Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer
Abstract
:1. Introduction
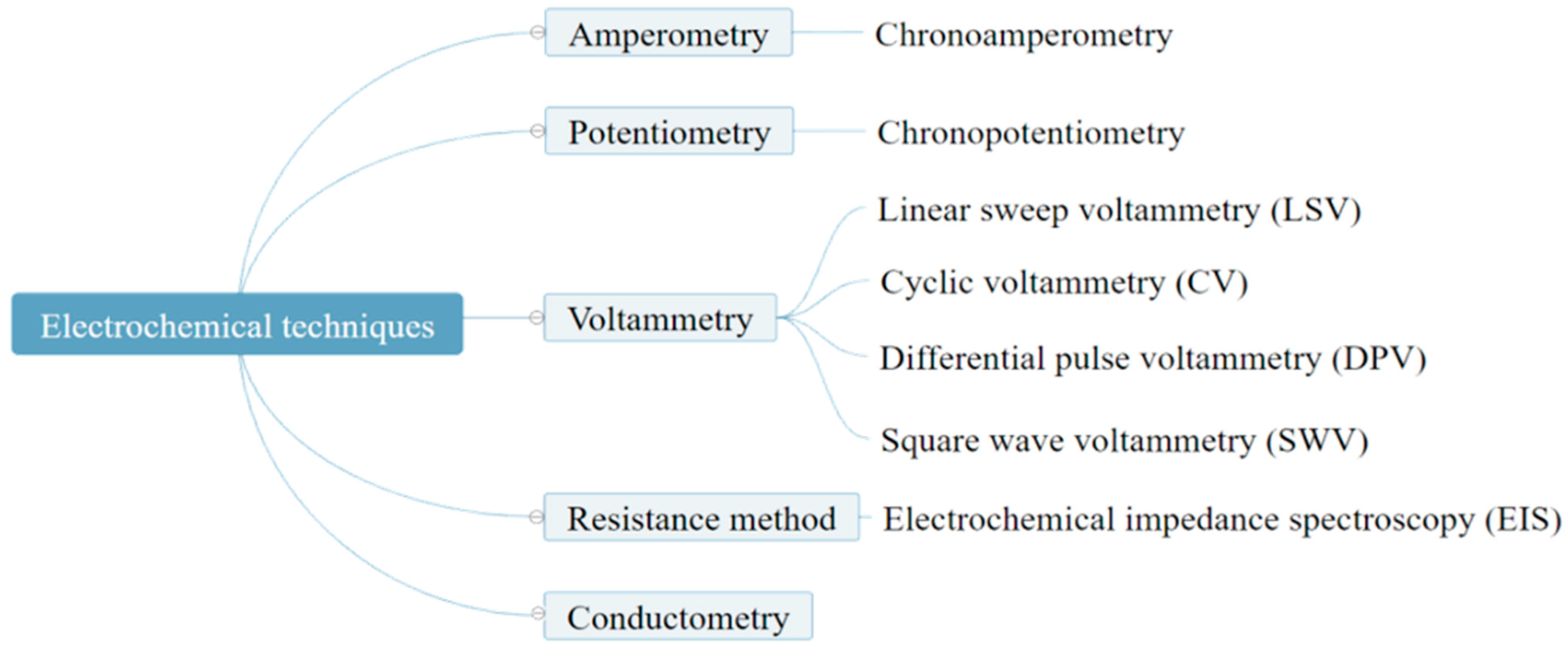
2. Polypyrrole Biosensors
2.1. Physical and Chemical Characteristics of PPy
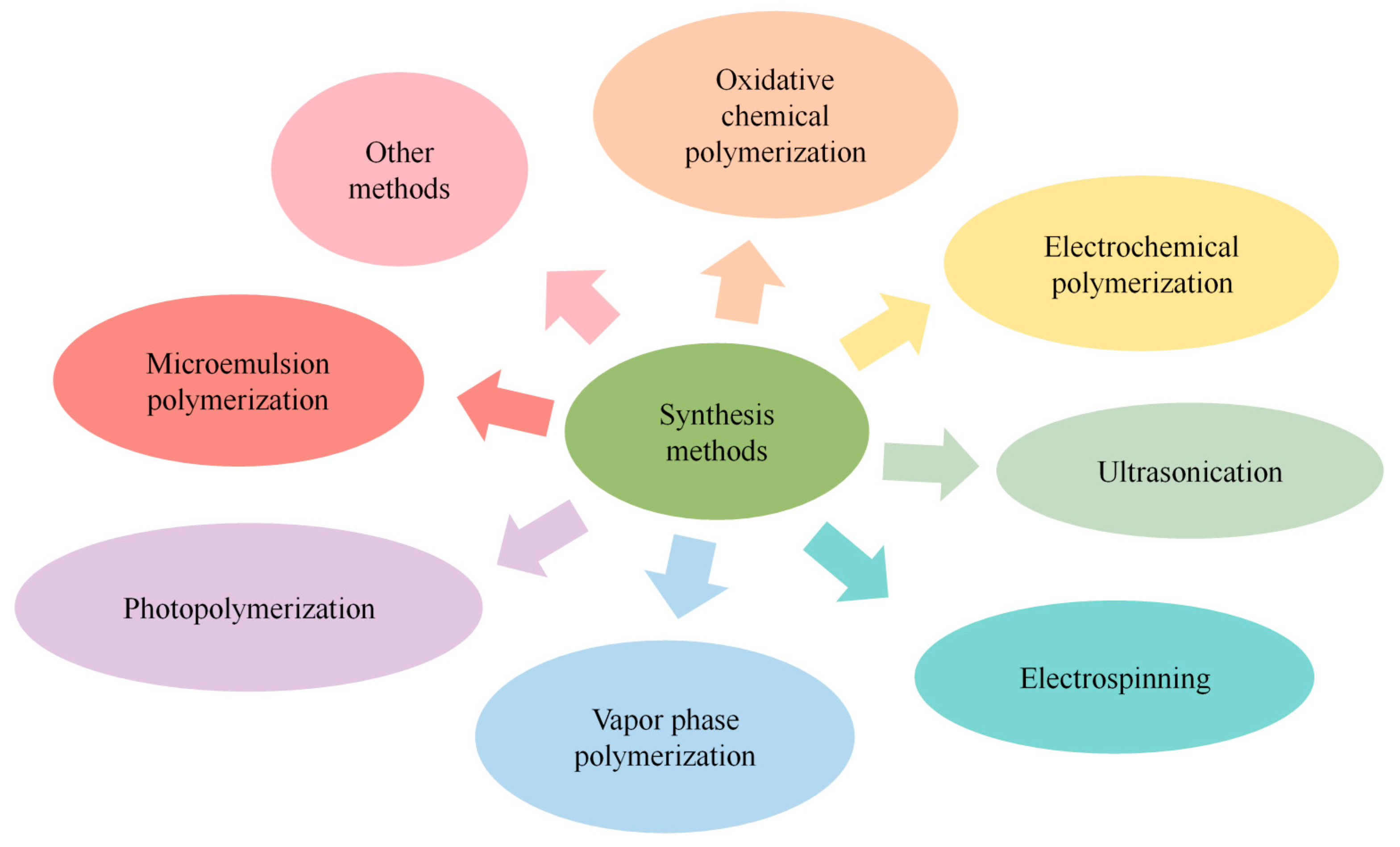
2.2. Synthesis and Modification of PPy
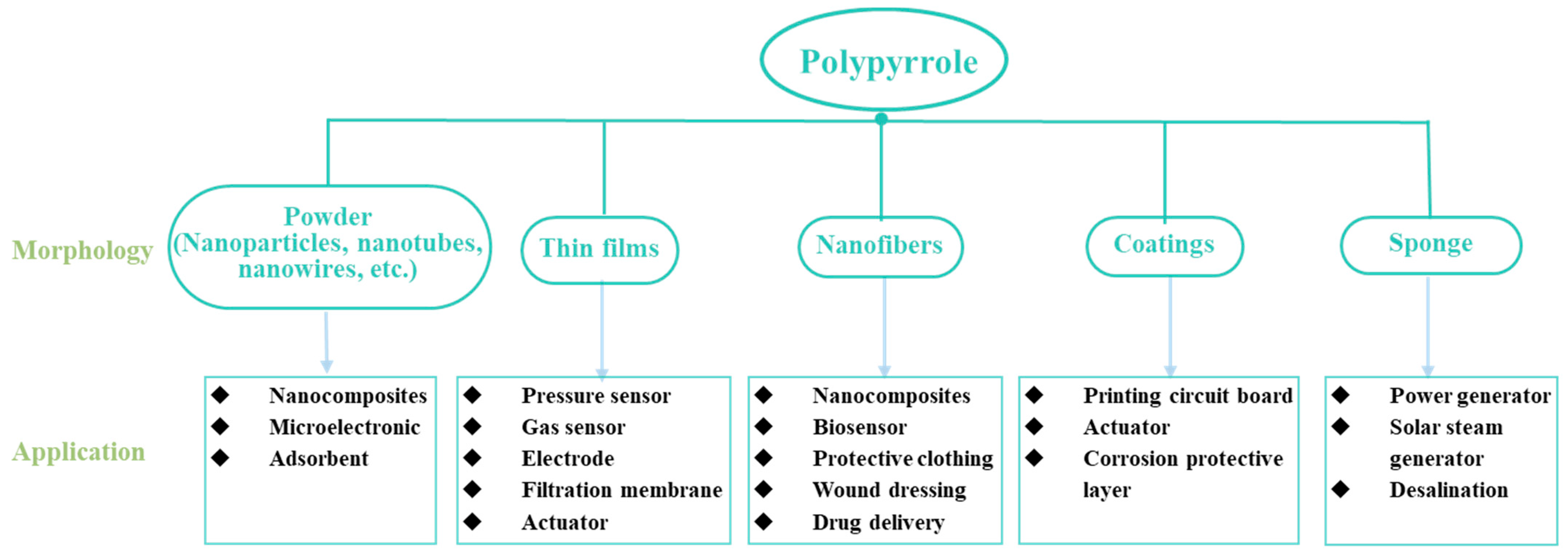
2.3. Applications of PPy
2.4. Application of PPy-Based Biosensors
2.4.1. Enzyme-Based Biosensors
2.4.2. Immunobiosensors
2.4.3. Aptamer-Based Biosensors
2.4.4. MIP-Based Biosensors
2.4.5. Nanocatalytic Biosensors
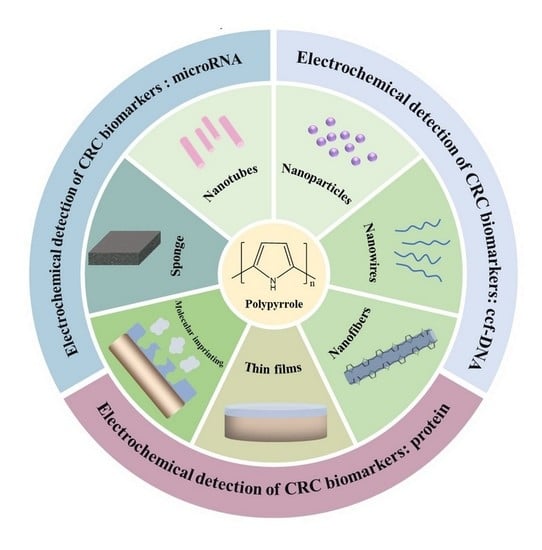
3. PPy-Based Biosensors for CRC Biomarker Detection
3.1. Circulating Cell-Free DNA (ccf-DNA)
3.1.1. DNA Mutation
3.1.2. DNA Methylation
3.2. MiRNA-Based Biomarkers
3.3. Specific Protein Biomarkers
3.3.1. Carcinoembryonic Antigen (CEA)
3.3.2. Carbohydrate Antigens
3.3.3. Interleukin-6 (IL-6)
3.3.4. Vascular Endothelial Growth Factor (VEGF)
3.3.5. Other CRC-Related Protein Biomarkers
3.4. Opportunities and Challenges Related to PPy-Based Sensors in CRC Diagnosis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Kanth, P.; Inadomi, J.M. Screening and prevention of colorectal cancer. BMJ 2021, 374, n1855. [Google Scholar] [CrossRef]
- Millien, V.O.; Mansour, N.M. Bowel Preparation for Colonoscopy in 2020: A Look at the Past, Present, and Future. Curr. Gastroenterol. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.-S.; Park, H.J. Adverse events related to colonoscopy: Global trends and future challenges. World J. Gastroenterol. 2019, 25, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Vuik, F.E.R.; Nieuwenburg, S.A.V.; Moen, S.; Spada, C.; Senore, C.; Hassan, C.; Pennazio, M.; Rondonotti, E.; Pecere, S.; Kuipers, E.J.; et al. Colon capsule endoscopy in colorectal cancer screening: A systematic review. Endoscopy 2021, 53, 815–824. [Google Scholar] [CrossRef]
- Bai, W.; Yu, D.; Zhu, B.; Yu, X.; Duan, R.; Li, Y.; Yu, W.; Hua, W.; Kou, C. Diagnostic accuracy of computed tomography colonography in patients at high risk for colorectal cancer: A meta-analysis. Color. Dis. 2020, 22, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, P.; Duncan, A. CT colonography: For screening and monitoring disease. Radiol. Technol. 2021, 92, 595CT–608CT. [Google Scholar]
- Wilkins, T.; McMechan, D.; Talukder, A. Colorectal cancer screening and prevention. Am. Fam. Physician 2018, 97, 658–665. [Google Scholar]
- Ladabaum, U.; Dominitz, J.A.; Kahi, C.; Schoen, R.E. Strategies for Colorectal Cancer Screening. Gastroenterology 2020, 158, 418–432. [Google Scholar] [CrossRef]
- Li, J.N.; Yuan, S.Y. Fecal occult blood test in colorectal cancer screening. J. Dig. Dis. 2019, 20, 62–64. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, G.; Chen, J.; Wang, L.; Hu, Q.; Wu, J.; Zhang, W.; Song, M.; Qiao, J.; Xu, C. Electrochemical biosensors for measurement of colorectal cancer biomarkers. Anal. Bioanal. Chem. 2021, 413, 2407–2428. [Google Scholar] [CrossRef] [PubMed]
- Nikolouzakis, T.K.; Vassilopoulou, L.; Fragkiadaki, P.; Sapsakos, T.M.; Papadakis, G.Z.; Spandidos, D.; Tsatsakis, A.; Tsiaoussis, J. Improving diagnosis, prognosis and prediction by using biomarkers in CRC patients (Review). Oncol. Rep. 2018, 39, 2455–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaou, S.; Qiu, S.; Fiorentino, F.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. Systematic review of blood diagnostic markers in colorectal cancer. Tech. Coloproctol. 2018, 22, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Zygulska, A.L.; Pierzchalski, P. Novel Diagnostic Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef]
- Monsalve-Lancheros, A.; Ibáñez-Pinilla, M.; Ramírez-Clavijo, S. Detection of mammagloblin by RT-PCR as a biomarker for lymph node metastasis in breast cancer patients: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0216989. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors 2018, 18, 2010. [Google Scholar] [CrossRef]
- Li, S.; Wu, H.; Mai, H.; Zhen, J.; Li, G.; Chen, S. Microarray-based analysis of whole-genome DNA methylation profiling in early detection of breast cancer. J. Cell. Biochem. 2019, 120, 658–670. [Google Scholar] [CrossRef]
- Oltedal, S.; Skaland, I.; Maple-Grødem, J.; Tjensvoll, K.; Janssen, E.A.M.; Gilje, B.; Smaaland, R.; Heikkilä, R.; Nordgård, O. Expression profiling and intracellular localization studies of the novel Proline-, Histidine-, and Glycine-rich protein 1 suggest an essential role in gastro-intestinal epithelium and a potential clinical application in colorectal cancer diagnostics. BMC Gastroenterol. 2018, 18, 26. [Google Scholar] [CrossRef]
- Kondo, T. Cancer biomarker development and two-dimensional difference gel electrophoresis (2D-DIGE). Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 2–8. [Google Scholar] [CrossRef]
- El-Bayoumy, A.S.A.-B.; Keshta, A.T.H.; Sallam, K.M.; Ebeid, N.H.; Elsheikh, H.M.; Bayoumy, B.E.-S. Extraction, purification of prostate-specific antigen (PSA), and establishment of radioimmunoassay system as a diagnostic tool for prostate disorders. J. Immunoass. Immunochem. 2018, 39, 12–29. [Google Scholar] [CrossRef]
- Sukswai, N.; Khoury, J.D. Immunohistochemistry Innovations for Diagnosis and Tissue-Based Biomarker Detection. Curr. Hematol. Malign. Rep. 2019, 14, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Neubert, H.; Shuford, C.M.; Olah, T.V.; Garofolo, F.; Schultz, G.A.; Jones, B.R.; Amaravadi, L.; Laterza, O.F.; Xu, K.; Ackermann, B.L. Protein Biomarker Quantification by Immunoaffinity Liquid Chromatography–Tandem Mass Spectrometry: Current State and Future Vision. Clin. Chem. 2020, 66, 282–301. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Kabashima, T.; Zhu, Q.; Shibata, T.; Kai, M. Fluorescence assay of dihydroorotate dehydrogenase that may become a cancer biomarker. Sci. Rep. 2017, 7, 40670. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, R.; Wu, X.; Shi, N.; Duan, T.; Xu, K.; Huang, T. Fluorescence turn-on immunosensing of HE4 biomarker and ovarian cancer cells based on target-triggered metal-enhanced fluorescence of carbon dots. Anal. Chim. Acta 2021, 1187, 339160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, J.; Li, S.; Zhang, L.; Huang, Y.; Zhao, S. A novel microchip electrophoresis-based chemiluminescence immunoassay for the detection of alpha-fetoprotein in human serum. Talanta 2017, 165, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, H.; Zhao, W.-W.; Ai, S. Electrochemical, electrochemiluminescent and photoelectrochemical bioanalysis of epigenetic modifiers: A comprehensive review. Coord. Chem. Rev. 2020, 424, 213519. [Google Scholar] [CrossRef]
- Dzulkurnain, N.A.; Mokhtar, M.; Rashid, J.I.A.; Knight, V.F.; Yunus, W.M.Z.W.; Ong, K.K.; Kasim, N.A.M.; Noor, S.A.M. A Review on Impedimetric and Voltammetric Analysis Based on Polypyrrole Conducting Polymers for Electrochemical Sensing Applications. Polymers 2021, 13, 2728. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Narayan, T.; Solanki, S.; Malhotra, B.D. Recent Advances of Conducting Polymers and Their Composites for Electrochemical Biosensing Applications. J. Funct. Biomater. 2020, 11, 71. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Lara, S.; Perez-Potti, A. Applications of Nanomaterials for Immunosensing. Biosensors 2018, 8, 104. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R.A. Recent Trends and Developments in Conducting Polymer Nanocomposites for Multifunctional Applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef] [PubMed]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J. A sensitive and label-free electrochemical microRNA biosensor based on Polyamidoamine Dendrimer functionalized Polypyrrole nanowires hybrid. Microchim. Acta 2021, 188, 173. [Google Scholar] [CrossRef] [PubMed]
- Puerres, J.; Ortiz, P.; Cortés, M. Effect of Electrosynthesis Potential on Nucleation, Growth, Adhesion, and Electronic Properties of Polypyrrole Thin Films on Fluorine-Doped Tin Oxide (FTO). Polymers 2021, 13, 2419. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Rouabhia, M.; Zhang, Z. Surface modification by assembling: A modular approach based on the match in nanostructures. J. Mater. Chem. B 2019, 7, 755–762. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Xing, Y.; Zhou, W.; Cai, K. Interface-Hybridization-Enhanced Photothermal Performance of Polypyrrole/Polydopamine Heterojunctions on Porous Nanoparticles. Macromol. Rapid Commun. 2019, 40, e1900263. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, W.; Luo, S.-X.L.; Bezdek, M.J.; Peraire-Bueno, A.; Swager, T.M. Wireless Lateral Flow Device for Biosensing. J. Am. Chem. Soc. 2022, 144, 15786–15792. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Lu, Q.; Li, X.; Weng, C.; Yan, X.; Hong, J.; Zhou, X. A versatile fluorometric aptasensing scheme based on the use of a hybrid material composed of polypyrrole nanoparticles and DNA-silver nanoclusters: Application to the determination of adenosine, thrombin, or interferon-gamma. Microchim. Acta 2019, 186, 356. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.-Z.; Zheng, G.; Zhang, H.; Zhou, M.; Li, S.; Kong, Y. Dual-template molecularly imprinted electrochemical biosensor for IgG-IgM combined assay based on a dual-signal strategy. Bioelectrochemistry 2022, 148, 108267. [Google Scholar] [CrossRef]
- Nguyet, N.T.; Yen, L.T.H.; Doan, V.Y.; Hoang, N.L.; Van Thu, V.; Lan, H.; Trung, T.; Pham, V.-H.; Tam, P.D. A label-free and highly sensitive DNA biosensor based on the core-shell structured CeO2-NR@Ppy nanocomposite for Salmonella detection. Mater. Sci. Eng. C 2019, 96, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xue, H.; Zhao, H.; Fei, T.; Liu, S.; Chen, Q.; Gao, B.; Zhang, T. A dual-functional polyaniline film-based flexible electrochemical sensor for the detection of pH and lactate in sweat of the human body. Talanta 2022, 242, 123289. [Google Scholar] [CrossRef] [PubMed]
- Uzunçar, S.; Meng, L.; Turner, A.P.; Mak, W.C. Processable and nanofibrous polyaniline:polystyrene-sulphonate (nano-PANI:PSS) for the fabrication of catalyst-free ammonium sensors and enzyme-coupled urea biosensors. Biosens. Bioelectron. 2021, 171, 112725. [Google Scholar] [CrossRef]
- Malmir, M.; Arjomandi, J.; Khosroshahi, A.G.; Moradi, M.; Shi, H. Label-free E-DNA biosensor based on PANi-RGO-G*NPs for detection of cell-free fetal DNA in maternal blood and fetal gender determination in early pregnancy. Biosens. Bioelectron. 2021, 189, 113356. [Google Scholar] [CrossRef] [PubMed]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Polythiophene supported MnO2 nanoparticles as nano-stabilizer for simultaneously electrostatically immobilization of d-amino acid oxidase and hemoglobin as efficient bio-nanocomposite in fabrication of dopamine bi-enzyme biosensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Senel, M.; Sagir, T.; Isik, S. Highly sensitive detection of cancer cells with an electrochemical cytosensor based on boronic acid functional polythiophene. Biosens. Bioelectron. 2017, 90, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Senel, M.; Cevik, E. Novel impedimetric dopamine biosensor based on boronic acid functional polythiophene modified electrodes. Mater. Sci. Eng. C 2017, 72, 641–649. [Google Scholar] [CrossRef]
- Zahed, A.; Barman, S.C.; Das, P.S.; Sharifuzzaman; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly flexible and conductive poly (3, 4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration. Biosens. Bioelectron. 2020, 160, 112220. [Google Scholar] [CrossRef]
- Qin, J.; Cho, M.; Lee, Y. Ultrasensitive Detection of Amyloid-β Using Cellular Prion Protein on the Highly Conductive Au Nanoparticles–Poly(3,4-ethylene dioxythiophene)–Poly(thiophene-3-acetic acid) Composite Electrode. Anal. Chem. 2019, 91, 11259–11265. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Alagar, S.; Mariappan, R.; Piraman, S. Glassy carbon electrodes modified with reduced graphene oxide-MoS2-poly (3,4-ethylene dioxythiophene) nanocomposites for the non-enzymatic detection of nitrite in water and milk. Anal. Chim. Acta 2020, 1093, 93–105. [Google Scholar] [CrossRef]
- Baghdadi, N.; Zoromba, M.; Abdel-Aziz, M.; Al-Hossainy, A.; Bassyouni, M.; Salah, N. One-Dimensional Nanocomposites Based on Polypyrrole-Carbon Nanotubes and Their Thermoelectric Performance. Polymers 2021, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Huang, J.; Yan, P.; Wan, F.; Zhu, Y.; Cheng, H. Preparation and Properties of Waterborne Polypyrrole/Cement Composites. Materials 2021, 14, 5166. [Google Scholar] [CrossRef] [PubMed]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Rahaman, M.; Aldalbahi, A.; Almoiqli, M.; Alzahly, S. Chemical and Electrochemical Synthesis of Polypyrrole Using Carrageenan as a Dopant: Polypyrrole/Multi-Walled Carbon Nanotube Nanocomposites. Polymers 2018, 10, 632. [Google Scholar] [CrossRef]
- Manickavasagan, A.; Ramachandran, R.; Chen, S.-M.; Velluchamy, M. Ultrasonic assisted fabrication of silver tungstate encrusted polypyrrole nanocomposite for effective photocatalytic and electrocatalytic applications. Ultrason. Sonochem. 2020, 64, 104913. [Google Scholar] [CrossRef]
- Kang, T.S.; Lee, S.W.; Joo, J.; Lee, J.Y. Electrically conducting polypyrrole fibers spun by electrospinning. Synth. Met. 2005, 153, 61–64. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ataei, A.A.; Pirsa, S. Nanostructured conducting polypyrrole film prepared by chemical vapor deposition on the interdigital electrodes at room temperature under atmospheric condition and its application as gas sensor. J. Iran. Chem. Soc. 2015, 12, 1585–1594. [Google Scholar] [CrossRef]
- Kopecký, D.; Vrňata, M.; Vysloužil, F.; Fitl, P.; Ekrt, O.; Seidl, J.; Myslík, V.; Hofmann, J.; Náhlík, J.; Vlček, J.; et al. Doped polypyrrole for MAPLE deposition: Synthesis and characterization. Synth. Met. 2010, 160, 1081–1085. [Google Scholar] [CrossRef]
- Leonavicius, K.; Ramanaviciene, A.; Ramanavicius, A. Polymerization Model for Hydrogen Peroxide Initiated Synthesis of Polypyrrole Nanoparticles. Langmuir ACS J. Surf. Colloids 2011, 27, 10970–10976. [Google Scholar] [CrossRef]
- Imae, I.; Krukiewicz, K. Self-doped conducting polymers in biomedical engineering: Synthesis, characterization, current applications and perspectives. Bioelectrochemistry 2022, 146, 108127. [Google Scholar] [CrossRef]
- Peng, H.; Zheng, J.; Zhang, B.; Xu, J.; Zhang, M. Fe doped MoS2/polypyrrole microtubes towards efficient peroxidase mimicking and colorimetric sensing application. Dalton Trans. 2021, 50, 15380–15388. [Google Scholar] [CrossRef] [PubMed]
- Andriukonis, E.; Ramanaviciene, A.; Ramanavicius, A. Synthesis of Polypyrrole Induced by [Fe(CN)6]3− and Redox Cycling of [Fe(CN)6]4−/[Fe(CN)6]3−. Polymers 2018, 10, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.; Zhang, Z. Polypyrrole as electrically conductive biomaterials: Synthesis, biofunctionalization, potential applications and challenges. Adv. Exp. Med. Biol. 2018, 1078, 347–370. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic Formation of Polyaniline, Polypyrrole, and Polythiophene Nanoparticles with Embedded Glucose Oxidase. Nanomaterials 2019, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation of Polyaniline and Polypyrrole Nanocomposites with Embedded Glucose Oxidase and Gold Nanoparticles. Polymers 2019, 11, 377. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer—Polypyrrole—For the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef]
- Sherman, H.G.; Hicks, J.M.; Jain, A.; Titman, J.J.; Alexander, C.; Stolnik, S.; Rawson, F.J.; Hicks, J. Mammalian-Cell-Driven Polymerisation of Pyrrole. Chembiochem 2019, 20, 1008–1013. [Google Scholar] [CrossRef]
- Fani, M.; Rezayi, M.; Pourianfar, H.R.; Meshkat, Z.; Makvandi, M.; Gholami, M.; Rezaee, S.A. Rapid and label-free electrochemical dna biosensor based on a facile one-step electrochemical synthesis of rgo-ppy-(l-cys)-aunps nanocomposite for the htlv-1 oligonucleotide detection. Biotechnol. Appl. Biochem. 2021, 68, 626–635. [Google Scholar] [CrossRef]
- Ayenimo, J.G.; Adeloju, S.B. Amperometric detection of glucose in fruit juices with polypyrrole-based biosensor with an integrated permselective layer for exclusion of interferences. Food Chem. 2017, 229, 127–135. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Gajbhiye, V.; Bodas, D. Development of nano-immunosensor with magnetic separation and electrical detection of Escherichia coli using antibody conjugated Fe3O4@Ppy. Nanotechnology 2021, 32, 085603. [Google Scholar] [CrossRef]
- Xu, T.; Chi, B.; Gao, J.; Chu, M.; Fan, W.; Yi, M.; Xu, H.; Mao, C. Novel electrochemical immune sensor based on Hep-PGA-PPy nanoparticles for detection of α-Fetoprotein in whole blood. Anal. Chim. Acta 2017, 977, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pruna, A.I.; Rosas-Laverde, N.M.; Mataix, D.B. Effect of Deposition Parameters on Electrochemical Properties of Polypyrrole-Graphene Oxide Films. Materials 2020, 13, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesküla, A.; Heinmaa, I.; Tamm, T.; Aydemir, N.; Travas-Sejdic, J.; Peikolainen, A.-L.; Kiefer, R. Improving the Electrochemical Performance and Stability of Polypyrrole by Polymerizing Ionic Liquids. Polymers 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Mametov, R.; Sagandykova, G.; Monedeiro, F.; Buszewski, B. Development of controlled film of polypyrrole for solid-phase microextraction fiber by electropolymerization. Talanta 2021, 232, 122394. [Google Scholar] [CrossRef]
- Glosz, K.; Stolarczyk, A.; Jarosz, T. Electropolymerised Polypyrroles as Active Layers for Molecularly Imprinted Sensors: Fabrication and Applications. Materials 2021, 14, 1369. [Google Scholar] [CrossRef]
- Ramanavičius, S.; Morkvėnaitė-Vilkončienė, I.; Samukaitė-Bubnienė, U.; Ratautaitė, V.; Plikusienė, I.; Viter, R.; Ramanavičius, A. Electrochemically Deposited Molecularly Imprinted Polymer-Based Sensors. Sensors 2022, 22, 1282. [Google Scholar] [CrossRef]
- Deore, B.; Chen, Z.; Nagaoka, T. Potential-Induced Enantioselective Uptake of Amino Acid into Molecularly Imprinted Overoxidized Polypyrrole. Anal. Chem. 2000, 72, 3989–3994. [Google Scholar] [CrossRef]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP sensors—The electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef]
- Kowalski, D.; Ueda, M.; Ohtsuka, T. The effect of ultrasonic irradiation during electropolymerization of polypyrrole on corrosion prevention of the coated steel. Corros. Sci. 2008, 50, 286–291. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Conductive Electrospun Nanofiber Mats. Materials 2019, 13, 152. [Google Scholar] [CrossRef]
- Lawal, A.T.; Wallace, G.G. Vapour phase polymerisation of conducting and non-conducting polymers: A review. Talanta 2014, 119, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Kasisomayajula, S.; Jadhav, N.; Gelling, V.J. Conductive polypyrrole and acrylate nanocomposite coatings: Mechanistic study on simultaneous photopolymerization. Prog. Org. Coat. 2016, 101, 440–454. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Ding, T.; Tang, J.; Xie, X.; Xing, Y.; Wang, L.; Zhang, J.; Cai, K. Interfacial Engineering of Hybrid Polydopamine/Polypyrrole Nanosheets with Narrow Band Gaps for Fluorescence Sensing of MicroRNA. ACS Appl. Mater. Interfaces 2021, 13, 42183–42194. [Google Scholar] [CrossRef]
- Okamura, N.; Maeda, T.; Fujiwara, H.; Soman, A.; Unni, K.N.N.; Ajayaghosh, A.; Yagi, S. Photokinetic study on remarkable excimer phosphorescence from heteroleptic cyclometalated platinum(ii) complexes bearing a benzoylated 2-phenylpyridinate ligand. Phys. Chem. Chem. Phys. 2017, 20, 542–552. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Dispersed Conducting Polymer Nanocomposites with Glucose Oxidase and Gold Nanoparticles for the Design of Enzymatic Glucose Biosensors. Polymers 2021, 13, 2173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.X.; Spinks, G.M.; Gorkin, R.; Sangian, D.; Di Bella, C.; Quigley, A.F.; Kapsa, R.; Wallace, G.G.; Choong, P.F.M. In vivo biocompatibility of porous and non-porous polypyrrole based trilayered actuators. J. Mater. Sci. Mater. Med. 2017, 28, 172. [Google Scholar] [CrossRef]
- Zhao, P.; Tang, Q.; Zhao, X.; Tong, Y.; Liu, Y. Highly stable and flexible transparent conductive polymer electrode patterns for large-scale organic transistors. J. Colloid Interface Sci. 2018, 520, 58–63. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, L.; Zhai, J.; Dong, S. Water/Oxygen Circulation-Based Biophotoelectrochemical System for Solar Energy Storage and Release. J. Am. Chem. Soc. 2019, 141, 16416–16421. [Google Scholar] [CrossRef]
- Mills, I.N.; Porras, J.A.; Bernhard, S. Judicious Design of Cationic, Cyclometalated Ir(III) Complexes for Photochemical Energy Conversion and Optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. [Google Scholar] [CrossRef]
- Rafique, S.; Rashid, I.; Sharif, R. Cost effective dye sensitized solar cell based on novel Cu polypyrrole multiwall carbon nanotubes nanocomposites counter electrode. Sci. Rep. 2021, 11, 14830. [Google Scholar] [CrossRef]
- Han, Y.; Sun, L.; Wen, C.; Wang, Z.; Dai, J.; Shi, L. Flexible conductive silk-PPy hydrogel toward wearable electronic strain sensors. Biomed. Mater. 2022, 17, 024107. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Li, Y.; Zhou, J.; Wang, T.; Zhang, R.; Cao, J.; Luo, M.; Li, N.; Zhang, N.; Gong, H.; et al. Implantable and Biodegradable Micro-Supercapacitor Based on a Superassembled Three-Dimensional Network Zn@PPy Hybrid Electrode. ACS Appl. Mater. Interfaces 2021, 13, 8285–8293. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zhao, L.; Li, M.; Li, Y.; Yang, W.; Ren, J. Polypyrrole-doped conductive self-healing multifunctional composite hydrogels with a dual crosslinked network. Soft Matter 2021, 17, 8363–8372. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2020, 13, 49. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Lü, H.; Hui, N. Electrochemical sensor based on Prussian blue/multi-walled carbon nanotubes functionalized polypyrrole nanowire arrays for hydrogen peroxide and microRNA detection. Microchim. Acta 2021, 188, 25. [Google Scholar] [CrossRef]
- Rong, Y.; Yan, W.; Wang, Z.; Hao, X.; Guan, G. An electroactive montmorillonite/polypyrrole ion exchange film: Ultrahigh uptake capacity and ion selectivity for rapid removal of lead ions. J. Hazard. Mater. 2022, 437, 129366. [Google Scholar] [CrossRef]
- Mohamed, F.; Abukhadra, M.R.; Shaban, M. Removal of safranin dye from water using polypyrrole nanofiber/Zn-Fe layered double hydroxide nanocomposite (Ppy NF/Zn-Fe LDH) of enhanced adsorption and photocatalytic properties. Sci. Total Environ. 2018, 640–641, 352–363. [Google Scholar] [CrossRef]
- Fan, Y.; Bai, W.; Mu, P.; Su, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, A. Conductively monolithic polypyrrole 3-D porous architecture with micron-sized channels as superior salt-resistant solar steam generators. Sol. Energy Mater. Sol. Cells 2020, 206, 110347. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review). Polymers 2021, 13, 974. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Cârâc, G.; Bahrim, G.-E.; Camurlu, P. Utilization of enzyme extract self-encapsulated within polypyrrole in sensitive detection of catechol. Enzym. Microb. Technol. 2019, 128, 34–39. [Google Scholar] [CrossRef]
- Dutta, R.R.; Puzari, P. Amperometric biosensing of organophosphate and organocarbamate pesticides utilizing polypyrrole entrapped acetylcholinesterase electrode. Biosens. Bioelectron. 2014, 52, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Song, Y.; Lin, N.; Zhou, S.; Cai, X. An ascorbic acid amperometric sensor using over-oxidized polypyrrole and palladium nanoparticles composites. Biosens. Bioelectron. 2012, 38, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Lin, L.-Y.; Lee, T.-H.; Liu, X.; He, J.-H.; Chung, R.-J. Disposable and cost-effective label-free electrochemical immunosensor for prolactin based on bismuth sulfide nanorods with polypyrrole. Bioelectrochemistry 2022, 143, 107948. [Google Scholar] [CrossRef]
- Tang, C.; Wang, P.; Zhou, K.; Ren, J.; Wang, S.; Tang, F.; Li, Y.; Liu, Q.; Xue, L. Electrochemical immunosensor based on hollow porous Pt skin AgPt alloy/NGR as a dual signal amplification strategy for sensitive detection of Neuron-specific enolase. Biosens. Bioelectron. 2022, 197, 113779. [Google Scholar] [CrossRef]
- Zou, Y.; Liang, J.; She, Z.; Kraatz, H.-B. Gold nanoparticles-based multifunctional nanoconjugates for highly sensitive and enzyme-free detection of E.coli K12. Talanta 2019, 193, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Wang, N.; Huang, Z.; Dai, H.; Xu, L.; Sun, S.; Ma, H.; Lin, M. Electrochemical endotoxin aptasensor based on a metal-organic framework labeled analytical platform. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110501. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.; Xu, K.; Hui, N.; Wang, D. Electrochemical assay of acetamiprid in vegetables based on nitrogen-doped graphene/polypyrrole nanocomposites. Microchim. Acta 2022, 189, 395. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Li, S.; Ruan, X.; Fei, M.; Zhou, Y.; Niu, X.; Zhu, W.; Du, D.; Lin, Y. Molecularly imprinted polypyrrole nanotubes based electrochemical sensor for glyphosate detection. Biosens. Bioelectron. 2021, 191, 113434. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Lu, Y.; Jia, M.; Yan, J.; Bian, X. Facile Preparation of a Bacteria Imprinted Artificial Receptor for Highly Selective Bacterial Recognition and Label-Free Impedimetric Detection. Anal. Chem. 2019, 91, 1027–1033. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Development of molecularly imprinted polymer based phase boundaries for sensors design (review). Adv. Colloid Interface Sci. 2022, 305, 102693. [Google Scholar] [CrossRef]
- Moncer, F.; Adhoum, N.; Catak, D.; Monser, L. Electrochemical sensor based on MIP for highly sensitive detection of 5-hydroxyindole-3-acetic acid carcinoid cancer biomarker in human biological fluids. Anal. Chim. Acta 2021, 1181, 338925. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Y.; Lv, X.; Xia, H.; Shi, H.; Yang, X.; Zhang, J.; Wang, Y. Controllable anchoring of gold nanoparticles to polypyrrole nanofibers by hydrogen bonding and their application in nonenzymatic glucose sensors. Biosens. Bioelectron. 2012, 38, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Yoo, J.; Park, S.; Lu, J.; Park, S.; Lee, J. Non-Enzymatic Glucose Biosensor Based on Highly Pure TiO2 Nanoparticles. Biosensors 2021, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Shi, W.; Sun, Y.; Zhu, X.; Wu, G.; Ruan, C.; Liu, X.; Ge, D. Nonenzymatic biosensor based on CuxO nanoparticles deposited on polypyrrole nanowires for improving detectionrange. Biosens. Bioelectron. 2013, 42, 141–147. [Google Scholar] [CrossRef] [PubMed]
- German, N.; Ramanavicius, A.; Voronovic, J.; Ramanaviciene, A. Glucose biosensor based on glucose oxidase and gold nanoparticles of different sizes covered by polypyrrole layer. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 224–230. [Google Scholar] [CrossRef]
- Meng, L.; Turner, A.P.; Mak, W.C. Soft and flexible material-based affinity sensors. Biotechnol. Adv. 2019, 39, 107398. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feng, Y.; Pan, G.; Liu, L. Advances in Molecularly Imprinting Technology for Bioanalytical Applications. Sensors 2020, 19, 177. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal- and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Bag, S.; Baksi, A.; Nandam, S.H.; Wang, D.; Ye, X.-L.; Ghosh, J.; Pradeep, T.; Hahn, H. Nonenzymatic Glucose Sensing Using Ni60Nb40 Nanoglass. ACS Nano 2020, 14, 5543–5552. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, L.; Dai, L.; Wang, Y.; Tian, Y. Nonenzymatic Electrochemical Sensor with Ratiometric Signal Output for Selective Determination of Superoxide Anion in Rat Brain. Anal. Chem. 2021, 93, 5570–5576. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Bellio, H.; Fumet, J.; Ghiringhelli, F. Targeting BRAF and RAS in Colorectal Cancer. Cancers 2021, 13, 2201. [Google Scholar] [CrossRef] [PubMed]
- Aghabozorgi, A.S.; Bahreyni, A.; Soleimani, A.; Bahrami, A.; Khazaei, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Role of adenomatous polyposis coli (APC) gene mutations in the pathogenesis of colorectal cancer; current status and perspectives. Biochimie 2018, 157, 64–71. [Google Scholar] [CrossRef]
- Liebl, M.; Hofmann, T. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; He, P.; Fang, Y. Sensitive detection of p53 tumor suppressor gene using an enzyme-based solid-state electrochemiluminescence sensing platform. Biosens. Bioelectron. 2011, 26, 3608–3613. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Zhu, K.; Xu, Q.; Tang, M. Novel electrochemical biosensor based on functional composite nanofibers for sensitive detection of p53 tumor suppressor gene. Anal. Chim. Acta 2013, 765, 63–69. [Google Scholar] [CrossRef]
- Sun, J.; Fei, F.; Zhang, M.; Li, Y.; Zhang, X.; Zhu, S.; Zhang, S. The role of mSEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC Cancer 2019, 19, 450. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Fu, J.; Huang, H.; Sun, S.; Zhang, D.; Zhao, L.; Onwuka, J.U.; Zhao, Y.; Cui, B. SCTR hypermethylation is a diagnostic biomarker in colorectal cancer. Cancer Sci. 2020, 111, 4558–4566. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, Y.; Li, H.; Li, S.; Zhu, Y.; Liu, X.; Xiong, S.; Liu, Y.; Miao, J.; Fei, S.; et al. A novel plasma based early colorectal cancer screening assay base on methylated SDC2 and SFRP2. Clin. Chim. Acta 2020, 503, 84–89. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Q.; Wei, W.; Zheng, L.; Yi, S.; Li, G.; Wang, W.; Sheng, H.; Pu, H.; Mo, H.; et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci. Transl. Med. 2020, 12, eaax7533. [Google Scholar] [CrossRef] [PubMed]
- Mujica, M.L.; Gallay, P.A.; Perrachione, F.; Montemerlo, A.E.; Tamborelli, L.A.; Vaschetti, V.M.; Reartes, D.F.; Bollo, S.; Rodríguez, M.C.; Dalmasso, P.R.; et al. New trends in the development of electrochemical biosensors for the quantification of microRNAs. J. Pharm. Biomed. Anal. 2020, 189, 113478. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Bahreyni, A.; Rezaei, M.; Bahrami, A.; Khazaei, M.; Fiuji, H.; Ryzhikov, M.; Ferns, G.A.; Avan, A.; Hassanian, S.M. Diagnostic, prognostic, and therapeutic potency of microRNA 21 in the pathogenesis of colon cancer, current status and prospective. J. Cell. Physiol. 2019, 234, 8075–8081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, D.; Cui, Y.; Qiu, Y.; Miao, C.; Lu, X. Identification of microRNA-451a as a Novel Circulating Biomarker for Colorectal Cancer Diagnosis. BioMed Res. Int. 2020, 2020, 5236236. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Pretorius, A.; Klein, A. Biomarkers for Stratification in Colorectal Cancer: MicroRNAs. Cancer Control J. Moffitt Cancer Cent. 2019, 26, 1073274819862784. [Google Scholar] [CrossRef]
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Guraya, S.Y.; Bendardaf, R.; Hamoudi, R. Integrative systematic review meta-analysis and bioinformatics identifies MicroRNA-21 and its target genes as biomarkers for colorectal adenocarcinoma. Int. J. Surg. 2019, 73, 113–122. [Google Scholar] [CrossRef]
- Liu, T.; Liu, D.; Guan, S.; Dong, M. Diagnostic role of circulating MiR-21 in colorectal cancer: A update meta-analysis. Ann. Med. 2020, 53, 87–102. [Google Scholar] [CrossRef]
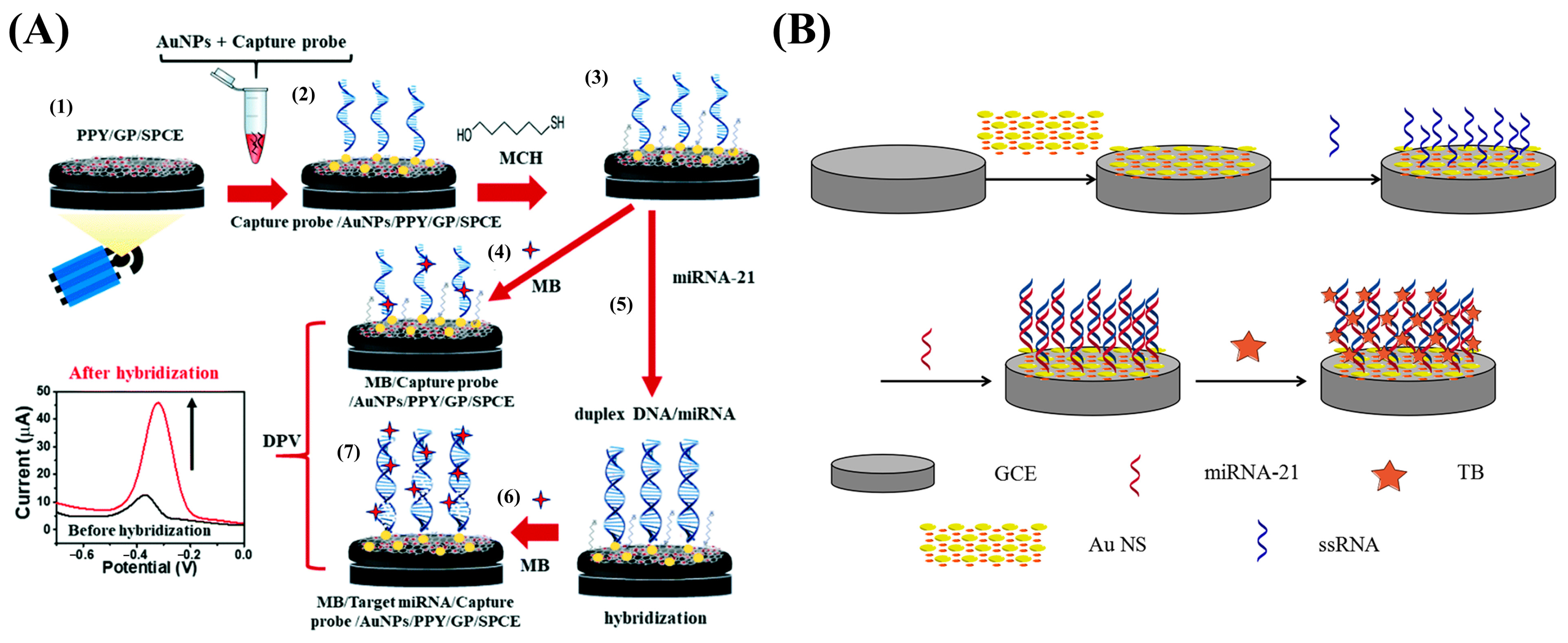
- Pothipor, C.; Aroonyadet, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. A highly sensitive electrochemical microRNA-21 biosensor based on intercalating methylene blue signal amplification and a highly dispersed gold nanoparticles/graphene/polypyrrole composite. Analyst 2021, 146, 2679–2688. [Google Scholar] [CrossRef]
- Tian, L.; Qian, K.; Qi, J.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosens. Bioelectron. 2018, 99, 564–570. [Google Scholar] [CrossRef]
- Kaplan, M.; Kilic, T.; Guler, G.; Mandli, J.; Amine, A.; Ozsoz, M. A novel method for sensitive microRNA detection: Electropolymerization based doping. Biosens. Bioelectron. 2017, 92, 770–778. [Google Scholar] [CrossRef] [PubMed]
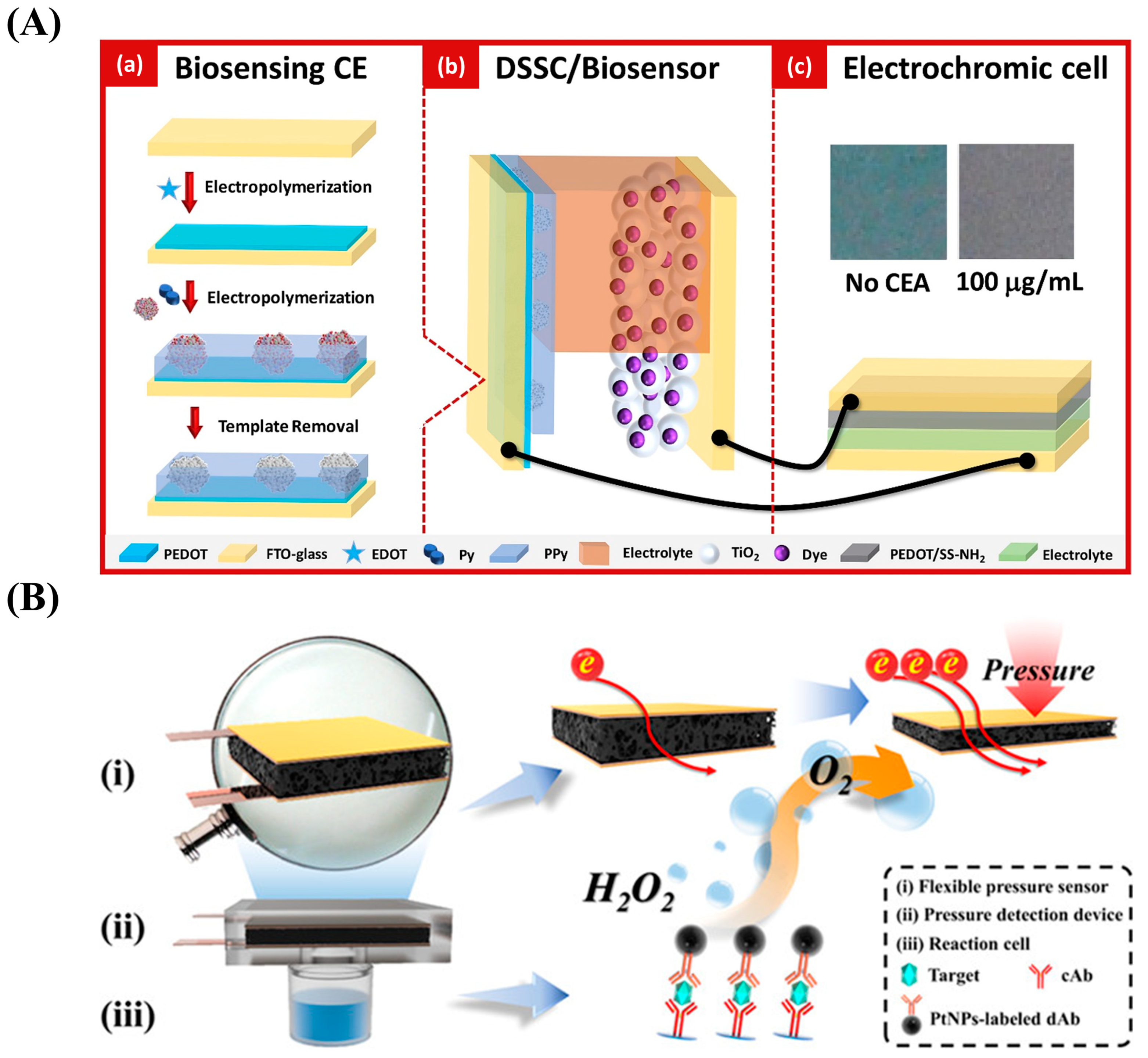
- Song, J.; Teng, H.; Xu, Z.; Liu, N.; Xu, L.; Liu, L.; Gao, F.; Luo, X. Free-standing electrochemical biosensor for carcinoembryonic antigen detection based on highly stable and flexible conducting polypyrrole nanocomposite. Microchim. Acta 2021, 188, 217. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cai, G.; Liu, X.; Tang, D. Platinum Nanozyme-Triggered Pressure-Based Immunoassay Using a Three-Dimensional Polypyrrole Foam-Based Flexible Pressure Sensor. ACS Appl. Mater. Interfaces 2020, 12, 40133–40140. [Google Scholar] [CrossRef] [PubMed]
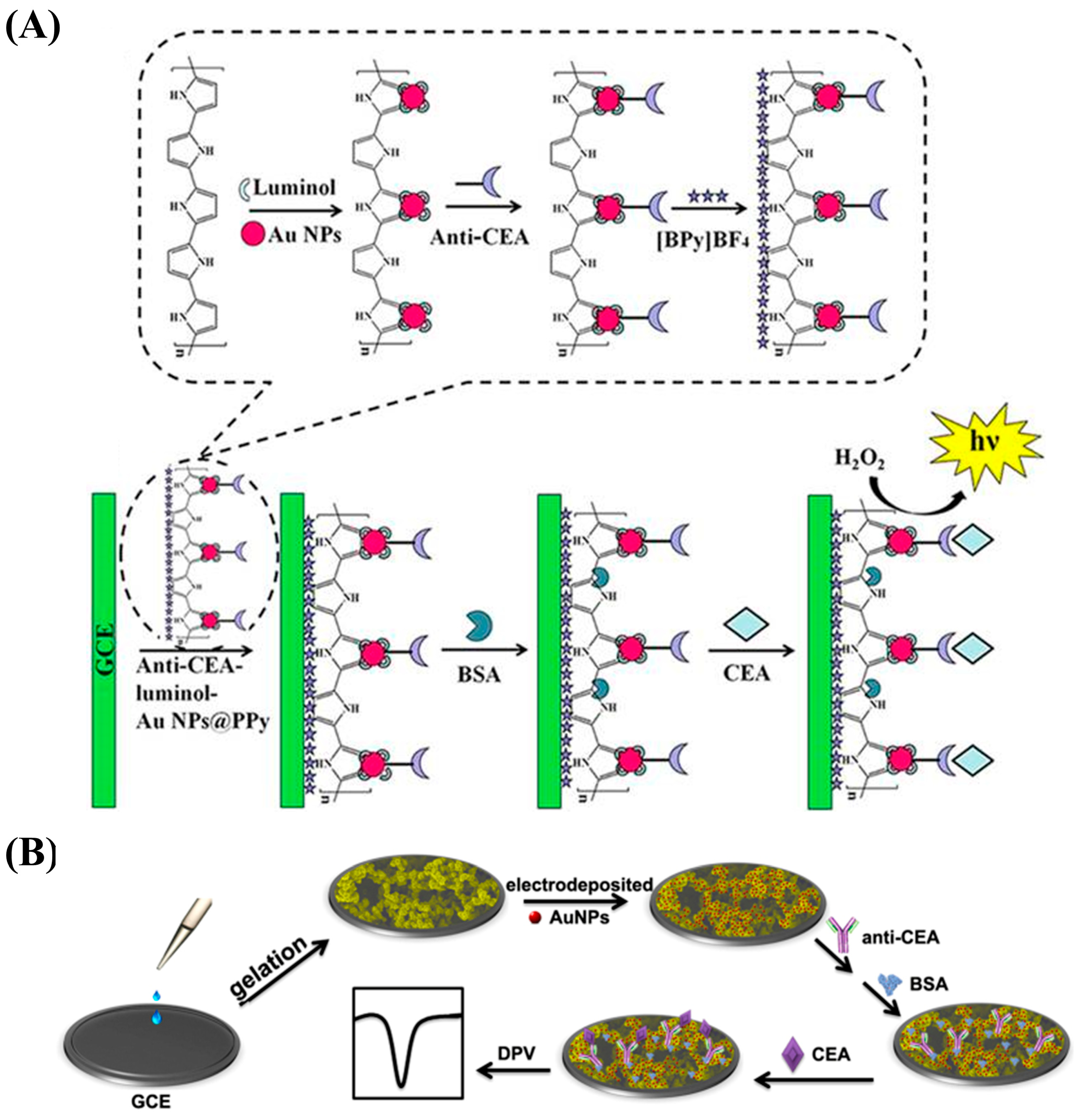
- Zhu, W.; Wang, Q.; Ma, H.; Lv, X.; Wu, D.; Sun, X.; Du, B.; Wei, Q. Single-step cycle pulse operation of the label-free electrochemiluminescence immunosensor based on branched polypyrrole for carcinoembryonic antigen detection. Sci. Rep. 2016, 6, 24599. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Shen, L.; Yu, J.; Ge, S.; Yan, M. Electrochemiluminescence behavior of AgNCs and its application in immunosensors based on PANI/PPy-Ag dendrite-modified electrode. Analyst 2017, 142, 2587–2594. [Google Scholar] [CrossRef]
- Pei, F.; Wang, P.; Ma, E.; Yu, H.; Gao, C.; Yin, H.; Li, Y.; Liu, Q.; Dong, Y. A sandwich-type amperometric immunosensor fabricated by Au@Pd NDs/Fe2+-CS/PPy NTs and Au NPs/NH2-GS to detect CEA sensitively via two detection methods. Biosens. Bioelectron. 2018, 122, 231–238. [Google Scholar] [CrossRef]
- Rong, Q.; Han, H.; Feng, F.; Ma, Z. Network nanostructured polypyrrole hydrogel/Au composites as enhanced electrochemical biosensing platform. Sci. Rep. 2015, 5, 11440. [Google Scholar] [CrossRef]
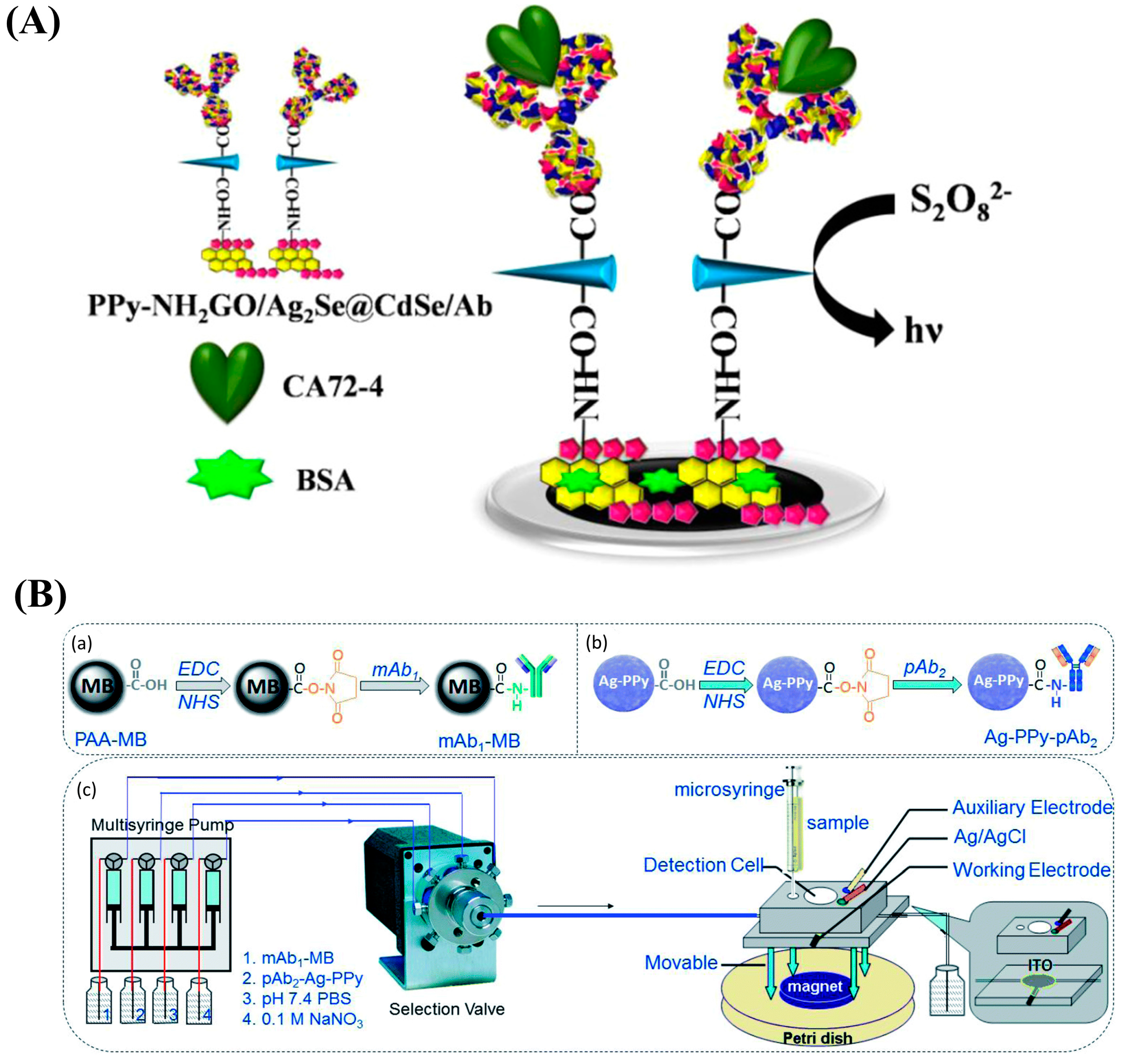
- Lv, X.; Pang, X.; Li, Y.; Yan, T.; Cao, W.; Du, B.; Wei, Q. Electrochemiluminescent Immune-Modified Electrodes Based on Ag2Se@CdSe Nanoneedles Loaded with Polypyrrole Intercalated Graphene for Detection of CA72-4. ACS Appl. Mater. Interfaces 2015, 7, 867–872. [Google Scholar] [CrossRef]
- Huang, J.; Huang, C.; Zhong, W.; Lin, Y. A magneto-controlled microfluidic device for voltammetric immunoassay of carbohydrate antigen-125 with silver–polypyrrole nanotags. Anal. Methods 2020, 12, 4211–4219. [Google Scholar] [CrossRef]
- Rebelo, T.S.C.R.; Costa, R.; Brandão, A.T.S.C.; Silva, A.F.; Sales, M.G.F.; Pereira, C.M. Molecularly imprinted polymer SPE sensor for analysis of CA-125 on serum. Anal. Chim. Acta 2019, 1082, 126–135. [Google Scholar] [CrossRef]
- Tertiş, M.; Ciui, B.; Suciu, M.; Săndulescu, R.; Cristea, C. Label-free electrochemical aptasensor based on gold and polypyrrole nanoparticles for interleukin 6 detection. Electrochim. Acta 2017, 258, 1208–1218. [Google Scholar] [CrossRef]
- Aydın, E.B. Highly sensitive impedimetric immunosensor for determination of interleukin 6 as a cancer biomarker by using conjugated polymer containing epoxy side groups modified disposable ITO electrode. Talanta 2020, 215, 120909. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A novel electrochemical immunosensor based on acetylene black/epoxy-substituted-polypyrrole polymer composite for the highly sensitive and selective detection of interleukin 6. Talanta 2021, 222, 121596. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cruz, A.; Nessark, F.; Lee, M.; Zine, N.; Sigaud, M.; Pruna, R.; Lopez, M.; Marote, P.; Bausells, J.; Jaffrezic-Renault, N.; et al. Efficient fabrication of poly(pyrrole)-nanowires through innovative nanocontact printing, using commercial CD as mold, on flexible thermoplastics substrates: Application for cytokines immunodetection. Sens. Actuators B Chem. 2018, 255, 2520–2530. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Jang, J. A high-performance VEGF aptamer functionalized polypyrrole nanotube biosensor. Biomaterials 2010, 31, 4740–4747. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Hong, J.-Y.; Han, A.-R.; Lee, J.S.; Lee, J.S.; Oh, J.H.; Jang, J. Flexible FET-Type VEGF Aptasensor Based on Nitrogen-Doped Graphene Converted from Conducting Polymer. ACS Nano 2012, 6, 1486–1493. [Google Scholar] [CrossRef]
- Lakemeyer, L.; Sander, S.; Wittau, M.; Henne-Bruns, D.; Kornmann, M.; Lemke, J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases 2021, 9, 21. [Google Scholar] [CrossRef]
- Tavares, A.P.; Truta, L.; Moreira, F.; Carneiro, L.P.; Sales, M.G.F. Self-powered and self-signalled autonomous electrochemical biosensor applied to cancinoembryonic antigen determination. Biosens. Bioelectron. 2019, 140, 111320. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhou, Y.; Sheng, S.; Qian, S.Y.; Huo, X. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci. Rep. 2018, 8, 2732. [Google Scholar] [CrossRef]
- Björkman, K.; Mustonen, H.; Kaprio, T.; Kekki, H.; Pettersson, K.; Haglund, C.; Böckelman, C. CA125: A superior prognostic biomarker for colorectal cancer compared to CEA, CA19-9 or CA242. Tumor Biol. J. Int. Soc. Oncodev. Biol. Med. 2021, 43, 57–70. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J. Expression Levels and Clinical Significance of Serum miR-497, CEA, CA24-2, and HBsAg in Patients with Colorectal Cancer. BioMed Res. Int. 2022, 2022, 3541403. [Google Scholar] [CrossRef] [PubMed]
- Nogués, A.; Gallardo-Vara, E.; Zafra, M.P.; Mate, P.; Marijuan, J.L.; Alonso, A.; Botella, L.M.; Prieto, M.I. Endoglin (CD105) and VEGF as potential angiogenic and dissemination markers for colorectal cancer. World J. Surg. Oncol. 2020, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Hazgui, M.; Weslati, M.; Boughriba, R.; Ounissi, D.; Bacha, D.; Bouraoui, S. MUC1 and MUC5AC implication in Tunisian colorectal cancer patients. Turk. J. Med. Sci. 2021, 51, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y.; Rokkas, T.; Niv, Y.; Rokkas, T. Mucin Expression in Colorectal Cancer (CRC). J. Clin. Gastroenterol. 2019, 53, 434–440. [Google Scholar] [CrossRef]
- Huang, J.; Luo, X.; Lee, I.; Hu, Y.; Cui, X.T.; Yun, M. Rapid real-time electrical detection of proteins using single conducting polymer nanowire-based microfluidic aptasensor. Biosens. Bioelectron. 2011, 30, 306–309. [Google Scholar] [CrossRef]
- Yu, D.; Sun, J.; Weng, Y.; Luo, L.; Sheng, J.; Xu, Z. Serum angiogenin as a potential biomarker for early detection of colorectal adenomas and colorectal cancer. Anti-Cancer Drugs 2021, 32, 703–708. [Google Scholar] [CrossRef]
- Chen, X.; Sun, J.; Wang, X.; Yuan, Y.; Cai, L.; Xie, Y.; Fan, Z.; Liu, K.; Jiao, X. A Meta-Analysis of Proteomic Blood Markers of Colorectal Cancer. Curr. Med. Chem. 2021, 28, 1176–1196. [Google Scholar] [CrossRef]
- Song, Y.F.; Xu, Z.B.; Zhu, X.J.; Tao, X.; Liu, J.L.; Gao, F.L.; Wu, C.L.; Song, B.; Lin, Q. Serum Cyr61 as a potential biomarker for diagnosis of colorectal cancer. Clin. Transl. Oncol. 2017, 19, 519–524. [Google Scholar] [CrossRef]
- Pączek, S.; Łukaszewicz-Zając, M.; Gryko, M.; Mroczko, P.; Kulczyńska-Przybik, A.; Mroczko, B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2040. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Wang, M.; Xuan, M.; Han, S.; Li, C.; Li, M.; Sun, X.; Yu, W.; Zhao, Z. ITGB4 as a novel serum diagnosis biomarker and potential therapeutic target for colorectal cancer. Cancer Med. 2021, 10, 6823–6834. [Google Scholar] [CrossRef]
- Huang, X.; Lan, Y.; Li, E.; Li, J.; Deng, Q.; Deng, X. Diagnostic values of MMP-7, MMP-9, MMP-11, TIMP-1, TIMP-2, CEA, and CA19-9 in patients with colorectal cancer. J. Int. Med. Res. 2021, 49, 03000605211012570. [Google Scholar] [CrossRef] [PubMed]
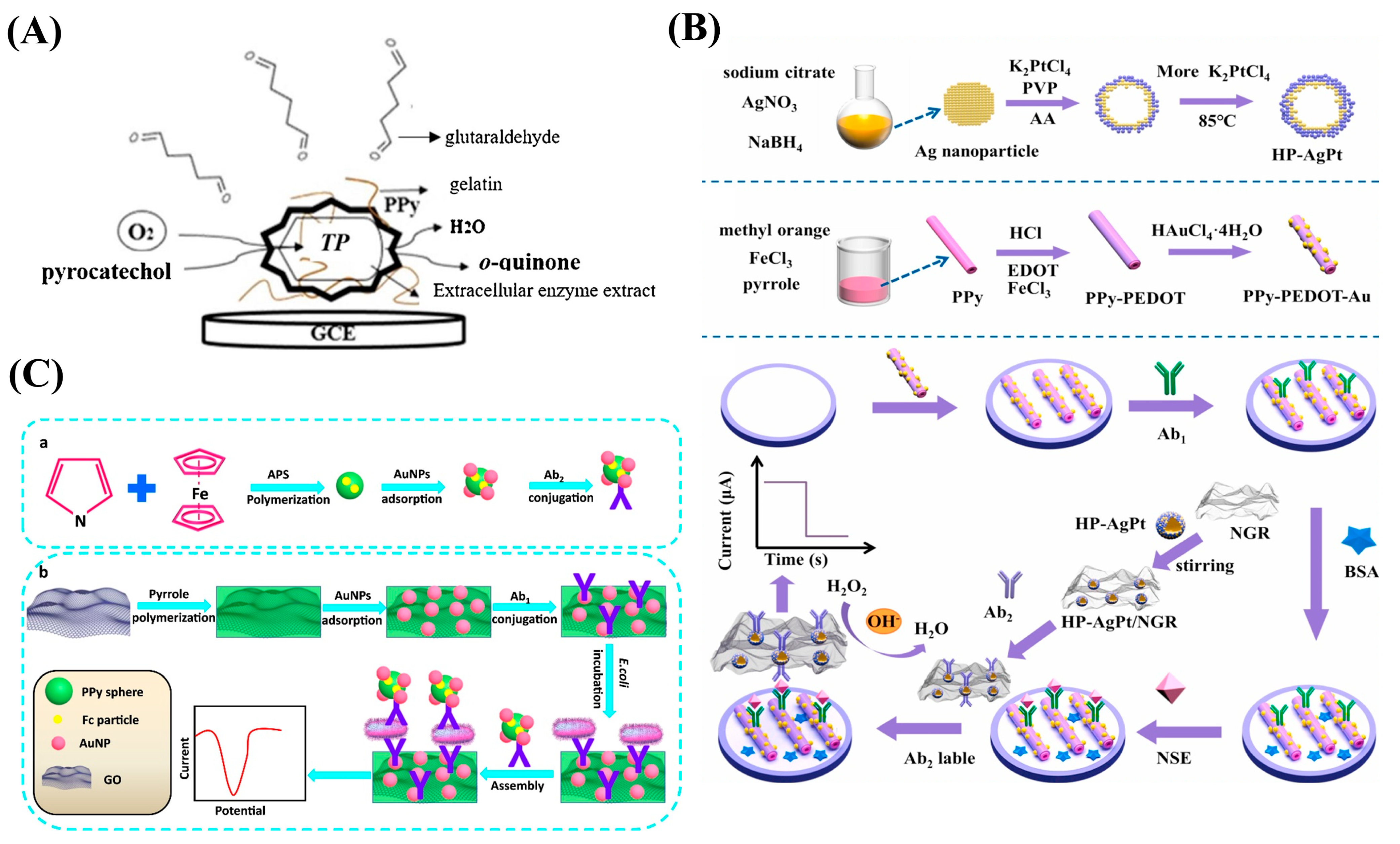
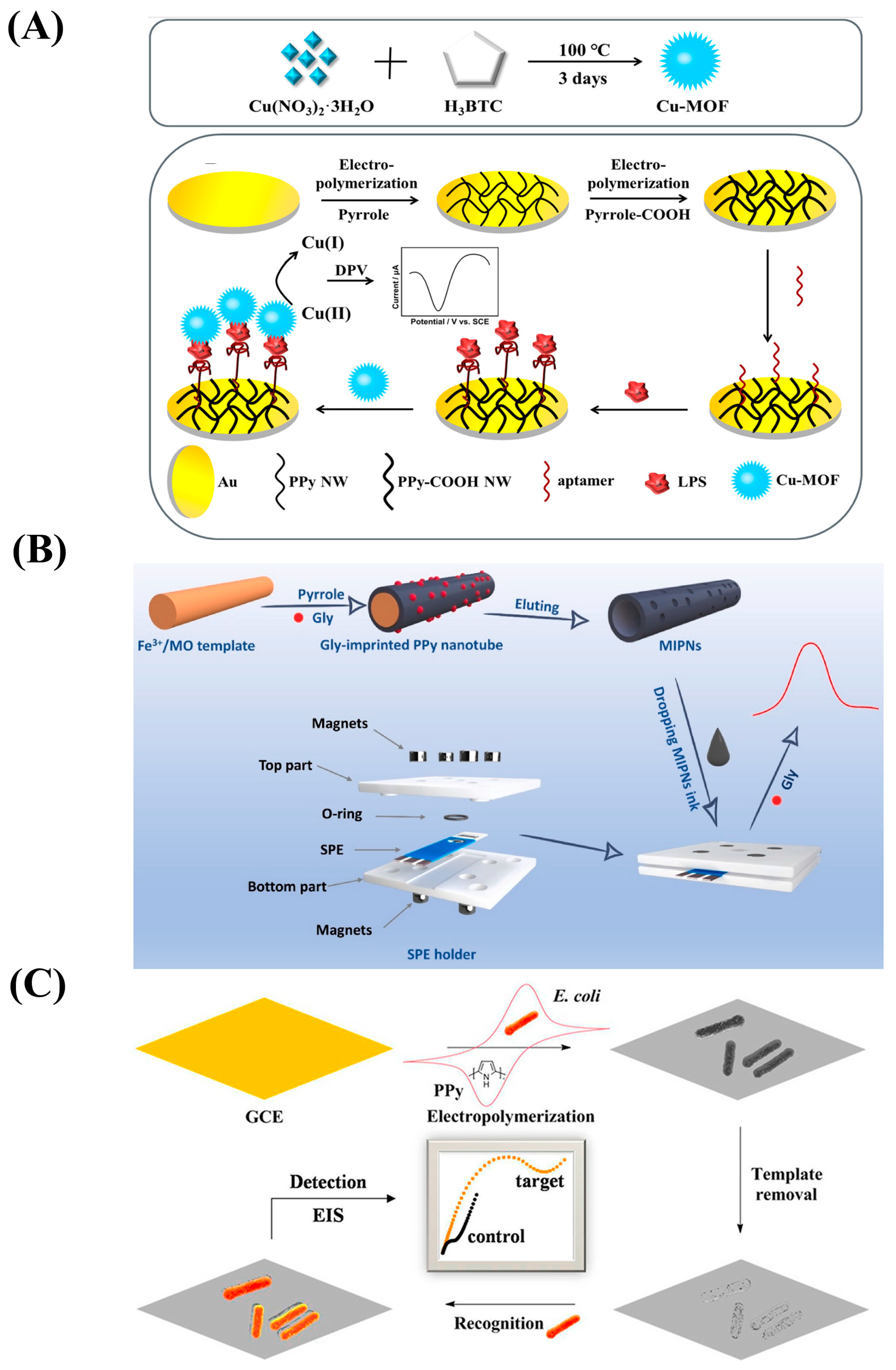
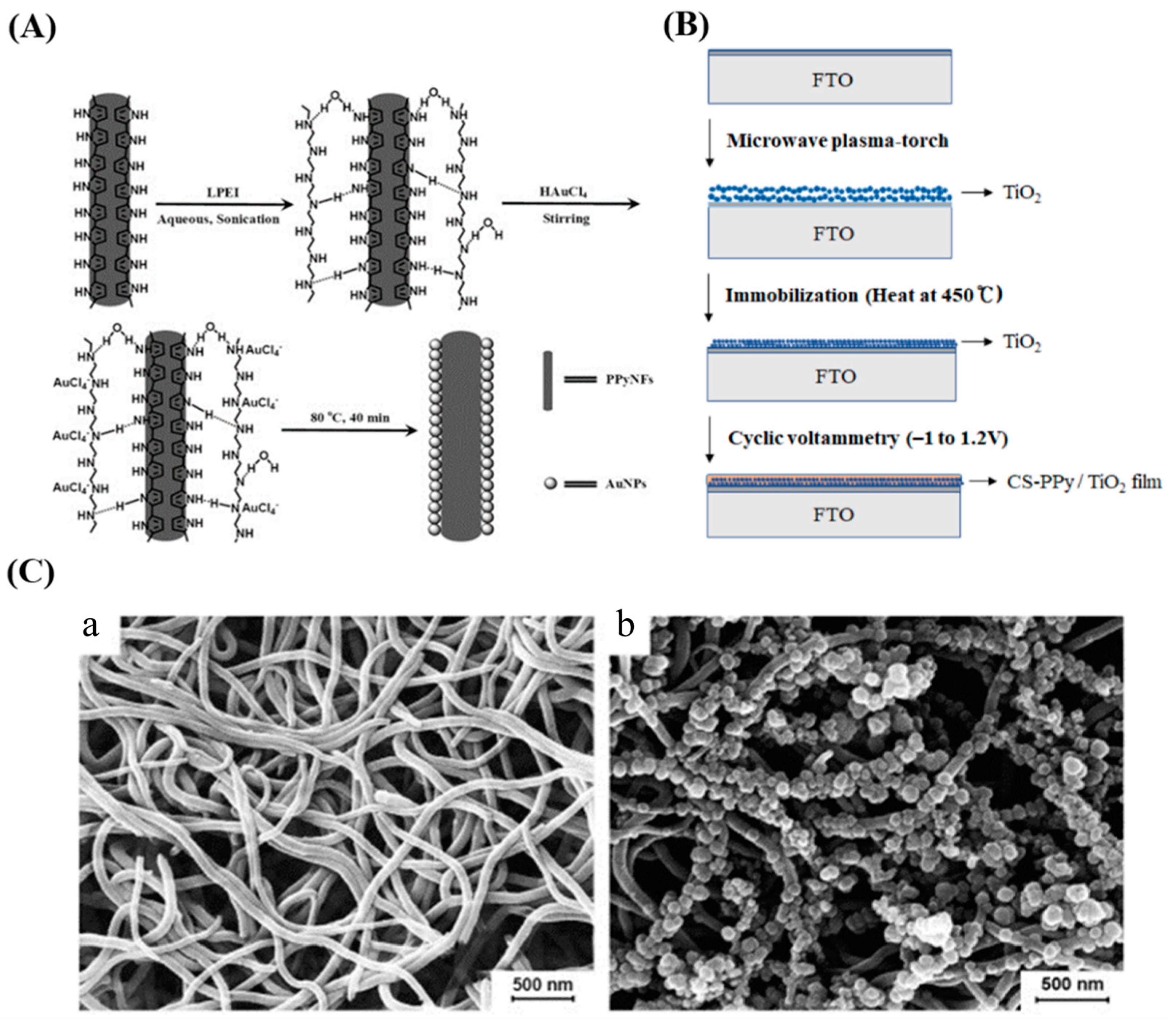
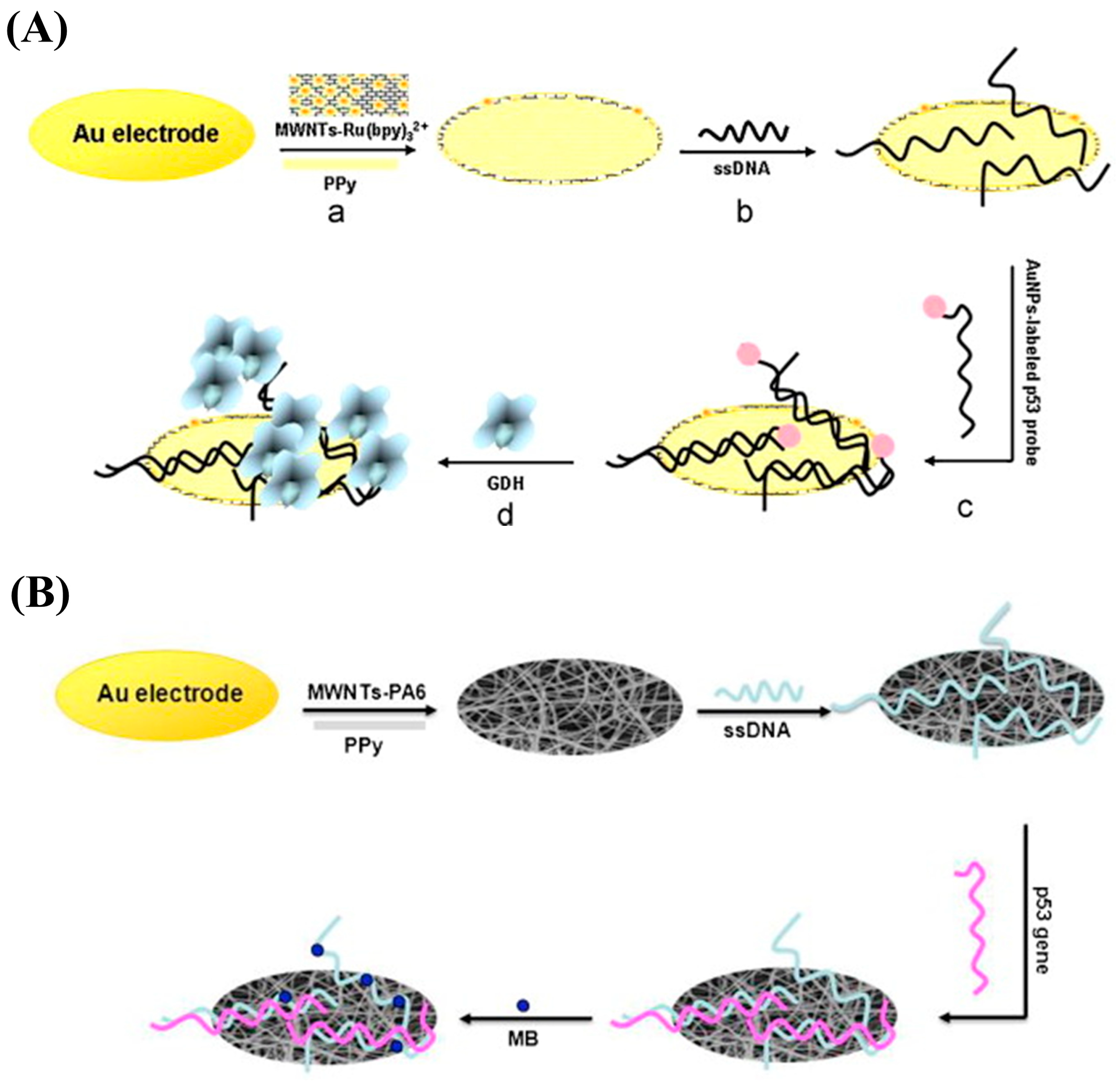


| Conductive Polymers | Ref. |
|---|---|
| Polypyrrole (PPy) | [38,39,40,41] |
| Polyaniline (PANI) | [42,43,44] |
| Polythiophene (PTh) | [45,46,47] |
| Poly(3,4-ethylene dioxythiophene) (PEDOT) | [48,49,50] |
| Type of Biosensor | Function of PPy | Ref. |
|---|---|---|
| Enzyme-based biosensors |
| [100,101,102] |
| Immunobiosensors |
| [103,104,105] |
| Aptamer-based biosensors |
| [106,107] |
| MIP-based biosensors |
| [108,109,110,111] |
| Nanocatalytic biosensors |
| [112,113,114] |
| Protein Biomarkers | Biosensor Components | Detection Method | Detection Range | LOD I | Ref. |
|---|---|---|---|---|---|
| CEA II | 2-NS-PPy III/PEE IV-PPy/2-NS-PPy/AuNP/Apt/CEA | EIS | 10−1–103 ng/mL | 0.033 ng/mL | [142] |
| PPy foam/Cu/ITO V/PET VI/Kapton/PDMS VII/cAb VIII/CEA/PtNP-labeled dAb IX | Resistance determination | 0.2–60 ng/mL | 0.13 ng/mL | [143] | |
| GCE X/PPy@AuNP-luminol-anti-CEA/CEA | ECL | 10−5–10 ng/mL | 3 fg/mL | [144] | |
| ITO/PANI/PPy-Ag/Ab1/CEA/ZnO@AgNC XI-Ab2 | ECL | 10−3–100 ng/mL | 0.4 pg/mL | [145] | |
| AuNP/NH2-GS XII/Ab1/CEA/Au@PdND XIII/Fe2+-CS/PPy NT/Ab2 | i-t/SWV | 5 × 10−5–50 ng/mL | 17 fg/mL | [146] | |
| GCE/PPy hydrogel/AuNP/anti-CEA/CEA | DPV | 10−6–200 ng/mL | 0.16 fg/mL | [147] | |
| CA72-4 XIV | GCE/PPy-NH2GO-Ag2Se@CdSe/Ab/CEA | ECL | 10−4–20 U/mL | 2.1 × 10−5 U/mL | [148] |
| CA125 XV | ITO/MB-mAb1/CEA/PPy-Ag-pAb2 | LSV | 0.001–300 U/mL | 7.6 mU/mL | [149] |
| Au-SPE/MIPPy XVI/CA125 | SWV/SPR XVII | 0.01–500 U/mL | 0.01 U/mL | [150] | |
| IL-6 XVIII | SPGE XIX/PPy/AuPts/Apt XX/IL-6 | EIS | 10−6–15 μg/mL | 0.33 pg/mL | [151] |
| ITO/PPCE XXI/IL-6 receptor/IL-6 | EIS/CV | 0.02–16 pg/mL | 6.0 fg/mL | [152] | |
| ITO/AB XXII/EpxS-PPyr XXIII/IL-6 receptor/IL-6 | EIS/CV | 0.01–50 pg/mL | 3.2 fg/mL | [153] | |
| PEEK XXIV/PETE XXV/PPyNW/mAb/IL-6 | EIS | 1–50 pg/mL | 0.36 pg/mL | [154] | |
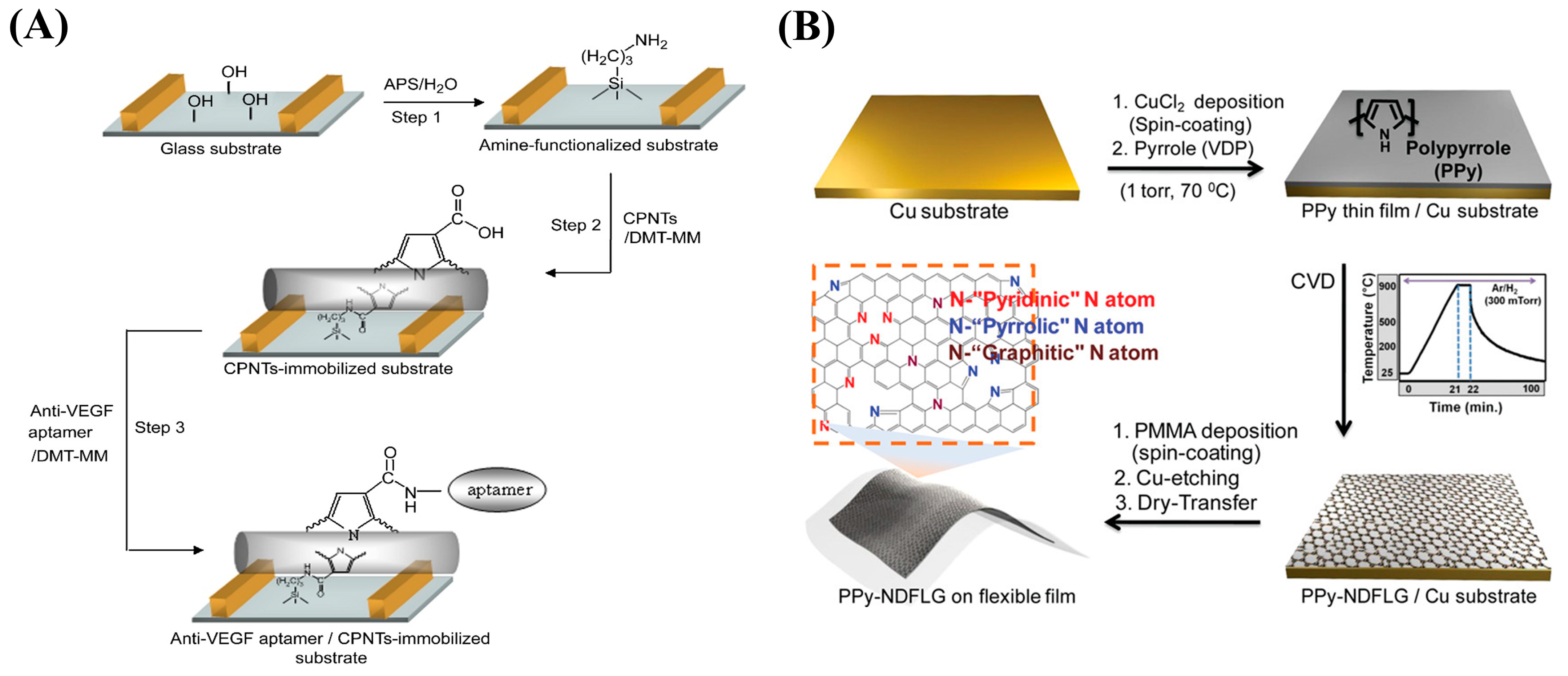
| VEGF XXVI | Glass substrate/CPNT XXVII/Apt/VEGF | FET XXVIII | - | 400 fM | [155] |
| Flexible substrate/PPy-NDFLG XXIX/Apt/VEGF | FET | - | 100 fM | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Tan, X.; Wang, P.; Qin, J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials 2023, 13, 674. https://doi.org/10.3390/nano13040674
Zhang X, Tan X, Wang P, Qin J. Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials. 2023; 13(4):674. https://doi.org/10.3390/nano13040674
Chicago/Turabian StyleZhang, Xindan, Xiao Tan, Ping Wang, and Jieling Qin. 2023. "Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer" Nanomaterials 13, no. 4: 674. https://doi.org/10.3390/nano13040674
APA StyleZhang, X., Tan, X., Wang, P., & Qin, J. (2023). Application of Polypyrrole-Based Electrochemical Biosensor for the Early Diagnosis of Colorectal Cancer. Nanomaterials, 13(4), 674. https://doi.org/10.3390/nano13040674