Enhancing Anticorrosion Resistance of Aluminum Alloys Using Femtosecond Laser-Based Surface Structuring and Coating
Abstract
:1. Introduction
2. Methodology
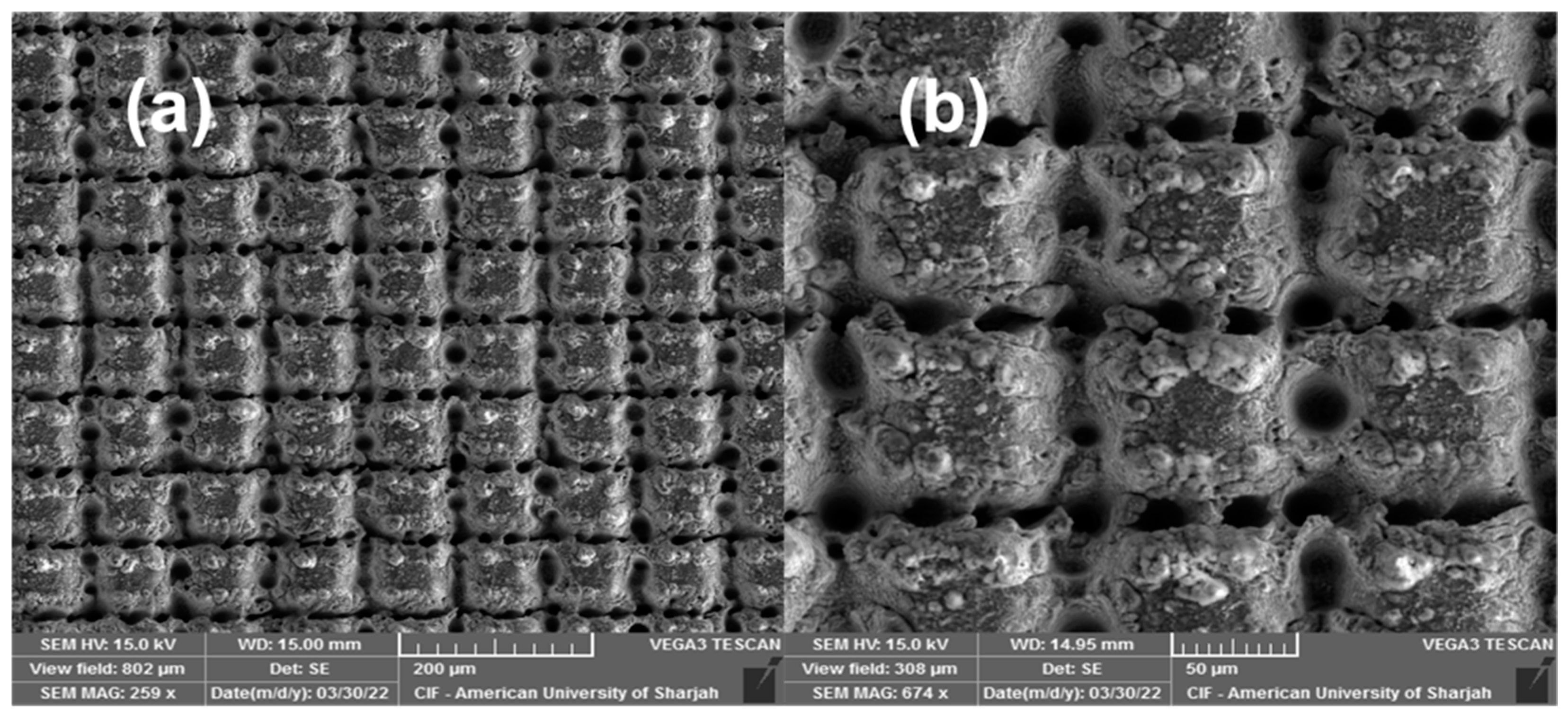
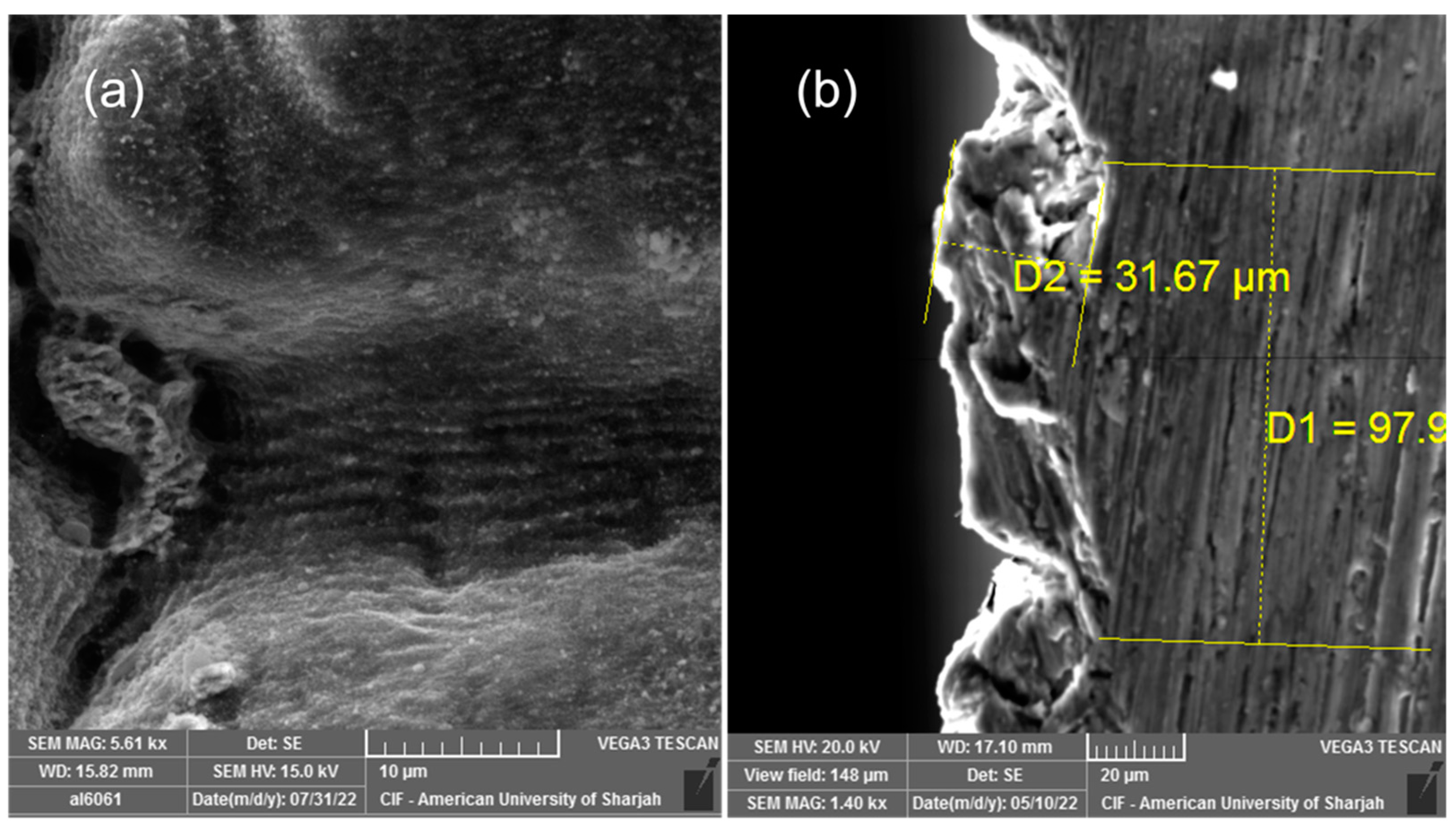
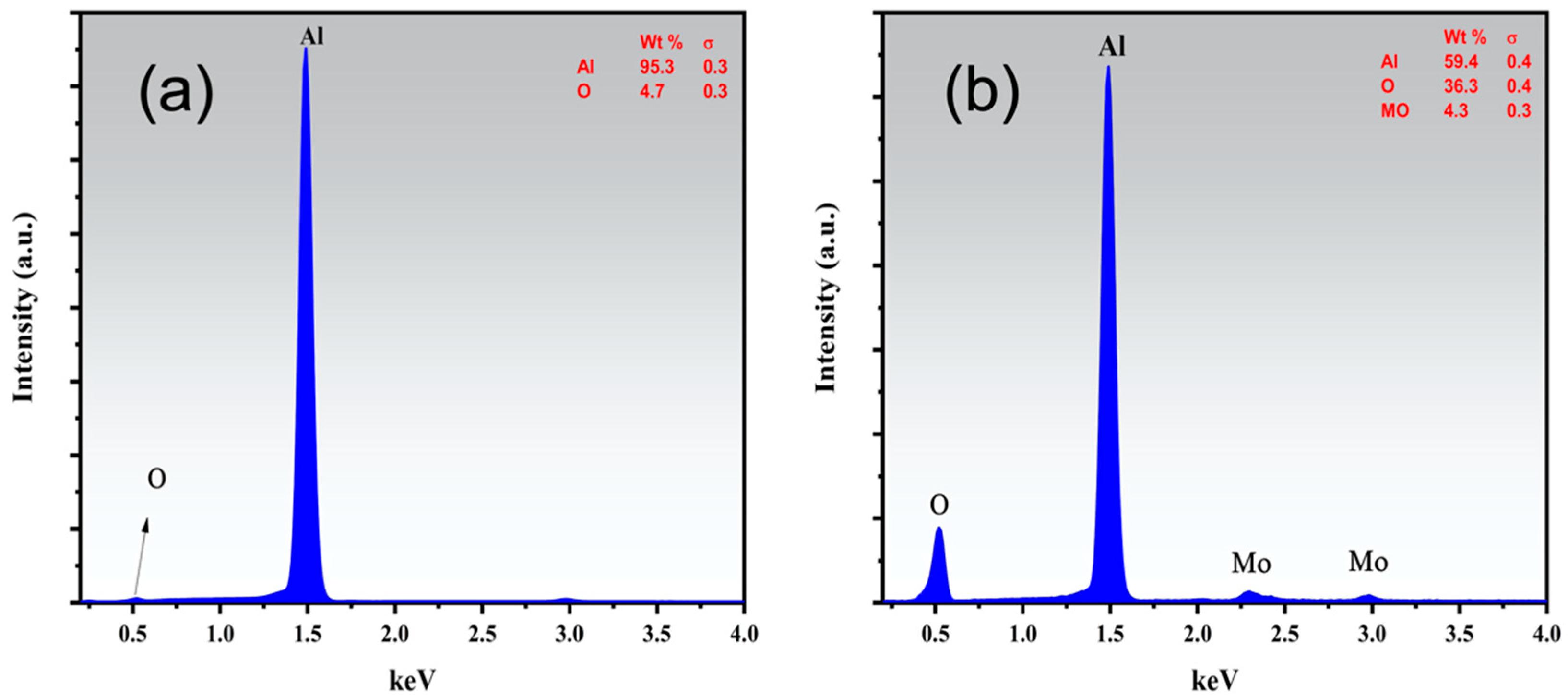
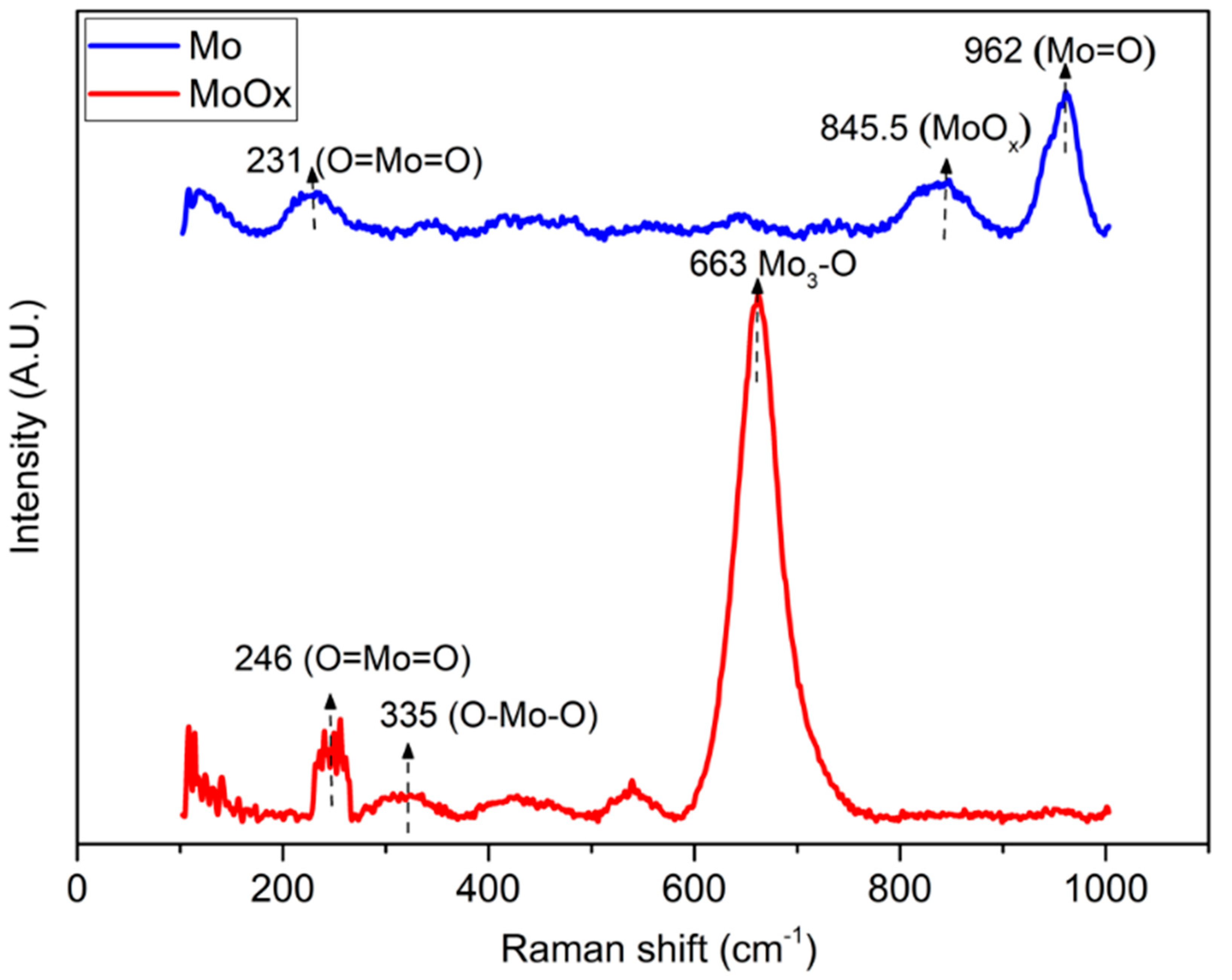
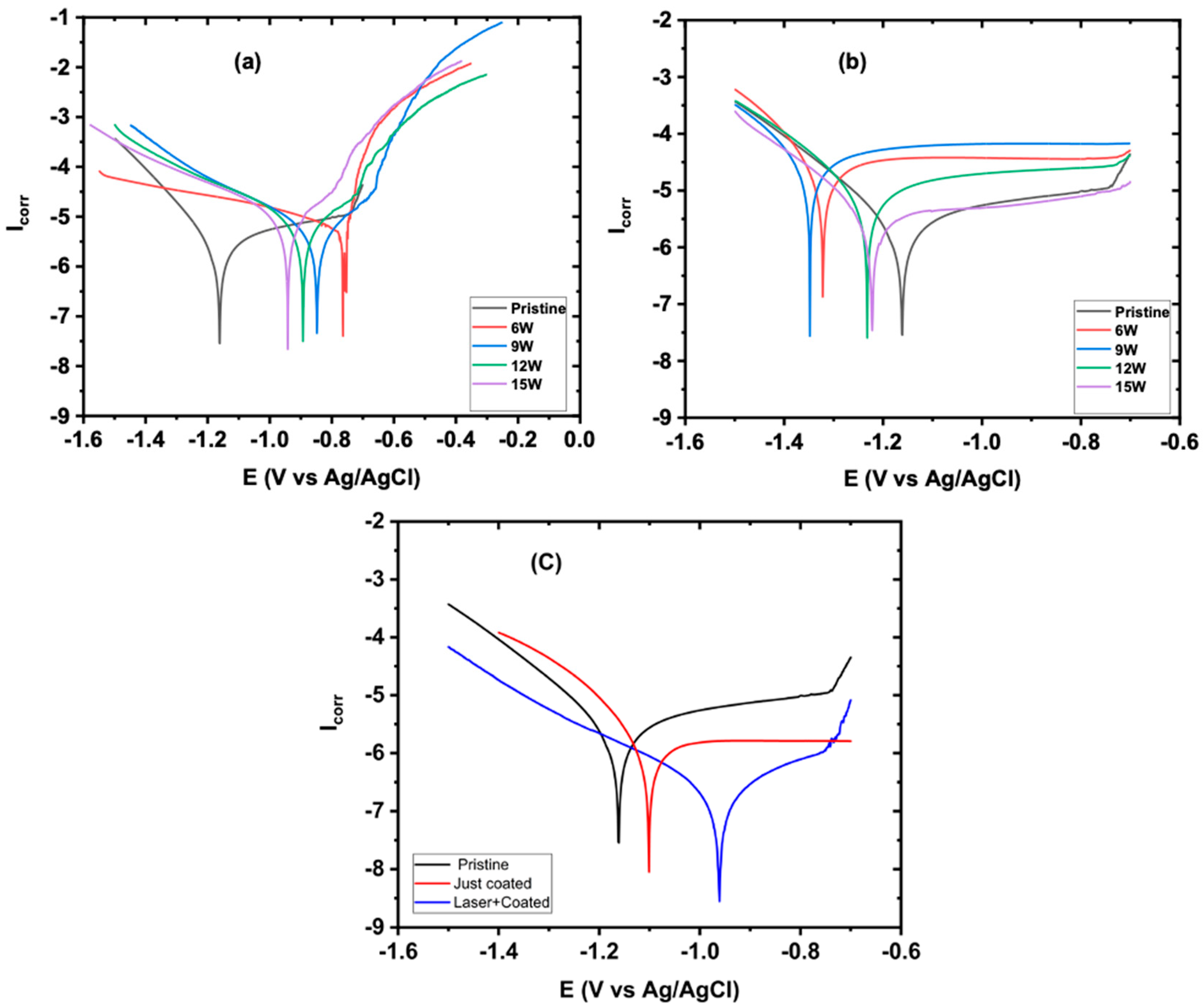
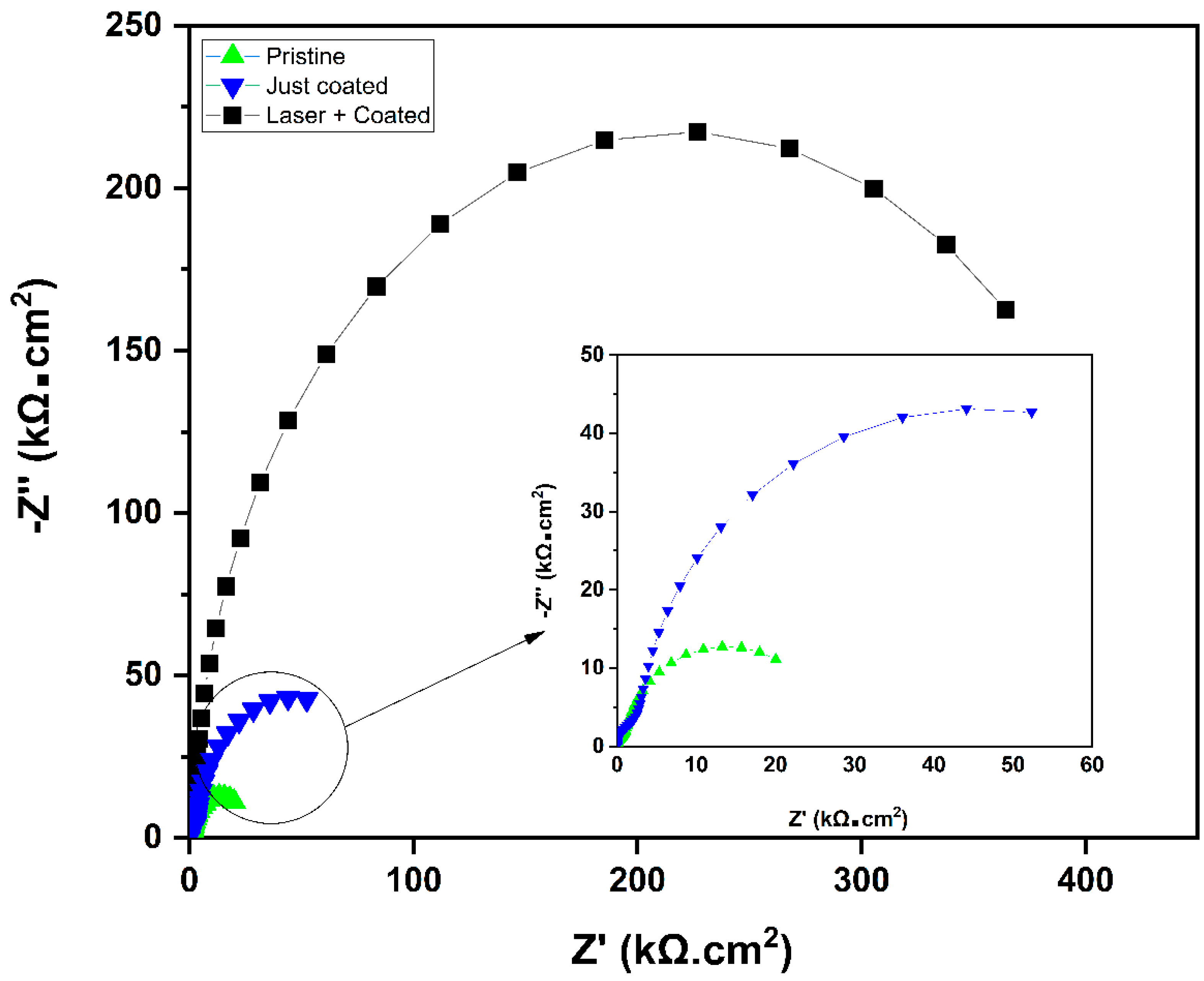
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rosliza, R. Improvement of Corrosion Resistance of Aluminium Alloy by Natural Products; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, W.; Abu Baker, A.; Egilmez, M.; Abuzaid, W.; Orhan, M.F.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. Enhancement of the corrosion resistance of mild steel with femtosecond laser- nanostructuring and CrCoNi medium entropy alloy coating. Appl. Surf. Sci. Adv. 2022, 12, 100321. [Google Scholar] [CrossRef]
- Xin, G.; Wu, C.; Liu, W.; Rong, Y.; Huang, Y. Anti-corrosion superhydrophobic surfaces of Al alloy based on micro-protrusion array structure fabricated by laser direct writing. J. Alloys Compd. 2021, 881, 160649. [Google Scholar] [CrossRef]
- Esquivel, J.; Gupta, R.K. Review—Corrosion-Resistant Metastable Al Alloys: An Overview of Corrosion Mechanisms. J. Electrochem. Soc. 2020, 167, 081504. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Liang, N.; Yang, F.; Jiang, J.; Sun, J.; Wu, G.; Ma, A.; Ma, X. Simultaneously improving corrosion resistance and mechanical properties of a magnesium alloy via equal-channel angular pressing and post water annealing. Mater. Des. 2019, 166, 107621. [Google Scholar] [CrossRef]
- Sukiman, N.L.; Zhou, X.; Birbilis, N.; Hughes, A.E.; Mol, J.M.C.; Garcia, S.J.; Zhou, X.; Thompson, G.E. Durability and Corrosion of Aluminium and Its Alloys: Overview, Property Space, Techniques and Developments. Alum. Alloy New Trends Fabr. Appl. 2012, 5, 47–97. [Google Scholar] [CrossRef]
- Godard, H.P.; Jepson, W.B.; Bothwell, M.R.; Kane, R.L. The Corrosion of Light Metals; John Wiley and Sons Inc.: New York, NY, USA, 1967; p. 7073. [Google Scholar]
- Guillaumin, V.; Mankowski, G. Localized corrosion of 2024 T351 aluminium alloy in chloride media. Corros. Sci. 1998, 41, 421–438. [Google Scholar] [CrossRef]
- Blanc, C.; Gastaud, S.; Mankowski, G. Mechanistic Studies of the Corrosion of 2024 Aluminum Alloy in Nitrate Solutions. J. Electrochem. Soc. 2003, 150, B396–B404. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2020, 11, 4. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Davis, J. Corrosion of Aluminum and Aluminum Alloys; ASM International: Almere, The Netherlands, 1999. [Google Scholar]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Polmear, I. Light Alloys: From Traditional Alloys to Nanocrystals; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Reboul, M.C.; Warner, T.J.; Mayet, H.; Baroux, B. A ten step mechanism for the pitting corrosion of aluminium alloys. Corros. Rev. 1997, 15, 471–496. [Google Scholar] [CrossRef]
- Vashi, R. Corrosion study of metals in marine environment. HindawiCom 2009, 6, 1240–1246. [Google Scholar] [CrossRef]
- Reboul, M.; Warner, T. A ten-step mechanism for the pitting corrosion of aluminium. In Materials Science Forum; Trans Tech Publication: Zurich, Switzerland, 1996. [Google Scholar]
- Guan, F.; Zhai, X.; Duan, J.; Zhang, J.; Li, K.; Hou, B. Influence of sulfate-reducing bacteria on the corrosion behavior of 5052 aluminum alloy. Surf. Coat. Technol. 2017, 316, 171–179. [Google Scholar] [CrossRef]
- Yao, X.; Wen, L.; Yu, Z.; Guo, W.; Huang, F.; Qiang, Y.; Jin, Y. Study on corrosion behavior and mechanism of 5A06 aluminum alloy in N2O4 medium. J. Alloys Compd. 2023, 931, 167544. [Google Scholar] [CrossRef]
- Zhang, W.; Frankel, G. Transitions between pitting and intergranular corrosion in AA2024. Electrochim. Acta 2003, 48, 1193–1210. [Google Scholar] [CrossRef]
- Rajan, R.A.; Konda, S.R.; Saraj, C.S.; Lai, Y.H.; Verma, G.; Yu, Z.; Yu, W.; Yan, D.; Yang, J. Long-term seawater anti-corrosion properties of Al alloy triggered by femtosecond laser structuring with phase change. Appl. Surf. Sci. 2021, 573, 151612. [Google Scholar] [CrossRef]
- Moukwa, M. Penetration of chloride ions from sea water into mortars under different exposure conditions. Cem. Concr. Res. 1989, 19, 894–904. [Google Scholar] [CrossRef]
- Du, Q.; Poole, W.; Wells, M.; Parson, N. Microstructure evolution during homogenization of Al–Mn–Fe–Si alloys: Modeling and experimental results. Acta Mater. 2013, 61, 4961–4973. [Google Scholar] [CrossRef]
- Chen, X.; Gong, Y.; Suo, X.; Huang, J.; Liu, Y.; Li, H. Construction of mechanically durable superhydrophobic surfaces by thermal spray deposition and further surface modification. Appl. Surf. Sci. 2015, 356, 639–644. [Google Scholar] [CrossRef]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic Fabrics Produced by Electrospinning and Chemical Vapor Deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Xu, W.; Liu, H.; Lu, S.; Xi, J.; Wang, Y. Fabrication of Superhydrophobic Surfaces with Hierarchical Structure through a Solution-Immersion Process on Copper and Galvanized Iron Substrates. Langmuir 2008, 24, 10895–10900. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.; Cannelli, O.; Boccia, A.; Zanoni, R.; Raimondo, M.; Caldarelli, A.; Veronesi, F. A mechanistic explanation of the peculiar am-phiphobic properties of hybrid organic-inorganic coatings by combining XPS characterization and DFT modeling. ACS Appl. Mater. Interfaces 2015, 7, 19941–19947. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Egilmez, M.; Iqbal, M.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. Pulsed Laser Deposited Zeolite Coatings on Femtosecond Laser-Nanostructured Steel Meshes for Durable Superhydrophilic/Oleophobic Functionalities. Front. Chem. 2021, 9, 1027. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Piatkowski, P.; Nawaz, T.; Ahmad, S.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. A Two-Step Femtosecond Laser-Based Deposition of Robust Corrosion-Resistant Molybdenum Oxide Coating. Materials 2023, 16, 909. [Google Scholar] [CrossRef]
- Ahmad, S.; Egilmez, M.; Kannan, A.M.; Alnaser, A. Oxygen evolution reaction enhancement of copper electrodes in alkaline medium using ultrafast femtosecond laser structuring. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Jagdheesh, R. Fabrication of a Superhydrophobic Al2O3 Surface Using Picosecond Laser Pulses. Langmuir 2014, 30, 12067–12073. [Google Scholar] [CrossRef]
- Ma, Q.; Tong, Z.; Wang, W.; Dong, G. Fabricating robust and repairable superhydrophobic surface on carbon steel by nanosecond laser texturing for corrosion protection. Appl. Surf. Sci. 2018, 455, 748–757. [Google Scholar] [CrossRef]
- Park, J.; Han, H.-S.; Park, J.; Seo, H.; Edwards, J.; Kim, Y.-C.; Ok, M.-R.; Seok, H.-K.; Jeon, H. Corrosion behavior of biodegradable Mg-based alloys via femtosecond laser surface melting. Appl. Surf. Sci. 2018, 448, 424–434. [Google Scholar] [CrossRef]
- Trdan, U.; Sano, T.; Klobčar, D.; Sano, Y.; Grum, J.; Šturm, R. Improvement of corrosion resistance of AA2024-T3 using femtosecond laser peening without protective and confining medium. Corros. Sci. 2018, 143, 46–55. [Google Scholar] [CrossRef]
- Wang, H.; Jürgensen, J.; Decker, P.; Hu, Z.; Yan, K.; Gurevich, E.; Ostendorf, A. Corrosion behavior of NiTi alloy subjected to femtosecond laser shock peening without protective coating in air environment. Appl. Surf. Sci. 2019, 501, 144338. [Google Scholar] [CrossRef]
- Sciancalepore, C.; Gemini, L.; Romoli, L.; Bondioli, F. Study of the wettability behavior of stainless steel surfaces after ultrafast laser texturing. Surf. Coat. Technol. 2018, 352, 370–377. [Google Scholar] [CrossRef]
- Pou-Álvarez, P.; Riveiro, A.; Nóvoa, X.; Fernández-Arias, M.; del Val, J.; Comesaña, R.; Boutinguiza, M.; Lusquiños, F.; Pou, J. Nanosecond, picosecond and femtosecond laser surface treatment of magnesium alloy: Role of pulse length. Surf. Coat. Technol. 2021, 427, 127802. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, Y.; Ngo, C.-V.; Guo, C. Rapid fabrication of anti-corrosion and self-healing superhydrophobic aluminum surfaces through environmentally friendly femtosecond laser processing. Opt. Express 2020, 28, 35636–35650. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tian, J. Superhydrophobicity: Is it really better than hydrophobicity on anti-corrosion? Colloids Surf. A Physicochem. Eng. Asp. 2014, 445, 75–78. [Google Scholar] [CrossRef]
- Boinovich, L.; Gnedenkov, S.; Alpysbaeva, D.; Egorkin, V.; Emelyanenko, A.; Sinebryukhov, S.; Zaretskaya, A. Corrosion resistance of composite coatings on low-carbon steel containing hydrophobic and superhydrophobic layers in combination with oxide sublayers. Corros. Sci. 2012, 55, 238–245. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Torabinejad, V.; Alimadadi, H.; Ashrafi, A. Electrodeposition mechanism and corrosion behavior of multilayer nanocrystalline nickel-tungsten alloy. Electrochim. Acta 2017, 258, 883–899. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Rouhaghdam, A.S.; Allahyarzadeh, M. Tribological properties of Ni-Fe-Co multilayer coatings fabricated by pulse electrodeposition. Tribol. Int. 2017, 106, 34–40. [Google Scholar] [CrossRef]
- Mahidashti, Z.; Aliofkhazraei, M.; Lotfi, N. Review of Nickel-Based Electrodeposited Tribo-Coatings. Trans. Indian Inst. Met. 2017, 71, 257–295. [Google Scholar] [CrossRef]
- Liu, J.; Pei, Z.; Shi, W.; Liu, Y.; Gong, J.; Sun, C. Studies on preparation, microstructure, mechanical properties and corrosion resistance of Ni Mo/micron-sized diamond composite coatings. Surf. Coatings Technol. 2020, 385, 125451. [Google Scholar] [CrossRef]
- Yan, J.; He, Z.; Wang, Y.; Qiu, J.; Wang, Y. Microstructure and Wear Resistance of Plasma-Sprayed Molybdenum Coating Rein-forced by MoSi2 Particles. J. Therm. Spray Technol. 2016, 25, 1322–1329. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, G.; Wang, T.; Ren, S.; Cao, Q.; Bai, Z.; Liu, Z. Microstructure and wear resistance of Mo coating deposited by plasma transferred arc process. Mater. Charact. 2017, 131, 517–525. [Google Scholar] [CrossRef]
- Mousavi, R.; Bahrololoom, M.; Deflorian, F. Preparation, corrosion, and wear resistance of Ni-Mo/Al composite coating reinforced with Al particles. Mater. Des. 2016, 110, 456–465. [Google Scholar] [CrossRef]
- Ahn, J.; Hwang, B.; Lee, S. Improvement of Wear Resistance of Plasma-Sprayed Molybdenum Blend Coatings. J. Therm. Spray Technol. 2005, 14, 251–257. [Google Scholar] [CrossRef]
- Sergevnin, V.; Blinkov, I.; Volkhonskii, A.; Belov, D.; Kuznetsov, D.; Gorshenkov, M.; Skryleva, E. Wear behaviour of wear-resistant adaptive nano-multilayered Ti-Al-Mo-N coatings. Appl. Surf. Sci. 2016, 388, 13–23. [Google Scholar] [CrossRef]
- Serin, I.G.; Göksenli, A. Effect of annealing temperature on hardness and wear resistance of electroless Ni–B–Mo coatings. Surf. Rev. Lett. 2015, 22, 1550058. [Google Scholar] [CrossRef]
- Romankov, S.; Hayasaka, Y.; Kasai, E.; Yoon, J.-M. Fabrication of nanostructured Mo coatings on Al and Ti substrates by ball impact cladding. Surf. Coatings Technol. 2010, 205, 2313–2321. [Google Scholar] [CrossRef]
- Chwa, S.O.; Klein, D.; Liao, H.; Dembinski, L.; Coddet, C. Temperature dependence of microstructure and hardness of vacuum plasma sprayed Cu–Mo composite coatings. Surf. Coatings Technol. 2006, 200, 5682–5686. [Google Scholar] [CrossRef]
- Melo, R.L.; Casciano, P.N.S.; Correia, A.N.; de Lima-Neto, P. Characterisation of electrodeposited and heat-treated Ni−Mo−P coatings. J. Braz. Chem. Soc. 2012, 23, 328–334. [Google Scholar] [CrossRef]
- Santa, J.; Espitia, L.; Blanco, J.; Romo, S.; Toro, A. Slurry and cavitation erosion resistance of thermal spray coatings. Wear 2009, 267, 160–167. [Google Scholar] [CrossRef]
- Fu, Q.; Shan, Y.; Cao, C.; Li, H.; Li, K. Oxidation and erosion resistant property of SiC/Si–Mo–Cr/MoSi2 multi-layer coated C/C composites. Ceram. Int. 2015, 41, 4101–4107. [Google Scholar] [CrossRef]
- Tabakoff, W. Erosion resistance of superalloys and different coatings exposed to particulate flows at high temperature. Surf. Coatings Technol. 1999, 120–121, 542–547. [Google Scholar] [CrossRef]
- Feng, T.; Li, H.; Fu, Q.; Shi, X.; Hu, M.; Liu, L. Erosion resistance of Mo–Si–Cr coating-modified C/C composites in a wind tunnel at 1873 K. J. Alloys Compd. 2014, 622, 1049–1054. [Google Scholar] [CrossRef]
- Renevier, N.; Hamphire, J.; Fox, V.; Witts, J.; Allen, T.; Teer, D. Advantages of using self-lubricating, hard, wear-resistant MoS2-based coatings. Surf. Coatings Technol. 2001, 142–144, 67–77. [Google Scholar] [CrossRef]
- Chen, J.; An, Y.; Yang, J.; Zhao, X.; Yan, F.; Zhou, H.; Chen, J. Tribological properties of adaptive NiCrAlY–Ag–Mo coatings prepared by atmospheric plasma spraying. Surf. Coatings Technol. 2013, 235, 521–528. [Google Scholar] [CrossRef]
- Bin Yaqub, T.; Vuchkov, T.; Evaristo, M.; Cavaleiro, A. DCMS Mo-Se-C solid lubricant coatings—Synthesis, structural, mechanical and tribological property investigation. Surf. Coatings Technol. 2019, 378, 124992. [Google Scholar] [CrossRef]
- Min, Y.; Penkov, O.V.; Khadem, M.; Kim, D.-E. Effects of molybdenum-based substrate coatings on tribological performance of graphene films. Carbon 2021, 176, 488–499. [Google Scholar] [CrossRef]
- Yang, J.-F.; Parakash, B.; Hardell, J.; Fang, Q.-F. Tribological properties of transition metal di-chalcogenide based lubricant coatings. Front. Mater. Sci. 2012, 6, 116–127. [Google Scholar] [CrossRef]
- Singh, R. Heat Treatment of Steels. In Applied Welding Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 111–124. [Google Scholar] [CrossRef]
- Ali, A.; Piatkowski, P.A.; Alawadhi, H.; Alnaser, A.S. Reducing the Cut-In Voltage of a Silicon Carbide/p-Silicon Heterojunction Diode Using Femtosecond Laser Ablation. ACS Appl. Electron. Mater. 2022, 4, 6076–6086. [Google Scholar] [CrossRef]
- Dongil, A.B.; Conesa, J.M.; Pastor-Pérez, L.; Sepúlveda-Escribano, A.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Carbothermally generated copper–molybdenum carbide supported on graphite for the CO2 hydrogenation to methanol. Catal. Sci. Technol. 2021, 11, 4051–4059. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Jiang, Z.; Li, Y.; Wen, C.; Zhang, D.; Lian, J.; Zhang, Z. Improvement of corrosion resistance of H59 brass through fabricating superhydrophobic surface using laser ablation and heating treatment. Corros. Sci. 2020, 180, 109186. [Google Scholar] [CrossRef]
- Zhao, D.; Gierse, N.; Wegner, J.; Pretzler, G.; Oelmann, J.; Brezinsek, S.; Liang, Y.; Neubauer, O.; Rasinski, M.; Linsmeier, C.; et al. Ablation mass features in multi-pulses femtosecond laser ablate molybdenum target. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 418, 54–59. [Google Scholar] [CrossRef]
- Liverani, E.; Li, Y.; Ascari, A.; Zhao, X.; Fortunato, A. The effect of femto-second laser shock peening on the microstructures and surface roughness of AlSi10Mg samples produced with selective laser melting (SLM). Procedia CIRP 2022, 108, 77–81. [Google Scholar] [CrossRef]
- Schmitz, C. Handbook of Aluminium Recycling; Vulkan-Verlag GmbH: Essen, Germany, 2006. [Google Scholar]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Synergistic Effect of Superhydropho-bicity and Oxidized Layers on Corrosion Resistance of Aluminum Alloy Surface Textured by Nanosecond Laser Treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [PubMed]
- Libenson, M.N.; Shandybina, G.D.; Shakhmin, A. Chemical analysis of products obtained by nanosecond laser ablation. Tech. Phys. 2000, 45, 1219–1222. [Google Scholar] [CrossRef]
- Dieterle, M.; Weinberg, G.; Mestl, G. Raman spectroscopy of molybdenum oxides. Phys. Chem. Chem. Phys. 2002, 4, 812–821. [Google Scholar] [CrossRef]
- Cano-Lara, M.; Camacho-López, S.; Esparza-García, A.; Camacho-López, M. Laser-induced molybdenum oxide formation by low energy (nJ)–high repetition rate (MHz) femtosecond pulses. Opt. Mater. 2011, 33, 1648–1653. [Google Scholar] [CrossRef]
- Oloye, F. Raman spectroscopy and XRD study on molybdenum oxide supported titania. Results Mater. 2020, 5, 100064. [Google Scholar] [CrossRef]
- Cuando-Espitia, N.; Redenius, J.; Mensink, K.; Camacho-López, M.; Camacho-López, S.; Aguilar, G. Influence of oxygen pressure on the fs laser-induced oxidation of molybdenum thin films. Opt. Mater. Express 2018, 8, 581–596. [Google Scholar] [CrossRef]
- Kumari, L.; Ma, Y.-R.; Tsai, C.-C.; Lin, Y.-W.; Wu, S.Y.; Cheng, K.-W.; Liou, Y. X-ray diffraction and Raman scattering studies on large-area array and nanobranched structure of 1D MoO2nanorods. Nanotechnology 2007, 18, 115717. [Google Scholar] [CrossRef]
- Klinbumrung, A.; Thongtem, T.; Thongtem, S. Characterization of Orthorhombic α-MoO3 Microplates Produced by a Microwave Plasma Process. J. Nanomater. 2012, 2012, 930763. [Google Scholar] [CrossRef]
- Zheng, Z.X.; Yang, H.; Cao, Y.; Dai, Z.Y. Laser-Induced Wettability Gradient Surface of the Aluminum Matrix Used for Direc-tional Transportation and Collection of Underwater Bubbles. ACS Omega 2020, 5, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Volpe, A.; Gaudiuso, C.; Venere, L.; Licciulli, F.; Giordano, F.; Ancona, A. Direct Femtosecond Laser Fabrication of Superhydrophobic Aluminum Alloy Surfaces with Anti-icing Properties. Coatings 2020, 10, 587. [Google Scholar] [CrossRef]
- Volpe, A.; Covella, S.; Gaudiuso, C.; Ancona, A. Improving the Laser Texture Strategy to Get Superhydrophobic Aluminum Alloy Surfaces. Coatings 2021, 11, 369. [Google Scholar] [CrossRef]
- Zhang, Z.; Qian, Y.; Xu, J.; Zuo, J.; Li, M. Effect of annealing on microstructure evolution and corrosion resistance of an amorphous Cr-Al-C coating. Corros. Sci. 2020, 178, 109062. [Google Scholar] [CrossRef]
- Högström, J.; Andersson, M.; Jansson, U.; Björefors, F.; Nyholm, L. On the Evaluation of Corrosion Resistances of Amorphous Chromium-Carbon Thin-Films. Electrochim. Acta 2014, 122, 224–233. [Google Scholar] [CrossRef]
- Sweitzer, J.; Shiflet, G.; Scully, J.R. Localized corrosion of Al90Fe5Gd5 and Al87Ni8.7Y4.3 alloys in the amorphous, nanocrystalline and crystalline states: Resistance to micrometer-scale pit formation. Electrochim. Acta 2003, 48, 1223–1234. [Google Scholar] [CrossRef]
- Poimenidis, I.A.; Papakosta, N.; Manousaki, A.; Klini, A.; Farsari, M.; Moustaizis, S.D.; Loukakos, P.A. Electrodeposited laser—Nanostructured electrodes for increased hydrogen production. Int. J. Hydrogen Energy 2022, 47, 9527–9536. [Google Scholar] [CrossRef]
- Schenato, M.; Ricardo, C.L.A.; Scardi, P.; Edla, R.; Miotello, A.; Orlandi, M.; Morrish, R. Effect of annealing and nanostructuring on pulsed laser deposited WS2 for HER catalysis. Appl. Catal. A Gen. 2016, 510, 156–160. [Google Scholar] [CrossRef]


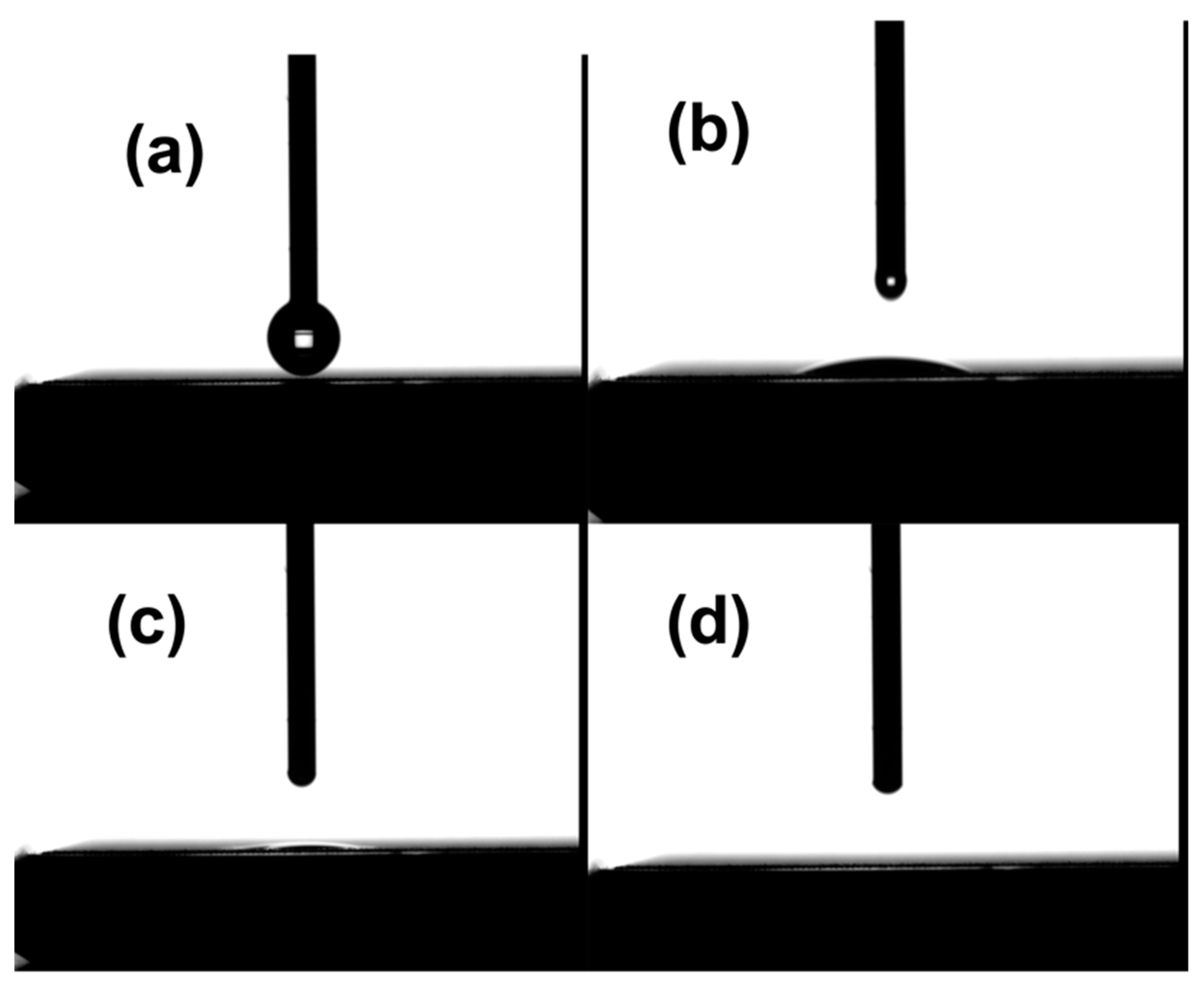
| Laser Parameter | Value |
|---|---|
| Nano-Structuring | |
| Fluence range | 5–15 J/cm2 |
| Frequency | 50 kHz |
| Scanning speed | 20 mm/s |
| Pulse duration | 40 fs |
| Step size | 100 µm |
| Beam wavelength | 1030 nm |
| Coating and post treatment | |
| Fluence | 3 J/cm2 for deposition process, 1.5, and 0.25 J/cm2 for post-deposition treatment |
| Frequency | 150 kHz for deposition and 50 kHz for post-deposition treatment |
| Scanning speed | 3325 mm/s for post-deposition treatment |
| Pulse duration | 240 fs for deposition process and 40 fs for post-deposition treatment |
| Step size | 10 µm for post-deposition treatment |
| Beam wavelength | 1030 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, T.; Ali, A.; Ahmad, S.; Piatkowski, P.; Alnaser, A.S. Enhancing Anticorrosion Resistance of Aluminum Alloys Using Femtosecond Laser-Based Surface Structuring and Coating. Nanomaterials 2023, 13, 644. https://doi.org/10.3390/nano13040644
Nawaz T, Ali A, Ahmad S, Piatkowski P, Alnaser AS. Enhancing Anticorrosion Resistance of Aluminum Alloys Using Femtosecond Laser-Based Surface Structuring and Coating. Nanomaterials. 2023; 13(4):644. https://doi.org/10.3390/nano13040644
Chicago/Turabian StyleNawaz, Tahir, Asghar Ali, Shahbaz Ahmad, Piotr Piatkowski, and Ali S. Alnaser. 2023. "Enhancing Anticorrosion Resistance of Aluminum Alloys Using Femtosecond Laser-Based Surface Structuring and Coating" Nanomaterials 13, no. 4: 644. https://doi.org/10.3390/nano13040644






