Surface Tamm States of 2–5 nm Nanodiamond via Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Computational Details
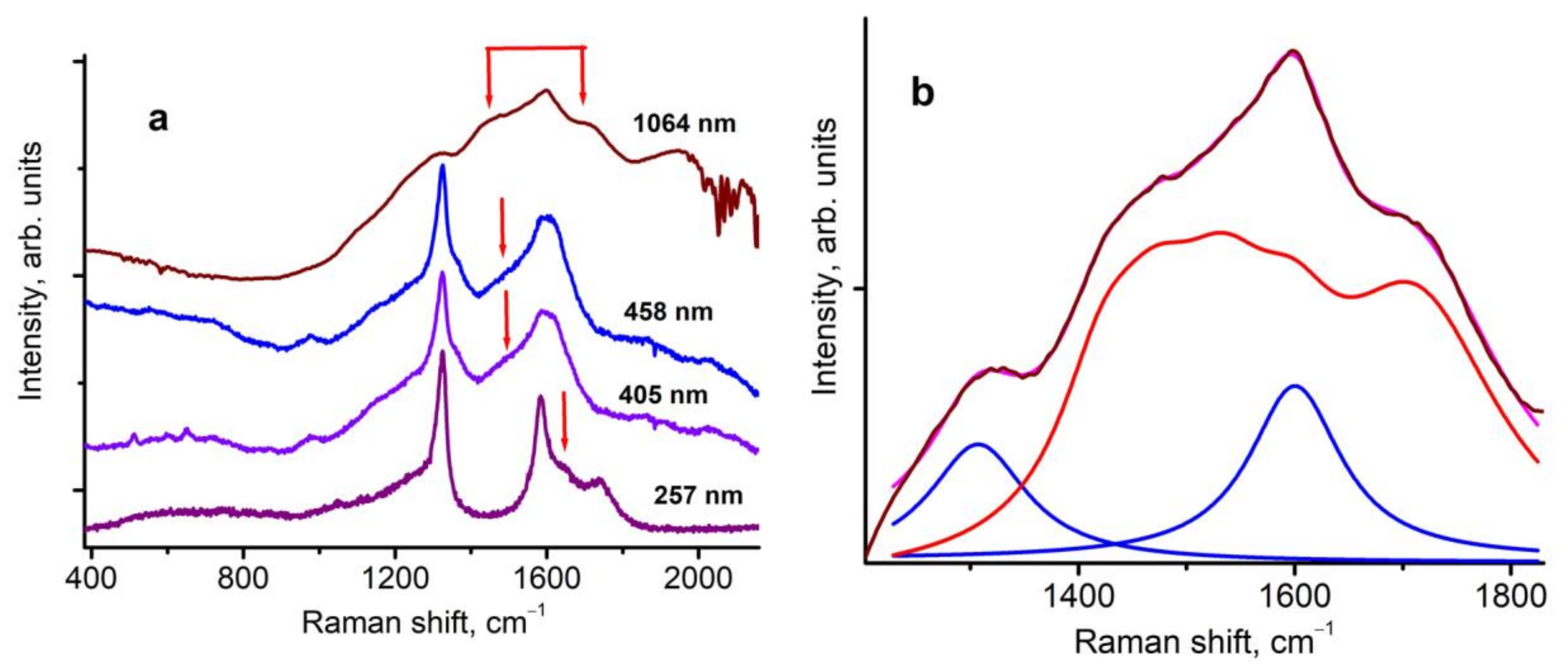
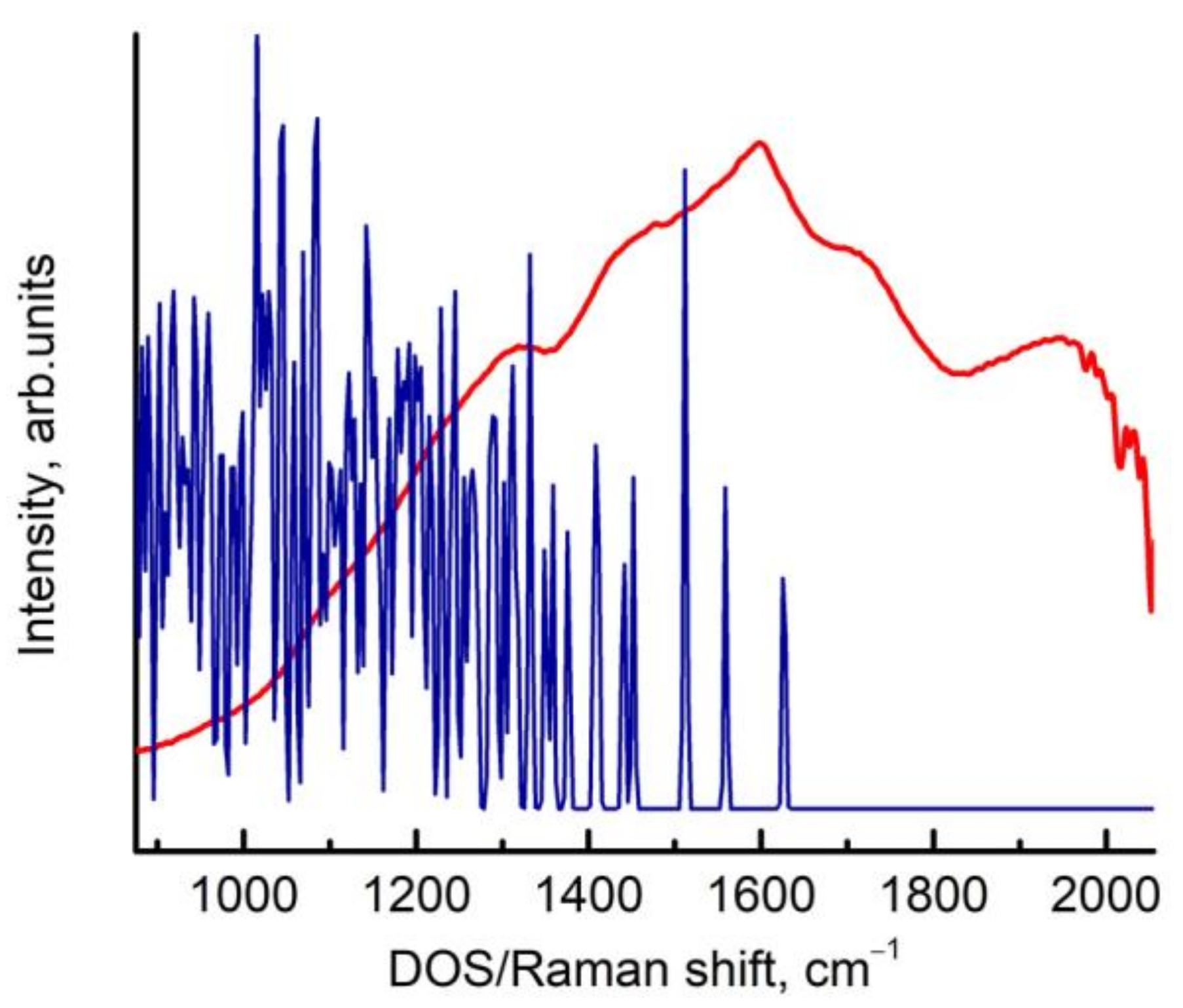
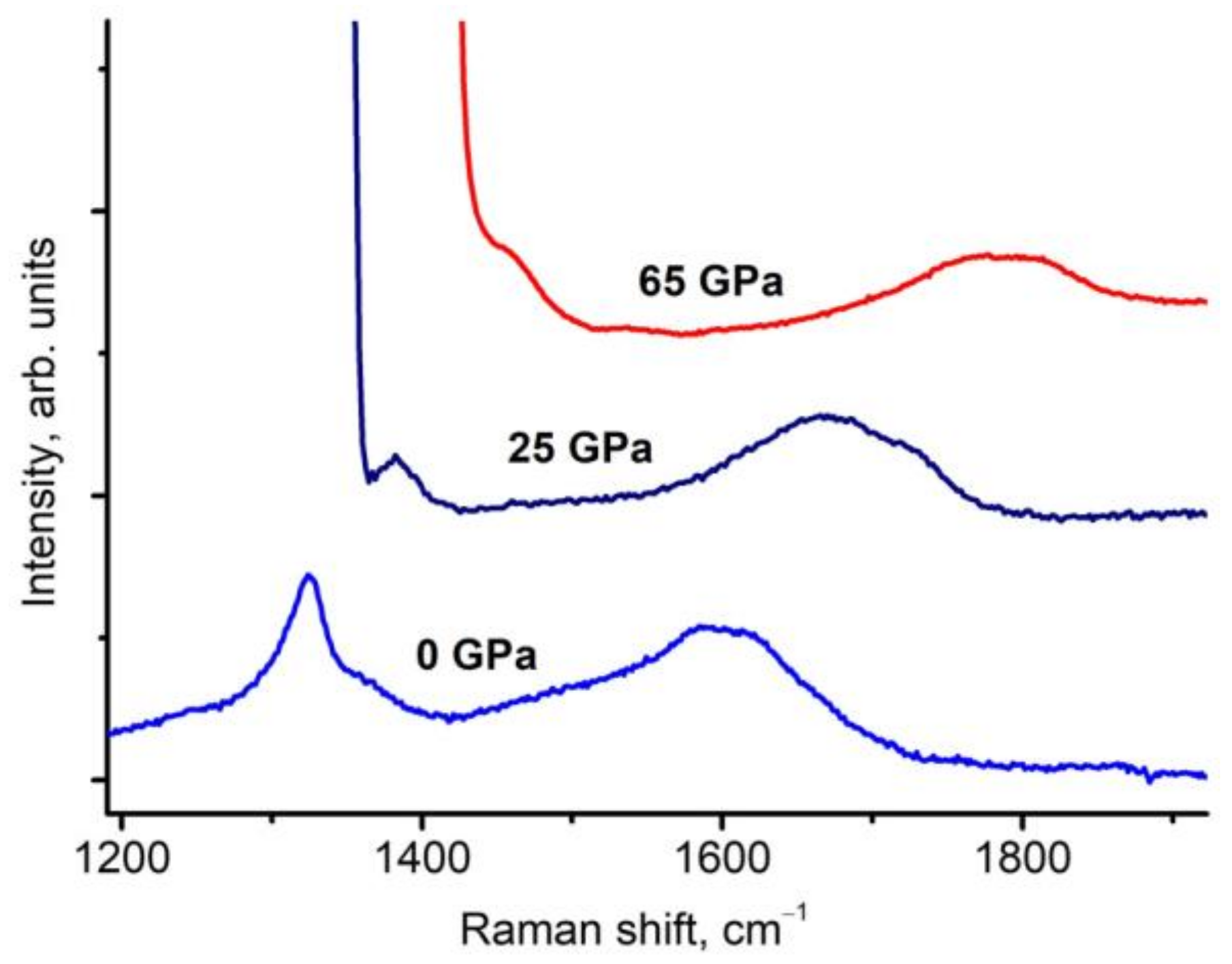
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popov, M.; Churkin, V.; Ovsyannikov, D.; Khabibrakhmanov, A.; Kirichenko, A.; Skryleva, E.; Parkhomenko, Y.; Kuznetsov, M.; Nosukhin, S.; Sorokin, P.; et al. Ultrasmall diamond nanoparticles with unusual incompressibility. Diam. Relat. Mater. 2019, 96, 52–57. [Google Scholar] [CrossRef]
- Popov, M.; Churkin, V.; Kirichenko, A.; Denisov, V.; Ovsyannikov, D.; Kulnitskiy, B.; Perezhogin, I.; Aksenenkov, V.; Blank, V. Raman spectra and bulk modulus of nanodiamond in a size interval of 2-5 nm. Nanoscale Res. Lett. 2017, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Popov, M.; Kulnitskiy, B.; Blank, V. Superhard materials, based on fullerenes and nanotubes. In Comprehensive Hard Materials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 515–538. [Google Scholar]
- Huang, Q.; Yu, D.; Xu, B.; Hu, W.; Ma, Y.; Wang, Y.; Zhao, Z.; Wen, B.; He, J.; Liu, Z.; et al. Nanotwinned diamond with unprecedented hardness and stability. Nature 2014, 510, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yaan, X.; Yu, P.; Hu, Q.; Wang, M.; Yao, Y.; Wu, L.; Zou, Q.; Ke, Y.; Zhao, Y.; et al. Revealing the formation mechanism of ultrahard nanotwinned diamond from onion carbon. Carbon 2018, 129, 159–167. [Google Scholar] [CrossRef]
- Vlk, A.; Ledinsky, M.; Shiryaev, A.; Ekimov, E.; Stehlik, S. Nanodiamond Size from Low-Frequency Acoustic Raman Modes. J. Phys. Chem. C 2022, 126, 6318–6324. [Google Scholar] [CrossRef]
- Ekimov, E.; Shiryaev, A.A.; Grigoriev, Y.; Averin, A.; Shagieva, E.; Stehlik, S.; Kondrin, M. Size-Dependent Thermal Stability and Optical Properties of Ultra-Small Nanodiamonds Synthesized under High Pressure. Nanomaterials 2022, 12, 351. [Google Scholar] [CrossRef]
- Mermoux, M.; Chang, S.; Girard, H.A.; Arnault, J.-C. Raman spectroscopy study of detonation nanodiamond. Diam. Relat. Mater. 2018, 87, 248–260. [Google Scholar] [CrossRef]
- Profeta, M.; Mauri, F. Theory of resonant Raman scattering of tetrahedral amorphous carbon. Phys. Rev. B 2001, 63, 245415. [Google Scholar] [CrossRef]
- Belobrov, P.I.; Bursill, L.A.; Maslakov, K.I.; Dementjev, A.P. Electron spectroscopy of nanodiamond surface states. Appl. Surf. Sci. 2003, 215, 169–177. [Google Scholar] [CrossRef]
- Fang, X.; Mao, J.; Levin, E.M.; Schmidt-Rohr, K. Nonaromatic Core-Shell structure of nanodiamond from Solid-State NMR spectroscopy. J. Am. Chem. Soc. 2009, 131, 1426–1435. [Google Scholar] [CrossRef]
- Tamm, I. On the possible bound states of electrons on a crystal surface. Phys. Z. Sowjetunion 1932, 1, 733–735. [Google Scholar]
- Pepper, S.V. Diamond (111) studied by electron energy loss spectroscopy in the characteristic loss region. Surf. Sci. 1982, 123, 47–60. [Google Scholar] [CrossRef]
- Namba, H.; Masuda, M.; Kuroda, H. Electronic states of 2 × 1 reconstructed surfaces of diamond(111) studied by UPS and EELS. Appl. Surf. Sci. 1988, 33–34, 187–192. [Google Scholar] [CrossRef]
- Graupner, R.; Hollering, M.; Ziegler, A.; Ristein, J.; Ley, L.; Stampfl, A. Dispersions of surface states on diamond (100) and (111). Phys. Rev. B 1997, 55, 10841–10847. [Google Scholar] [CrossRef]
- Cardona, M. Resonance Phenomena. In Light Scattering in Solids II; Cardona, M., Guntherodt, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; Volume II, pp. 19–178. [Google Scholar]
- Popov, M. Pressure measurements from Raman spectra of stressed diamond anvils. J. Appl. Phys. 2004, 95, 5509–5514. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal--amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Shapeev, A.V. Moment Tensor Potentials: A Class of Systematically Improvable Interatomic Potentials. Multiscale Model. Simul. 2015, 14, 1153–1173. [Google Scholar] [CrossRef]
- Novikov, I.S.; Gubaev, K.; Podryabinkin, E.V.; Shapeev, A.V. The MLIP package: Moment tensor potentials with MPI and active learning. Mach. Learn. Sci. Technol. 2020, 2, 025002. [Google Scholar] [CrossRef]
- Schütt, A.K.T.; Kindermans, A.P.-J.; Sauceda, A.H.E.; Chmiela, A.S.; Tkatchenko, A.A.; Müller, A.K.-R. A continuous-filter convolutional neural network for modeling quantum interactions. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; pp. 992–1002. [Google Scholar]
- Botu, V.; Batra, R.; Chapman, J.; Ramprasad, R. Machine Learning Force Fields: Construction, Validation, and Outlook. J. Phys. Chem. C 2017, 121, 511–522. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, C.; Li, X.; Deng, Z.; Chen, Y.; Behler, J.; Csányi, G.; Shapeev, A.V.; Thompson, A.P.; Wood, M.A.; et al. Performance and Cost Assessment of Machine Learning Interatomic Potentials. J. Phys. Chem. A 2020, 124, 731–745. [Google Scholar] [CrossRef]
- Mortazavi, B.; Podryabinkin, E.V.; Novikov, I.S.; Rabczuk, T.; Zhuang, X.; Shapeev, A.V. Accelerating first-principles estimation of thermal conductivity by machine-learning interatomic potentials: A MTP/ShengBTE solution. Comput. Phys. Commun. 2021, 258, 107583. [Google Scholar] [CrossRef]
- Novikov, I. MLIP_Phonopy GitLab [Electronic Resource] GitLab. Available online: https://gitlab.com/ivannovikov/mlip_phonopy (accessed on 30 October 2022).
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Korepanov, V.I.; Hamaguchi, H.-o.; Osawa, E.; Ermolenkov, V.; Lednev, I.K.; Etzold, B.J.M.; Levinson, O.; Zousman, B.; Epperla, C.P.; Chang, H.-C. Carbon structure in nanodiamonds elucidated from Raman spectroscopy. Carbon 2017, 121, 322–329. [Google Scholar] [CrossRef]
- Khorobrykh, F.; Kulnitskiy, B.; Churkin, V.; Skryleva, E.; Parkhomenko, Y.; Zholudev, S.; Blank, V.; Popov, M. The effect of C60 fullerene polymerization processes on the mechanical properties of clusters forming ultrahard structures of 3D C60 polymers. Diam. Relat. Mater. 2022, 124, 108911. [Google Scholar] [CrossRef]
- Blank, V.D.; Buga, S.G.; Dubitsky, G.A.; Serebryanaya, N.R.; Sulyanov, S.N.; Popov, M.Y.; Denisov, V.N.; Ivlev, A.N.; Mavrin, B.N. Phase transformations in solid C60 at high pressure-high temperature treatment and structure of 3D polymerized fullerites. Phys. Lett. A 1996, 220, 149–157. [Google Scholar] [CrossRef]
- Popov, M.; Mordkovich, V.; Perfilov, S.; Kirichenko, A.; Kulnitskiy, B.; Perezhogin, I.; Blank, V. Synthesis of ultrahard fullerite with a catalytic 3D polymerization reaction of C60. Carbon 2014, 76, 250–256. [Google Scholar] [CrossRef]
- Piscanec, S.; Mauri, F.; Ferrari, A.C.; Lazzeri, M.; Robertson, J. Ab initio resonant Raman spectra of diamond-like carbons. Diam. Relat. Mater. 2005, 14, 1078–1083. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, L.; Mao, H.-k.; Chow, P.; Xiao, Y.; Baldini, M.; Shu, J.; Mao, W.L. Amorphous Diamond: A High-Pressure Superhard Carbon Allotrope. Phys. Rev. Lett. 2011, 107, 175504. [Google Scholar] [CrossRef]
- Pashkin, E.Y.; Pankov, A.M.; Kulnitskiy, B.A.; Perezhogin, I.A.; Karaeva, A.R.; Mordkovich, V.Z.; Popov, M.Y.; Sorokin, P.B.; Blank, V.D. The unexpected stability of multiwall nanotubes under high pressure and shear deformation. Appl. Phys. Lett. 2016, 109, 081904. [Google Scholar] [CrossRef]
- Yao, M.; Fan, X.; Zhang, W.; Bao, Y.; Liu, R.; Sundqvist, B.; Liu, B. Uniaxial-stress-driven transformation in cold compressed glassy carbon. Appl. Phys. Lett. 2017, 111, 101901. [Google Scholar] [CrossRef]
- Yang, X.; Yao, M.; Wu, X.; Liu, S.; Chen, S.; Yang, K.; Liu, R.; Cui, T.; Sundqvist, B.; Liu, B. Novel Superhard sp3 Carbon Allotrope from Cold-Compressed C70 Peapods. Phys. Rev. Lett. 2017, 118, 245701. [Google Scholar] [CrossRef]
- Shiell, T.B.; de Tomas, C.; McCulloch, D.G.; McKenzie, D.R.; Basu, A.; Suarez-Martinez, I.; Marks, N.A.; Boehler, R.; Haberl, B.; Bradby, J.E. In situ analysis of the structural transformation of glassy carbon under compression at room temperature. Phys. Rev. B 2019, 99, 024114. [Google Scholar] [CrossRef]
- Odake, S.; Zinin, P.V.; Hellebrand, E.; Prakapenka, V.; Liu, Y.; Hong, S.; Burgess, K.; Ming, L.-C. Formation of the high pressure graphite and BC8 phases in a cold compression experiment by Raman scattering. J. Raman Spectrosc. 2013, 44, 1596–1602. [Google Scholar] [CrossRef]
- Pankov, A.M.; Bredikhina, A.S.; Kulnitskiy, B.A.; Perezhogin, I.A.; Skryleva, E.A.; Parkhomenko, Y.N.; Popov, M.Y.; Blank, V.D. Transformation of multiwall carbon nanotubes to onions with layers cross-linked by sp3 bonds under high pressure and shear deformation. AIP Adv. 2017, 7, 085218. [Google Scholar] [CrossRef] [Green Version]
- Mao, W.L.; Mao, H.-k.; Eng, P.J.; Trainor, T.P.; Newville, M.; Kao, C.-c.; Heinz, D.L.; Shu, J.; Meng, Y.; Hemley, R.J. Bonding Changes in Compressed Superhard Graphite. Science 2003, 302, 425–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vladimir, D.B.; Valentin, D.; Churkin, B.; Kulnitskiy, I.; Perezhogin, A.; Kirichenko, N.; Denisov, V.N.; Erohin, S.V.; Sorokin, P.B.; Popov, M.Y. Phase diagram of carbon and the factors limiting the quantity and size of natural diamonds. Nanotechnology 2018, 29, 115603. [Google Scholar]
- Goncharov, A. Graphite under high-pressure-pseudomelting at 44 gpa. Zhurnal Eksperimentalnoi I Teor. Fiz. 1990, 98, 1824–1827. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, M.; Khorobrykh, F.; Klimin, S.; Churkin, V.; Ovsyannikov, D.; Kvashnin, A. Surface Tamm States of 2–5 nm Nanodiamond via Raman Spectroscopy. Nanomaterials 2023, 13, 696. https://doi.org/10.3390/nano13040696
Popov M, Khorobrykh F, Klimin S, Churkin V, Ovsyannikov D, Kvashnin A. Surface Tamm States of 2–5 nm Nanodiamond via Raman Spectroscopy. Nanomaterials. 2023; 13(4):696. https://doi.org/10.3390/nano13040696
Chicago/Turabian StylePopov, Mikhail, Fedor Khorobrykh, Sergei Klimin, Valentin Churkin, Danila Ovsyannikov, and Alexander Kvashnin. 2023. "Surface Tamm States of 2–5 nm Nanodiamond via Raman Spectroscopy" Nanomaterials 13, no. 4: 696. https://doi.org/10.3390/nano13040696
APA StylePopov, M., Khorobrykh, F., Klimin, S., Churkin, V., Ovsyannikov, D., & Kvashnin, A. (2023). Surface Tamm States of 2–5 nm Nanodiamond via Raman Spectroscopy. Nanomaterials, 13(4), 696. https://doi.org/10.3390/nano13040696





