Construction of Nanostructured Glass-Zirconia to Improve the Interface Stability of Dental Bilayer Zirconia
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoscale Glass Powder
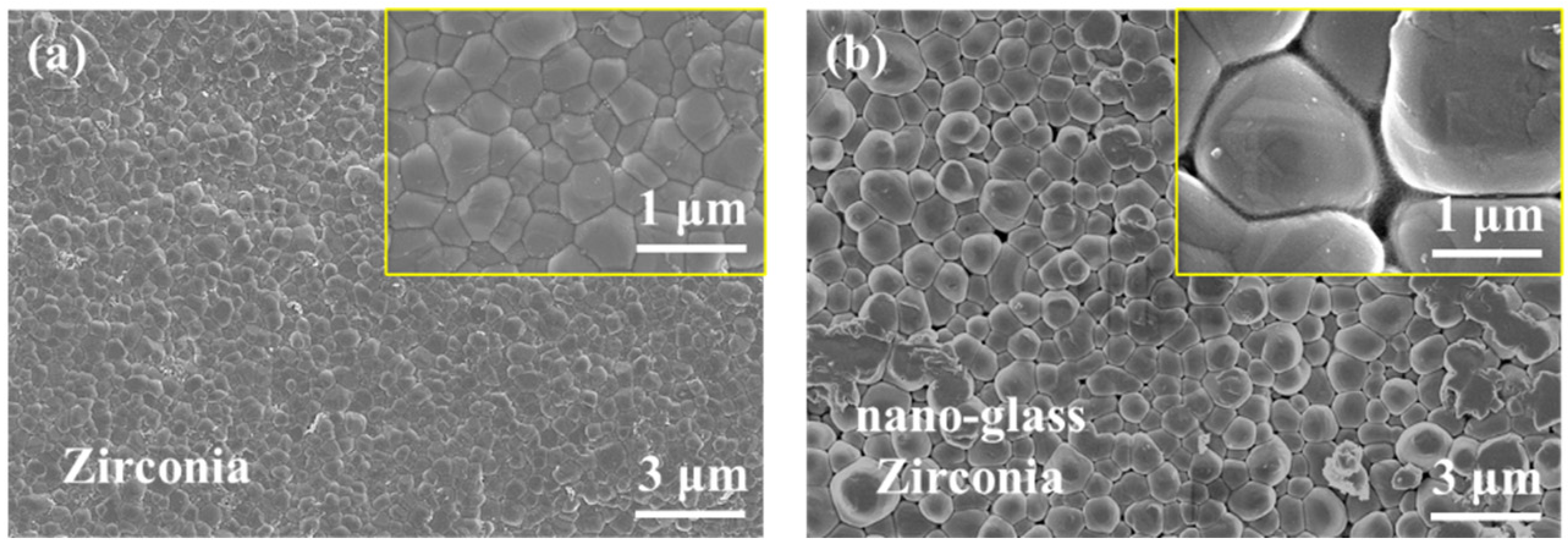
2.2. Construction of Nanostructured Glass-Zirconia
2.3. Characterization of Nanostructured Glass-Zirconia
2.3.1. Observation of the Microscopic Morphology
2.3.2. X-ray Diffraction Spectroscopy (XRD)
2.3.3. Roughness Assessment
2.3.4. Wettability
2.4. Fabrication of Bilayer Zirconia Specimens
2.5. Shear Bonding Strength (SBS) and Failure Mode Analysis
2.6. Evaluation of Bonding Interface
2.7. Statistical Analysis
3. Results and Discussion
- (a)
- Adhesive failure: the failure occurred at interface of zirconia and porcelain.
- (b)
- Cohesive failure: the failure occurred inside the porcelain.
- (c)
- Mixed failure: both a and b failure modes exist simultaneously.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spitznagel, F.A.; Boldt, J.; Gierthmuehlen, P.C. CAD/CAM ceramic restorative materials for natural teeth. J. Dent. Res. 2018, 97, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Meng, M.; Chai, Z.Z.; Zhang, Y.M.; Li, D.; Niu, L.A.; Jia, Y.M.; Zhang, S.F.; Wang, F. Dynamic wear characteristics and fracture strength of high-translucent monolithic zirconia crowns. Ceram. Int. 2022, 48, 11298–11303. [Google Scholar] [CrossRef]
- Shelar, P.; Abdolvand, H.; Butler, S. On the behaviour of zirconia-based dental materials: A review. J. Mech. Behav. Biomed. Mater. 2021, 124, 104861. [Google Scholar] [CrossRef] [PubMed]
- Shahmiri, R.; Standard, O.C.; Hart, J.N.; Sorrell, C.C. Optical properties of zirconia ceramics for esthetic dental restorations: A systematic review. J. Prosthet. Dent. 2018, 119, 36–46. [Google Scholar] [CrossRef] [PubMed]
- De Lima, E.; Meira, J.B.C.; Özcan, M.; Cesar, P.F. Chipping of veneering ceramics in zirconium dioxide fixed dental prosthesis. Curr. Oral Health Rep. 2015, 2, 169–173. [Google Scholar] [CrossRef]
- Marefati, M.T.; Hadian, A.M.; Hooshmand, T.; Yekta, B.E.; Koohkan, R. Wettability of zirconia by feldspathic veneer in dental restorations: Effect of firing atmosphere and surface roughness. Ceram. Int. 2018, 44, 4307–4312. [Google Scholar] [CrossRef]
- Yan, Y.; Ji, Y.; Yan, J.; Hu, X.; Zhang, Q.; Liu, M.; Zhang, F. Atomic layer deposition SiO2 films over dental ZrO2 towards strong adhesive to resin. J. Mech. Behav. Biomed. Mater. 2021, 114, 104197. [Google Scholar] [CrossRef]
- Li, K.; Kou, H.; Rao, J.; Liu, C.; Ning, C. Fabrication of enamel-like structure on polymer-infiltrated zirconia ceramics. Dent. Mater. 2021, 37, e245–e255. [Google Scholar] [CrossRef]
- Silva-Herzog Rivera, D.; Pozos-Guillen, A.; Aragon-Pina, A.; Cerda-Cristerna, B.I.; Masuoka-Ito, D.; Sanchez-Vargas, L.O. Glass coatings to enhance the interfacial bond strength between veneering ceramic and zirconia. Odontology 2020, 108, 415–423. [Google Scholar] [CrossRef]
- Abdullah, A.O.; Hui, Y.; Sun, X.; Pollington, S.; Muhammed, F.K.; Liu, Y. Effects of different surface treatments on the shear bond strength of veneering ceramic materials to zirconia. J. Adv. Prosthodont. 2019, 11, 65–74. [Google Scholar] [CrossRef]
- Moon, J.E.; Kim, S.H.; Lee, J.B.; Han, J.S.; Yeo, I.S.; Ha, S.R. Effects of airborne-particle abrasion protocol choice on the surface characteristics of monolithic zirconia materials and the shear bond strength of resin cement. Ceram. Int. 2016, 42, 1552–1562. [Google Scholar] [CrossRef]
- Garcia Fonseca, R.; de Oliveira Abi-Rached, F.; dos Santos Nunes Reis, J.M.; Rambaldi, E.; Baldissara, P. Effect of particle size on the flexural strength and phase transformation of an airborne-particle abraded yttria-stabilized tetragonal zirconia polycrystal ceramic. J. Prosthet. Dent. 2013, 110, 510–514. [Google Scholar] [CrossRef]
- Kim, H.K.; Yoo, K.W.; Kim, S.J.; Jung, C.H. Phase transformations and subsurface changes in three dental zirconia grades after sandblasting with various Al2O3 particle sizes. Materials 2021, 14, 5321. [Google Scholar] [CrossRef]
- Ji, M.; Xu, J.; Chen, M.; El Mansori, M. Enhanced hydrophilicity and tribological behavior of dental zirconia ceramics based on picosecond laser surface texturing. Ceram. Int. 2020, 46, 7161–7169. [Google Scholar] [CrossRef]
- Santos, R.L.P.; Silva, F.S.; Nascimento, R.M.; Souza, J.C.M.; Motta, F.V.; Carvalho, O.; Henriques, B. Shear bond strength of veneering porcelain to zirconia: Effect of surface treatment by CNC-milling and composite layer deposition on zirconia. J. Mech. Behav. Biomed. Mater. 2016, 60, 547–556. [Google Scholar] [CrossRef]
- Ruyter, E.I.; Vajeeston, N.; Knarvang, T.; Kvam, K. A novel etching technique for surface treatment of zirconia ceramics to improve adhesion of resin-based luting cements. Acta Biomater. Odontol. Scand. 2017, 3, 36–46. [Google Scholar] [CrossRef]
- Yoon, H.I.; Yeo, I.S.; Yi, Y.J.; Kim, S.H.; Lee, J.B.; Han, J.S. Effect of various intermediate ceramic layers on the interfacial stability of zirconia core and veneering ceramics. Acta Odontol. Scand. 2015, 73, 488–495. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, C.J.; Cho, L.R.; Huh, Y.H. Evaluation of the ceramic liner bonding effect between zirconia and lithium disilicate. J. Prosthet. Dent. 2018, 120, 282–289. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Bian, C.; Kong, H. Effect of zirconia surface treatment on zirconia/veneer interfacial toughness evaluated by fracture mechanics method. J. Dent. 2014, 42, 808–815. [Google Scholar] [CrossRef]
- Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L. Adhesion to zirconia: A systematic review of current conditioning methods and bonding materials. Dent. J. 2019, 7, 74. [Google Scholar] [CrossRef]
- Bitencourt, S.B.; Dos Santos, D.M.; da Silva, E.V.F.; Barao, V.A.R.; Rangel, E.C.; da Cruz, N.C.; de Souza, G.M.; Goiato, M.C.; Pesqueira, A.A. Characterisation of a new plasma-enhanced film to improve shear bond strength between zirconia and veneering ceramic. Mat. Sci. Eng. C Mater. 2018, 92, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.Y.; Kang, C.M.; Feng, S.W.; Hung, C.Y.; Iwaguro, S.; Lin, D.J. Effects of glass-ceramic spray deposition manipulation on the surface characteristics of zirconia dental restorations. Ceram. Int. 2022, 48, 29873–29881. [Google Scholar] [CrossRef]
- Ali, N.; Safwat, A.; Aboushelib, M. The effect of fusion sputtering surface treatment on microshear bond strength of zirconia and MDP-containing resin cement. Dent. Mater. 2019, 35, e107–e112. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N. Fusion sputtering for bonding to zirconia-based materials. J. Adhes. Dent. 2012, 14, 323–328. [Google Scholar]
- Zhang, Y.; Kim, J.W. Graded structures for damage resistant and aesthetic all-ceramic restorations. Dent. Mater. 2009, 25, 781–790. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, H.; Lee, J.J.W.; Lawn, B.R. Chipping resistance of graded zirconia ceramics for dental crowns. J. Dent. Res. 2012, 91, 311–315. [Google Scholar] [CrossRef]
- Chai, H.; Lee, J.J.W.; Mieleszko, A.J.; Chu, S.J.; Zhang, Y. On the interfacial fracture of porcelain/zirconia and graded zirconia dental structures. Acta Biomater. 2014, 10, 3756–3761. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L. Optimization of ceramic strength using elastic gradients. Acta Mater. 2009, 57, 2721–2729. [Google Scholar] [CrossRef]
- Zhang, Y.; Chai, H.; Lawn, B.R. Graded structures for all-ceramic restorations. J. Dent. Res. 2010, 89, 417–421. [Google Scholar] [CrossRef]
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration curve for quantitative analysis of the monoclinic-tetragonal ZrO2 system by X-ray diffraction. J. Am. Ceram. Soc. 1984, 67, C-119–C-121. [Google Scholar]
- Garvie, R.C.; Nicholson, P.S. Phase analysis in zirconia systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- Tong, H.; Tanaka, C.B.; Kaizer, M.R.; Zhang, Y. Characterization of three commercial Y-TZP ceramics produced for their high-translucency, high-strength and high-surface area. Ceram. Int. 2016, 42, 1077–1085. [Google Scholar] [CrossRef]
- Catramby, M.F.; do Vale, A.L.; Dos Santos, H.E.S.; Elias, C.N. Effect of sintering process on microstructure, 4-point flexural strength, and grain size of yttria-stabilized tetragonal zirconia polycrystal for use in monolithic dental restorations. J. Prosthet. Dent. 2021, 125, 824.e1–824.e8. [Google Scholar] [CrossRef]
- Chai, H.; Mieleszko, A.J.; Chu, S.J.; Zhang, Y. Using glass-graded zirconia to increase delamination growth resistance in porcelain/zirconia dental structures. Dent. Mater. 2018, 34, e8–e14. [Google Scholar] [CrossRef]
- Yan, M.; Csík, A.; Yang, C.C.; Luo, Y.; Fodor, T.; Ding, S.J. Synergistic reinforcement of surface modification on improving the bonding of veneering ceramics to zirconia. Ceram. Int. 2018, 44, 19665–19671. [Google Scholar] [CrossRef]
- Mahadik, D.B.; Rao, A.V.; Rao, A.P.; Wagh, P.B.; Ingale, S.V.; Gupta, S.C. Effect of concentration of trimethylchlorosilane (TMCS) and hexamethyldisilazane (HMDZ) silylating agents on surface free energy of silica aerogels. J. Colloid Interface Sci. 2011, 356, 298–302. [Google Scholar] [CrossRef]
- Neis, C.A.; Albuquerque, N.L.; Albuquerque Ide, S.; Gomes, E.A.; Souza-Filho, C.B.; Feitosa, V.P.; Spazzin, A.O.; Bacchi, A. Surface treatments for repair of feldspathic, leucite - and lithium disilicate-reinforced glass ceramics using composite resin. Braz. Dent. J. 2015, 26, 152–155. [Google Scholar] [CrossRef]
- Jin, C.; Wang, J.; Huang, Y.; Yu, P.; Xiong, Y.; Yu, H.; Gao, S. Effects of hydrofluoric acid concentration and etching time on the bond strength to ceramic-coated zirconia. J. Adhes. Dent. 2022, 24, 125–136. [Google Scholar]
- Bitencourt, S.B.; Hatton, B.D.; Bastos-Bitencourt, N.A.; Dos Santos, D.M.; Pesqueira, A.A.; De Souza, G.M. Silica deposition on zirconia via room-temperature atomic layer deposition (RT-ALD): Effect on bond strength to veneering ceramic. J. Mech. Behav. Biomed. Mater. 2022, 129, 105142. [Google Scholar] [CrossRef]
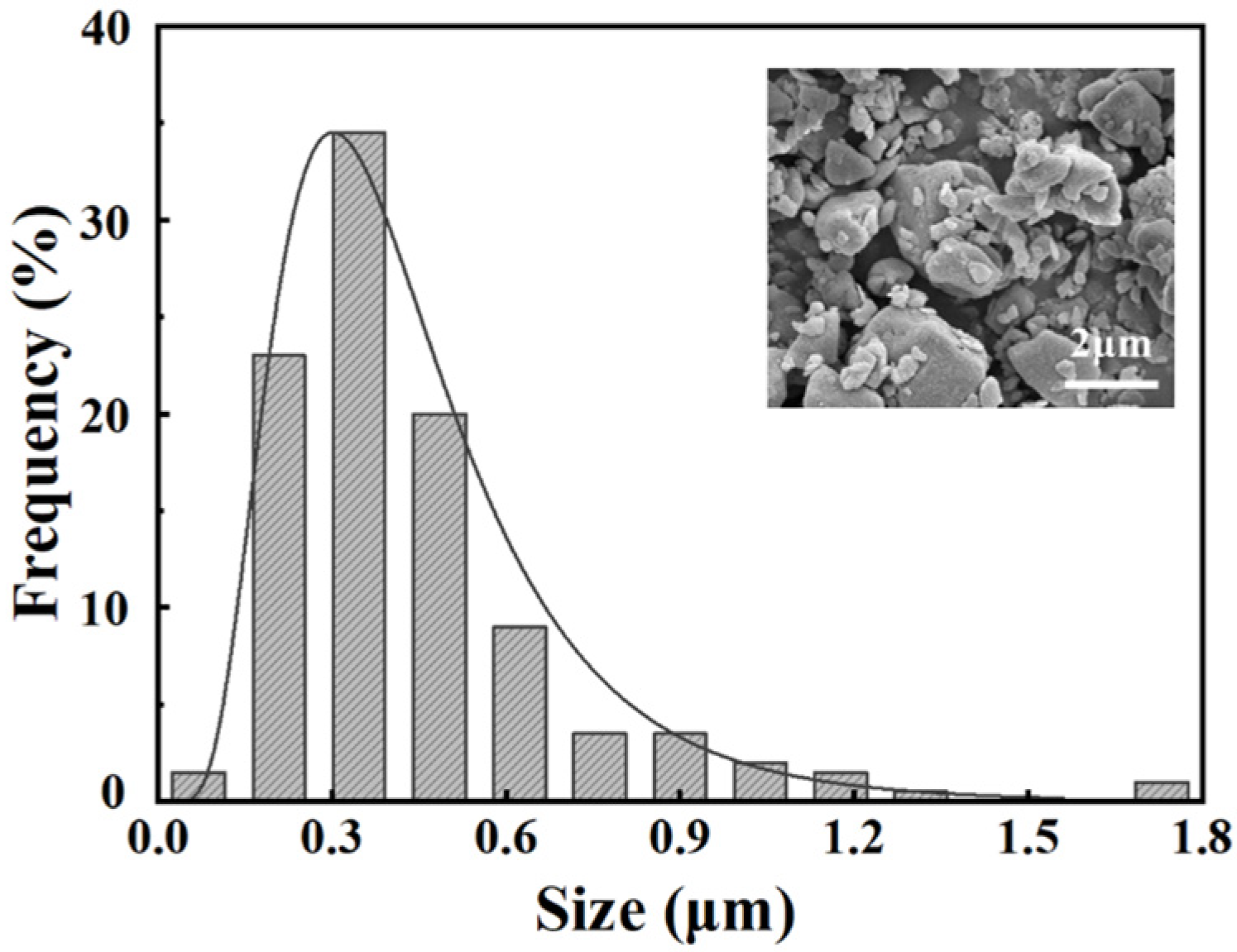






| SiO2 | Li2CO3 | Al2O3 | K2CO3 | ZrO2 | P2O5 | Others |
|---|---|---|---|---|---|---|
| 58.0 | 27.0 | 10.0 | 1.8 | 1.5 | 1.2 | 0.5 |
| Temperature (°C) | 20~900 | 900~900 | 900~1500 | 1500~1500 | 1500~900 | 900~20 |
|---|---|---|---|---|---|---|
| Time (h) | 1.5 | 0.5 | 3 | 2 | 1 | 1.8 |
| Groups | Surface Treatment | Veneering Techniques |
|---|---|---|
| A (ngZirL) | Glass infiltration | hand-layering |
| B (ngZirP) | Glass infiltration | heat-pressing |
| C (sZirL) | Sandblast | hand-layering |
| D (sZirP) | Sandblast | heat-pressing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Zhang, X.; Zhang, Y.; Li, D.; Zhao, Z.; Wang, Q.; Tang, K.; Niu, L.; Wang, F. Construction of Nanostructured Glass-Zirconia to Improve the Interface Stability of Dental Bilayer Zirconia. Nanomaterials 2023, 13, 678. https://doi.org/10.3390/nano13040678
Zhou M, Zhang X, Zhang Y, Li D, Zhao Z, Wang Q, Tang K, Niu L, Wang F. Construction of Nanostructured Glass-Zirconia to Improve the Interface Stability of Dental Bilayer Zirconia. Nanomaterials. 2023; 13(4):678. https://doi.org/10.3390/nano13040678
Chicago/Turabian StyleZhou, Ming, Xiaoyu Zhang, Yaming Zhang, Ding Li, Zhe Zhao, Qing Wang, Kai Tang, Lina Niu, and Fu Wang. 2023. "Construction of Nanostructured Glass-Zirconia to Improve the Interface Stability of Dental Bilayer Zirconia" Nanomaterials 13, no. 4: 678. https://doi.org/10.3390/nano13040678
APA StyleZhou, M., Zhang, X., Zhang, Y., Li, D., Zhao, Z., Wang, Q., Tang, K., Niu, L., & Wang, F. (2023). Construction of Nanostructured Glass-Zirconia to Improve the Interface Stability of Dental Bilayer Zirconia. Nanomaterials, 13(4), 678. https://doi.org/10.3390/nano13040678







