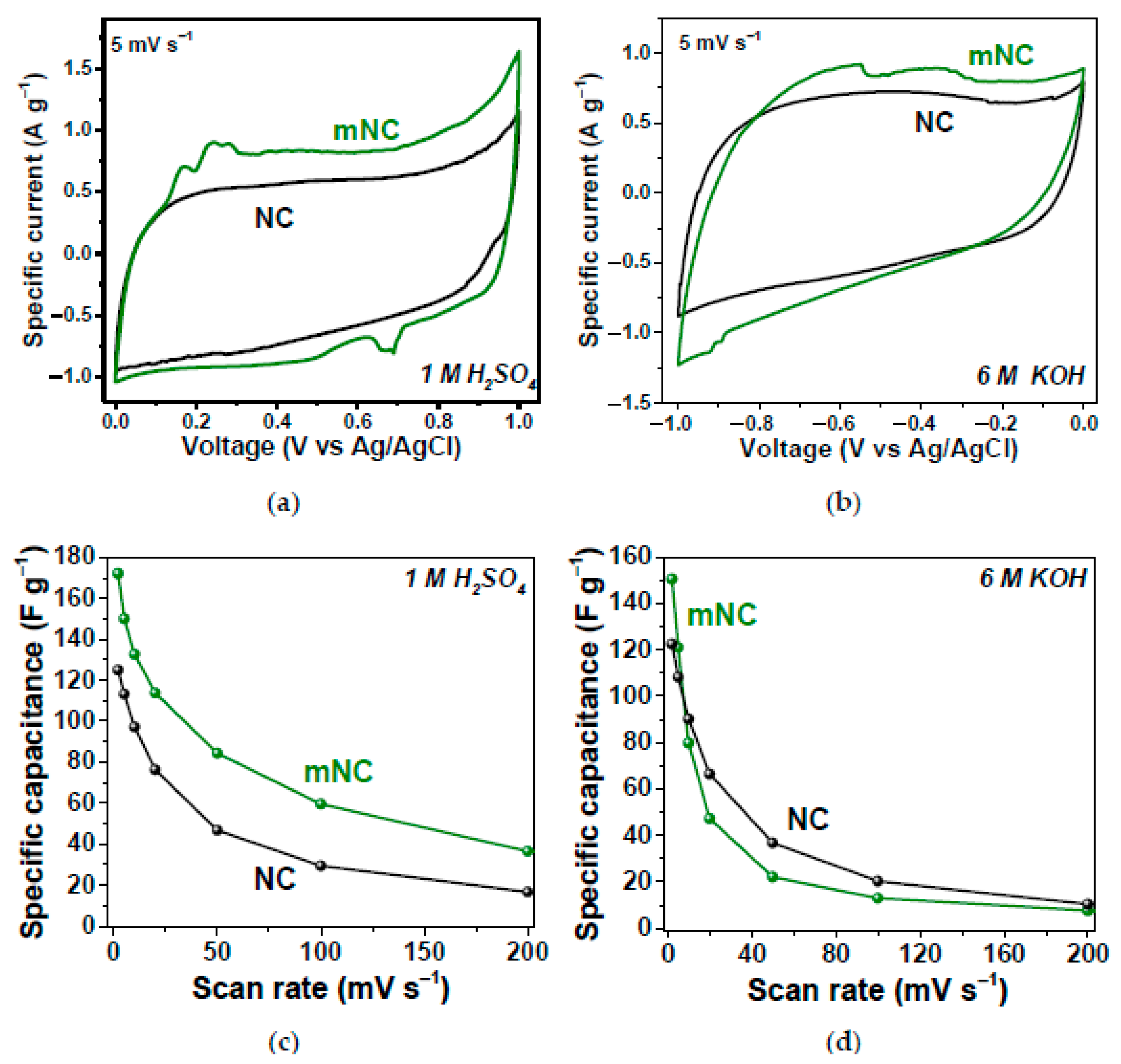
In Ref. [268], the nitrogen-doped carbon (NC) and modified-with-ammonia nitrogen-doped carbon (mNC) were tested as working electrodes of supercapacitors in three-electrode cells using 1 M H2SO4 and 6 M KOH electrolytes. Figure 24 shows the electrochemical performance of the samples, which show excellent characteristics [268].
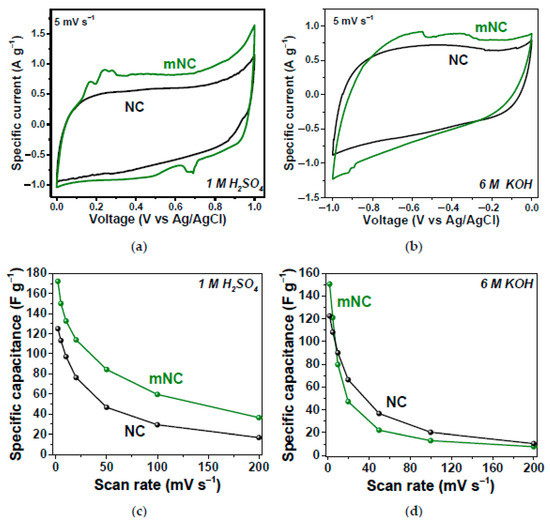
Figure 24.
Specific current vs. voltage dependence of NC and mNC in 1 M H2SO4 (a) and 6 M KOH (b); specific capacitance vs. scan rate dependence of NC and mNC in 1 M H2SO4 (c) and 6 M KOH (d). Copyright 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [268].
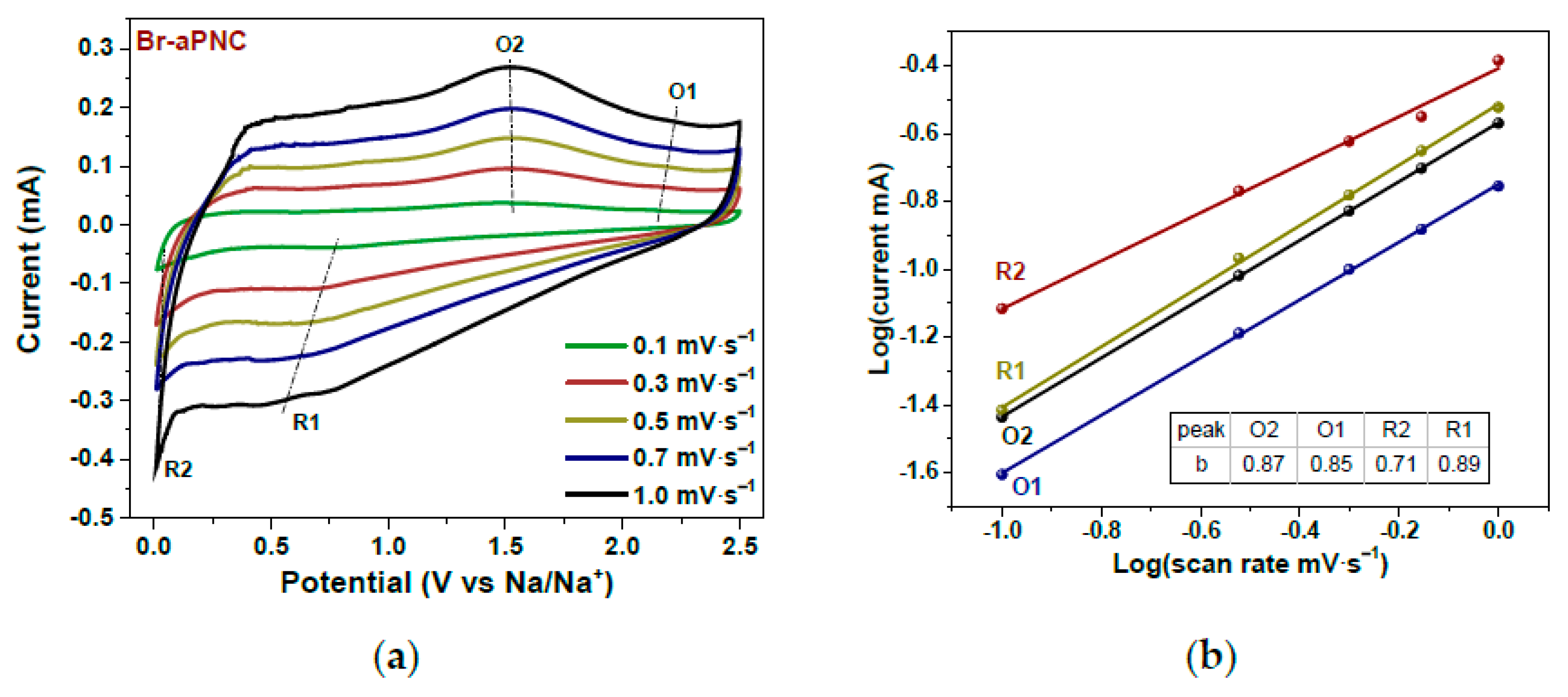
In Ref. [262], the kinetics of sodium storage were studied in brominated activated in alkali porous nitrogen-doped carbon. Figure 25 shows the current–potential curves of Br-aPNC (Figure 25a) measured at scan rates of 0.1–1.0 mV s−1 and the log(i)–log(v) plots (Figure 25b) obtained for oxidation and reduction peaks [262]. This behavior improves the storage and high-rate capability of carbon materials in sodium-ion batteries.
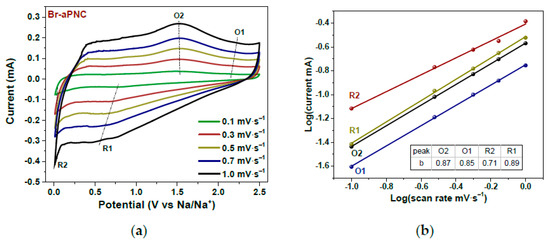
Figure 25.
Current vs. potential curves of Br-aPNC (a) measured at scan rates of 0.1–1.0 mV s−1 and log(current)–log(scan rate) plots (b) plotted for oxidation (O) and reduction (R) peaks. Copyright 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [262].
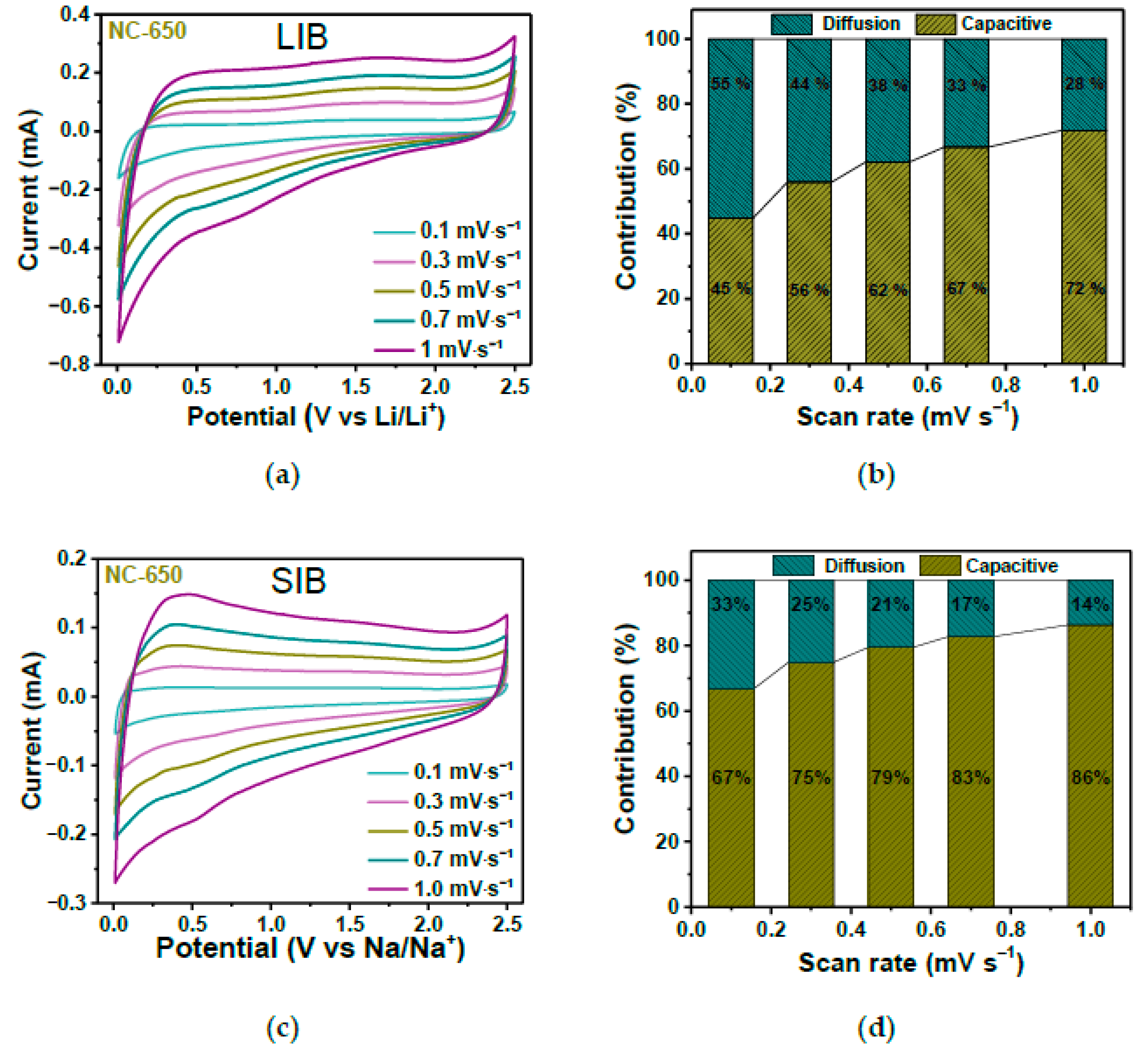
In Ref. [263], annealed nitrogen-doped carbon showed an excellent performance in both lithium-ion batteries and sodium-ion batteries. Figure 26a,c shows the current vs. potential measurements at various scanning rates. The diffusion and pseudocapacitive contributions to the electrochemical storage for different scan rates are shown in Figure 26b,d. It is visible that by increasing the scan rate, the pseudocapacitive contribution increases [263].
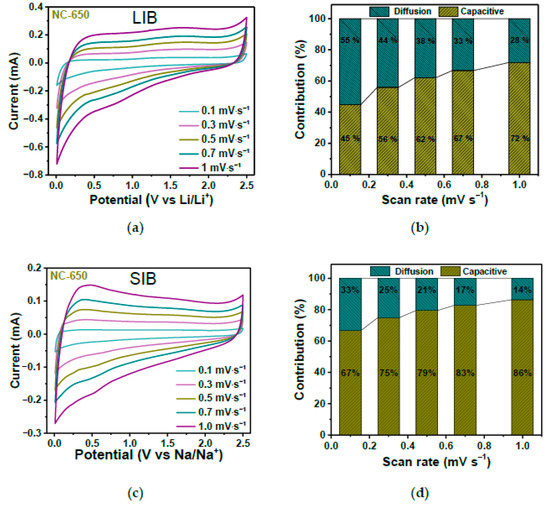
Figure 26.
The current vs. potential measurements (a,c) and diffusion and pseudocapacitive contributions to the electrochemical storage at various scanning rates (b,d) in lithium-ion batteries and sodium-ion batteries. Copyright 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [263].
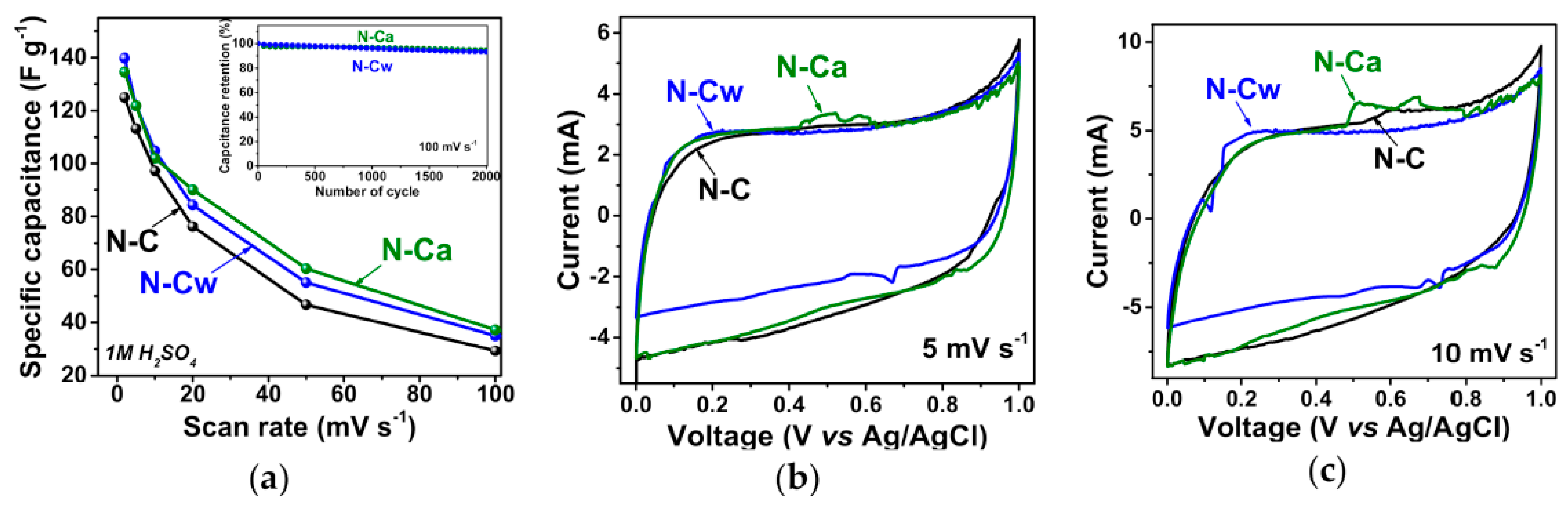
In Ref. [275], the electrochemical properties of nitrogen-doped carbon material were investigated. Figure 27a shows the specific capacitance plotted vs. scan rate for pristine nitrogen-doped carbon (N-C) and that after hydrothermal treatment in water (N-Cw) and ammonia solution (N-Ca). Figure 27b,c show the current vs. voltage measurements at scan rates of 5 and 10 mV s−1 [275].
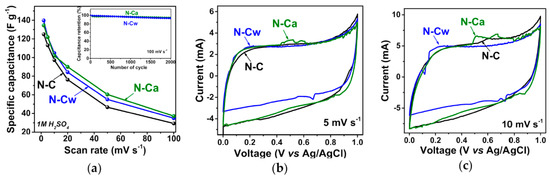
Figure 27.
(a) Specific capacitances and current vs. voltage curves at scan rates of (b) 5 and (c) 10 mV s−1 of N-C, N-Cw, and N-Ca samples. The inset in (a) presents capacitance retention plots for N-C and N-Ca during 2000 cycles at 100 mV s−1. Copyright 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [275].
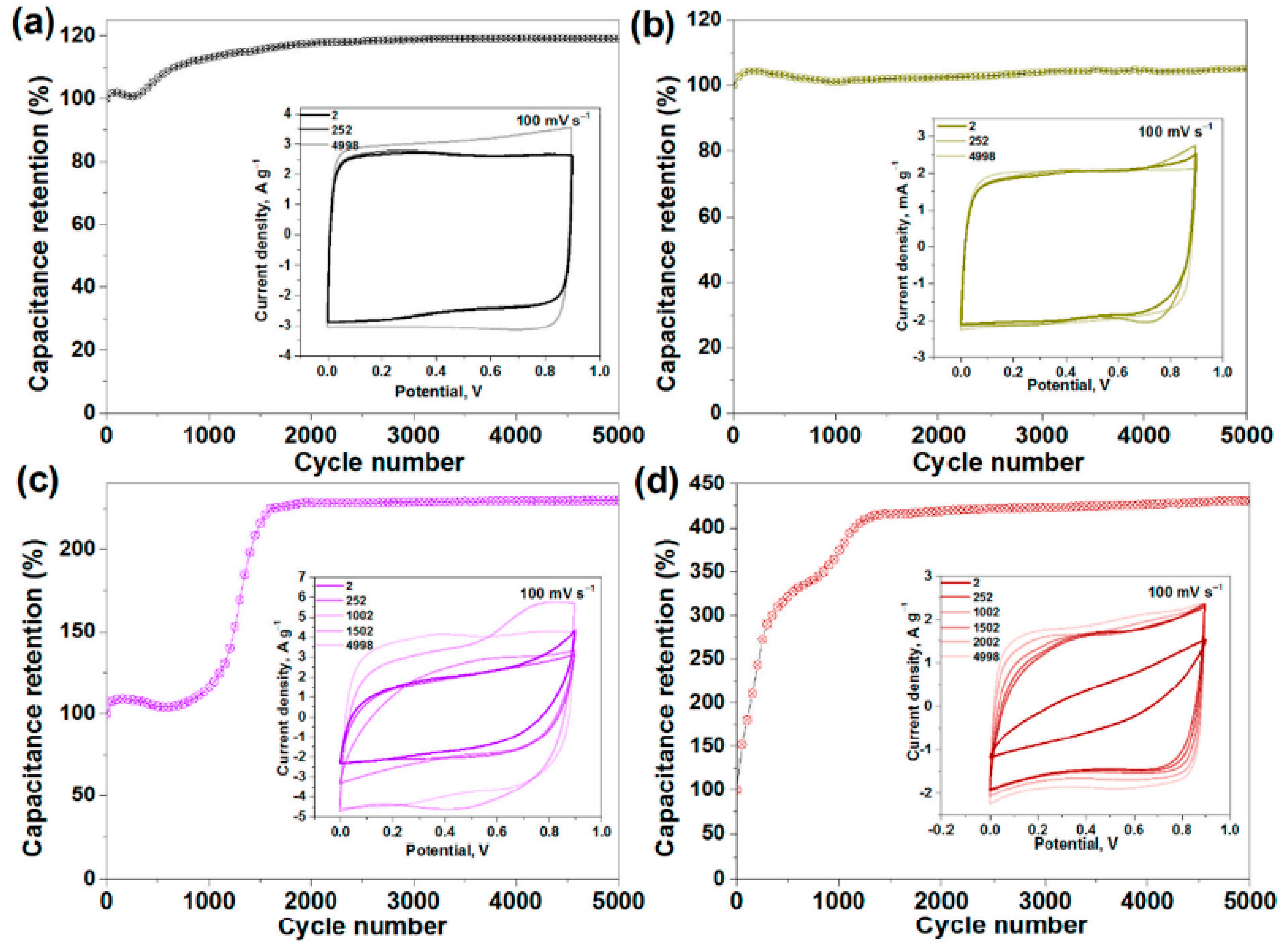
In Ref. [276], the electrochemical properties of pristine, sonicated, and fluorinated SWCNTs were investigated. Figure 28 shows the current density vs. potential measurements for pristine (SW), split (SW_DC), and fluorinated F-SW, and the F-SW_DC electrodes at a scan rate of 100 mV s−1 show a nearly rectangular shape. Figure 28 also shows the capacitance retention vs. cycle number measurements [276].
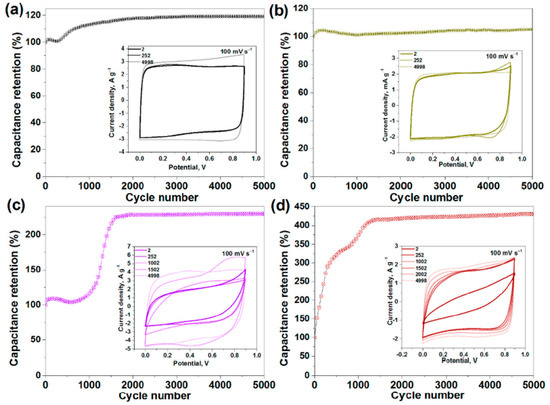
Figure 28.
Capacitance retention versus cycle number measurements, with insets showing the current density versus potential measurements for pristine (SW) (a), split (SW_DC) (b), and fluorinated F-SW (c) and F-SW_DC (d) electrodes at a scan rate of 100 mV s−1. Copyright 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [276].
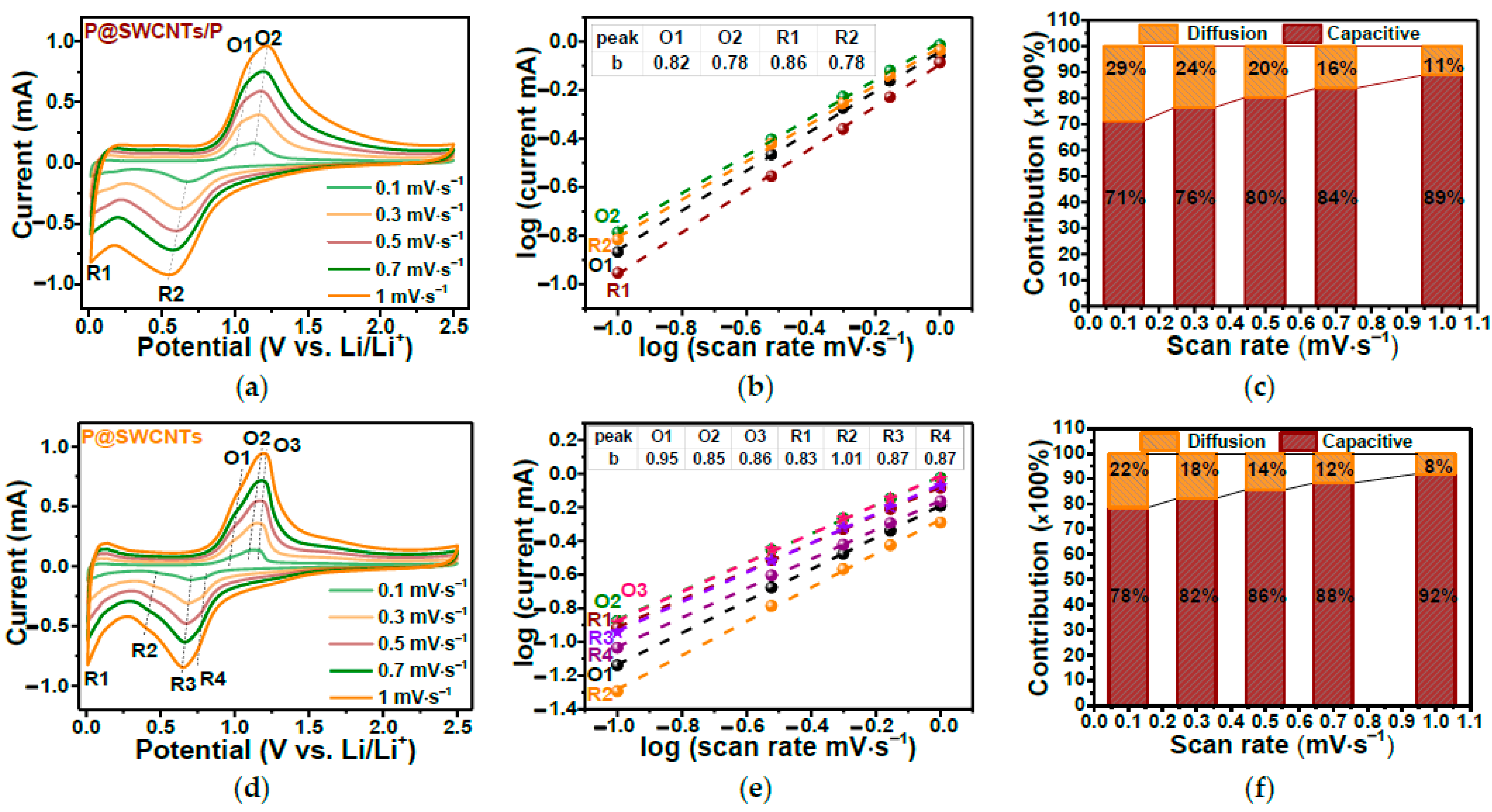
In Ref. [277], the electrochemical properties of SWCNTs filled with red phosphorous were investigated. Figure 29a,d show the current vs. potential dependence of P-filled SWCNTs (P@SWCNT/P) and those treated by filtering, sonication, and drying samples (P@SWCNT). Figure 29b,e show the log(current) vs. log(scan rate). Figure 29c,f demonstrate the diffusion and capacitive contributions for different scan rates [277].
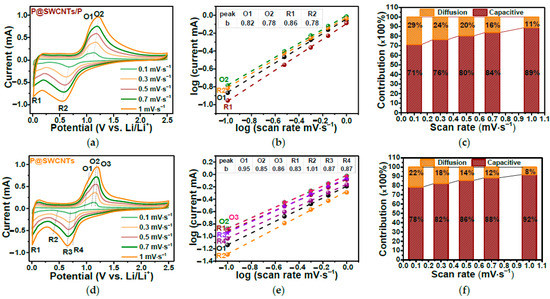
Figure 29.
The current versus potential dependence (a,d), the log(current) vs. log(scan rate) (b,e), and the diffusion and capacitive contributions for different scan rates (c,f) of P-filled SWCNTs (P@SWCNT/P) and treated P@SWCNT. Copyright 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license [277].
7. Conclusions
The filled nanotubes show great promise in many areas [13,253] and are set to advance functional materials of the future. For instance, carbon nanotubes have been used with metal oxides as cheap and stable nanocatalysts in the alcohol oxidation processes for fuel cells [282]. There are many filling materials [6,7,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181] that can create devices with an appropriate efficiency, intensity, and color. They are not patented yet because their construction requires five to ten years. I think that the filling of single-walled carbon nanotubes is a very promising achievement; there are no other ways to create devices than to start from laboratory synthesis in a flask, and the applications in car lights are the most recent. The state corporations in Russia, Europe, the USA, and different countries are interested in developments [283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302].
Author Contributions
The manuscript was written solely by M.V.K. C.K.—editing. All authors have read and agreed to the published version of the manuscript.
Funding
These studies were partly performed during the implementation of the project “Building-up Center for advanced materials application of the Slovak Academy of Sciences”, ITMS project code 313021T081, supported by the Research & Innovation Operational Program, funded by the ERDF. The APC was funded by Nanomaterials as an invited paper.
Data Availability Statement
Data are available on request to the first author (M.V.K.).
Acknowledgments
M.V.K. acknowledges the coauthors of the reviewed paper.
Conflicts of Interest
The author may have a conflict of interest with Andrei Eliseev (Lomonosov Moscow State University). The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Jeon, I.; Xiang, R.; Shawky, A.; Matsuo, Y.; Maruyama, S. Single-Walled Carbon Nanotubes in Emerging Solar Cells: Synthesis and Electrode Applications. Adv. Energ. Mater. 2019, 9, 1801312. [Google Scholar] [CrossRef]
- Ferguson, V.; Silva, S.R.P.; Zhang, W. Carbon Materials in Perovskite Solar Cells: Prospects and Future Challenges. Energ. Environ. Mater. 2019, 2, 107–118. [Google Scholar] [CrossRef]
- Liu, B.L.; Wu, F.Q.; Gui, H.; Zheng, M.; Zhou, C.W. Chirality-Controlled Synthesis and Applications of Single-Wall Carbon Nanotubes. ACS Nano 2017, 11, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Bati, A.S.R.; Yu, L.P.; Batmunkh, M.; Shapter, J.G. Recent advances in applications of sorted single-walled carbon nanotubes. Adv. Funct. Mater. 2019, 29, 1902273. [Google Scholar] [CrossRef]
- Moore, K.E.; Tune, D.D.; Flavel, B.S. Double-walled carbon nanotube processing. Adv. Mater. 2015, 27, 3105–3137. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Electronic properties of pristine and modified single-walled carbon nanotubes. Physics-Uspekhi 2013, 56, 1047–1073. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Advances in tailoring the electronic properties of single-walled carbon nanotubes. Prog. Mater. Sci. 2016, 77, 125–211. [Google Scholar] [CrossRef]
- Botos, A.; Biskupek, J.; Chamberlain, T.W.; Rance, G.A.; Stoppiello, C.T.; Sloan, J.; Liu, Z.; Suenaga, K.; Kaiser, U.; Khlobystov, A.N. Carbon Nanotubes as Electrically Active Nanoreactors for Multi-Step Inorganic Synthesis: Sequential Trans-formations of Molecules to Nanoclusters and Nanoclusters to Nanoribbons. J. Am. Chem. Soc. 2016, 138, 8175–8183. [Google Scholar] [CrossRef]
- Jordan, J.W.; Lowe, G.A.; McSweeney, R.L.; Stoppiello, C.T.; Lodge, R.W.; Skowron, S.T.; Biskupek, J.; Rance, G.A.; Kaiser, U.; Walsh, D.A.; et al. Host–Guest Hybrid Redox Materials Self-Assembled from Polyoxo-metalates and Single-Walled Carbon Nanotubes. Adv. Mater. 2019, 31, 1904182. [Google Scholar] [CrossRef]
- Li, L.J.; Khlobystov, A.N.; Wiltshire, J.G.; Briggs, G.A.; Nicholas, R.J. Diameter-selective encapsulation of metallocenes in single-walled carbon nanotubes. Nat. Mater. 2005, 4, 481–485. [Google Scholar] [CrossRef]
- Philp, E.; Sloan, J.; Kirkland, A.I.; Meyer, R.R.; Friedrichs, S.; Hutchison, J.L.; Green, M.L.H. An encapsulated helical one-dimensional cobalt iodide nanostructure. Nat. Mater. 2003, 2, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, P.V.C.; Marks, S.; Wynn, J.M.; Vasylenko, A.; Ramasse, Q.M.; Quigley, D.; Sloan, J.; Morris, A.J. Single-Atom Scale Structural Selectivity in Te Nanowires Encapsulated Inside Ultranarrow, Single-Walled Carbon Nanotubes. ACS Nano 2017, 11, 6178–6185. [Google Scholar] [CrossRef]
- Kashtiban, R.J.; Burdanova, M.G.; Vasylenko, A.; Wynn, J.; Medeiros, P.V.C.; Ramasse, Q.; Morris, A.J.; Quigley, D.; Lloyd-Hughes, J.; Sloan, J. Linear and Helical Cesium Iodide Atomic Chains in Ultranarrow Single-Walled Carbon Nanotubes: Impact on Optical Properties. ACS Nano 2021, 15, 13389–13398. [Google Scholar] [CrossRef]
- McSweeney, R.L.; Chamberlain, T.W.; Baldoni, M.; Lebedeva, M.A.; Davies, E.S.; Besley, E.; Khlobystov, A.N. Direct Measurement of Electron Transfer in Nanoscale Host-Guest Systems: Metallocenes in Carbon nanotubes. Chem. Eur. J. 2016, 22, 3540–3549. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.W.; Townsend, W.J.V.; Johnson, L.R.; Walsh, D.A.; Newton, G.N.; Khlobystov, A.N. Electrochemistry of redox-active molecules confined within narrow carbon nanotubes. Chem. Soc. Rev. 2021, 50, 10895. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, R.; Ogawa, D.; Kobayashi, K.; Saito, T.; Ohshima, S.; Nakamura, T.; Yoshikawa, H.; Awaga, K.; Shinohara, H. High yield synthesis and characterization of the structural and magnetic properties of crystalline ErCl3 nanowires in single-walled carbon nanotube templates. Nano Res. 2008, 1, 152–157. [Google Scholar] [CrossRef]
- Satishkumar, B.C.; Taubert, A.; Luzzi, D.E. Filling single-wall carbon nanotubes with d- and f-metal chloride and metal nanowires. J. Nanosci. Nanotechnol. 2003, 3, 159–163. [Google Scholar] [CrossRef]
- Xu, C.; Sloan, J.; Brown, G.; Bailey, S.; Williams, V.C.; Friedrichs, S.; Coleman, K.S.; Flahaut, E.; Green, M.L.H.; Hutchison, J.L.; et al. 1D lanthanide halide crystals inserted into single-walled carbon nanotubes. Chem. Commun. 2000, 2427–2428. [Google Scholar] [CrossRef]
- Santidrián, A.; Kierkowicz, M.; Pach, E.; Darvasiová, D.; Ballesteros, B.; Tobias, G.; Kalbáč, M. Charge transfer in steam purified arc discharge single walled carbon nanotubes filled with lutetium halides. Phys. Chem. Chem. Phys. 2020, 22, 10063–10075. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Bailey, S.R.; Sloan, J.; Xu, C.; Friedrichs, S.; Flahaut, E.; Coleman, K.S.; Green, M.L.H.; Hutchison, J.L.; Dunin-Borkowski, R.E. Electron beam induced in situ clusterisation of 1D ZrCl4 chains within single-walled carbon nanotubes. Chem. Commun. 2001, 845–846. [Google Scholar] [CrossRef]
- Brown, G.; Bailey, S.; Novotny, M.; Carter, R.; Flahaut, E.; Coleman, K.; Hutchison, J.; Green, M.; Sloan, J. High yield incorporation and washing properties of halides incorporated into single walled carbon nanotubes. Appl. Phys. A 2003, 76, 457–462. [Google Scholar] [CrossRef]
- Kirkland, A.I.; Meyer, R.R.; Sloan, J.; Hutchison, J. Structure Determination of Atomically Controlled Crystal Architectures Grown within Single Wall Carbon Nanotubes. Microsc. Microanal. 2005, 11, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.; Friedrichs, S.; Meyer, R.R.; Kirkland, A.I.; Hutchison, J.L.; Green, M.L.H. Structural changes induced in nanocrystals of binary compounds confined within single walled carbon nanotubes: A brief review. Inorg. Chim. Acta 2002, 330, 1–12. [Google Scholar] [CrossRef]
- Sloan, J.; Kirkland, A.I.; Hutchison, J.L.; Green, M.L. Aspects of crystal growth within carbon nanotubes. Comptes Rendus Phys. 2003, 4, 1063–1074. [Google Scholar] [CrossRef]
- Bendall, J.S.; Ilie, A.; Welland, M.E.; Sloan, J.; Green, M.L.H. Thermal Stability and Reactivity of Metal Halide Filled Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 6569–6573. [Google Scholar] [CrossRef]
- Chernysheva, M.V.; Eliseev, A.A.; Lukashin, A.V.; Tretyakov, Y.D.; Savilov, S.V.; Kiselev, N.A.; Zhigalina, O.M.; Kumskov, A.S.; Krestinin, A.V.; Hutchison, J.L. Filling of single-walled carbon nanotubes by Cul nanocrystals via capillary technique. Physica E 2007, 37, 62–65. [Google Scholar] [CrossRef]
- Hutchison, J.L.; Sloan, J.; Kirkland, A.I.; Green, M.L.H. Growing and characterizing one-dimensional crystals within single-walled carbon nanotubes. J. Electron. Microsc. 2004, 53, 101–106. [Google Scholar] [CrossRef]
- Kiselev, N.; Zakalyukin, R.; Zhigalina, O.; Grobert, N.; Kumskov, A.; Grigoriev, Y.V.; Chernysheva, M.; Eliseev, A.; Krestinin, A.; Tretyakov, Y.; et al. The structure of 1D CuI crystals inside SWNTs. J. Microsc. 2008, 232, 335–342. [Google Scholar] [CrossRef]
- Kiselev, N.; Kumskov, A.; Zakalyukin, R.; Vasiliev, A.; Chernisheva, M.; Eliseev, A.; Krestinin, A.; Freitag, B.; Hutchison, J. The structure of nanocomposite 1D cationic conductor crystal@SWNT. J. Microsc. 2012, 246, 309–321. [Google Scholar] [CrossRef]
- Kumskov, A.; Zhigalina, V.; Chuvilin, A.; Verbitskiy, N.; Ryabenko, A.; Zaytsev, D.; Eliseev, A.; Kiselev, N. The structure of 1D and 3D CuI nanocrystals grown within 1.5–2.5 nm single wall carbon nanotubes obtained by catalyzed chemical vapor deposition. Carbon 2012, 50, 4696–4704. [Google Scholar] [CrossRef]
- Meyer, R.R.; Sloan, J.; Dunin-Borkowski, R.E.; Kirkland, A.I.; Novotny, M.C.; Bailey, S.R.; Hutchison, J.L.; Green, M.L.H. Discrete Atom Imaging of One-Dimensional Crystals Formed Within Single-Walled Carbon Nanotubes. Science 2000, 289, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.; Novotny, M.; Bailey, S.; Brown, G.; Xu, C.; Williams, V.; Friedrichs, S.; Flahaut, E.; Callender, R.; York, A.; et al. Two layer 4:4 co-ordinated KI crystals grown within single walled carbon nanotubes. Chem. Phys. Lett. 2000, 329, 61–65. [Google Scholar] [CrossRef]
- Flahaut, E.; Sloan, J.; Friedrichs, S.; Kirkland, A.I.; Coleman, K.S.; Williams, V.C.; Hanson, N.; Hutchison, J.L.; Green, M.L.H. Crystallization of 2H and 4H PbI2 in Carbon Nanotubes of Varying Diameters and Morphologies. Chem. Mater. 2006, 18, 2059–2069. [Google Scholar] [CrossRef]
- Kharlamova, V.; Mochalin, V.N.; Lukatskaya, M.R.; Niu, J.J.; Presser, V.; Mikhalovsky, S.; Gogotsi, Y. Adsorption of proteins in channels of carbon nanotubes: Effect of surface chemistry. Mater. Express 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Sloan, J.; Grosvenor, S.J.; Friedrichs, S.; Kirkland, A.I.; Hutchison, J.L.; Green, M.L.H. A one-dimensional BaI2 chain with five- and six-coordination, formed within a single-walled carbon nanotube. Angew. Chem. Int. Ed. 2002, 41, 1156. [Google Scholar] [CrossRef]
- Friedrichs, S.; Falke, U.; Green, M.L.H. Phase separation of Lal(3) inside single-walled carbon nanotubes. Chemphyschem 2005, 6, 300–305. [Google Scholar] [CrossRef]
- Friedrichs, S.; Kirkland, A.I.; Meyer, R.R.; Sloan, J.; Green, M.L.H. LaI2@(18,3)SWNT: The unprecedented structure of a LaI2 “Crystal” encapsulated within a single-walled carbon nanotube. Microsc. Microanal. 2005, 11, 421–430. [Google Scholar] [CrossRef]
- Sloan, J.; Terrones, M.; Nufer, S.; Friedrichs, S.; Bailey, S.R.; Woo, H.G.; Ruhle, M.; Hutchison, J.L.; Green, M.L.H. Metastable one-dimensional AgCl1-xIx solid-solution wurzite “tunnel” crystals formed within single-walled carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 2116–2117. [Google Scholar] [CrossRef]
- Falaleev, N.S.; Kumskov, A.S.; Zhigalina, V.G.; Verbitskiy, I.I.; Vasiliev, A.L.; Makarova, A.A.; Vyalikh, D.V.; Kiselev, N.A.; Eliseev, A.A. Capsulate structure effect on SWNTs doping in RbxAg1-xI@SWNT composites. Crystengcomm 2017, 19, 3063–3070. [Google Scholar] [CrossRef]
- Sloan, J.; Kirkland, A.I.; Hutchison, J.L.; Green, M.L.H. Integral atomic layer architectures of 1D crystals inserted into single walled carbon nanotubes. Chem. Commun. 2002, 1319–1332. [Google Scholar] [CrossRef]
- Eremina, V.A.; Fedotov, P.V.; Obraztsova, E.D. Copper chloride functionalization of semiconducting and metallic fractions of single-walled carbon nanotubes. J. Nanophotonics 2015, 10, 012515. [Google Scholar] [CrossRef]
- Fedotov, P.V.; Tonkikh, A.A.; Obraztsova, E.A.; Nasibulin, A.G.; Kauppinen, E.I.; Chuvilin, A.L.; Obraztsova, E.D. Optical properties of single-walled carbon nanotubes filled with CuCl by gas-phase technique. Phys. Status Solidi 2014, 251, 2466–2470. [Google Scholar] [CrossRef]
- Fedotov, P.V.; Eremina, V.A.; Tonkikh, A.A.; Chernov, A.I.; Obraztsova, E.D. Enhanced optical transparency of films formed from sorted metallic or semiconducting single-walled carbon nanotubes filled with CuCl. Phys. Status Solidi 2016, 253, 2400–2405. [Google Scholar] [CrossRef]
- Nakanishi, R.; Kitaura, R.; Ayala, P.; Shiozawa, H.; de Blauwe, K.; Hoffmann, P.; Choi, D.; Miyata, Y.; Pichler, T.; Shinohara, H. Electronic structure of Eu atomic wires encapsulated inside single-wall carbon nanotubes. Phys. Rev. B 2012, 86, 115445. [Google Scholar] [CrossRef]
- Ayala, P.; Kitaura, R.; Nakanishi, R.; Shiozawa, H.; Ogawa, D.; Hoffmann, P.; Shinohara, H.; Pichler, T. Templating rare-earth hybridization via ultrahigh vacuum annealing of ErCl3 nanowires inside carbon nanotubes. Phys. Rev. B 2011, 83, 085407. [Google Scholar] [CrossRef]
- Zakalyukin, R.; Mavrin, B.; Dem’Yanets, L.; Kiselev, N. Synthesis and characterization of single-walled carbon nanotubes filled with the superionic material SnF2. Carbon 2008, 46, 1574–1578. [Google Scholar] [CrossRef]
- Burteaux, B.; Claye, A.; Smith, B.W.; Monthioux, M.; Luzzi, D.E.; Fischer, J.E. Abundance of encapsulated C60 in single-wall carbon nanotubes. Chem. Phys. Lett. 1999, 310, 21–24. [Google Scholar] [CrossRef]
- Chamberlain, T.W.; Popov, A.M.; Knizhnik, A.A.; Samoilov, G.E.; Khlobystov, A.N. The Role of Molecular Clusters in the Filling of Carbon Nanotubes. ACS Nano 2010, 4, 5203–5210. [Google Scholar] [CrossRef]
- Hirahara, K.; Suenaga, K.; Bandow, S.; Kato, H.; Okazaki, T.; Shinohara, H.; Iijima, S. One-Dimensional Metallofullerene Crystal Generated Inside Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2000, 85, 5384–5387. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-H.; Hirata, T.; Hatakeyama, R.; Tohji, K.; Motomiya, K. C60 encapsulation inside single-walled carbon nanotubes using alkali–fullerene plasma method. Carbon 2002, 40, 2247–2253. [Google Scholar] [CrossRef]
- Kataura, H.; Maniwa, Y.; Kodama, T.; Kikuchi, K.; Hirahara, K.; Suenaga, K.; Iijima, S.; Suzuki, S.; Achiba, Y.; Krätschmer, W. High-yield fullerene encapsulation in single-wall carbon nanotubes. Synth. Met. 2001, 121, 1195–1196. [Google Scholar] [CrossRef]
- Kataura, H.; Maniwa, Y.; Abe, M.; Fujiwara, A.; Kodama, T.; Kikuchi, K.; Imahori, H.; Misaki, Y.; Suzuki, S.; Achiba, Y. Optical properties of fullerene and non-fullerene peapods. Appl. Phys. A 2002, 74, 349–354. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Britz, D.A.; Wang, J.; O’Neil, S.A.; Poliakoff, M.; Briggs, G.A.D. Low temperature assembly of fullerene arrays in single-walled carbon nanotubes using supercritical fluids. J. Mater. Chem. 2004, 14, 2852–2857. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Porfyrakis, K.; Kanai, M.; Britz, D.A.; Ardavan, A.; Shinohara, H.; Dennis, T.J.S.; Briggs, G.A.D. Molecular motion of endohedral fullerenes in single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2004, 43, 1386–1389. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Britz, D.A.; Briggs, G.A.D. Molecules in carbon nanotubes. Accounts Chem. Res. 2005, 38, 901–909. [Google Scholar] [CrossRef]
- Luzzi, D.E.; Smith, B.W. Carbon cage structures in single wall carbon nanotubes: A new class of materials. Carbon 2000, 38, 1751–1756. [Google Scholar] [CrossRef]
- Monthioux, M.; Smith, B.; Burteaux, B.; Claye, A.; Fischer, J.; Luzzi, D. Sensitivity of single-wall carbon nanotubes to chemical processing: An electron microscopy investigation. Carbon 2001, 39, 1251–1272. [Google Scholar] [CrossRef]
- Shimada, T.; Ohno, Y.; Okazaki, T.; Sugai, T.; Suenaga, K.; Kishimoto, S.; Mizutani, T.; Inoue, T.; Taniguchi, R.; Fukui, N.; et al. Transport properties of C-78, C-90 and Dy@C-82 fullerenes-nanopeapods by field effect transistors. Physica E 2004, 21, 1089–1092. [Google Scholar] [CrossRef]
- Shiozawa, H.; Ishii, H.; Kihara, H.; Sasaki, N.; Nakamura, S.; Yoshida, T.; Takayama, Y.; Miyahara, T.; Suzuki, S.; Achiba, Y.; et al. Photoemission and inverse photoemission study of the electronic structure of C60 fullerenes encapsulated in single-walled carbon nanotubes. Phys. Rev. B 2006, 73, 075406. [Google Scholar] [CrossRef]
- Simon, F.; Kuzmany, H.; Rauf, H.; Pichler, T.; Bernardi, J.; Peterlik, H.; Korecz, L.; Fülöp, F.; Jánossy, A. Low temperature fullerene encapsulation in single wall carbon nanotubes: Synthesis of N@C60@SWCNT. Chem. Phys. Lett. 2004, 383, 362–367. [Google Scholar] [CrossRef]
- Sloan, J.; Dunin-Borkowski, R.E.; Hutchison, J.L.; Coleman, K.S.; Williams, V.C.; Claridge, J.B.; York, A.P.; Xu, C.; Bailey, S.R.; Brown, G.; et al. The size distribution, imaging and obstructing properties of C60 and higher fullerenes formed within arc-grown single walled carbon nanotubes. Chem. Phys. Lett. 2000, 316, 191–198. [Google Scholar] [CrossRef]
- Smith, B.W.; Monthioux, M.; Luzzi, D.E. Carbon nanotube encapsulated fullerenes: A unique class of hybrid materials. Chem. Phys. Lett. 1999, 315, 31–36. [Google Scholar] [CrossRef]
- Smith, B.W.; Luzzi, D.E. Formation mechanism of fullerene peapods and coaxial tubes: A path to large scale synthesis. Chem. Phys. Lett. 2000, 321, 169–174. [Google Scholar] [CrossRef]
- Yudasaka, M.; Ajima, K.; Suenaga, K.; Ichihashi, T.; Hashimoto, A.; Iijima, S. Nano-extraction and nano-condensation for C60 incorporation into single-wall carbon nanotubes in liquid phases. Chem. Phys. Lett. 2003, 380, 42–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Iijima, S.; Shi, Z.; Gu, Z. Defects in arc-discharge-produced single-walled carbon nanotubes. Philos. Mag. Lett. 1999, 79, 473–479. [Google Scholar] [CrossRef]
- Simon, F.; Kramberger, C.; Pfeiffer, R.; Kuzmany, H.; Zólyomi, V.; Kürti, J.; Singer, P.M.; Alloul, H. Isotope Engineering of Carbon Nanotube Systems. Phys. Rev. Lett. 2005, 95, 017401. [Google Scholar] [CrossRef]
- Simon, F.; Kukovecz, A.; Kramberger, C.; Pfeiffer, R.; Hasi, F.; Kuzmany, H.; Kataura, H. Diameter selective reaction processes of single-wall carbon nanotubes. Phys. Rev. B 2005, 71, 165439. [Google Scholar] [CrossRef]
- Ashino, M.; Obergfell, D.; Haluška, M.; Yang, S.; Khlobystov, A.N.; Roth, S.; Wiesendanger, R. Atomically resolved mechanical response of individual metallofullerene molecules confined inside carbon nanotubes. Nat. Nanotechnol. 2008, 3, 337–341. [Google Scholar] [CrossRef]
- Chiu, P.W.; Gu, G.; Kim, G.T.; Philipp, G.; Roth, S.; Yang, S.F. Temperature-induced change from p to n conduction in metallofullerene nanotube peapods. Appl. Phys. Lett. 2001, 79, 3845–3847. [Google Scholar] [CrossRef]
- Gloter, A.; Suenaga, K.; Kataura, H.; Fujii, R.; Kodama, T.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Suzuki, S.; Achiba, Y.; et al. Structural evolutions of carbon nano-peapods under electron microscopic observation. Chem. Phys. Lett. 2004, 390, 462–466. [Google Scholar] [CrossRef]
- Kitaura, R.; Imazu, N.; Kobayashi, K.; Shinohara, H. Fabrication of metal nanowires in carbon nanotubes via versatile nano-template reaction. Nano Lett. 2008, 8, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Suenaga, K.; Hirahara, K.; Bandow, S.; Iijima, S.; Shinohara, H. Real Time Reaction Dynamics in Carbon Nanotubes. J. Am. Chem. Soc. 2001, 123, 9673–9674. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Suenaga, K.; Hirahara, K.; Bandow, S.; Iijima, S.; Shinohara, H. Electronic and geometric structures of metallofullerene peapods. Phys. B Condens. Matter 2002, 323, 97–99. [Google Scholar] [CrossRef]
- Pichler, T.; Kramberger, C.; Ayala, P.; Shiozawa, H.; Knupfer, M.; Rümmeli, M.H.; Batchelor, D.; Kitaura, R.; Imazu, N.; Kobayashi, K.; et al. Bonding environment and electronic structure of Gd metallofullerene and Gd nanowire filled single-wall carbon nanotubes. Phys. Status Solidi 2008, 245, 2038–2041. [Google Scholar] [CrossRef]
- Suenaga, K.; Tencé, M.; Mory, C.; Colliex, C.; Kato, H.; Okazaki, T.; Shinohara, H.; Hirahara, K.; Bandow, S.; Iijima, S. Element-Selective Single Atom Imaging. Science 2000, 290, 2280–2282. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Kitaura, R.; Kramberger, C.; Shiozawa, H.; Imazu, N.; Kobayashi, K.; Mowbray, D.J.; Hoffmann, P.; Shinohara, H.; Pichler, T. A Resonant Photoemission Insight to the Electronic Structure of Gd Nanowires Templated in the Hollow Core of SWCNTs. Mater. Express 2011, 1, 30–35. [Google Scholar] [CrossRef]
- Débarre, A.; Jaffiol, R.; Julien, C.; Richard, A.; Nutarelli, D.; Tchénio, P. Antenna effect in dimetallofullerene peapods. Chem. Phys. Lett. 2003, 380, 6–11. [Google Scholar] [CrossRef]
- Fan, X.; Dickey, E.C.; Eklund, P.C.; Williams, K.A.; Grigorian, L.; Buczko, R.; Pantelides, S.T.; Pennycook, S.J. Atomic Arrangement of Iodine Atoms inside Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2000, 84, 4621–4624. [Google Scholar] [CrossRef]
- Guan, L.; Suenaga, K.; Shi, Z.; Gu, Z.; Iijima, S. Polymorphic Structures of Iodine and Their Phase Transition in Confined Nanospace. Nano Lett. 2007, 7, 1532–1535. [Google Scholar] [CrossRef]
- Kissell, K.R.; Hartman, K.B.; Van der Heide, P.A.W.; Wilson, L.J. Preparation of I-2@ SWNTs: Synthesis and spectroscopic characterization of I-2-loaded SWNTs. J. Phys. Chem. B 2006, 110, 17425–17429. [Google Scholar] [CrossRef]
- Tonkikh, A.; Tsebro, V.; Obraztsova, E.; Suenaga, K.; Kataura, H.; Nasibulin, A.; Kauppinen, E. Metallization of single-wall carbon nanotube thin films induced by gas phase iodination. Carbon 2015, 94, 768–774. [Google Scholar] [CrossRef]
- Hatakeyama, R.; Li, Y.F. Synthesis and electronic-property control of Cs-encapsulated single- and double-walled carbon nanotubes by plasma ion irradiation. J. Appl. Phys. 2007, 102, 034309. [Google Scholar] [CrossRef]
- Jeong, G.-H.; Hatakeyama, R.; Hirata, T.; Tohji, K.; Motomiya, K.; Yaguchi, T.; Kawazoe, Y. Formation and structural observation of cesium encapsulated single-walled carbon nanotubesElectronic supplementary information (ESI) available: Bright-field STEM images and their corresponding Z-contrast images composed of Cs inside filled and outside doped SWNTs are given. Chem. Commun. 2002, 152–153. [Google Scholar] [CrossRef]
- Nishide, D.; Dohi, H.; Wakabayashi, T.; Nishibori, E.; Aoyagi, S.; Ishida, M.; Kikuchi, S.; Kitaura, R.; Sugai, T.; Sakata, M.; et al. Single-wall carbon nanotubes encaging linear chain C10H2 polyyne molecules inside. Chem. Phys. Lett. 2006, 428, 356–360. [Google Scholar] [CrossRef]
- Chancolon, J.; Archaimbault, F.; Pineau, A.; Bonnamy, S. Filling of carbon nanotubes with selenium by vapor phase process. J. Nanosci. Nanotech. 2006, 6, 82–86. [Google Scholar] [CrossRef]
- Chernysheva, M.; Kiseleva, E.; Verbitskii, N.; Eliseev, A.; Lukashin, A.; Tretyakov, Y.; Savilov, S.; Kiselev, N.; Zhigalina, O.; Kumskov, A.; et al. The electronic properties of SWNTs intercalated by electron acceptors. Phys. E Low-Dimens. Syst. Nanostruct. 2007, 40, 2283–2288. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, T.; Hayashi, T.; Endo, M.; Hong, S.Y.; et al. Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef]
- Hart, M.; White, E.R.; Chen, J.; McGilvery, C.M.; Pickard, C.J.; Michaelides, A.; Sella, A.; Shaffer, M.S.P.; Salzmann, C.G. Encapsulation and Polymerization of White Phosphorus Inside Single-Wall Carbon Nanotubes. Angew. Chem. Int. Ed. 2017, 56, 8144–8148. [Google Scholar] [CrossRef]
- Hart, M.; Chen, J.; Michaelides, A.; Sella, A.; Shaffer, M.S.P.; Salzmann, C.G. One-Dimensional Arsenic Allotropes: Polymerization of Yellow Arsenic Inside Single-Wall Carbon Nanotubes. Angew. Chem. Int. Ed. 2018, 57, 11649–11653. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Z.; Gu, Z. Synthesis of single-walled carbon nanotube/metal nanoparticle hybrid materials from potassium-filled nanotubes. Carbon 2010, 48, 443–446. [Google Scholar] [CrossRef]
- Kiang, C.H.; Choi, J.S.; Tran, T.T.; Bacher, A.D. Molecular nanowires of 1 nm diameter from capillary filling of single-walled carbon nanotubes. J. Phys. Chem. B 1999, 103, 7449–7451. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Mendoza, E.; Bachmatiuk, A.; Rummeli, M.; Gemming, T.; Nogues, J.; Skumryev, V.; Kalenczuk, R.; Pichler, T.; Silva, S. Iron filled single-wall carbon nanotubes—A novel ferromagnetic medium. Chem. Phys. Lett. 2006, 421, 129–133. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Bachmatiuk, A.; Rümmeli, M.H.; Gemming, T.; Pichler, T.; Kalenczuk, R. Iron filled singlewalled carbon nanotubes—Synthesis and characteristic properties. Phys. Status Solidi 2006, 243, 3277–3280. [Google Scholar] [CrossRef]
- Cui, T.; Pan, X.; Dong, J.; Miao, S.; Miao, D.; Bao, X. A versatile method for the encapsulation of various non-precious metal nanoparticles inside single-walled carbon nanotubes. Nano Res. 2018, 11, 3132–3144. [Google Scholar] [CrossRef]
- Li, Y.; Kaneko, T.; Ogawa, T.; Takahashi, M.; Hatakeyama, R. Novel Properties of Single-Walled Carbon Nanotubes with Encapsulated Magnetic Atoms. Jpn. J. Appl. Phys. 2008, 47, 2048–2055. [Google Scholar] [CrossRef]
- Domanov, O.; Weschke, E.; Saito, T.; Peterlik, H.; Pichler, T.; Eisterer, M.; Shiozawa, H. Exchange coupling in a frustrated trimetric molecular magnet reversed by a 1D nano-confinement. Nanoscale 2019, 11, 10615–10621. [Google Scholar] [CrossRef]
- Sloan, J.; Hammer, J.; Zwiefka-Sibley, M.; Green, M.L.H. The opening and filling of single walled carbon nanotubes (SWTs). Chem. Commun. 1998, 347–348. [Google Scholar] [CrossRef]
- Govindaraj, A.; Satishkumar, B.C.; Nath, M.; Rao, C.N.R. Metal Nanowires and Intercalated Metal Layers in Single-Walled Carbon Nanotube Bundles. Chem. Commun. 2003, 334–337. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Ruemmeli, M.H.; Gemming, T.; Pichler, T.; Kalenczuk, R.J.; Silva, S.R.P. Silver filled single-wall carbon nanotubes—Synthesis, structural and electronic properties. Nanotechnology 2006, 17, 2415–2419. [Google Scholar] [CrossRef]
- Corio, P.; Santos, A.; Santos, P.; Temperini, M.; Brar, V.; Pimenta, M.; Dresselhaus, M. Characterization of single wall carbon nanotubes filled with silver and with chromium compounds. Chem. Phys. Lett. 2004, 383, 475–480. [Google Scholar] [CrossRef]
- Sloan, J.; Wright, D.M.; Bailey, S.; Brown, G.; York, A.P.E.; Coleman, K.S.; Green, M.L.H.; Hutchison, J.L.; Woo, H.-G. Capillarity and silver nanowire formation observed in single walled carbon nanotubes. Chem. Commun. 1999, 699–700. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Li, B.; Shi, Z.J.; Gu, Z.N.; Xue, Z.Q.; Peng, L.-M. Filling of single-walled carbon nanotubes with silver. J. Mater. Res. 2000, 15, 2658–2661. [Google Scholar] [CrossRef]
- Chamberlain, T.W.; Zoberbier, T.; Biskupek, J.; Botos, A.; Kaiser, U.; Khlobystov, A.N. Formation of uncapped nanometre-sized metal particles by decomposition of metal carbonyls in carbon nanotubes. Chem. Sci. 2012, 3, 1919–1924. [Google Scholar] [CrossRef]
- Costa, P.M.F.J.; Sloan, J.; Rutherford, T.; Green, M.L.H. Encapsulation of RexOy clusters within single-walled carbon nanotubes and their in tubulo reduction and sintering to Re metal. Chem. Mater. 2005, 17, 6579–6582. [Google Scholar] [CrossRef]
- Zoberbier, T.; Chamberlain, T.W.; Biskupek, J.; Kuganathan, N.; Eyhusen, S.; Bichoutskaia, E.; Kaiser, U.; Khlobystov, A.N. Interactions and Reactions of Transition Metal Clusters with the Interior of Single-Walled Carbon Nanotubes Imaged at the Atomic Scale. J. Am. Chem. Soc. 2012, 134, 3073–3079. [Google Scholar] [CrossRef]
- Kitaura, R.; Nakanishi, R.; Saito, T.; Yoshikawa, H.; Awaga, K.; Shinohara, H. High-Yield Synthesis of Ultrathin Metal Nanowires in Carbon Nanotubes. Angew. Chem. Int. Ed. 2009, 48, 8298–8302. [Google Scholar] [CrossRef]
- Yanagi, K.; Miyata, Y.; Kataura, H. Highly stabilized beta-carotene in carbon nanotubes. Adv. Mater. 2006, 18, 437. [Google Scholar] [CrossRef]
- Takenobu, T.; Takano, T.; Shiraishi, M.; Murakami, Y.; Ata, M.; Kataura, H.; Achiba, Y.; Iwasa, Y. Stable and controlled amphoteric doping by encapsulation of organic molecules inside carbon nanotubes. Nat. Mater. 2003, 2, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Fukumaru, T.; Fujigaya, T.; Nakashima, N. Development of n-type cobaltocene-encapsulated carbon nanotubes with remarkable thermoelectric property. Sci. Rep. 2015, 5, 7951. [Google Scholar] [CrossRef]
- Claye, A.S.; Nemes, N.M.; Jánossy, A.; Fischer, J.E. Structure and electronic properties of potassium-doped single-wall carbon nanotube. Phys. Rev. B 2000, 62, R4845. [Google Scholar] [CrossRef]
- Grigorian, L.; Sumanasekera, G.U.; Loper, A.L.; Fang, S.; Allen, J.L.; Eklund, P.C. Transport properties of alkali-metal-doped single-wall carbon nanotubes. Phys. Rev. B 1998, 58, R4195. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Electrochemistry of carbon nanotubes. In Carbon Nanotubes; Jorio, A., Dresselhaus, G., Dresselhaus, M.S., Eds.; Topics in Applied Physics; Springer: Berlin, Germany, 2008; Volume 111, p. 567. [Google Scholar]
- Cronin, S.B.; Barnett, R.; Tinkham, M.; Chou, S.G.; Rabin, O.; Dresselhaus, M.S.; Swan, A.K.; Ünlü, M.S.; Goldberg, B.B. Electrochemical gating of individual single-wall carbon nanotubes observed by electron transport measurements and resonant Raman spectroscopy. Appl. Phys. Lett. 2004, 84, 2052. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Bard, A.J.; Wudl, F.; Weitz, I.; Heath, J.R. Electrochemical Characterization of Films of Single-Walled Carbon Nanotubes and Their Possible Application in Supercapacitors. Electrochem. Solid-State Lett. 1999, 2, 577. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937. [Google Scholar] [CrossRef]
- Frackowiak, E.; Beguin, F. Electrochemical storage of energy in carbon nanotubes and nanostructured carbons. Carbon 2002, 40, 1775. [Google Scholar] [CrossRef]
- Heller, I.; Kong, J.; Heering, H.A.; Williams, K.A.; Lemay, S.G.; Dekker, C. Individual Single-Walled Carbon Nanotubes as Nanoelectrodes for Electrochemistry. Nano Lett. 2005, 5, 137. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Rapta, P.; Dunsch, L. In situ Raman and Vis-NIR spectroelectrochemistry at single-walled carbon nanotubes. Chem. Phys. Lett. 2000, 328, 363. [Google Scholar] [CrossRef]
- Kavan, L.; Rapta, P.; Dunsch, L.; Bronikowski, M.J.; Willis, P.; Smalley, R.E. Electrochemical Tuning of Electronic Structure of Single-Walled Carbon Nanotubes: In-situ Raman and Vis-NIR Study. J. Phys. Chem. B 2001, 105, 10764. [Google Scholar] [CrossRef]
- An, C.; Vardeny, Z.; Iqbal, Z.; Spinks, G.; Baughman, R.; Zakhidov, A. Raman scattering study of electrochemically doped single wall nanotubes. Synthet. Met. 2001, 116, 411. [Google Scholar] [CrossRef]
- Corio, P.; Santos, P.S.; Brar, V.W.; Samsonidze, G.G.; Chou, S.G.; Dresselhaus, M.S. Potential dependent surface Raman spectroscopy of single wall carbon nanotube films on platinum electrodes. Chem. Phys. Lett. 2003, 370, 675. [Google Scholar] [CrossRef]
- Corio, P.; Jorio, A.; Demir, N.; Dresselhaus, M.S. Spectro-electrochemical studies of single wall carbon nanotubes films. Chem. Phys. Lett. 2004, 392, 396. [Google Scholar] [CrossRef]
- Ghosh, S.; Sood, A.K.; Rao, C.N.R. Electrochemical tuning of band structure of single-walled carbon nanotubes probed by in situ resonance Raman scattering. J. Appl. Phys. 2002, 92, 1165. [Google Scholar] [CrossRef]
- Gupta, S.; Hughes, M.; Windle, A.H.; Robertson, J. Charge transfer in carbon nanotube actuators investigated using in situ Raman spectroscopy. J. Appl. Phys. 2004, 95, 2038. [Google Scholar] [CrossRef]
- Gupta, S.; Robertson, J. Ion transport and electrochemical tuning of Fermi level in single-wall carbon nanotube probed by in situ Raman scattering. J. Appl. Phys. 2006, 100, 083711. [Google Scholar] [CrossRef]
- Gupta, S. Electrochemical tuning and investigations on actuator mechanism of single-wall carbon nanotubes. Diamond Relat. Mater. 2006, 15, 378. [Google Scholar] [CrossRef]
- Kalbac, M.; Kavan, L.; Zukalova, M.; Dunsch, L. The identification of dispersive and non-dispersive intermediate frequency modes of HiPco single walled carbon nanotubes by in situ Raman spectroelectrochemistry. Phys. Status Solidi B 2006, 243, 3134. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Diameter-Selective Electrochemical Doping of HiPco Single-Walled Carbon Nanotubes. Nano Lett. 2003, 3, 969. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Ionic Liquid for in situ Vis/NIR and Raman Spectroelectrochemistry: Doping of Carbon Nanostructures. ChemPhysChem 2003, 4, 944. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbáč, M.; Zukalová, M.; Dunsch, L. Electrochemical Doping of Chirality-Resolved Carbon Nanotubes. J. Phys. Chem. B 2005, 109, 19613. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbáč, M.; Zukalová, M.; Dunsch, L. Raman spectroelectrochemistry of index-identified metallic carbon nanotubes: The resonance rule revisited. Phys. Status Solidi B 2006, 243, 3130. [Google Scholar] [CrossRef]
- Kazaoui, S.; Minami, N.; Kataura, H.; Achiba, Y. Absorption spectroscopy of single-wall carbon nanotubes: Effects of chemical and electrochemical doping. Synthet. Met. 2001, 121, 1201. [Google Scholar] [CrossRef]
- Kazaoui, S.; Minami, N.; Matsuda, N.; Kataura, H.; Achiba, Y. Electrochemical tuning of electronic states in single-wall carbon nanotubes studied by in situ absorption spectroscopy and ac resistance. Appl. Phys. Lett. 2001, 78, 3433. [Google Scholar] [CrossRef]
- Murakoshi, K.; Okazaki, K. Electrochemical potential control of isolated single-walled carbon nanotubes on gold electrode. Electrochim. Acta 2005, 50, 3069. [Google Scholar] [CrossRef]
- Okazaki, K.; Nakato, Y.; Murakoshi, K. Absolute potential of the Fermi level of isolated single-walled carbon nanotubes. Phys. Rev. B 2003, 68, 035434. [Google Scholar] [CrossRef]
- Okazaki, K.; Nakato, Y.; Murakoshi, K. Characteristics of Raman features of isolated single-walled carbon nanotubes under electrochemical potential control. Surf. Sci. 2004, 566, 436. [Google Scholar] [CrossRef]
- Rafailov, P.M.; Maultzsch, J.; Thomsen, C.; Kataura, H. Electrochemical switching of the Peierls-like transition in metallic single-walled carbon nanotubes. Phys. Rev. B 2005, 72, 045411. [Google Scholar] [CrossRef]
- Rafailov, P.M.; Thomsen, C.J. Raman spectroscopy on electrochemically doped carbon nanotubes. Optoelectron. Adv. Mater. 2005, 7, 461. [Google Scholar]
- Stoll, M.; Rafailov, P.; Frenzel, W.; Thomsen, C. Electrochemical and Raman measurements on single-walled carbon nanotubes. Chem. Phys. Lett. 2003, 375, 625. [Google Scholar] [CrossRef]
- Wang, Z.; Pedrosa, H.; Krauss, T.; Rothberg, L. Determination of the Exciton Binding Energy in Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2006, 96, 047403. [Google Scholar] [CrossRef]
- Kavan, L.; Dunsch, L. Spectroelectrochemistry of Carbon Nanostructures. Chem. Phys. Chem. 2007, 8, 974. [Google Scholar] [CrossRef]
- Sumanasekera, G.U.; Pradhan, B.K.; Romero, H.E.; Adu, K.W.; Eklund, P.C. Giant Thermopower Effects from Molecular Physisorption on Carbon Nanotubes. Phys. Rev. Lett. 2002, 89, 166801. [Google Scholar] [CrossRef] [PubMed]
- Claye, A.; Rahman, S.; Fischer, J.; Sirenko, A.; Sumanasekera, G.; Eklund, P. In situ Raman scattering studies of alkali-doped single wall carbon nanotubes. Chem. Phys. Lett. 2001, 333, 16. [Google Scholar] [CrossRef]
- Frackowiak, E.; Gautier, S.; Gaucher, H.; Bonnamy, S.; Beguin, F. Electrochemical storage of lithium in multiwalled carbon nanotubes. Carbon 1999, 37, 61–69. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Brzhezinskaya, M.M.; Vinogradov, A.S.; Suzdalev, I.P.; Maksimov, Y.V.; Imshennik, V.K.; Novichikhin, S.; Krestinin, A.V.; Yashina, L.; Lukashin, A.V.; et al. The forming and properties of one-dimensional FeHal2 (Hal=Cl, Br, I) nanocrystals in channels of single-walled carbon nanotubes. Russ. Nanotechnol. 2009, 4, 77–87. [Google Scholar] [CrossRef]
- Kharlamova, M.; Eliseev, A.A.; Yashina, L.V.; Petukhov, D.I.; Liu, C.; Wang, C.; Semenenko, D.A.; Belogorokhov, A.I. Study of the electronic structure of single-walled carbon nanotubes filled with cobalt bromide. JETP Lett. 2010, 91, 196–200. [Google Scholar] [CrossRef]
- Kharlamova, M.; Yashina, L.V.; Eliseev, A.A.; Volykhov, A.A.; Neudachina, V.S.; Brzhezinskaya, M.M.; Zyubina, T.S.; Lukashin, A.V.; Tretyakov, Y.D. Single-walled carbon nanotubes filled with nickel halogenides: Atomic structure and doping effect. Phys. Status Solidi B 2012, 249, 2328–2332. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Raman Spectroscopy Study of the Doping Effect of the Encapsulated Iron, Cobalt, and Nickel Bromides on Single-Walled Carbon Nanotubes. Spectroscopy 2015, 2015, 653848. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Electronic properties of single-walled carbon nanotubes filled with manganese halogenides. Appl. Phys. A 2016, 122, 791. [Google Scholar] [CrossRef]
- Kharlamova, M.; Yashina, L.V.; Volykhov, A.A.; Niu, J.J.; Neudachina, V.S.; Brzhezinskaya, M.M.; Zyubina, T.S.; Belogorokhov, A.I.; Eliseev, A.A. Acceptor doping of single-walled carbon nanotubes by encapsulation of zinc halogenides. Eur. Phys. J. B 2012, 85, 34. [Google Scholar] [CrossRef]
- Kharlamova, M. Comparison of influence of incorporated 3d-, 4d- and 4f- metal chlorides on electronic properties of single-walled carbon nanotubes. Appl. Phys. A 2013, 111, 725–731. [Google Scholar] [CrossRef]
- Kharlamova, M.; Yashina, L.V.; Lukashin, A.V. Charge transfer in single-walled carbon nanotubes filled with cadmium halogenides. J. Mater. Sci. 2013, 48, 8412–8419. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Pichler, T. Semiconducting response in single-walled carbon nanotubes filled with cadmium chloride. Phys. Status Solidi B 2016, 253, 2433–2439. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Fermi level engineering of metallicity sorted metallic single-walled carbon nanotubes by encapsulation of few-atom thick crystals of silver chloride. J. Mater. Sci. 2018, 53, 13018–13029. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Mittelberger, A.; Yanagi, K.; Pichler, T.; Eder, D. Silver chloride encapsulation-induced modifications of Raman modes of metallicity sorted semiconducting single-walled carbon nanotubes. J. Spectrosc. 2018, 2018, 5987428. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Saito, T.; Yanagi, K.; Pichler, T.; Eder, D. Comparison of doping levels of single-walled carbon nanotubes synthesized by arc-discharge and chemical vapor deposition methods by encapsulated silver chloride. Phys. Status Solidi B 2018, 255, 1800178. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Volykhov, A.A.; Yashina, L.V.; Egorov, A.V.; Lukashin, A.V. Experimental and theoretical studies on the electronic properties of praseodymium chloride-filled single-walled carbon nanotubes. J. Mater. Sci. 2015, 50, 5419–5430. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Mittelberger, A. Raman spectroscopy study of the doping effect of the encapsulated terbium halogenides on single-walled carbon nanotubes. Appl. Phys. A 2017, 123, 239. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Rare-earth metal halogenide encapsulation-induced modifications in Raman spectra of single-walled carbon nanotubes. Appl. Phys. A 2014, 118, 27–35. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Rudatis, P.; Pichler, T.; Eder, D. Revealing the doping effect of encapsulated lead halogenides on single walled carbon nanotubes. Appl. Phys. A 2019, 125, 320. [Google Scholar] [CrossRef]
- Kharlamova, M.; Yashina, L.V.; Lukashin, A.V. Comparison of modification of electronic properties of single-walled carbon nanotubes filled with metal halogenide, chalcogenide and pure metal. Appl. Phys. A 2013, 112, 297–304. [Google Scholar] [CrossRef]
- Kharlamova, M. Novel approach to tailoring the electronic properties of single-walled carbon nanotubes by the encap-sulation of high-melting gallium selenide using a single-step process. JETP Lett. 2013, 98, 272–277. [Google Scholar] [CrossRef]
- Kharlamova, M. Comparative analysis of electronic properties of tin, gallium, and bismuth chalcogenide-filled sin-gle-walled carbon nanotubes. J. Mater. Sci. 2014, 49, 8402–8411. [Google Scholar] [CrossRef]
- Kharlamova, M.; Niu, J.J. Donor doping of single-walled carbon nanotubes by filling of channels with silver. J. Exp. Theor. Phys. 2012, 115, 485–491. [Google Scholar] [CrossRef]
- Kharlamova, M.; Niu, J.J. New method of the directional modification of the electronic structure of single-walled carbon nanotubes by filling channels with metallic copper from a liquid phase. JETP Lett. 2012, 95, 314–319. [Google Scholar] [CrossRef]
- Kharlamova, M.; Niu, J.J. Comparison of metallic silver and copper doping effects on single-walled carbon nanotubes. Appl. Phys. A 2012, 109, 25–29. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Rudatis, P.; Yanagi, K.; Eder, D. Characterization of the electronic properties of sin-gle-walled carbon nanotubes filled with an electron donor-rubidium iodide: Multifrequency Raman and X-ray photoelectron spectroscopy studies. Phys. Status Solidi B 2019, 256, 1900209. [Google Scholar] [CrossRef]
- Kharlamova, M.; Sauer, M.; Saito, T.; Krause, S.; Liu, X.; Yanagi, K.; Pichler, T.; Shiozawa, H. Inner tube growth properties and electronic structure of ferrocene-filled large diameter single-walled carbon nanotubes. Phys. Status Solidi B 2013, 250, 2575–2580. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Saito, T.; Shiozawa, H.; Pichler, T. In situ Raman spectroscopy studies on time-dependent inner tube growth in ferrocene-filled large diameter single-walled carbon nanotubes. Phys. Status Solidi B 2014, 251, 2394–2400. [Google Scholar] [CrossRef]
- Kharlamova, M.; Sauer, M.; Egorov, A.; Saito, T.; Kramberger, C.; Pichler, T.; Shiozawa, H. Temperature-dependent inner tube growth and electronic structure of nickelocene-filled single-walled carbon nanotubes. Phys. Status Solidi B 2015, 252, 2485–2490. [Google Scholar] [CrossRef]
- Kharlamova, M.; Sauer, M.; Saito, T.; Sato, Y.; Suenaga, K.; Pichler, T.; Shiozawa, H. Doping of single-walled carbon nanotubes controlled via chemical transformation of encapsulated nickelocene. Nanoscale 2015, 7, 1383–1391. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Saito, T.; Shiozawa, H.; Pichler, T. Growth dynamics of inner tubes inside co-baltocene-filled single-walled carbon nanotubes. Appl. Phys. A 2016, 122, 749. [Google Scholar] [CrossRef]
- Kharlamova, M. Investigation of growth dynamics of carbon nanotubes: A review. Beilstein J. Nanotechnol. 2017, 8, 826–856. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.; Kramberger, C.; Saito, T.; Sato, Y.; Suenaga, K.; Picher, T.; Shiozawa, H. Chirality-dependent growth of single-wall carbon nanotubes as revealed inside nano-test tubes. Nanoscale 2017, 9, 7998–8006. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.; Kramberger, C.; Yanagi, K.; Sauer, M.; Saito, T.; Pichler, T. Separation of nickelocene-filled single-walled carbon nanotubes by conductivity type and diameter. Phys. Status Solidi B 2017, 254, 1700178. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Sauer, M.; Yanagi, K.; Saito, T.; Pichler, T. Inner tube growth and electronic properties of metallicity sorted nickelocene-filled semiconducting single-walled carbon nanotubes. Appl. Phys. A 2018, 124, 247. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Sato, Y.; Saito, T.; Suenaga, K.; Pichler, T.; Shiozawa, H. Chiral vector and metal cata-lyst-dependent growth kinetics of single-wall carbon nanotubes. Carbon 2018, 133, 283–292. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C.; Saito, T.; Pichler, T. Diameter and metal-dependent growth properties of inner tubes inside metallocene-filled single-walled carbon nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 20–26. [Google Scholar] [CrossRef]
- Kharlamova, M.V. Nickelocene-Filled Purely Metallic Single-Walled Carbon Nanotubes: Sorting and Tuning the Electronic Properties. Nanomaterials 2021, 11, 2500. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Metal cluster size-dependent activation energies of growth of single-chirality sin-gle-walled carbon nanotubes inside metallocene-filled single-walled carbon nanotubes. Nanomaterials 2021, 11, 2649. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Temperature-Dependent Growth of 36 Inner Nanotubes inside Nickelocene, Cobaltocene and Ferrocene-Filled Single-Walled Carbon Nanotubes. Nanomaterials 2021, 11, 2984. [Google Scholar] [CrossRef]
- Green, A.A.; Hersam, M.C. Properties and Application of Double-Walled Carbon Nanotubes Sorted by Outer-Wall Electronic Type. ASC Nano 2011, 5, 4927–4934. [Google Scholar] [CrossRef] [PubMed]
- Brozena, A.H.; Moskowitz, J.; Shao, B.; Deng, S.; Liao, H.; Gaskell, K.J.; Wang, Y. Outer Wall Selectively Oxidized, Water-Soluble Double-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2010, 132, 3932. [Google Scholar] [CrossRef] [PubMed]
- Bulusheva, L.G.; Fedoseeva, Y.; Okotrub, A.V.; Flahaut, E.; Asanov, I.P.; Koroteev, V.O.; Yaya, A.; Ewels, C.; Chuvilin, A.; Felten, A.; et al. Stability of Fluorinated Double-Walled Carbon Nanotubes Produced by Different Fluorination Techniques. Chem. Mater. 2010, 22, 4197–4203. [Google Scholar] [CrossRef]
- Piao, Y.; Chen, C.F.; Green, A.A.; Kwon, H.; Hersam, M.C.; Lee, C.S.; Schatz, G.C.; Wang, Y. Optical and Electrical Properties of Inner Tubes in Outer Wall-Selectively Functionalized Double-Wall Carbon Nanotubes. J. Phys. Chem. Lett. 2011, 2, 1577–1582. [Google Scholar] [CrossRef]
- Ellis, B.D.; Dyker, C.A.; Decken, A.; Macdonald, C.L.B. The synthesis, characterisation and electronic structure of N-heterocyclic carbene adducts of PI cations. Chem. Commun. 2005, 2002–2004. [Google Scholar] [CrossRef]
- Collins, P.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622. [Google Scholar] [CrossRef]
- Ayala, P.; Arenal, R.; Loiseau, A.; Rubio, A.; Pichler, T. The physical and chemical properties of heteronanotubes. Rev. Mod. Phys. 2010, 82, 1843. [Google Scholar] [CrossRef]
- Yi, J.-Y.; Bernholc, J. Atomic structure and doping of microtubules. Phys. Rev. B 1993, 47, 1708. [Google Scholar] [CrossRef]
- Ayala, P.; Grüneis, A.; Gemming, T.; Grimm, D.; Kramberger, C.; Rümmeli, M.H.; Freire, F.L., Jr.; Kuzmany, H.; Pfeiffer, R.; Barreiro, A.; et al. Tailoring N-Doped Single and Double Wall Carbon Nanotubes from a Nondiluted Carbon/Nitrogen Feedstock. J. Phys. Chem. C 2007, 111, 2879. [Google Scholar] [CrossRef]
- Elias, A.L.; Ayala, P.; Zamudio, A.; Grobosch, M.; Cruz-Silva, E.; Romo-Herrera, J.M.; Campos-Delgado, J.; Terrones, H.; Pichler, T.; Terrones, M. Spectroscopic characterization of N-doped single-walled carbon nanotube strands: An X-ray photoelectron spectroscopy and Raman study. J. Nanosci. Nanotechnol. 2010, 10, 3959. [Google Scholar] [CrossRef] [PubMed]
- Glerup, M.; Steinmetz, J.; Samaille, D.; Stéphan, O.; Enouz, S.; Loiseau, A.; Roth, S.; Bernier, P. Synthesis of N-doped SWNT using the arc-discharge procedure. Chem. Phys. Lett. 2004, 387, 193. [Google Scholar] [CrossRef]
- Keskar, G.; Rao, R.; Luo, J.; Hudson, J.; Chen, J.; Rao, A.M. Growth, nitrogen doping and characterization of isolated single-wall carbon nanotubes using liquid precursor. Chem. Phys. Lett. 2005, 412, 269. [Google Scholar] [CrossRef]
- Krstić, V.; Rikken, G.L.J.A.; Bernier, P.; Roth, S.; Glerup, M. Nitrogen doping of metallic single-walled carbon nanotubes:n-type conduction and dipole scattering. Europhys. Lett. 2007, 77, 37001. [Google Scholar] [CrossRef][Green Version]
- Lin, H.; Lagoute, J.; Chacon, C.; Arenal, R.; Stéphan, O.; Repain, V.; Girard, Y.; Enouz, S.; Bresson, L.; Rousset, S.; et al. Combined STM/STS, TEM/EELS investigation of CNx-SWNTs. Phys. Status Solidi B 2008, 245, 1986. [Google Scholar] [CrossRef]
- Lin, H.; Arenal, R.; Enouz-Vedrenne, S.; Stephan, O.; Loiseau, A. Nitrogen Configuration in Individual CNx-SWNTs Synthesized by Laser Vaporization Technique. J. Phys. Chem. C 2009, 113, 9509. [Google Scholar] [CrossRef]
- Maciel, I.O.; Anderson, N.; Pimenta, M.A.; Hartschuh, A.; Qian, H.; Terrones, M.; Terrones, H.; Campos-Delgado, J.; Rao, A.M.; Novotny, L.; et al. Electron and phonon renormalization near charged defects in carbon nanotubes. Nat. Mater. 2008, 7, 878. [Google Scholar] [CrossRef]
- Susi, T.; Zhu, Z.; Ruiz-Soria, G.; Arenal, R.; Ayala, P.; Nasibulin, A.G.; Lin, H.; Jiang, H.; Stephan, O.; Pichler, T.; et al. Nitrogen-doped SWCNT synthesis using ammonia and carbon monoxide. Phys. Status Solidi B 2010, 247, 2726. [Google Scholar] [CrossRef]
- Villalpando-Paez, F.; Zamudio, A.; Elias, A.L.; Son, H.; Barros, E.B.; Chou, S.G.; Kim, Y.A.; Muramatsu, H.; Hayashi, T.; Kong, J.; et al. Synthesis and characterization of long strands of nitrogen-doped single-walled carbon nanotubes. Chem. Phys. Lett. 2006, 424, 345. [Google Scholar] [CrossRef]
- Wiltshire, J.G.; Li, L.-J.; Herz, L.M.; Nicholas, R.J.; Glerup, M.; Sauvajol, J.-L.; Khlobystov, A.N. Chirality-dependent boron-mediated growth of nitrogen-doped single-walled carbon nanotubes. Phys. Rev. B 2005, 72, 205431. [Google Scholar] [CrossRef]
- Ayala, P.; Plank, W.; Grüneis, A.; Kauppinen, E.I.; Rümmeli, M.H.; Kuzmany, H.; Pichler, T. A one step approach to B-doped single-walled carbon nanotubes. J. Mater. Chem. 2008, 18, 5676. [Google Scholar] [CrossRef]
- Ayala, P.; Reppert, J.; Grobosch, M.; Knupfer, M.; Pichler, T.; Rao, A.M. Evidence for substitutional boron in doped single-walled carbon nanotubes. Appl. Phys. Lett. 2010, 96, 183110. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Pichler, T.; Fuentes, G.G.; Graff, A.; Kalenczuk, R.J.; Knupfer, M.; Fink, J. Efficient production of B-substituted single-wall carbon nanotubes. Chem. Phys. Lett. 2003, 378, 516. [Google Scholar] [CrossRef]
- Borowiak-Palen, E.; Pichler, T.; Graff, A.; Kalenczuk, R.J.; Knupfer, M.; Fink, J. Synthesis and electronic properties of B-doped single wall carbon nanotubes. Carbon 2004, 42, 1123. [Google Scholar] [CrossRef]
- Daothong, S.; Parjanne, J.; Kauppinen, E.; Valkeapää, M.; Pichler, T.; Singjai, P.; Ayala, P. Study of the role of Fe based catalysts on the growth of B-doped SWCNTs synthesized by CVD. Phys. Status Solidi B 2009, 246, 2518. [Google Scholar] [CrossRef]
- Fuentes, G.G.; Borowiak-Palen, E.; Knupfer, M.; Pichler, T.; Fink, J.; Wirtz, L.; Rubio, A. Formation and electronic properties of BC3 single-wall nanotubes upon boron substitution of carbon nanotubes. Phys. Rev. B 2004, 69, 245403. [Google Scholar] [CrossRef]
- Gai, P.L.; Stephan, O.; McGuire, K.; Rao, A.M.; Dresselhaus, M.S.; Dresselhaus, G.; Colliex, C. Structural systematics in boron-doped single wall carbon nanotube. J. Mater. Chem. 2004, 14, 669. [Google Scholar] [CrossRef]
- McGuire, K.; Gothard, N.; Gai, P.L.; Dresselhaus, M.S.; Sumanasekera, G.; Rao, A.M. Synthesis and Raman characterization of boron-doped single-walled carbon nanotubes. Carbon 2005, 43, 219. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, X.; Liu, R.; Shen, S.; Wu, F.; Xie, A. Tuning the Dielectric and Microwaves Absorption Properties of N-Doped Carbon Nanotubes by Boron Insertion. Nanomaterials 2021, 11, 1164. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution Properties of Single-Walled Carbon Nanotubes. Science 1998, 282, 95. [Google Scholar] [CrossRef]
- Rao, A.M.; Eklund, P.C.; Bandow, S.; Thess, A.; Smalley, R.E. Evidence for charge transfer in doped carbon nanotube bundles from Raman scattering. Nature 1997, 388, 257. [Google Scholar] [CrossRef]
- Lee, R.S.; Kim, H.J.; Fischer, J.E.; Thess, A.; Smalley, R.E. Conductivity enhancement in single-walled carbon nanotube bundles doped with K and Br. Nature 1997, 388, 255. [Google Scholar] [CrossRef]
- Bandow, S.; Rao, A.; Sumanasekera, G.; Eklund, P.; Kokai, F.; Takahashi, K.; Yudasaka, M.; Iijima, S. Evidence for anomalously small charge transfer in doped single-wall carbon nanohorn aggregates with Li, K and Br. Appl. Phys. A 2000, 71, 561. [Google Scholar] [CrossRef]
- Kukovecz, A.; Pichler, T.; Pfeiffer, R.; Kramberger, C.; Kuzmany, H. Diameter selective doping of single wall carbon nanotubes. Phys. Chem. Chem. Phys. 2003, 5, 582. [Google Scholar] [CrossRef]
- Pichler, T.; Kukovecz, A.; Kuzmany, H.; Kataura, H. Charge transfer in doped single-walled carbon nanotubes. Synthet. Met. 2003, 136, 717. [Google Scholar] [CrossRef]
- De Blauwe, K.; Kramberger, C.; Plank, W.; Kataura, H.; Pichler, T. Raman response of FeCl3 intercalated single-wall carbon nanotubes at high doping. Phys. Status Solidi B 2009, 246, 2732. [Google Scholar] [CrossRef]
- Grigorian, L.; Williams, K.A.; Fang, S.; Sumanasekera, G.U.; Loper, A.L.; Dickey, E.C.; Pennycook, S.J.; Eklund, P.C. Reversible Intercalation of Charged Iodine Chains into Carbon Nanotube Ropes. Phys. Rev. Lett. 1998, 80, 5560. [Google Scholar] [CrossRef]
- Sumanasekera, G.U.; Allen, J.L.; Fang, S.L.; Loper, A.L.; Rao, A.M.; Eklund, P.C. Electrochemical Oxidation of Single Wall Carbon Nanotube Bundles in Sulfuric Acid. J. Phys. Chem. B 1999, 103, 4292. [Google Scholar] [CrossRef]
- Kukovecz, A.; Pichler, T.; Pfeiffera, R.; Kuzmany, H. Diameter selective charge transfer in p- and n-doped single wall carbon nanotubes synthesized by the HiPCO method. Chem. Commun. 2002, 1730. [Google Scholar] [CrossRef]
- Bendiab, N.; Anglaret, E.; Bantignies, J.-L.; Zahab, A.; Sauvajol, J.L.; Petit, P.; Mathis, C.; Lefrant, S. Stoichiometry dependence of the Raman spectrum of alkali-doped single-wall carbon nanotubes. Phys. Rev. B 2001, 64, 245424. [Google Scholar] [CrossRef]
- Iwasa, Y.; Fudo, H.; Yatsu, Y.; Mitani, T.; Kataura, H.; Achiba, Y. Phase stability of doped carbon nanotubes. Synthet. Met. 2001, 121, 1203. [Google Scholar] [CrossRef]
- Rao, A.; Bandow, S.; Richter, E.; Eklund, P. Raman spectroscopy of pristine and doped single wall carbon nanotubes. Thin Solid Films 1998, 331, 141. [Google Scholar] [CrossRef]
- Kazaoui, S.; Minami, N.; Jacquemin, R.; Kataura, H.; Achiba, Y. Amphoteric doping of single-wall carbon-nanotube thin films as probed by optical absorption spectroscopy. Phys. Rev. B 1999, 60, 13339. [Google Scholar] [CrossRef]
- Jouguelet, E.; Mathis, C.; Petit, P. Controlling the electronic properties of single-wall carbon nanotubes by chemical doping. Chem. Phys. Lett. 2000, 318, 561. [Google Scholar] [CrossRef]
- Petit, P.; Mathis, C.; Journet, C.; Bernier, P. Tuning and monitoring the electronic structure of carbon nanotubes. Chem. Phys. Lett. 1999, 305, 370. [Google Scholar] [CrossRef]
- Yuan, J.; Ji, G.; Chen, X.; Wei, D.; Zhao, F.; Wu, Q. Phase transition, thermodynamics properties and IR spectrum of α- and γ-RDX: First principles and MD studies. Chem. Phys. Lett. 2016, 644, 250–254. [Google Scholar] [CrossRef]
- Jacquemin, R.; Kazaoui, S.; Yu, D.; Hassanien, A.; Minami, N.; Kataura, H.; Achiba, Y. Doping mechanism in single-wall carbon nanotubes studied by optical absorption. Synthet. Met. 2000, 115, 283. [Google Scholar] [CrossRef]
- Minami, N.; Kazaoui, S.; Jacquemin, R.; Yamawaki, H.; Aoki, K.; Kataura, H.; Achiba, Y. Optical properties of semiconducting and metallic single wall carbon nanotubes: Effects of doping and high pressure. Synthet. Met. 2001, 116, 405–409. [Google Scholar] [CrossRef]
- Bower, C.; Suzuki, S.; Tanigaki, K.; Zhou, O. Synthesis and structure of pristine and alkali-metal-intercalated single-walled carbon nanotubes. Appl. Phys. A 1998, 67, 47. [Google Scholar] [CrossRef]
- Liu, X.; Pichler, T.; Knupfer, M.; Fink, J. Electronic and optical properties of alkali-metal-intercalated single-wall carbon nanotubes. Phys. Rev. B 2003, 67, 125403. [Google Scholar] [CrossRef]
- Yanagi, K.; Iakoubovskii, K.; Matsui, H.; Okamoto, H.; Miyata, Y.; Maniwa, Y.; Kazaoui, S.; Minami, N.; Kataura, H. Photosensitive Function of Encapsulated Dye in Carbon Nanotubes. Am. Chem. Soc. 2007, 129, 4992–4997. [Google Scholar] [CrossRef]
- Pichler, T.; Sing, M.; Knupfer, M.; Golden, M.; Fink, J. Potassium intercalated bundles of single-wall carbon nanotubes: Electronic structure and optical properties. Solid State Commun. 1999, 109, 721. [Google Scholar] [CrossRef]
- Suzuki, S.; Bower, C.; Zhou, O. In-situ TEM and EELS studies of alkali–metal intercalation with single-walled carbon nanotubes. Chem. Phys. Lett. 1998, 285, 230. [Google Scholar] [CrossRef]
- Liu, X.; Pichler, T.; Knupfer, M.; Fink, J.; Kataura, H. Electronic properties of FeCl3-intercalated single-wall carbon nanotubes. Phys. Rev. B 2004, 70, 205405. [Google Scholar] [CrossRef]
- Ruzicka, B.; Degiorgi, L.; Gaal, R.; Thien-Nga, L.; Bacsa, R.; Salvetat, J.-P.; Forró, L. Optical and dc conductivity study of potassium-doped single-walled carbon nanotube films. Phys. Rev. B 2000, 61, R2468. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Phemenology of Filling, Investigation of Growth Kinetics and Electronic Properties for Applications of Filled Single-Walled Carbon Nanotubes. Nanomaterials 2023, 13, 314. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Kinetics, Electronic Properties of Filled Carbon Nanotubes Investigated with Spec-troscopy for Applications. Nanomaterials 2023, 13, 176. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Gurova, O.A.; Makarova, A.A.; Fedorenko, A.D.; Nikolenko, A.D.; Plyusnin, P.E.; Arenal, R.; Bulusheva, L.G.; Okotrub, A.V. Light-Induced Sulfur Transport inside Single-Walled Carbon Nanotubes. Nanomaterials 2020, 10, 818. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Analyt. Bioanalyt. Chem. 2006, 385, 452. [Google Scholar] [CrossRef]
- Gong, K.; Yan, Y.; Zhang, M.; Su, L.; Xiong, S.; Mao, L. Electrochemistry and electroanalytical applications of carbon nanotubes: A review. Anal. Sci. 2005, 21, 1383. [Google Scholar] [CrossRef][Green Version]
- Gooding, J.J. Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochim. Acta 2005, 50, 3049. [Google Scholar] [CrossRef]
- Sherigara, B.S.; Kutner, W.; D’Souza, F. Electrocatalytic Properties and Sensor Applications of Fullerenes and Carbon Nanotubes. Electroanalysis 2003, 15, 753. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Leventis, H.C.; Compton, R.G. Chemically Modified Carbon Nanotubes for Use in Electroanalysis. Microchim. Acta 2006, 152, 187. [Google Scholar] [CrossRef]
- Niessen, R.A.H.; de Jonge, J.; Notten, P.H.L.J. The Electrochemistry of Carbon Nanotubes: I. Aqueous Electrolyte. Electrochem. Soc. 2006, 153, A1484. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Dhathathreyan, K.; Govindaraj, A.; Satishkumar, B. Electrochemical investigation of single-walled carbon nanotubes for hydrogen storage. Electrochim. Acta 2000, 45, 4511. [Google Scholar] [CrossRef]
- Claye, A.S.; Fischer, J.E.; Huffman, C.B.; Rinzler, A.G.; Smalley, R.E. Solid-State Electrochemistry of the Li Single Wall Carbon Nanotube System. J. Electrochem. Soc. 2000, 147, 2845. [Google Scholar] [CrossRef]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421. [Google Scholar] [CrossRef]
- Wu, G.T.; Wang, C.S.; Zhang, X.B.; Yang, H.S.; Qi, Z.F.; He, P.M.; Li, W.Z. Structure and Lithium Insertion Properties of Carbon Nanotubes. J. Electrochem. Soc. 1999, 146, 1696. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Coroneus, J.G.; Khalap, V.R.; Kane, A.A.; Weiss, G.A.; Collins, P.G. Conductance-controlled point functionalization of single-walled carbon nanotubes. Science 2007, 315, 77. [Google Scholar] [CrossRef]
- Larrimore, L.; Nad, S.; Zhou, X.; Abruna, H.; McEuen, P.L. Probing Electrostatic Potentials in Solution with Carbon Nanotube Transistor. Nano Lett. 2006, 6, 1329. [Google Scholar] [CrossRef]
- Rosenblatt, S.; Yaish, Y.; Park, J.; Gore, J.; Sazonova, V.; McEuen, P.L. High Performance Electrolyte Gated Carbon Nanotube Transistors. Nano Lett. 2002, 2, 869. [Google Scholar] [CrossRef]
- Leng, C.; Fedoseeva, Y.V.; Zhao, Z.; Yan, B.; Okotrub, A.V.; Wang, X.; Fan, J.; Qiu, J. Rational-design heteroatom-doped cathode and ion modulation layer modified Zn anode for ultrafast zinc-ion hybrid capacitors with simultaneous high power and energy densities. J. Power Sources 2022, 536, 231484. [Google Scholar] [CrossRef]
- Faulques, E.; Kalashnyk, N.; Slade, C.A.; Sanchez, A.M.; Sloan, J.; Ivanov, V.G. Vibrational and electronic structures of tin selenide nanowires confined inside carbon nanotubes. Synth. Met. 2022, 284, 116968. [Google Scholar] [CrossRef]
- Zorn, N.F.; Zaumseil, J. Charge transport in semiconducting carbon nanotube networks. Appl. Phys. Rev. 2021, 8, 041318. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Metal and Metal Halogenide-Filled Single-Walled Carbon Nanotubes: Kinetics, Electronic Properties, Engineering the Fermi Level. Nanomaterials 2023, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Vorfolomeeva, A.A.; Pushkarevsky, N.A.; Koroteev, V.O.; Surovtsev, N.V.; Chuvilin, A.L.; Shlyakhova, E.V.; Plyusnin, P.E.; Makarova, A.A.; Okotrub, A.V.; Bulusheva, L.G. Doping of Carbon Nanotubes with Encapsulated Phosphorus Chains. Inorg. Chem. 2022, 61, 9605–9614. [Google Scholar] [CrossRef] [PubMed]
- González, M.C.R.; Leonhardt, A.; Stadler, H.; Eyley, S.; Thielemans, W.; De Gendt, S.; Mali, K.S.; De Feyter, S. Multicomponent Covalent Chemical Patterning of Graphene. ACS Nano 2021, 15, 13389–13398. [Google Scholar]
- Liang, Y.; Li, M.; Yang, Y.; Qiao, L.; Xu, H.; Guo, B. pH/Glucose Dual Responsive Metformin Release Hydrogel Dressings with Adhesion and Self-Healing via Dual-Dynamic Bonding for Athletic Diabetic Foot Wound Healing. ACS Nano 2022, 16, 6002–6012. [Google Scholar] [CrossRef]
- Hu, Z.; Breeze, B.; Kashtiban, R.J.; Sloan, J.; Lloyd-Hughes, J. Zigzag HgTe Nanowires Modify the Electron–Phonon Interaction in Chirality-Refined Single-Walled Carbon Nanotubes. ACS Nano 2022, 16, 6789–6800. [Google Scholar] [CrossRef]
- Kashtiban, R.J.; Patrick, C.E.; Ramasse, Q.; Walton, R.I.; Sloan, J. Picoperovskites: The Smallest Conceivable Isolated Halide Perovskite Structures Formed within Carbon Nanotubes. Adv. Mater. 2023. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Shlyakhova, E.V.; Stolyarova, S.G.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Makarova, A.A.; Shubin, Y.V.; Okotrub, A.V.; Bulusheva, L.G. Brominated Porous Nitrogen-Doped Carbon Materials for Sodium-Ion Storage. Batteries 2022, 8, 114. [Google Scholar] [CrossRef]
- Burdanova, M.G.; Kharlamova, M.V.; Kramberger, C.; Nikitin, M.P. Applications of Pristine and Functionalized Carbon Nanotubes, Graphene, and Graphene Nanoribbons in Biomedicine. Nanomaterials 2021, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, H.; Teng, Y.; Xiong, Y.; Chen, Z.; You, L.; Xiao, D. Recent advances in magnetic carbon nanotubes: Synthesis, challenges and highlighted applications. J. Mater. Chem. B 2021, 9, 9076. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, V.I.; Yamaletdinov, R.D.; Plyusnin, P.E.; Okotrub, A.V.; Bulusheva, L.G. Adsorption kinetics of NO2 gas on oxyfluorinated graphene film. Phys. Chem. Chem. Phys. 2023, 25, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Navrotskaya, A.G.; Aleksandrova, D.D.; Krivoshapkina, E.F.; Sillanpää, M.; Krivoshapkin, P.V. Hybrid Materials Based on Carbon Nanotubes and Nanofibers for Environmental Applications. Front. Chem. 2020, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Chen, H.; Yuan, R.; Zhang, X.; Chen, D. Co-Ni Basic Carbonate Nanowire/Carbon Nanotube Network with High Electrochemical Capacitive Performance via Electrochemical Conversion. Front. Chem. 2021, 9, 655025. [Google Scholar] [CrossRef] [PubMed]
- Queirós-Reis, L.; Gomes da Silva, P.; Gonçalves, J.; Brancale, A.; Bassetto, M.; Mesquita, J.R. SARS-CoV-2 Virus−Host Interaction: Currently Available Structures and Implications of Variant Emergence on Infectivity and Immune Response. Int. J. Mol. Sci. 2021, 22, 10836. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Arkhipov, V.E.; Popov, K.M.; Sysoev, V.I.; Makarova, A.A.; Okotrub, A.V. Electronic Structure of Nitrogen- and Phosphorus-Doped Graphenes Grown by Chemical Vapor Deposition Method. Materials 2020, 13, 1173. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Bulavskiy, M.O.; Pinakov, D.V.; Chekhova, G.N.; Asanov, I.P.; Gevko, P.N.; Bulusheva, L.G.; Okotrub, A.V. Chemiresistive Properties of Imprinted Fluorinated Graphene Films. Materials 2020, 13, 3538. [Google Scholar] [CrossRef]
- Gorodetskiy, D.V.; Gusel’Nikov, A.V.; Kurenya, A.G.; Smirnov, D.A.; Bulusheva, L.G.; Okotrub, A.V. Hydrogen Plasma Treatment of Aligned Multi-Walled Carbon Nanotube Arrays for Improvement of Field Emission Properties. Materials 2020, 13, 4420. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Gorodetskiy, D.V.; Gusel’Nikov, A.V.; Kondranova, A.M.; Bulusheva, L.G.; Korabovska, M.; Meija, R.; Erts, D. Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition. Materials 2022, 15, 6639. [Google Scholar] [CrossRef] [PubMed]
- Thauer, E.; Ottmann, A.; Schneider, P.; Möller, L.; Deeg, L.; Zeus, R.; Wilhelmi, F.; Schlestein, L.; Neef, C.; Ghunaim, R.; et al. Filled Carbon Nanotubes as Anode Materials for Lithium-Ion Batteries. Molecules 2020, 25, 1064. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.V.; Sysoev, V.I.; Gurova, O.A.; Ivanov, Y.P.; Koroteev, V.O.; Arenal, R.; Makarova, A.A.; Bulusheva, L.G.; Okotrub, A.V. Role of interface interactions in the sensitivity of sulfur-modified single-walled carbon nanotubes for nitrogen dioxide gas sensing. Carbon 2022, 186, 539. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Lobiak, E.V.; Shlyakhova, E.V.; Kovalenko, K.A.; Kuznetsova, V.R.; Vorfolomeeva, A.A.; Grebenkina, M.A.; Nishchakova, A.D.; Makarova, A.A.; Bulusheva, L.G.; et al. Hydrothermal Activation of Porous Nitrogen-Doped Carbon Materials for Electrochemical Capacitors and Sodium-Ion Batteries. Nanomaterials 2020, 10, 2163. [Google Scholar] [CrossRef] [PubMed]
- Gurova, O.; Sysoev, V.; Lobiak, E.; Makarova, A.; Asanov, I.; Okotrub, A.; Kulik, L.; Bulusheva, L. Enhancement of Volumetric Capacitance of Binder-Free Single-Walled Carbon Nanotube Film via Fluorination. Nanomaterials 2021, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Vorfolomeeva, A.A.; Stolyarova, S.G.; Asanov, I.P.; Shlyakhova, E.V.; Plyusnin, P.E.; Maksimovskiy, E.A.; Gerasimov, E.Y.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Single-Walled Carbon Nanotubes with Red Phosphorus in Lithium-Ion Batteries: Effect of Surface and Encapsulated Phosphorus. Nanomaterials 2022, 13, 153. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Chernov, A.I.; Lavrov, A.N.; Gurova, O.A.; Shubin, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Zvezdin, A.K.; Lähderanta, E.; Bulusheva, L.G.; et al. Magnetic Properties of 1D Iron–Sulfur Compounds Formed Inside Single-Walled Carbon Nanotubes. Phys. Status Solidi RRL 2020, 14, 2000291. [Google Scholar] [CrossRef]
- Tang, L.; Xiao, Q.; Mei, Y.; He, S.; Zhang, Z.; Wang, R.; Wang, W. Insights on functionalized carbon nanotubes for cancer theranostics. J. Nanobiotechnol. 2021, 19, 1–28. [Google Scholar] [CrossRef]
- Paukov, M.; Kramberger, C.; Begichev, I.; Kharlamova, M.; Burdanova, M. Functionalized Fullerenes and Their Applications in Electrochemistry, Solar Cells, and Nanoelectronics. Materials 2023, 16, 1276. [Google Scholar] [CrossRef]
- Singh, N.; Aul, G.D. Fabrication of cobalt filled multi-walled carbon nanotubes/polyurethane composite for microwave absorption. SN Appl. Sci. 2020, 2, 423. [Google Scholar] [CrossRef]
- Shojaeifar, M.; Askari, M.B.; Hashemi, S.R.S.; Di Bartolomeo, A. MnO2–NiO–MWCNTs nanocomposite as a catalyst for methanol and ethanol electrooxidation. J. Phys. D Appl. Phys. 2022, 55, 355502. [Google Scholar] [CrossRef]
- Kharlamova, M.; Kramberger, C. Applications of Filled Single-Walled Carbon Nanotubes: Progress, Challenges, and Perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Eliseev, A.A.; Yashina, L.V.; Lukashin, A.V.; Tretyakov, Y.D. Structure and electronic properties of nanocomposites on basis of intercalated single-walled carbon nanotubes. In Proceedings of the 4th International Forum on Nanotechnologies “Rusnanotech’11”, Moscow, Russia, 26–28 October 2011. [Google Scholar]
- Kharlamova, M.V.; Eliseev, A.A. The formation of one-dimensional structures on the basis of intercalated single-walled carbon nanotubes. In Proceedings of the 2nd International Forum on Nanotechnologies “Rusnanotech’09”, Moscow, Russia, 6–8 October 2009; pp. 532–533. (In Russian). [Google Scholar]
- Kharlamova, M.V.; Eliseev, A.A.; Yashina, L.V.; Lukashin, A.V.; Tretyakov, Y.D. The investigation of properties of the single-walled carbon nanotubes filled with inorganic compounds. In Proceedings of the XVIII International Conference for Undergraduate, Postgraduate Students and Young Scientists “Lomonosov” (Section “Fundamental Materials Science”), Lomonosov Moscow State University, Moscow, Russia, 11–15 April 2011. [Google Scholar]
- Kharlamova, M.V.; Eliseev, A.A.; Verbitskii, N.I.; Yashina, L.V.; Kiselev, N.A.; Lukashin, A.V.; Tretyakov, Y.D. Structure and electronic properties of single-walled carbon nanotubes filled with one-dimensional nanocrystals of inorganic chemical compounds. In Proceedings of the E-MRS 2011 Spring Meeting, Nice, France, 8–13 May 2011. [Google Scholar]
- Kharlamova, M.V.; Yashina, L.V.; Lukashin, A.V. The filling of single-walled carbon nanotube channels is a method of directional modification of their electronic properties. In Proceedings of the 11th International Conference “Advanced Carbon NanoStructures” (ACNS’2013), St. Petersburg, Russia, 1–5 July 2013. [Google Scholar]
- Kharlamova, M.; Eliseev, A.A.; Yashina, L.V.; Lukashin, A.V.; Tretyakov, Y.D. Modification of electronic properties of single-walled carbon nanotubes by filling with transition metal halogenides. In Proceedings of the 19th International Symposium on Metastable, Amorphous and Nanostructured Materials, Moscow, Russia, 18–22 June 2012. [Google Scholar]
- Kharlamova, M.V.; Eliseev, A.A. Formation of one-dimensional structures on the basis of intercalated single-walled carbon nanotubes. In Proceedings of the XIX Mendeleev Conference for Young Scientists, St. Petersburg, Russia, 29 June–3 July 2009. [Google Scholar]
- Kharlamova, M.V.; Eliseev, A.A.; Yashina, L.V.; Lukashin, A.V.; Tretyakov, Y.D. Directional modification of electronic structure of single-walled carbon nanotubes by filling their channels with substances of different chemical nature. In Proceedings of the XIX International Conference for Undergraduate, Postgraduate Students and Young Scientists “Lomonosov” (Section “Fundamental Materials Science”), Lomonosov Moscow State University, Moscow, Russia, 9–13 April 2012. [Google Scholar]
- Kharlamova, M.; Eliseev, A.A.; Yashina, L.V.; Lukashin, A.V.; Tretyakov, Y.D. Synthesis of nanocomposites on basis of single-walled carbon nanotubes. In Proceedings of the International Scientific and Technical Conference “The Nanotechnologies of Functional Materials (NFM’10)”, St. Petersburg, Russia, 22–24 September 2010. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Mittelberger, A.; Rudatis, P.; Yanagi, K.; Pichler, T.; Eder, D. Modification of the electronic properties of single-walled carbon nanotubes by encapsulation of electron acceptor and donor substances. In Proceedings of the 33th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 9–16 March 2019. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Domanov, O.; Mittelberger, A.; Yanagi, K.; Saito, T.; Pichler, T.; Eder, D. Electronic properties of single-walled carbon nanotubes filled with silver chloride. In Proceedings of the 32th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 17–24 March 2018. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Shiozawa, H.; Pichler, T. Revealing the growth mechanism of inner tubes inside metallocene-filled single-walled carbon nanotubes. In Proceedings of the 31th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 4–11 March 2017. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Saito, T.; Shiozawa, H.; Pichler, T. Revealing the growth mechanism of inner tubes inside metallocene-filled single-walled carbon nanotubes. In Proceedings of the 17th International Conference on the Science and Application of Nanotubes and Low-Dimensional Materials, Vienna, Austria, 7–13 August 2016. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Sauer, M.; Yanagi, K.; Saito, T.; Shiozawa, H.; Pichler, T. Inner tube growth properties and electronic structure of metallocene-filled single-walled carbon nanotubes. In Proceedings of the 30th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 13–20 February 2016. [Google Scholar]
- Kharlamova, M.V.; Kramberger, C.; Shiozawa, H.; Yanagi, K.; Saito, T.; Pichler, T. Synthesis and growth properties of inner tubes inside metallocene-filled single-walled carbon nanotubes. In Proceedings of the 29th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 7–14 March 2015. [Google Scholar]
- Kharlamova, M.; Sauer, M.; Saito, T.; Pichler, T.; Shiozawa, H. Metallocene-filled single-walled carbon nanotubes: Synthesis, inner tube growth properties and electronic structure. In Proceedings of the 28th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 8–15 March 2014. [Google Scholar]
- Kharlamova, M.V.; Sauer, M.; Saito, T.; Pichler, T.; Shiozawa, H. Synthesis and transformation of SWCNT nanohybrids filled with different transition metal metallocenes. In Proceedings of the 27th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 2–9 March 2013. [Google Scholar]
- Kharlamova, M.V.; Shiozawa, H.; Sauer, M.; Pichler, T. Tailoring the functionality of single-walled carbon nanotubes by metallocene filling. In Proceedings of the 5th Szeged International Workshop on Advances in Nanoscience (SIWAN), Szeged, Hungary, 24–27 October 2012. [Google Scholar]
- Kharlamova, M.V.; Eliseev, A.A.; Yashina, L.V.; Lukashin, A.V.; Tretyakov, Y.D. Electronic properties of single-walled carbon nanotubes intercalated by one-dimensional nanocrystals of inorganic chemical compounds. In Proceedings of the 26th International Winterschool on Electronic Properties of Novel Materials: “Molecular Nanostructures”, Kirchberg, Austria, 4–11 March 2012. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).